Adenosine A2B Receptor Antagonism Interferes with TGF-β Cellular Signaling Through SMAD2/-3 and p65-Nf-κB in Podocytes and Protects from Phenotypical Transformation in Experimental Diabetic Glomerulopathy
Abstract
1. Introduction
2. Materials and Methods
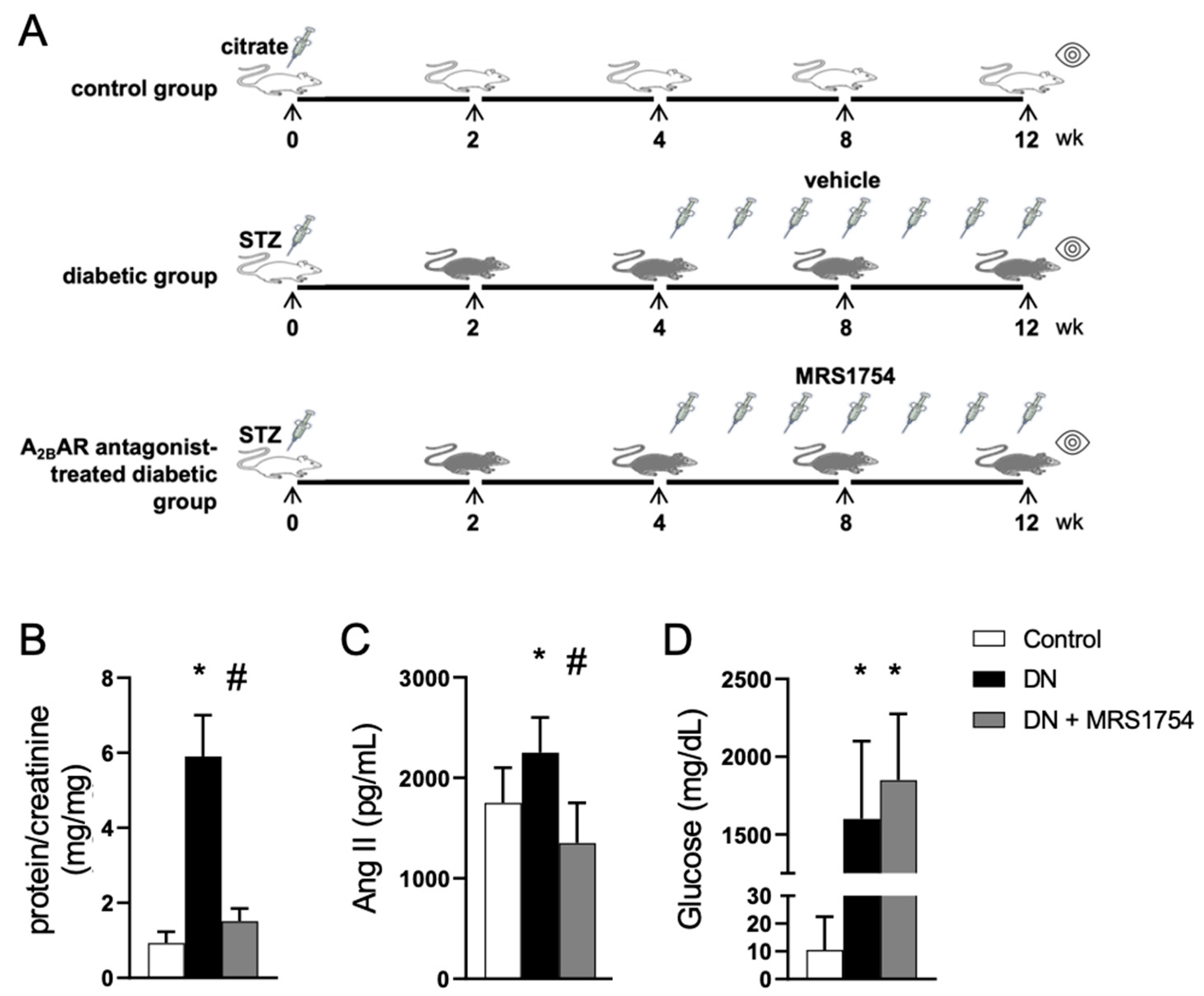
2.1. Experimental Diabetic Nephropathy
2.2. Urine Assays
2.3. Glomerulus Isolation
2.4. Histological Analysis
2.5. Purification of Cell Fractions
2.6. Quantification of MCP-1 and CCL3
2.7. Cell Culture and Treatments
2.8. Western Blots
2.9. Chromatin Immunoprecipitation (ChIP)
2.10. Transcriptomic Analysis
2.11. Statistics
3. Results
3.1. The Antagonism of A2BAR Attenuates Renal Alterations in Experimental DN
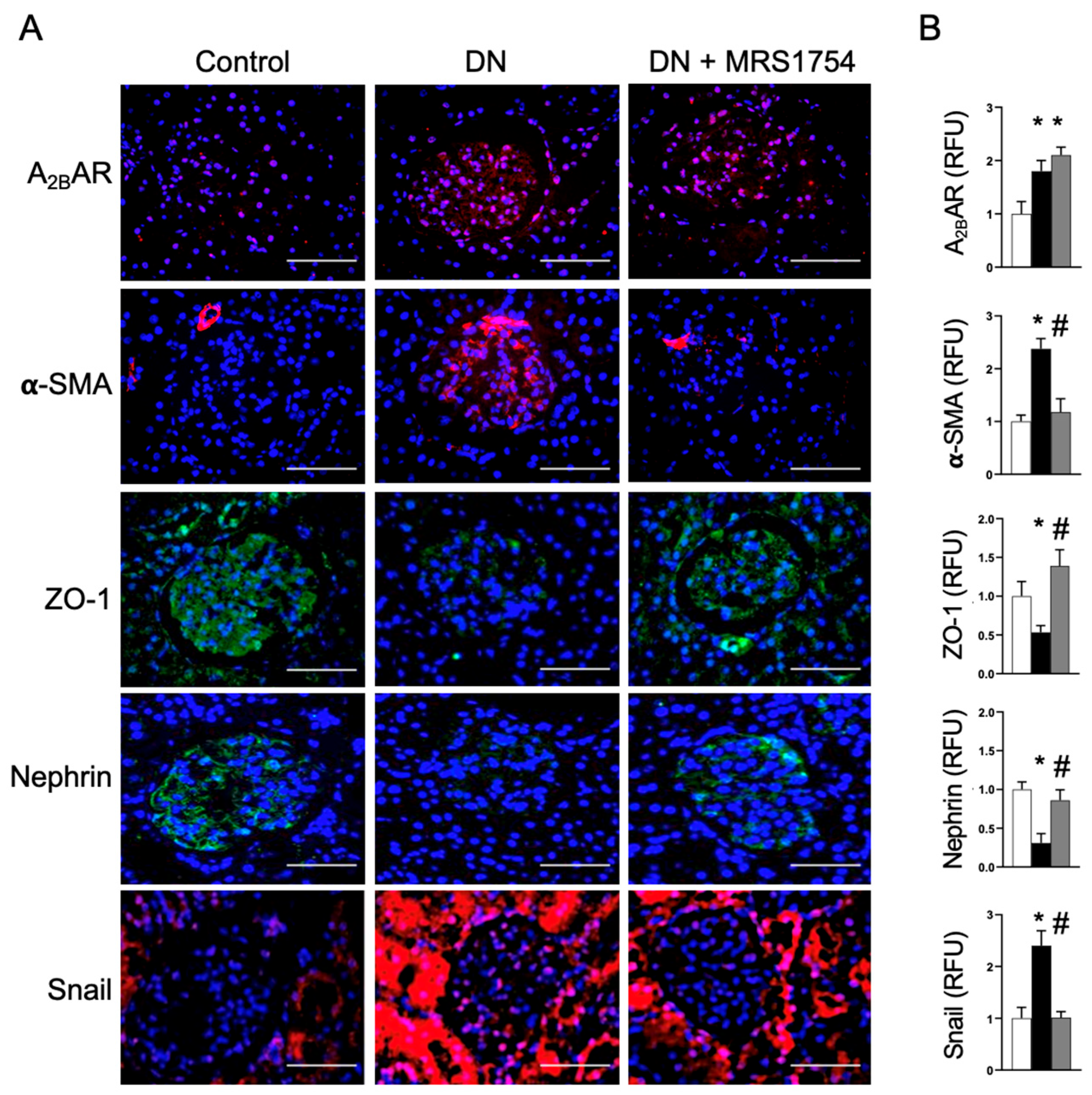
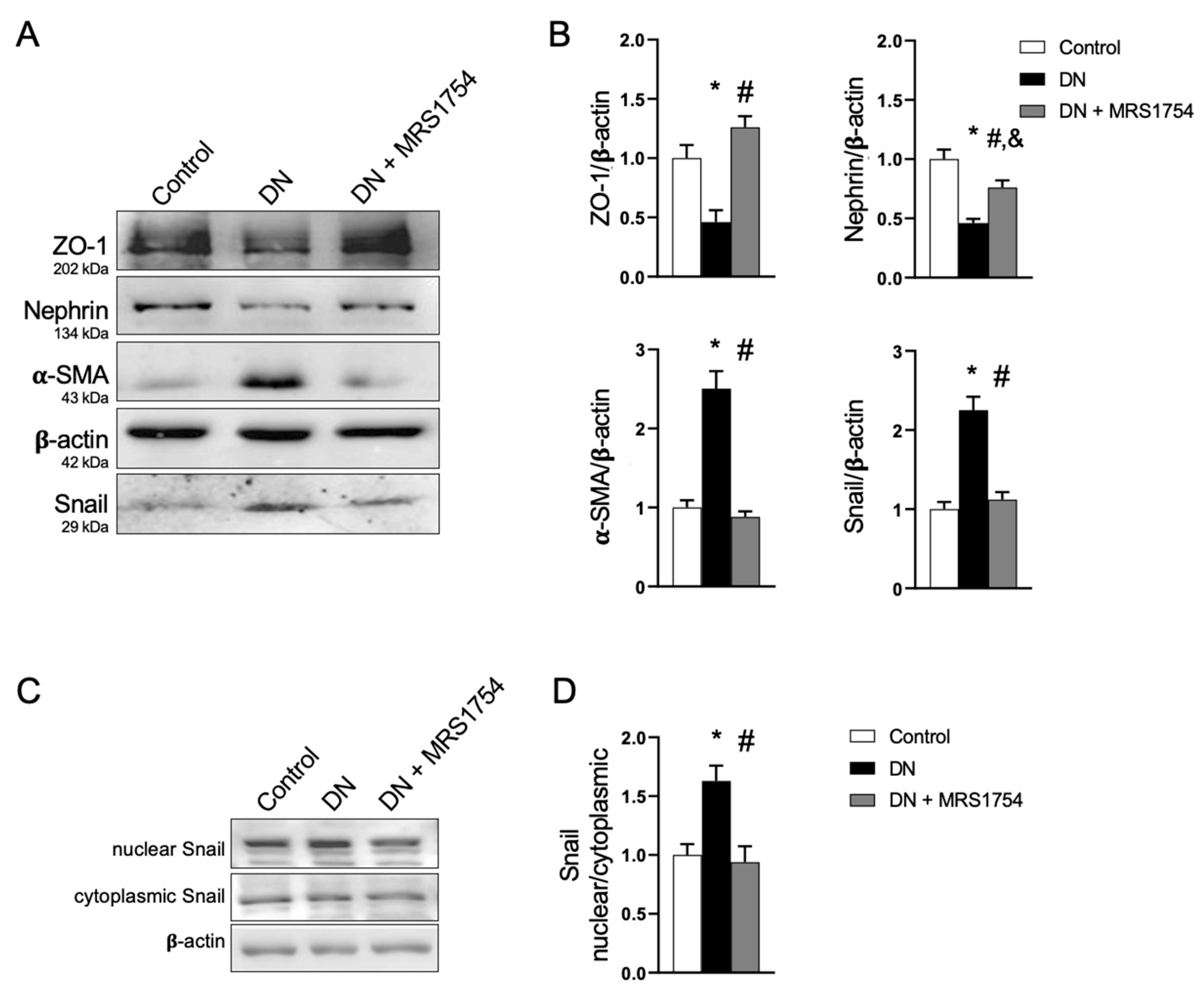
3.2. The Antagonism of A2BAR Attenuates Podocyte Dedifferentiation
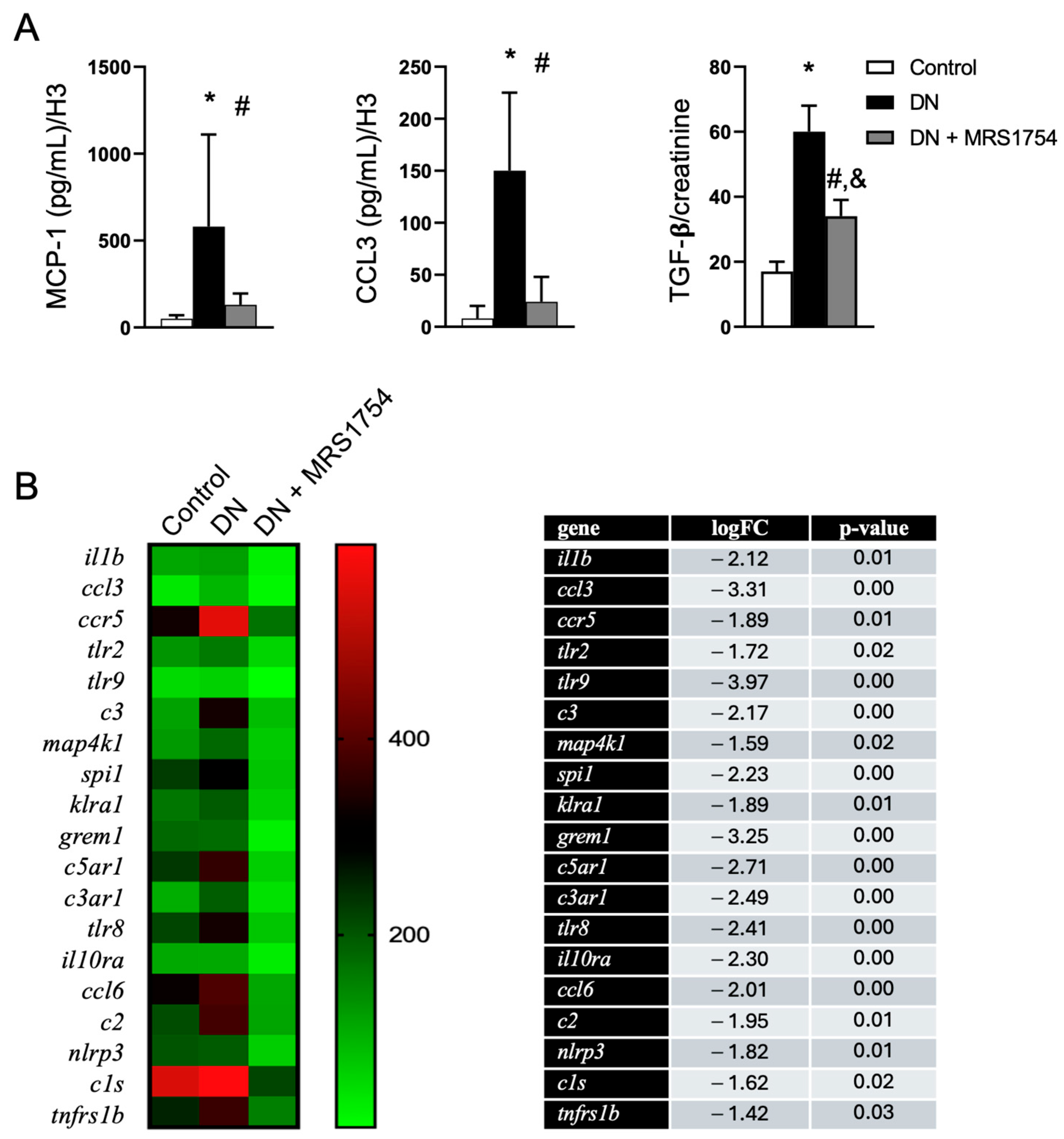
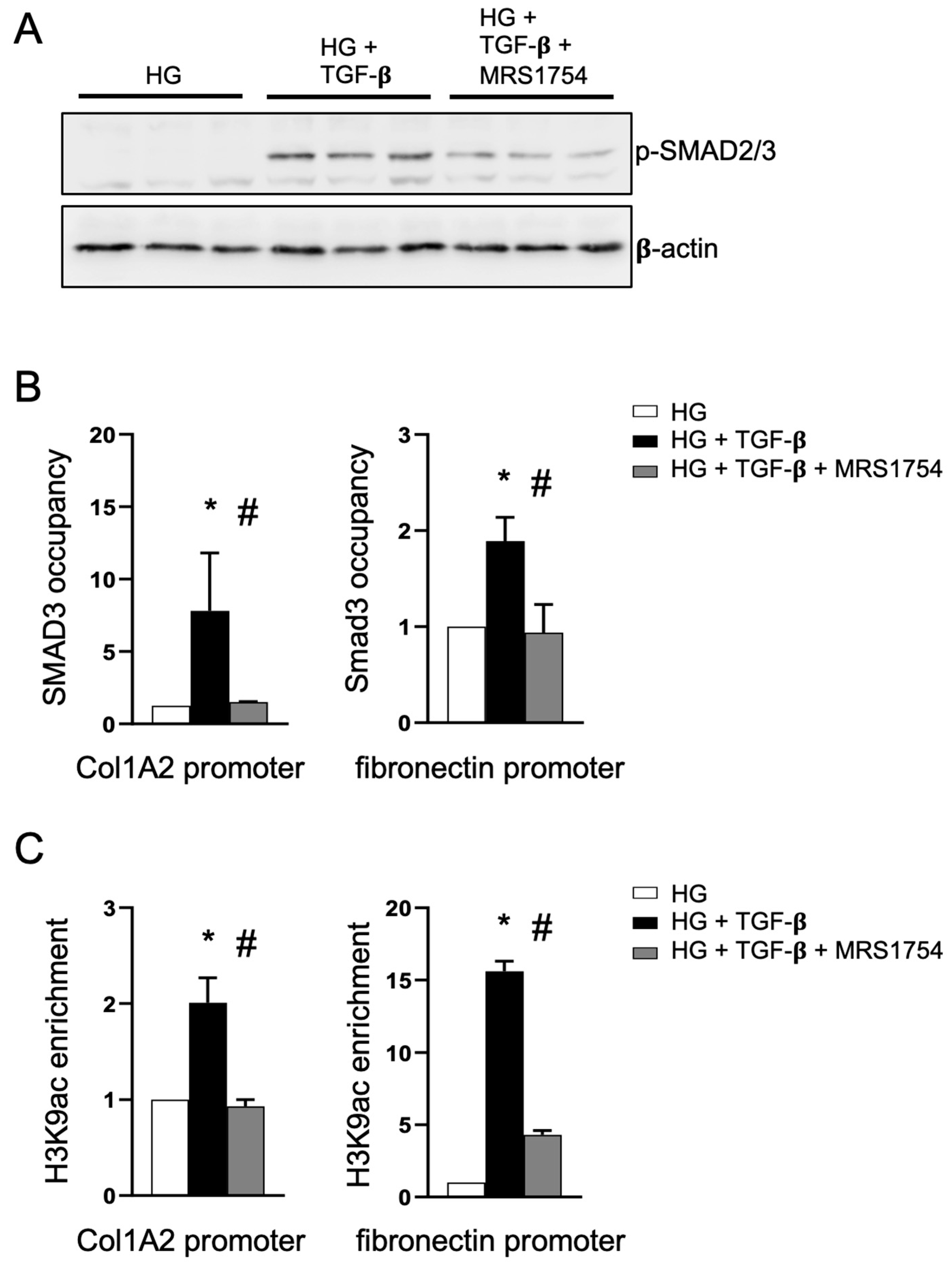
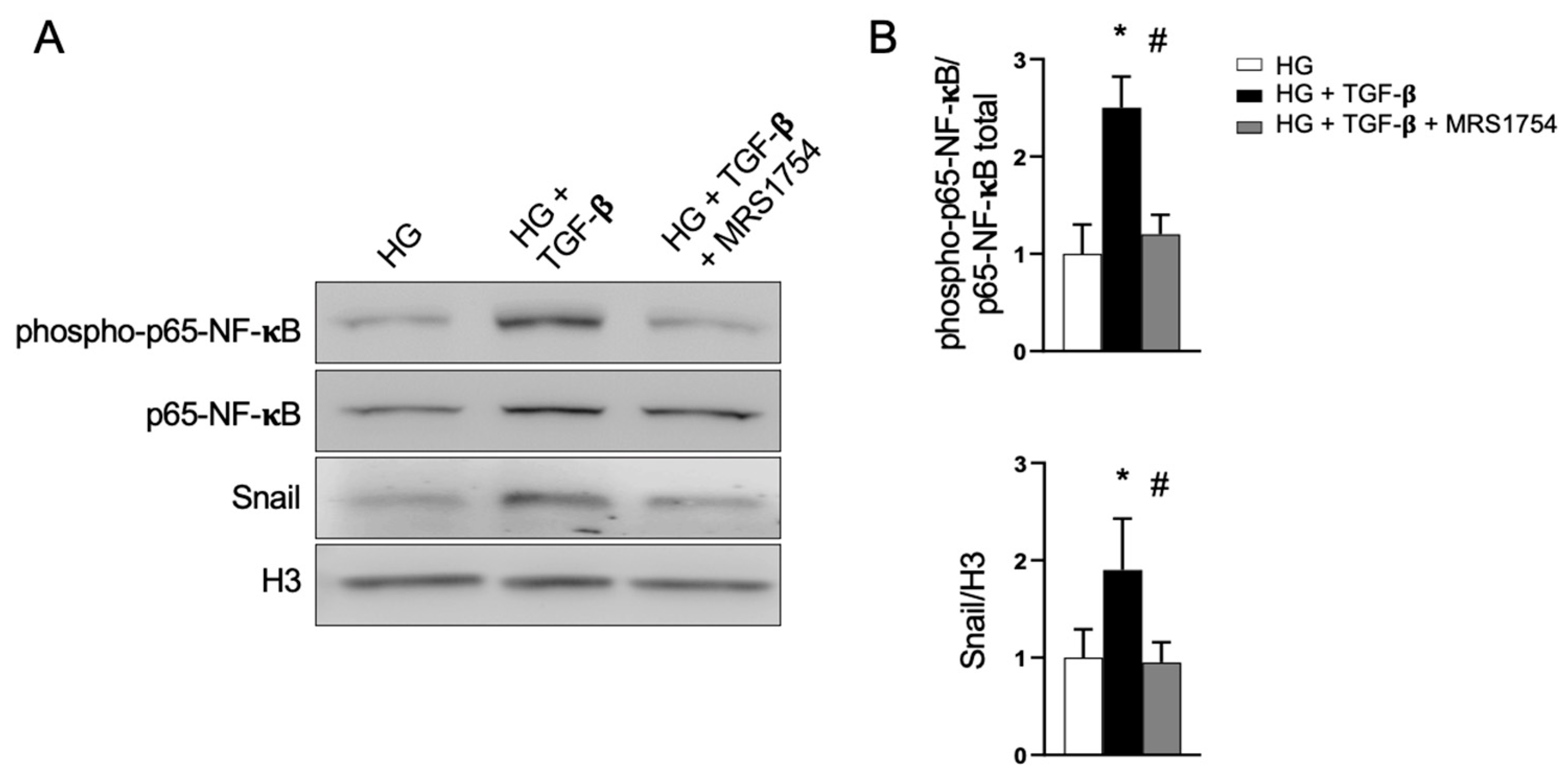
3.3. The Antagonism of A2BAR Blocks TGF-β Signaling in Podocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A2BAR | Adenosine A2B receptor |
| DN | Diabetic nephropathy |
| RAS | Renin–angiotensin system |
| α-SMA | Alfa-smooth muscle actin |
| STZ | Streptozotocin |
| TGF-β | Transforming growth factor beta 1 |
| NF-κB | Nuclear factor kappa B |
| SMAD | Small mother against decapentaplegic |
| ZO-1 | Zonula occludens 1 |
| IL-1β | Interleukin 1 beta |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MAPK | Mitogen-activated protein kinase |
| KDIGO | Kidney Disease: Improving Global Outcomes |
References
- Tuttle, K.R.; Agarwal, R.; Alpers, C.E.; Bakris, G.L.; Brosius, F.C.; Kolkhof, P.; Uribarri, J. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022, 102, 248–260. [Google Scholar] [CrossRef]
- Nath, K.A. The tubulointerstitium in progressive renal disease. Kidney Int. 1998, 54, 992–994. [Google Scholar] [CrossRef]
- Duffield, J.S. Cellular and Molecular Mechanisms in Kidney Fibrosis. J. Clin. Invest. 2014, 124, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Oyarzún, C.; Garrido, W.; Alarcón, S.; Yáñez, A.; Sobrevia, L.; Quezada, C.; San Martín, R. Adenosine contribution to normal renal physiology and chronic kidney disease. Mol. Asp. Med. 2017, 55, 75–89. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Taduri, G.; O’Connell, J.; Teng, Y.; Cooke, V.G.; Woda, C.; Sugimoto, H.; Kalluri, R. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 2013, 19, 1047–1053. [Google Scholar] [CrossRef]
- Grande, M.T.; Sánchez-Laorden, B.; López-Blau, C.; De Frutos, C.A.; Boutet, A.; Arévalo, M.; Rowe, R.G.; Weiss, S.J.; López-Novoa, J.M.; Nieto, M.A. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med. 2015, 21, 989–997. [Google Scholar] [CrossRef]
- Torres-Arévalo, Á.; Nahuelpán, Y.; Muñoz, K.; Jara, C.; Cappelli, C.; Taracha-Wiśniewska, A.; Quezada-Monrás, C.; Martín, R.S. A2BAR Antagonism Decreases the Glomerular Expression and Secretion of Chemoattractants for Monocytes and the Pro-Fibrotic M2 Macrophages Polarization during Diabetic Nephropathy. Int. J. Mol. Sci. 2023, 24, 10829. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.A.; Welsh, G.I.; Saleem, M.A.; Menon, R.K. Molecular and Cellular Events Mediating Glomerular Podocyte Dysfunction and Depletion in Diabetes Mellitus. Front. Endocrinol. (Lausanne) 2014, 5, 151. [Google Scholar]
- Li, J.J.; Kwak, S.J.; Jung, D.S.; Kim, J.J.; Yoo, T.H.; Ryu, D.R.; Han, S.H.; Choi, H.Y.; Lee, J.E.; Moon, S.J.; et al. Podocyte biology in diabetic nephropathy. Kidney Int. Suppl. 2007, 106, S36–S42. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Iwano, M.; Suzuki, D.; Nakatani, K.; Kimura, K.; Harada, K.; Kubo, A.; Akai, Y.; Toyoda, M.; Kanauchi, M.; et al. Epithelial-mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am. J. Kidney Dis. 2009, 54, 653–664. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.L.; Liu, T.T.; Lan, H.Y. TGF-Beta as a Master Regulator of Diabetic Nephropathy. Int. J. Mol. Sci. 2021, 22, 7881. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nakamura, T.; Noble, N.A.; Ruoslahti, E.; Border, W.A. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1993, 90, 1814–1818. [Google Scholar] [CrossRef]
- Park, I.S.; Kiyomoto, H.; Abboud, S.L.; Abboud, H.E. Expression of transforming growth factor-beta and type IV collagen in early streptozotocin-induced diabetes. Diabetes 1997, 46, 473–480. [Google Scholar] [CrossRef]
- Herman-Edelstein, M.; Thomas, M.C.; Thallas-Bonke, V.; Saleem, M.; Cooper, M.E.; Kantharidis, P. Dedifferentiation of immortalized human podocytes in response to transforming growth factor-β: A model for diabetic podocytopathy. Diabetes 2011, 60, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, X.; Xiao, W.; Fu, J.; Harris, R.C.; Lindenmeyer, M.; Cohen, C.D.; Guillot, N.; Baron, M.H.; Wang, N.; et al. BAMBI elimination enhances alternative TGF-beta signaling and glomerular dysfunction in diabetic mice. Diabetes 2015, 64, 2220–2233. [Google Scholar] [CrossRef]
- Chang, A.S.; Hathaway, C.K.; Smithies, O.; Kakoki, M. Transforming growth factor-β1 and diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2016, 310, F689–F696. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Liu, X.S.; Lan, H.Y. Therapeutic potential for renal fibrosis by targeting Smad3-dependent noncoding RNAs. Mol. Ther. 2024, 32, 313–324. [Google Scholar] [CrossRef]
- Tang, P.M.; Zhang, Y.Y.; Mak, T.S.; Tang, P.C.; Huang, X.R.; Lan, H.Y. Transforming growth factor-β signalling in renal fibrosis: From Smads to non-coding RNAs. J. Physiol. 2018, 596, 3493–3503. [Google Scholar] [CrossRef]
- Du, M.; Wang, Q.; Li, W.; Ma, X.; Wu, L.; Guo, F.; Zhao, S.; Huang, F.; Wang, H.; Qin, G. Overexpression of FOXO1 ameliorates the podocyte epithelial-mesenchymal transition induced by high glucose in vitro and in vivo. Biochem. Biophys. Res. Commun. 2016, 471, 416–422. [Google Scholar] [CrossRef]
- Lai, H.; Chen, A.; Cai, H.; Fu, J.; Salem, F.; Li, Y.; He, J.C.; Schlondorff, D.; Lee, K. Podocyte and endothelial-specific elimination of BAMBI identifies differential transforming growth factor-β pathways contributing to diabetic glomerulopathy. Kidney Int. 2020, 98, 601–614. [Google Scholar] [CrossRef]
- Williams, B.M.; Cliff, C.L.; Lee, K.; Squires, P.E.; Hills, C.E. The Role of the NLRP3 Inflammasome in Mediating Glomerular and Tubular Injury in Diabetic Nephropathy. Front. Physiol. 2022, 13, 907504. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 493–503. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Z.; Fu, J.; Fang, Z.; Zhang, W.; He, J.C.; Lee, K. LRG1 loss effectively restrains glomerular TGF-β signaling to attenuate diabetic kidney disease. Mol. Ther. 2024, 32, 3177–3193. [Google Scholar] [CrossRef] [PubMed]
- Suarez, R.; Villarreal, C.; Nahuelpán, Y.; Jara, C.; Oyarzún, C.; Alarcón, S.; Díaz-Encarnación, M.M.; Guillén-Gómez, E.; Quezada, C.; San Martín, R. Defective insulin-stimulated equilibrative nucleoside transporter-2 activity and altered subcellular transporter distribution drive the loss of adenosine homeostasis in diabetic kidney disease progression. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166890. [Google Scholar] [CrossRef]
- Mendoza-Soto, P.; Jara, C.; Torres-Arévalo, Á.; Oyarzún, C.; Mardones, G.A.; Quezada-Monrás, C.; San Martín, R. Pharmacological Blockade of the Adenosine A2B Receptor Is Protective of Proteinuria in Diabetic Rats, through Affecting Focal Adhesion Kinase Activation and the Adhesion Dynamics of Podocytes. Cells 2024, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.F.; Liang, Q.L.; Hu, P.; Wang, Y.M.; Li, P.; Luo, G.A. Correlations of six related purine metabolites and diabetic nephropathy in Chinese type 2 diabetic patients. Clin. Biochem. 2009, 42, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liang, Q.; Li, P.; Xia, J.; Wang, Y.; Hu, P.; Jiang, Z.; He, Y.; Pang, L.; Han, L.; et al. Biomarkers for early diagnosis of type 2 diabetic nephropathy: A study based on an integrated biomarker system. Mol. Biosyst. 2013, 9, 2134–2141. [Google Scholar] [CrossRef]
- Pak, E.S.; Cha, J.J.; Cha, D.R.; Kanasaki, K.; Ha, H. Adenosine receptors as emerging therapeutic targets for diabetic kidney disease. Kidney Res. Clin. Pract. 2022, 41, S74–S88. [Google Scholar] [CrossRef]
- Alarcón, S.; Garrido, W.; Vega, G.; Cappelli, C.; Suárez, R.; Oyarzún, C.; Quezada, C.; San Martín, R. Deficient Insulin-mediated Upregulation of the Equilibrative Nucleoside Transporter 2 Contributes to Chronically Increased Adenosine in Diabetic Glomerulopathy. Sci. Rep. 2017, 7, 9439. [Google Scholar] [CrossRef]
- Huang, D.Y.; Vallon, V.; Zimmermann, H.; Koszalka, P.; Schrader, J.; Osswald, H. Ecto-5’-nucleotidase (cd73) dependent and independent generation of adenosine participates in the mediation of tubuloglomerular feedback in vivo. Am. J. Physiol. Ren. Physiol. 2006, 291, F282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roberts, V.S.; Cowan, P.J.; Alexander, S.I.; Robson, S.C.; Dwyer, K.M. The role of adenosine receptors A2A and A2B signaling in renal fibrosis. Kidney Int. 2014, 86, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Torres, Á.; Muñoz, K.; Nahuelpán, Y.; RSaez, A.P.; Mendoza, P.; Jara, C.; Cappelli, C.; Suarez, R.; Oyarzún, C.; Quezada, C.; et al. Intraglomerular Monocyte/Macrophage Infiltration and Macrophage-Myofibroblast Transition during Diabetic Nephropathy Is Regulated by the A(2B) Adenosine Receptor. Cells 2020, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.A.; O’Hare, M.J.; Reiser, J.; Coward, R.J.; Inward, C.D.; Farren, T.; Xing, C.Y.; Ni, L.; Mathieson, P.W.; Mundel, P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002, 13, 630–638. [Google Scholar] [CrossRef]
- Hatzis, P.; Talianidis, I. Dynamics of enhancer-promoter communication during differentiation-induced gene activation. Mol. Cell 2002, 10, 1467–1477. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Singh, R.M.; Howarth, F.C.; Adeghate, E.; Bidasee, K.; Singh, J.; Waqar, T. Type 1 diabetes mellitus induces structural changes and molecular remodelling in the rat kidney. Mol. Cell. Biochem. 2018, 449, 9–25. [Google Scholar] [CrossRef]
- Naaman, S.C.; Bakris, G.L. Diabetic Nephropathy: Update on Pillars of Therapy Slowing Progression. Diabetes Care 2023, 46, 1574–1586. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef]
- Evans, J.V.; Suman, S.; Goruganthu, M.U.L.; Tchekneva, E.E.; Guan, S.; Arasada, R.R.; Antonucci, A.; Piao, L.; Ilgisonis, I.; Bobko, A.A.; et al. Improving combination therapies: Targeting A2B adenosine receptor to modulate metabolic tumor microenvironment and immunosuppression. J. Natl. Cancer Inst. 2023, 115, 1404–1419. [Google Scholar] [CrossRef]
- Voelker, J.; Berg, P.H.; Sheetz, M.; Duffin, K.; Shen, T.; Moser, B.; Greene, T.; Blumenthal, S.S.; Rychlik, I.; Yagil, Y.; et al. Anti-TGF-beta1 Antibody Therapy in Patients with Diabetic Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Yao, J.; Caruana, G.; Ricardo, S.D.; Yamamoto, Y.; Yamamoto, H.; Bertram, J.F. Blockade of Endothelial-Mesenchymal Transition by a Smad3 Inhibitor Delays the Early Development of Streptozotocin-Induced Diabetic Nephropathy. Diabetes 2010, 59, 2612–2624. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.-M.; Huang, X.R.; Lan, H.Y. The preventive and therapeutic implication for renal fibrosis by targetting TGF-β/Smad3 signaling. Clin. Sci. (Lond.) 2018, 132, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Zhu, H.; Song, W.; Su, J.H. Gremlin Regulates Podocyte Apoptosis via Transforming Growth Factor-β (TGF-β) Pathway in Diabetic Nephropathy. Med. Sci. Monit. 2018, 24, 183–189. [Google Scholar] [CrossRef]
- Wang, A.; Ziyadeh, F.N.; Lee, E.Y.; Pyagay, P.E.; Sung, S.H.; Sheardown, S.A.; Laping, N.J.; Chen, S. Interference with TGF-beta signaling by Smad3- knockout in mice limits diabetic glomerulosclerosis without affecting albuminuria. Am. J. Physiol. Ren. Physiol. 2007, 293, F1657–F1665. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Sinniah, R.; Hsu, S.I. In situ glomerular expression of activated NF-κB in human lupus nephritis and other non-proliferative proteinuric glomerulopathy. Virchows Arch. 2006, 448, 172–183. [Google Scholar] [CrossRef]
- Yamashita, M.; Yoshida, T.; Suzuki, S.; Homma, K.; Hayashi, M. Podocyte-specific NF-κB inhibition ameliorates proteinuria in adriamycin-induced nephropathy in mice. Clin. Exp. Nephrol. 2017, 21, 16–26. [Google Scholar] [CrossRef]
- Ren, N.; Wang, W.F.; Zou, L.; Zhao, Y.L.; Miao, H.; Zhao, Y.Y. The nuclear factor kappa B signaling pathway is a master regulator of renal fibrosis. Front. Pharmacol. 2024, 14, 1335094. [Google Scholar] [CrossRef]
- Luo, K. Signaling Cross Talk between TGF-β/Smad and Other Signaling Pathways. Cold Spring Harb. Perspect. Biol. 2017, 9, a022137. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, J.; Qiao, S.; Yang, Y.; Yun, C.; Li, Y.; Wang, F. Salvianolic acid B attenuates diabetic nephropathy through alleviating ADORA2B, NALP3 in flammasome, and NFκB activity. Can. J. Physiol. Pharmacol. 2024, 102, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.I.; Park, A.S.; Mohtat, D.; Thomas, D.B.; Pullman, J.M.; Susztak, K. Transcriptome analysis of human diabetic kidney disease. Diabetes 2011, 60, 2354–2369. [Google Scholar] [CrossRef]
- Tang, S.C.W.; Yiu, W.H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; Ziemann, M.; Thallas-Bonke, V.; Snelson, M.; Kumar, V.; Laskowski, A.; Nguyen, T.V.; Huynh, K.; Clarke, M.V.; Libianto, R.; et al. Complement C5a induces renal injury in diabetic kidney disease by disrupting mitochondrial metabolic agility. Diabetes 2020, 69, 83–98. [Google Scholar] [CrossRef]
- Lavoz, C.; Alique, M.; Rodrigues-Diez, R.; Pato, J.; Keri, G.; Mezzano, S.; Egido, J.; Ruiz-Ortega, M. Gremlin regulates renal inflammation via the vascular endothelial growth factor receptor 2 pathway. J. Pathol. 2015, 236, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, Y.; Wada, J.; Malakauskas, S.M.; Liu, M.; Ren, Y.; Du, C.; Duan, H.; Li, Y.; Li, Y.; et al. In vivo delivery of Gremlin siRNA plasmid reveals therapeutic potential against diabetic nephropathy by recovering bone morphogenetic protein-7. PLoS ONE 2010, 5, e11709. [Google Scholar] [CrossRef][Green Version]
- Higgins, D.F.; Ewart, L.M.; Masterson, E.; Tennant, S.; Grebnev, G.; Prunotto, M.; Pomposiello, S.; Conde-Knape, K.; Martin, F.M.; Godson, C. BMP7-induced-Pten inhibits Akt and prevents renal fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3095–3104. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, I.; Jara, C.; Mendoza-Soto, P.; Nahuelpán, Y.; Cappelli, C.; Oyarzún, C.; Carrillo-Beltrán, D.; Quezada-Monrás, C.; Torres-Arévalo, A.; San Martín, R. Adenosine A2B Receptor Antagonism Interferes with TGF-β Cellular Signaling Through SMAD2/-3 and p65-Nf-κB in Podocytes and Protects from Phenotypical Transformation in Experimental Diabetic Glomerulopathy. Cells 2025, 14, 890. https://doi.org/10.3390/cells14120890
Arias I, Jara C, Mendoza-Soto P, Nahuelpán Y, Cappelli C, Oyarzún C, Carrillo-Beltrán D, Quezada-Monrás C, Torres-Arévalo A, San Martín R. Adenosine A2B Receptor Antagonism Interferes with TGF-β Cellular Signaling Through SMAD2/-3 and p65-Nf-κB in Podocytes and Protects from Phenotypical Transformation in Experimental Diabetic Glomerulopathy. Cells. 2025; 14(12):890. https://doi.org/10.3390/cells14120890
Chicago/Turabian StyleArias, Ignacio, Claudia Jara, Pablo Mendoza-Soto, Yessica Nahuelpán, Claudio Cappelli, Carlos Oyarzún, Diego Carrillo-Beltrán, Claudia Quezada-Monrás, Angelo Torres-Arévalo, and Rody San Martín. 2025. "Adenosine A2B Receptor Antagonism Interferes with TGF-β Cellular Signaling Through SMAD2/-3 and p65-Nf-κB in Podocytes and Protects from Phenotypical Transformation in Experimental Diabetic Glomerulopathy" Cells 14, no. 12: 890. https://doi.org/10.3390/cells14120890
APA StyleArias, I., Jara, C., Mendoza-Soto, P., Nahuelpán, Y., Cappelli, C., Oyarzún, C., Carrillo-Beltrán, D., Quezada-Monrás, C., Torres-Arévalo, A., & San Martín, R. (2025). Adenosine A2B Receptor Antagonism Interferes with TGF-β Cellular Signaling Through SMAD2/-3 and p65-Nf-κB in Podocytes and Protects from Phenotypical Transformation in Experimental Diabetic Glomerulopathy. Cells, 14(12), 890. https://doi.org/10.3390/cells14120890






