The Consequence of the Presence of Ribonucleotide for ds-DNA’s Electronic Properties: Preliminary Theoretical Studies
Abstract
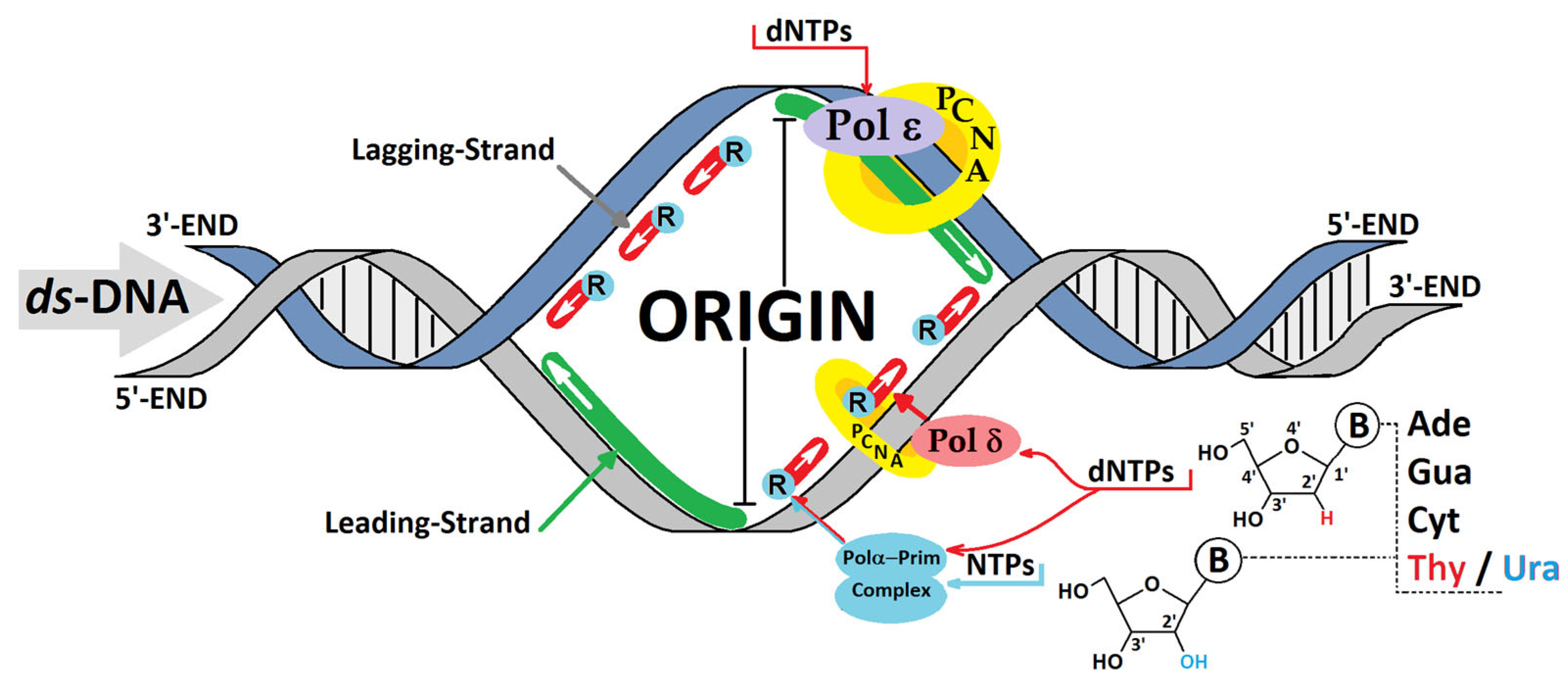
1. Introduction
2. Materials and Methods
3. Results and Discussion
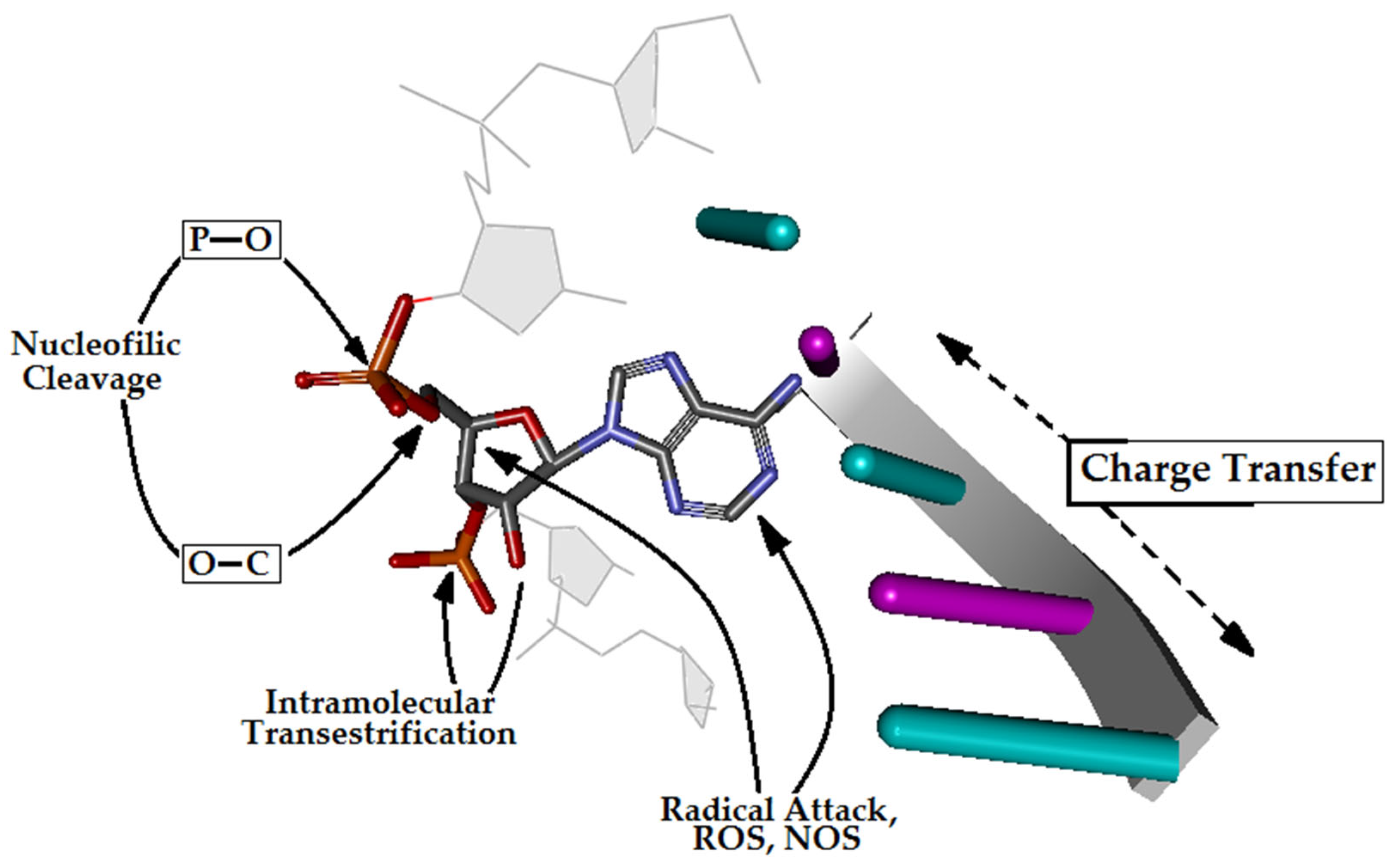
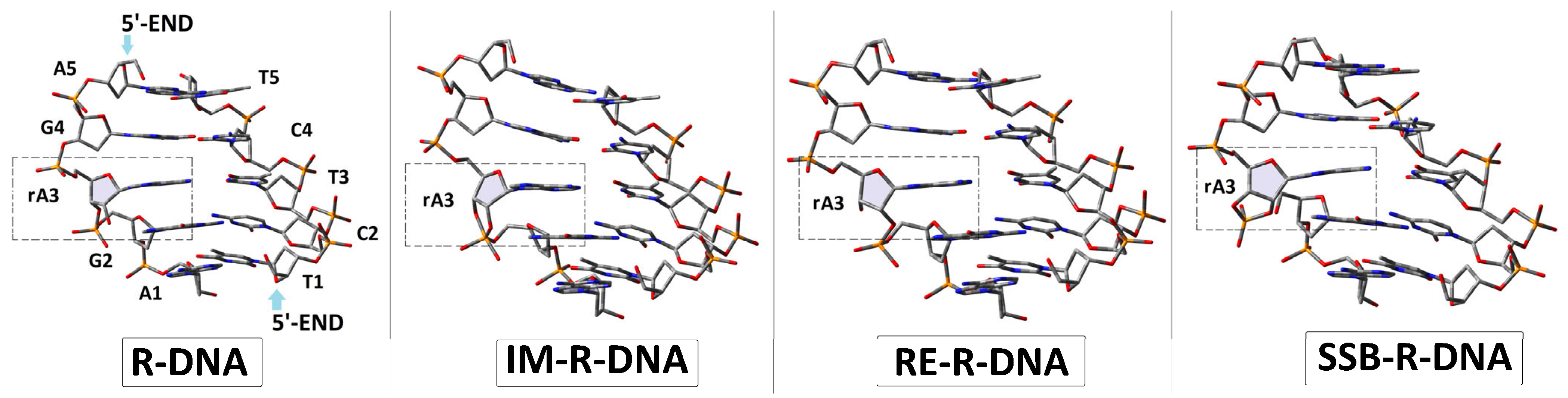
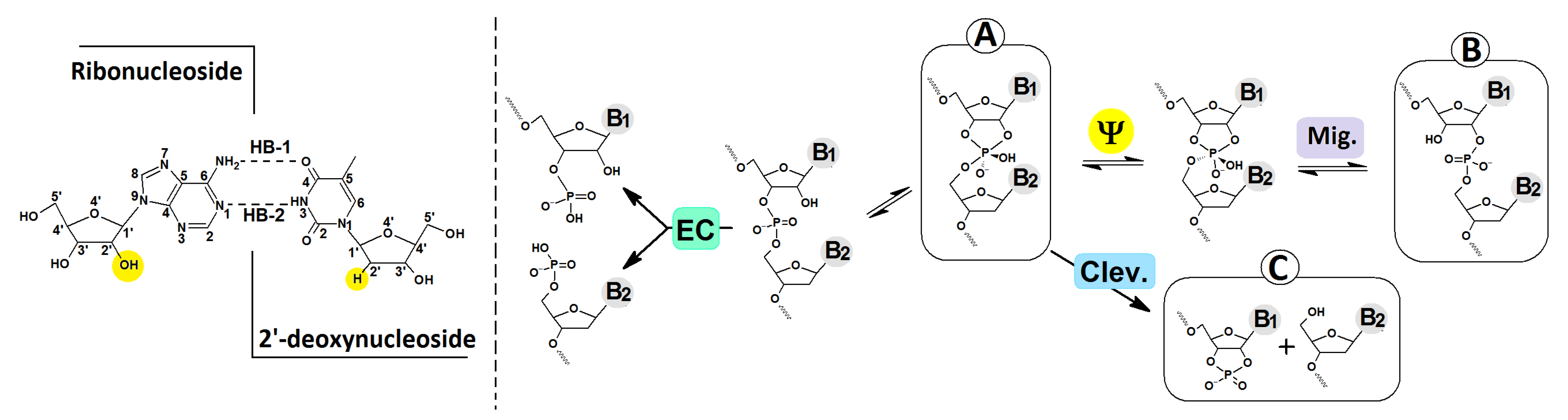
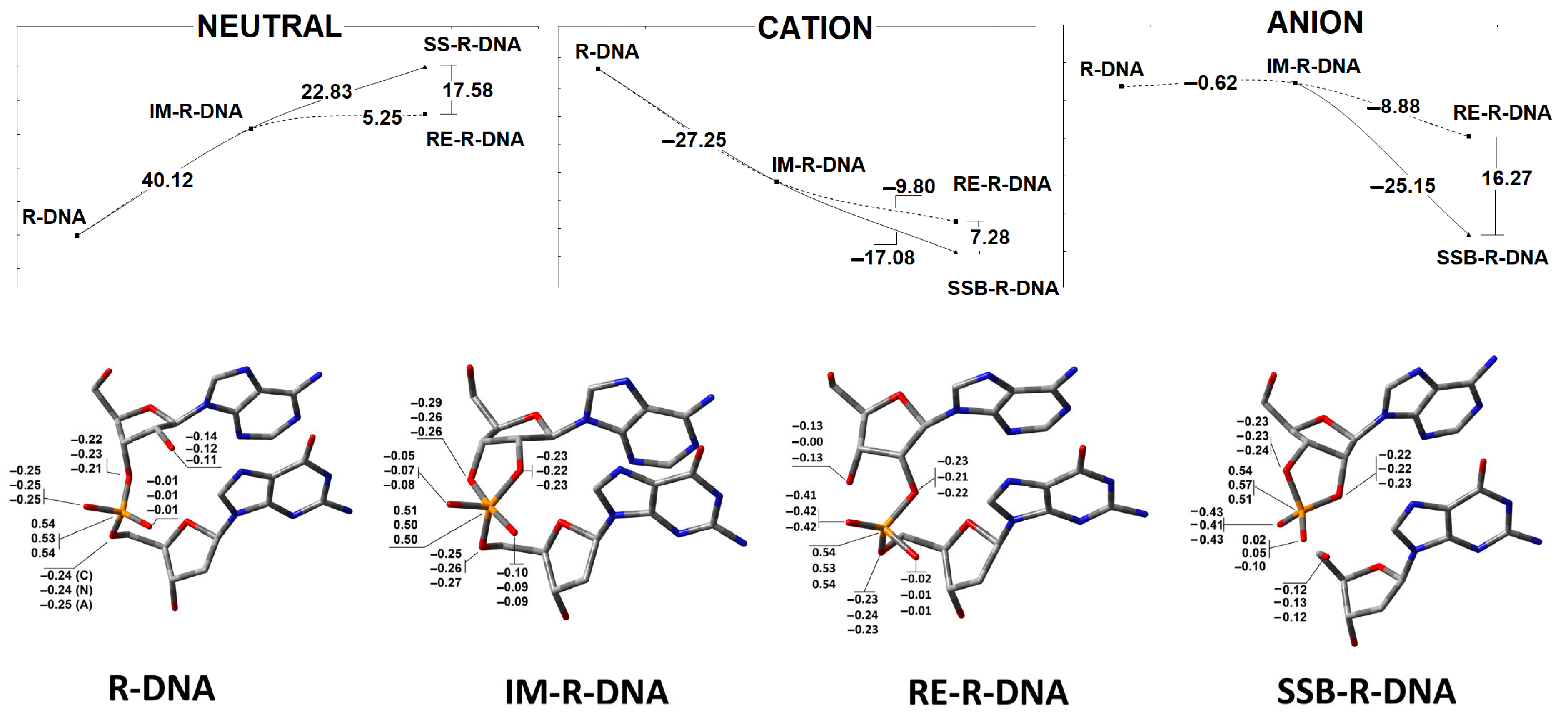
3.1. The Mechanistic Aspect or Internucleotide Phosphodiester Bond Cleavage and Migration
3.2. The Influence of a Ribonucleoside on ds-DNA’s Electronic Properties
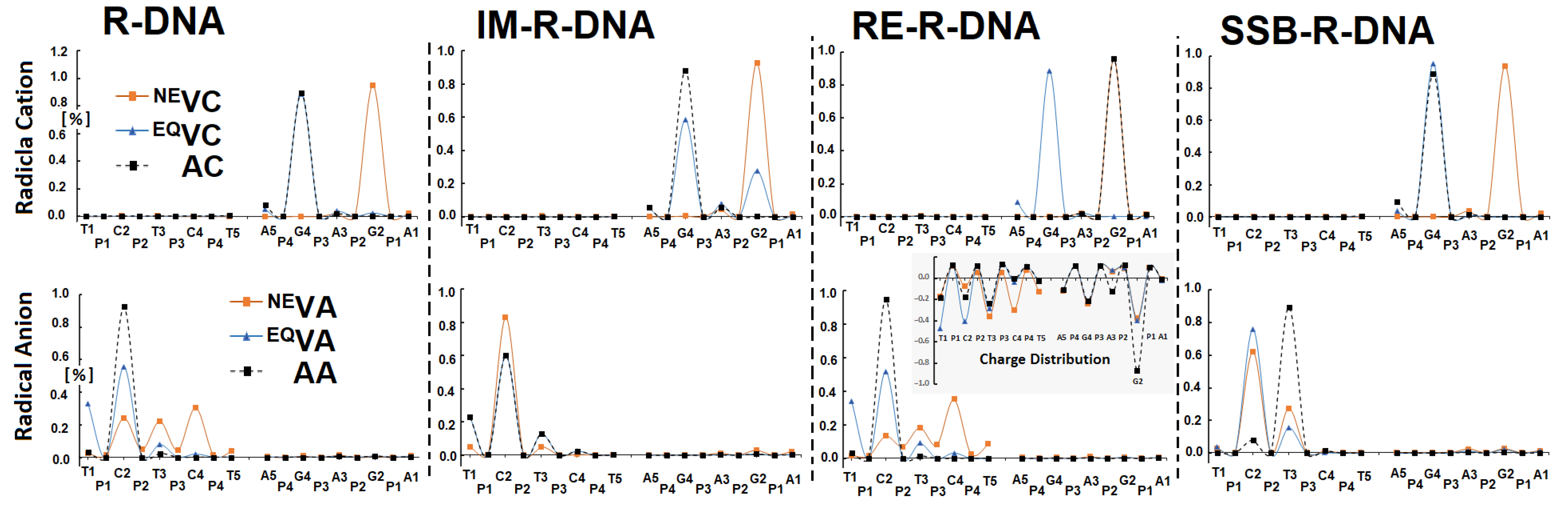
3.3. The Influence of Ribonucleoside’s Presence in ds-DNA on the Charge, Spin and Distribution
4. Conclusions
- The stability of the phosphodiester internucleotide bond between the ribonucleotide moiety and DNA decreases after a hole or electron appears in the ds-DNA structure, in comparison to the neutral form.
- Analysis of the electronic properties of an adiabatic radical cation and anion revealed the following order of the calculated AIP and AEA: →R-DNA > IM-R-DNA > RE-R-DNA > SSB-DNA and SSB-R-DNA > RE-R-DNA > IM-R-DNA > R-DNA, respectively. These results indicate that the formation of any structures other than the canonical 3′-5′ linkage causes the formation of an endpoint for the transfer of a hole (radical cation) or an extra electron (radical anion) through the double helix.
- The analysis of the Hirshfeld charge distribution showed that, in the non-equilibrated solvent–solute interaction mode, vertical cations accumulate exclusively on one nucleoside; namely, 2′-deoxyguanosine (G2). Further radical cation relaxation and adiabatic state achievement by the discussed ds-oligos elucidated the 2′-deoxyguanosine (G4) as the endpoint of the hole migration (except in R-DNA, where G2 was found to be more susceptible to radical cation adoption).
- The appearance of an additional electron in the double helix causes the non-equilibrated vertical radical anion state, where the spin is dispersed over three pyrimidines (C2, T3, and C4) of R-DNA and RE-R-DNA or two pyrimidines (C2 and T3) of IM-R-DNA and SSB-R-DNA. Relaxation of the solvent–solute interaction leads to the accumulation of unpaired electrons at the C2 moiety. The subsequent structure relaxation (adiabatic radical anion state) results in the spin being localised exclusively at 2′-deoxycytidine (C2), except for in SSB-R-DNA, where it accumulates at thymidine (T3).
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Diez-Castellnou, M.; Martinez, A.; Mancin, F. Phosphate Ester Hydrolysis: The Path From Mechanistic Investigation to the Realization of Artificial Enzymes. Adv. Phys. Org. Chem. 2017, 51, 129–186. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Shrinivas, S.A.; Shanta, S.H.; Prajakta, B.B. DNA: Damage and Repair Mechanisms in Humans. Glob. J. Pharm. Pharm. Sci. 2017, 3, 555613. [Google Scholar] [CrossRef][Green Version]
- Sancar, A.; Lindsey-Boltz, L.A.; Ünsal-Kaçmaz, K.; Linn, S. Molecular Mechanisms of Mammalian DNA Repair and the DNA Damage Checkpoints. Annu. Rev. Biochem. 2004, 73, 39–85. [Google Scholar] [CrossRef]
- Hegde, M.L.; Hazra, T.K.; Mitra, S. Early Steps in the DNA Base Excision/Single-Strand Interruption Repair Pathway in Mammalian Cells. Cell Res. 2008, 18, 27–47. [Google Scholar] [CrossRef]
- Boal, A.K.; Yavin, E.; Lukianova, O.A.; O’Shea, V.L.; David, S.S.; Barton, J.K. DNA-bound redox activity of DNA repair glycosylases containing [4Fe-4S] clusters. Biochemistry 2005, 44, 8397–8407. [Google Scholar] [CrossRef]
- Fuss, J.O.; Tsai, C.L.; Ishida, J.P.; Tainer, J.A. Emerging critical roles of Fe-S clusters in DNA replication and repair. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 1253–1271. [Google Scholar] [CrossRef]
- Syed, A.; Tainer, J.A. Charge Transport Communication through DNA by Protein Fe-S Clusters: How Far Is Not Too Far? ACS Cent. Sci. 2019, 5, 7–9. [Google Scholar] [CrossRef]
- Genereux, J.C.; Barton, J.K. Mechanisms for DNA charge transport. Chem. Rev. 2010, 110, 1642–1662. [Google Scholar] [CrossRef] [PubMed]
- Cerritelli, S.M.; Crouch, R.J. The Balancing Act of Ribonucleotides in DNA. Trends Biochem. Sci. 2016, 41, 434–445. [Google Scholar] [CrossRef]
- Korona, D.A.; Lecompte, K.G.; Pursell, Z.F. The high fidelity and unique error signature of human DNA polymerase ε. Nucleic Acids Res. 2011, 39, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Dalgaard, J.Z. Causes and consequences of ribonucleotide incorporation into nuclear DNA. Trends Genet. 2012, 28, 592–597. [Google Scholar] [CrossRef]
- Nick McElhinny, S.A.; Watts, B.E.; Kumar, D.; Watt, D.L.; Lundström, E.B.; Burgers, P.M.J.; Johansson, E.; Chabes, A.; Kunkel, T.A. Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases. Proc. Natl. Acad. Sci. USA 2010, 107, 4949–4954. [Google Scholar] [CrossRef]
- Beard, W.A.; Wilson, S.H. Structural Insights into the Origins of DNA Polymerase Fidelity. Structure 2003, 11, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Berti, M.; Cortez, D.; Lopes, M. The plasticity of DNA replication forks in response to clinically relevant genotoxic stress. Nat. Rev. Mol. Cell Biol. 2020, 21, 633–651. [Google Scholar] [CrossRef]
- Hiller, B.; Achleitner, M.; Glage, S.; Naumann, R.; Behrendt, R.; Roers, A. Mammalian RNase H2 removes ribonucleotides from DNA to maintain genome integrity. J. Exp. Med. 2012, 209, 1419–1426. [Google Scholar] [CrossRef]
- Sassa, A.; Yasui, M.; Honma, M. Current perspectives on mechanisms of ribonucleotide incorporation and processing in mammalian DNA. Genes Environ. 2019, 41, 3. [Google Scholar] [CrossRef]
- Kellner, V.; Luke, B. Molecular and physiological consequences of faulty eukaryotic ribonucleotide excision repair. EMBO J. 2020, 39, e102309. [Google Scholar] [CrossRef]
- BIOVIA. Discovery Studio Visualizer, v16.1.0.15350; BIOVIA: San Diego, CA, USA, 2015.
- Plumley, J.A.; Dannenberg, J.J. A comparison of the behavior of functional/basis set combinations for hydrogen-bonding in the water dimer with emphasis on basis set superposition error. J. Comput. Chem. 2011, 32, 1519–1527. [Google Scholar] [CrossRef]
- Zhao, Y.; Pu, J.; Lynch, B.J.; Truhlar, D.G. Tests of second-generation and third-generation density functionals for thermochemical kinetics. Phys. Chem. Chem. Phys. 2004, 6, 673. [Google Scholar] [CrossRef]
- Dapprich, S.; Komáromi, I.; Byun, K.S.; Morokuma, K.; Frisch, M.J. A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct. THEOCHEM 1999, 461–462, 1–21. [Google Scholar] [CrossRef]
- Nakagawa, S. Polarizable Model Potential Function for Nucleic Acid Bases. J. Comput. Chem. 2007, 28, 1539–1550. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. How Accurate Are the Minnesota Density Functionals for Noncovalent Interactions, Isomerization Energies, Thermochemistry, and Barrier Heights Involving Molecules Composed of Main-Group Elements? J. Chem. Theory Comput. 2016, 12, 4303–4325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other fun. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Chill, S.T.; Sherrill, C.D. Assessment of the performance of the M05-2X and M06-2X exchange correlation functionals for noncovalent interactions in biomolecules. J. Chem. Theory Comput. 2008, 4, 1996–2000. [Google Scholar] [CrossRef]
- Cammi, R.; Corni, S.; Mennucci, B.; Tomasi, J. Electronic excitation energies of molecules in solution: State specific and linear response methods for nonequilibrium continuum solvation models. J. Chem. Phys. 2005, 122, 104513. [Google Scholar] [CrossRef]
- Gu, J.; Wang, J.; Leszczynski, J. Electron attachment-induced DNA single-strand breaks at the pyrimidine sites. Nucleic Acids Res. 2010, 38, 5280–5290. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Karwowski, B.T. Ionisation potential and electron affinity of free 5′,8-cyclopurine-2′-deoxynucleosides. DFT study in gaseous and aqueous phase. Cent. Eur. J. Chem. 2010, 8, 70–76. [Google Scholar] [CrossRef]
- Miertus̃, S.; Tomasi, J. Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem. Phys. 1982, 65, 239–245. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2019.
- Kennedy, E.M.; Gavegnano, C.; Nguyen, L.; Slater, R.; Lucas, A.; Fromentin, E.; Schinazi, R.F.; Kim, B. Ribonucleoside triphosphates as substrate of human immunodeficiency virus type 1 reverse transcriptase in human macrophages. J. Biol. Chem. 2010, 285, 39380–39391. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, S.; Lönnberg, T.; Lönnberg, H. Phosphodiester models for cleavage of nucleic acids. Beilstein J. Org. Chem. 2018, 14, 803–837. [Google Scholar] [CrossRef] [PubMed]
- Westheimer, F.H. The hydrolysis of phosphate esters. Pure Appl. Chem. 1977, 49, 1059–1067. [Google Scholar] [CrossRef]
- Stephen Berry, R. Correlation of rates of intramolecular tunneling processes, with application to some group V compounds. J. Chem. Phys. 1960, 32, 933–938. [Google Scholar] [CrossRef]
- Silva López, C.; Nieto Faza, O.; Gregersen, B.A.; Lopez, X.; De Lera, A.R.; York, D.M. Pseudorotation of natural and chemically modified biological phosphoranes: Implications for RNA catalysis. ChemPhysChem 2004, 5, 1045–1049. [Google Scholar] [CrossRef]
- Kosonen, M.; Hakala, K.; Lönnberg, H. Hydrolysis and intramolecular transesterification of ribonucleoside 3′-phosphotriesters: The effect of alkyl groups on the general and specific acid–base-catalyzed reactions of 5′-O-pivaloyluridin-3′-yl dialkyl phosphates. J. Chem. Soc. Perkin Trans. 1998, 2, 663–670. [Google Scholar] [CrossRef]
- Altona, C.; Sundaralingam, M. Conformational Analysis of the Sugar Ring in Nucleosides and Nucleotides. a New Description Using the Concept of Pseudorotation. J. Am. Chem. Soc. 1972, 94, 8205–8212. [Google Scholar] [CrossRef]
- Giese, B. Long-distancee lectron transfer through DNA. Annu. Rev. Biochem. 2002, 71, 51–70. [Google Scholar] [CrossRef]
- Arnold, A.R.; Grodick, M.A.; Barton, J.K. DNA Charge Transport: From Chemical Principles to the Cell. Cell Chem. Biol. 2016, 23, 183–197. [Google Scholar] [CrossRef]
- Eriksen, K.A. Theoretical Biology and Medical Location of DNA damage by charge exchanging repair enzymes: Effects of cooperativity on location time. Theor. Biol. Med. Model. 2005, 2, 15. [Google Scholar] [CrossRef]
- Hall, D.; Holmlin, R.; Barton, J. Oxidative DNA damage through longrange electron transfer. Nature 1996, 382, 731–735. [Google Scholar] [CrossRef]
- Kelley, S.O.; Barton, J.K. Electron Transfer Between Bases in Double Helical DNA. Science 1999, 283, 375–381. [Google Scholar] [CrossRef]
- Boon, E.M.; Ceres, D.M.; Drummond, T.G.; Hill, M.G.; Barton, J.K. Mutation detection by electro catalysis at DNA-modified electrodes. Nat. Biotechnol. 2000, 18, 1096–1100. [Google Scholar] [CrossRef]
- Breslin, D.T.; Schuster, G.B. Anthraquinone photonucleases: Mechanisms for GG-selective and nonselective cleavage of double-stranded DNA. J. Am. Chem. Soc. 1996, 118, 554–558. [Google Scholar] [CrossRef]
- Karwowski, B.T. The Influence of Oxidized Imino-Allantoin in the Presence of OXOG on Double Helix Charge Transfer: A Theoretical Approach. Int. J. Mol. Sci. 2024, 25, 5962. [Google Scholar] [CrossRef]
- Wallace, B.D.; Williams, R.S. Ribonucleotide triggered DNA damage and RNA-DNA damage responses. RNA Biol. 2014, 11, 1340–1346. [Google Scholar] [CrossRef]
- Kumar, A.; Adhikary, A.; Sevilla, M.D.; Close, D.M. One-electron oxidation of ds(5′-GGG-3′) and ds(5′-G(8OG)G-3′) and the nature of hole distribution: A density functional theory (DFT) study. Phys. Chem. Chem. Phys. 2020, 22, 5078–5089. [Google Scholar] [CrossRef]
- Bredtmann, T.; Chelkowski, S.; Bandrauk, A.D. Effect of nuclear motion on molecular high order harmonic pump probe spectroscopy. J. Phys. Chem. A 2012, 116, 11398–11405. [Google Scholar] [CrossRef]
- Karwowski, B. How Clustered DNA Damage Can Change the Electronic Properties of ds-DNA, Differences between GAG, GAOXOG, and OXOGAOXOG. Biomolecules 2023, 13, 517. [Google Scholar] [CrossRef] [PubMed]
- Jalbout, A.F.; Adamowicz, L. Electron Attachment to DNA Base Complexes. Adv. Quantum Chem. 2007, 52, 231–251. [Google Scholar] [CrossRef]
- Caldecott, K.W. Causes and consequences of DNA single-strand breaks. Trends Biochem. Sci. 2024, 49, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Tyburski, R.; Liu, T.; Glover, S.D.; Hammarström, L. Proton-Coupled Electron Transfer Guidelines, Fair and Square. J. Am. Chem. Soc. 2021, 143, 560–576. [Google Scholar] [CrossRef]

| ds-Oligo | Neutral | Cation | Anion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HB-1 | HB-2 [Å] | P [°] | HB-1 | HB-2 | P | HB-1 | HB-2 | P | |
| DNA | 2.88 | 2.82 | 76.4 (0T4) | 2.89 | 2.85 | 71.9 (4T0) | 2.93 | 2.81 | 74.0 (0T4) |
| R-DNA | 2.90 | 2.88 | 156.6 (2T1) | 2.85 | 2.66 | 149.0 (2T1) | 2.92 | 2.93 | 146.4 (2T1) |
| IM-R-DNA | 2.89 | 2.84 | 117.4 (1T0) | 2.90 | 2.89 | 191.3 (3T2) | 2.93 | 2.88 | 130.0 (1T2) |
| RE-R-DNA | 3.00 | 2.80 | 97.7 (0T1) | 3.02 | 2.84 | 115.7 (1T0) | 2.90 | 2.92 | 127.5 (1T2) |
| SSB-R-DNA | 2.94 | 2.65 | 51.8 (4T3) | 2.93 | 3.03 | 90.5 (0E) | |||
| Neutral | Cation | Anion | |||||||
| P-O2′ | P-O3′ | P-O5′ | P-O2′ | P-O3′ | P-O5′ | P-O2′ | P-O3′ | P-O5′ | |
| DNA | 1.72 | 1.72 | 1.72 | 1.72 | 1.72 | 1.72 | |||
| R-DNA | 3.45 | 1.61 | 1.44 | 3.54 | 1.66 | 1.66 | 2.91 | 1.71 | 1.71 |
| IM-R-DNA | 1.75 | 1.77 | 1.74 | 1.65 | 1.74 | 1.66 | 1.75 | 1.77 | 1.74 |
| RE-R-DNA | 1.73 | 2.46 | 1.72 | 1.61 | 3.22 | 1.60 | 1.61 | 3.19 | 1.60 |
| SSB-R-DNA | 1.68 | 1.69 | 3.33 | 1.63 | 1.63 | 3.57 | 1.64 | 1.62 | 3.30 |
| ds-Oligo | rA::dT Nucleosides Pair | ds-oligonucleotides | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NEIP | EQIP | AIP | NEEA | EQEA | AEA | NEIP | EQIP | AIP | NEEA | EQEA | AEA | HOMO | LUMO | |
| R-DNA | 7.72 | 6.69 | 7.04 | 0.46 | 1.48 | 1.44 | 7.03 | 6.21 | 7.41 | 0.73 | 1.38 | 1.76 | 7.08 | 0.67 |
| IM-R-DNA | 7.73 | 6.70 | 6.44 | 0.46 | 1.48 | 1.69 | 6.91 | 6.25 | 4.49 | 0.68 | 1.42 | 3.47 | 6.96 | 0.67 |
| RE-R-DNA | 7.74 | 6.71 | 6.55 | 0.43 | 1.45 | 1.61 | 6.84 | 6.12 | 3.84 | 0.62 | 1.33 | 4.08 | 6.90 | 0.65 |
| SSB-R-DNA | 7.80 | 6.78 | 6.61 | 0.57 | 1.58 | 2.25 | 6.75 | 6.09 | 2.76 | 0.82 | 1.62 | 5.55 | 6.81 | 0.78 |
| ** SSB-R-DNA | 7.84 | 6.83 | 5.73 | 0.57 | 1.58 | 2.74 | ||||||||
| DNA [53] | 7.70 | 6.67 | 6.63 | 0.44 | 1.46 | 1.48 | 6.72 | 6.08 | 5.65 | 0.84 | 1.58 | 2.09 | 6.95 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karwowski, B.T. The Consequence of the Presence of Ribonucleotide for ds-DNA’s Electronic Properties: Preliminary Theoretical Studies. Cells 2025, 14, 881. https://doi.org/10.3390/cells14120881
Karwowski BT. The Consequence of the Presence of Ribonucleotide for ds-DNA’s Electronic Properties: Preliminary Theoretical Studies. Cells. 2025; 14(12):881. https://doi.org/10.3390/cells14120881
Chicago/Turabian StyleKarwowski, Boleslaw T. 2025. "The Consequence of the Presence of Ribonucleotide for ds-DNA’s Electronic Properties: Preliminary Theoretical Studies" Cells 14, no. 12: 881. https://doi.org/10.3390/cells14120881
APA StyleKarwowski, B. T. (2025). The Consequence of the Presence of Ribonucleotide for ds-DNA’s Electronic Properties: Preliminary Theoretical Studies. Cells, 14(12), 881. https://doi.org/10.3390/cells14120881






