Exogenous Ubiquitin Differentially Modulates the Phenotype and Function of M1 and M2 Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation of Peritoneal Macrophages
2.3. Macrophage Activation and Treatment
2.4. Measurement of Cytokines/Chemokines Levels in the Conditioned Media
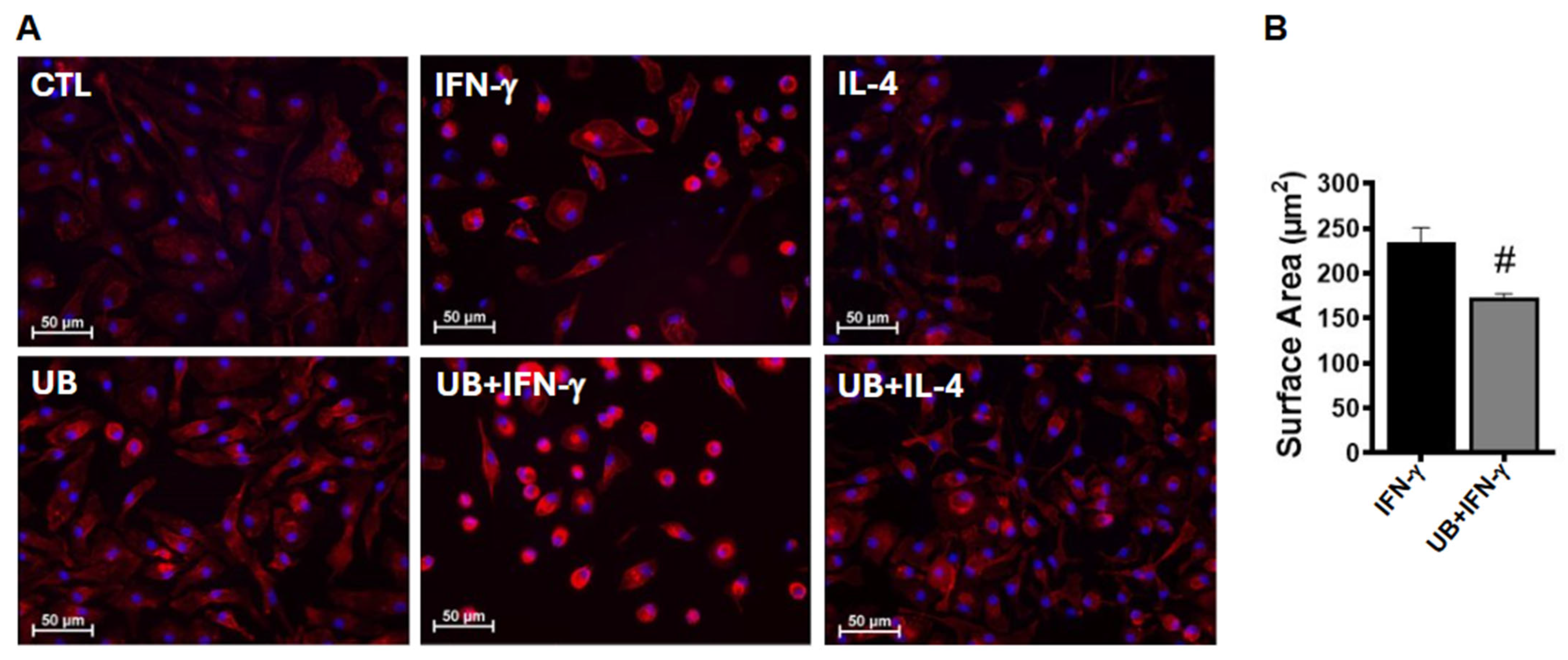
2.5. Cell Morphology
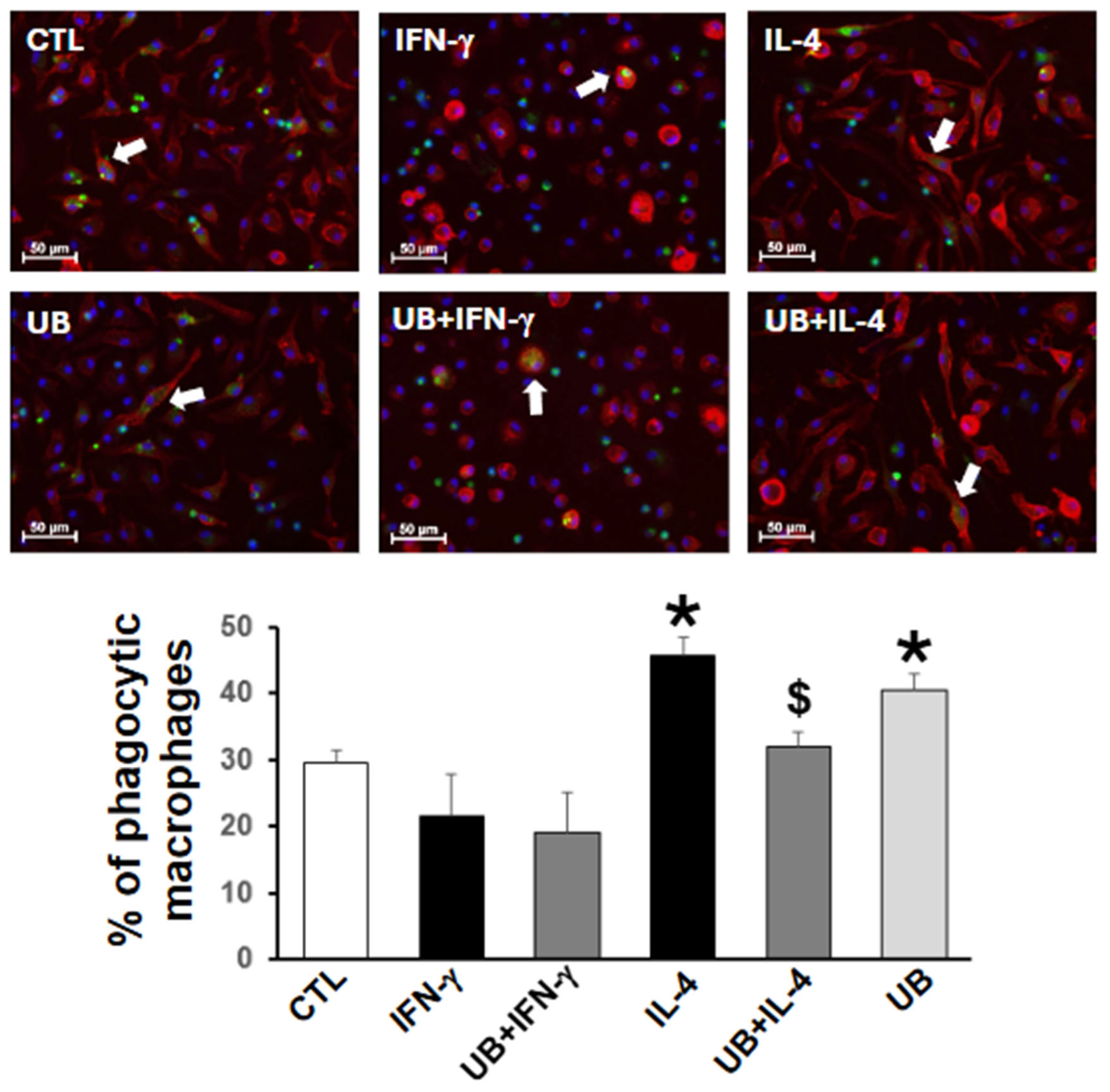
2.6. Efferocytosis Assay
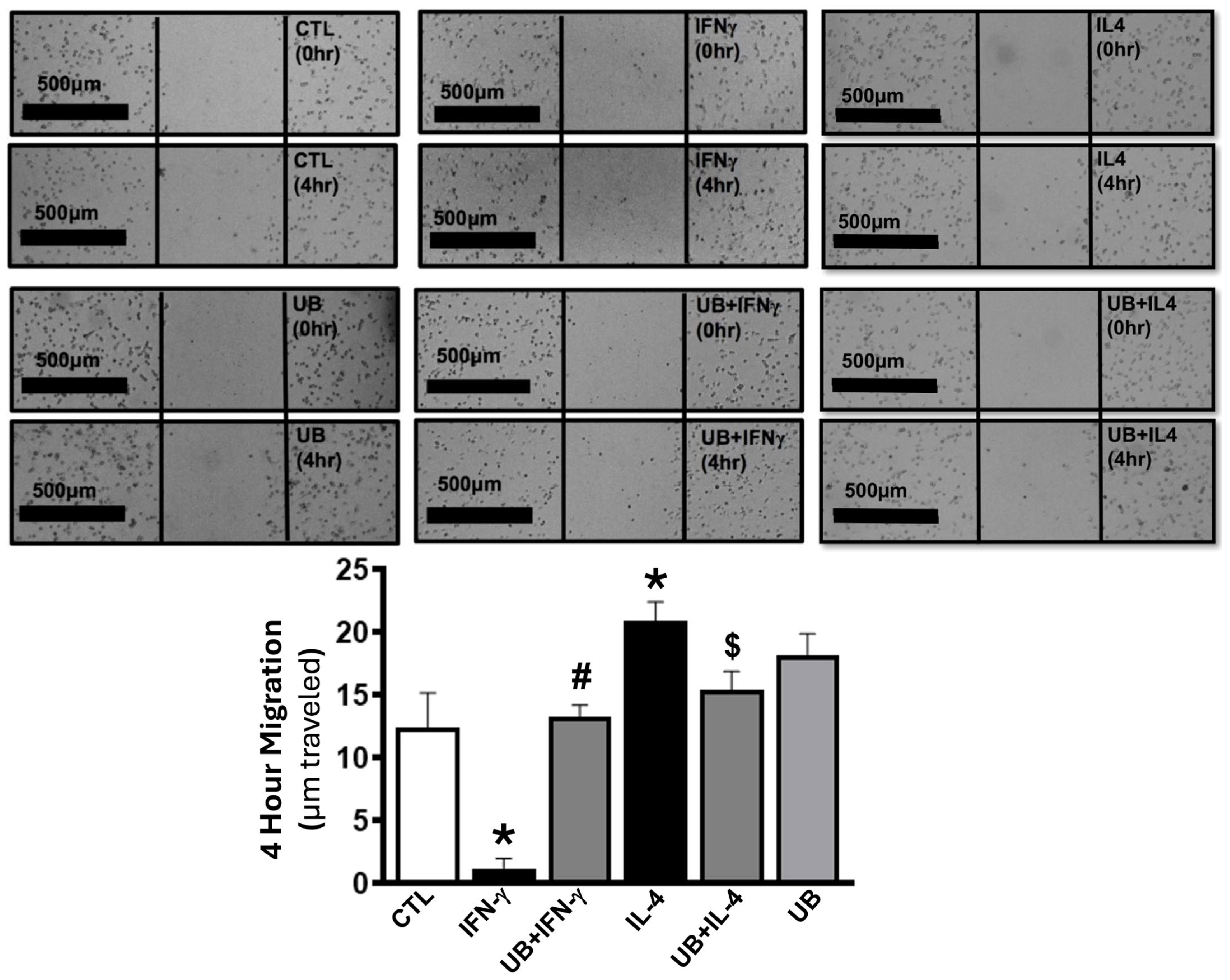
2.7. Two-Dimensional Migration Assay
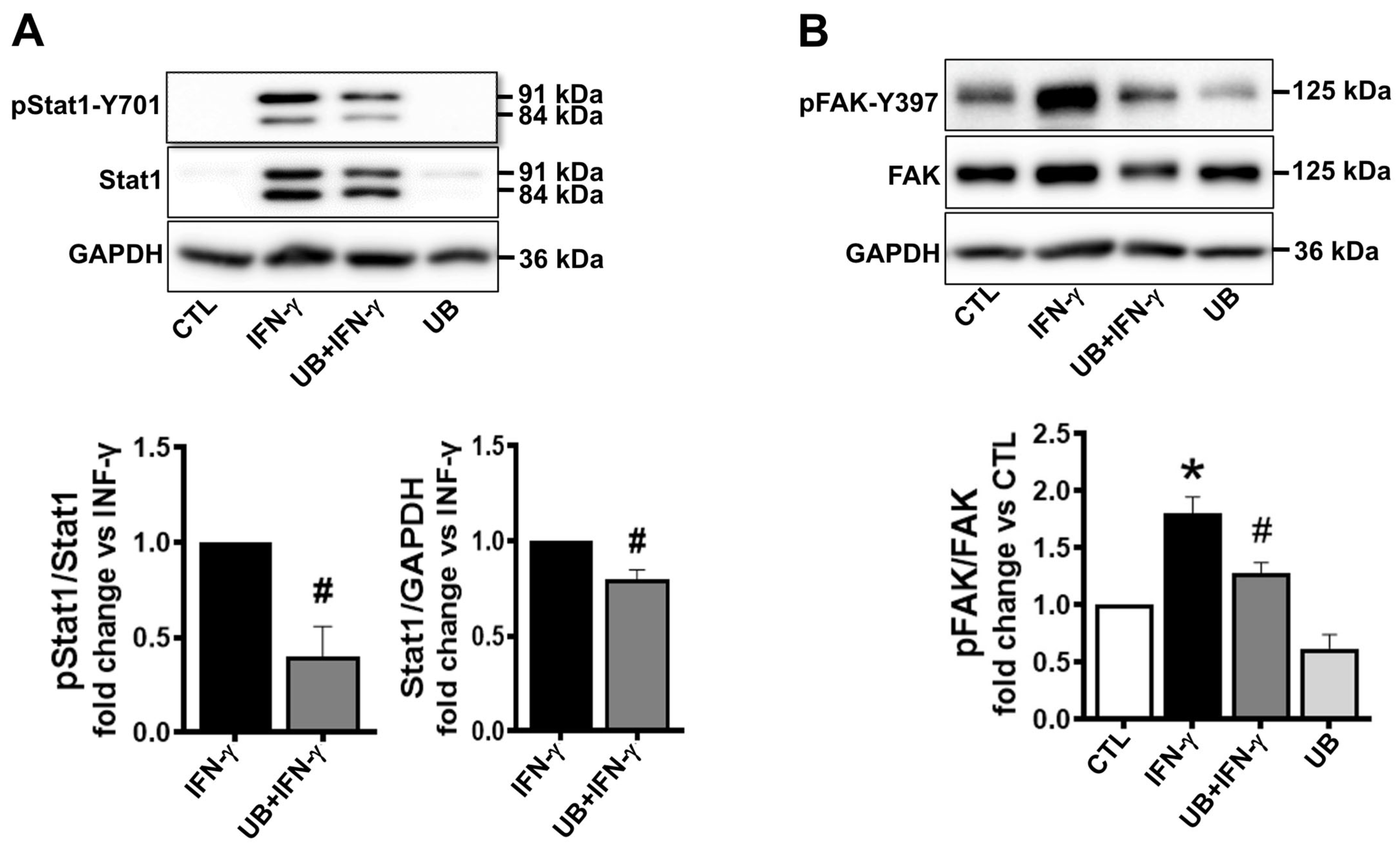
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Exogenous UB Decreases Cytokine Levels in the Conditioned Media
3.2. Exogenous UB Modulates Phenotype of Macrophages
3.3. Exogenous UB Decreases M2-Polarized Macrophage Efferocytosis
3.4. Exogenous UB Modulates Macrophage Migration
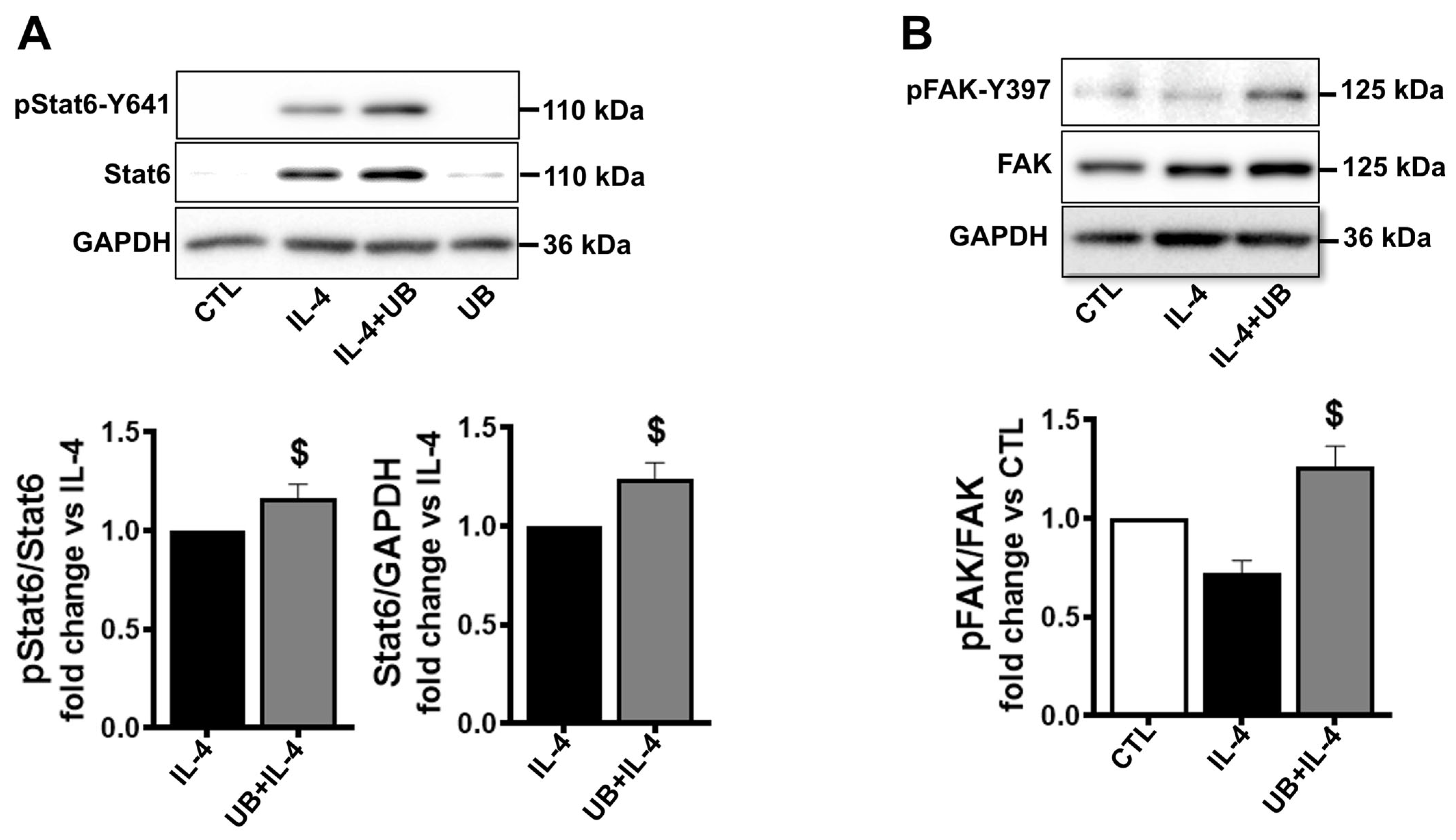
3.5. Exogenous UB Affects Intracellular Signaling
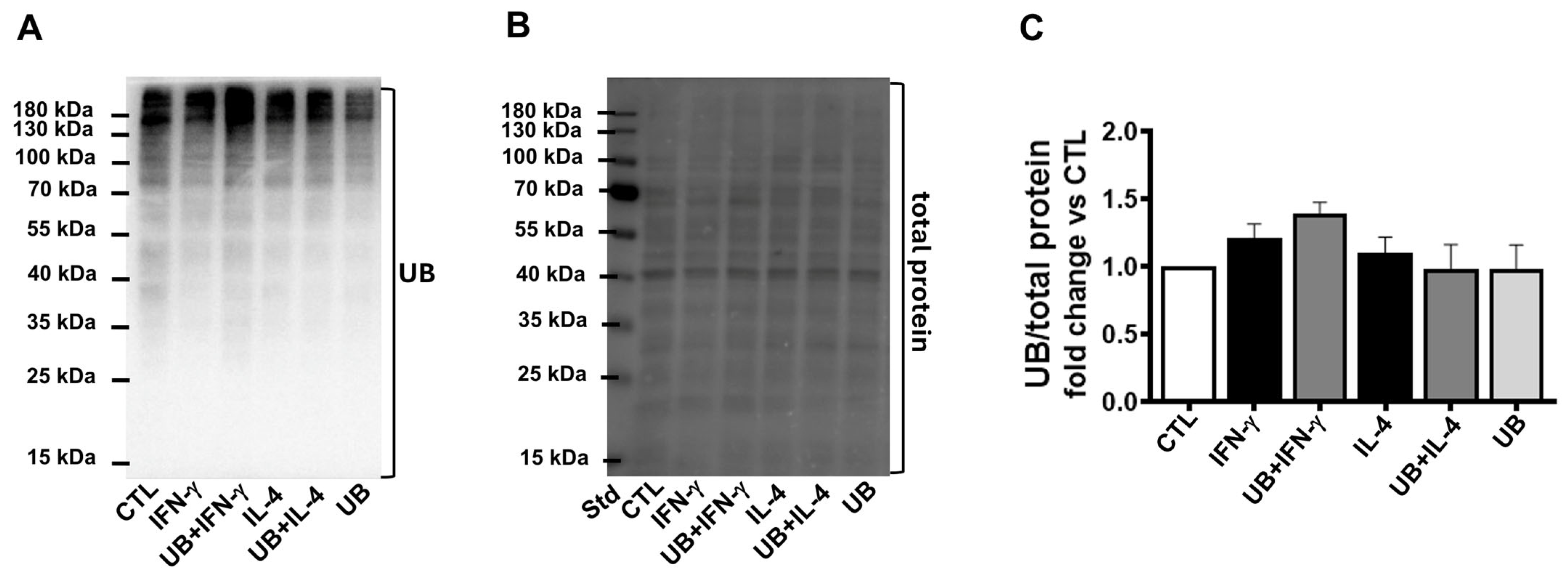
3.6. Exogenous UB Does Not Affect Ubiquitination of Cellular Proteins
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| eUB | Exogenous ubiquitin. |
| I/R | Post-ischemia/reperfusion. |
| MI | Myocardial infarction. |
| M1 | Pro-inflammatory macrophage. |
| M2 | Anti-inflammatory macrophage. |
| UB | Ubiquitin. |
| UPS | Ubiquitin–proteasome system. |
| DMEM | Dulbecco’s modified Eagle’s medium. |
| FBS | Fetal bovine serum. |
| CTL | Control. |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor. |
| IFN | Interferon. |
| TNF | Tumor necrosis factor. |
| PFA | Paraformaldehyde. |
| PMA | Phorbol-12-myristate-13-acetate. |
| ANOVA | One-way analysis of variance. |
| STAT1 | Activate signal transducer and activator of transcription 1. |
| STAT6 | Activate signal transducer and activator of transcription 6. |
| FAK | Focal adhesion kinase. |
References
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 13 January 2025).
- Valencia, A.; Burgess, J.H. Arterial Hypoxemia Following Acute Myocardial Infarction. Circulation 1969, 40, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair after Myocardial Infarction. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Westman, P.C.; Lipinski, M.J.; Luger, D.; Waksman, R.; Bonow, R.O.; Wu, E.; Epstein, S.E. Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 67, 2050–2060. [Google Scholar] [CrossRef]
- Lambert, J.M.; Lopez, E.F.; Lindsey, M.L. Macrophage Roles Following Myocardial Infarction. Int. J. Cardiol. 2008, 130, 147. [Google Scholar] [CrossRef]
- Pluijmert, N.J.; Atsma, D.E.; Quax, P.H.A. Post-Ischemic Myocardial Inflammatory Response: A Complex and Dynamic Process Susceptible to Immunomodulatory Therapies. Front. Cardiovasc. Med. 2021, 8, 647785. [Google Scholar] [CrossRef]
- Mouton, A.J.; Deleon-Pennell, K.Y.; Rivera Gonzalez, O.J.; Flynn, E.R.; Freeman, T.C.; Saucerman, J.J.; Garrett, M.R.; Ma, Y.; Harmancey, R.; Lindsey, M.L. Mapping Macrophage Polarization over the Myocardial Infarction Time Continuum. Basic. Res. Cardiol. 2018, 113, 26. [Google Scholar] [CrossRef]
- Damgaard, R.B. The Ubiquitin System: From Cell Signalling to Disease Biology and New Therapeutic Opportunities. Cell Death Differ. 2021, 28, 423–426. [Google Scholar] [CrossRef]
- Swatek, K.N.; Komander, D. Ubiquitin Modifications. Cell Res. 2016, 26, 399–422. [Google Scholar] [CrossRef]
- Majetschak, M. Extracellular Ubiquitin: Immune Modulator and Endogenous Opponent of Damage-Associated Molecular Pattern Molecules. J. Leukoc. Biol. 2011, 89, 205–219. [Google Scholar] [CrossRef]
- Dalal, S.; Shook, P.L.; Singh, M.; Singh, K. Post-Ischemic Cardioprotective Potential of Exogenous Ubiquitin in Myocardial Remodeling Late after Ischemia/Reperfusion Injury. Life Sci. 2023, 312. [Google Scholar] [CrossRef]
- Scofield, S.L.C.; Dalal, S.; Lim, K.A.; Thrasher, P.R.; Daniels, C.R.; Peterson, J.M.; Singh, M.; Singh, K.; Quillen, J.H. Exogenous Ubiquitin Reduces Inflammatory Response and Preserves Myocardial Function 3 Days Post-Ischemia-Reperfusion Injury. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Staren, D.M.; Ziarek, J.J.; Nashaat, Z.N.; Campbell, E.M.; Volkman, B.F.; Marchese, A.; Majetschak, M. The CXC Chemokine Receptor 4 Ligands Ubiquitin and Stromal Cell-Derived Factor-1α Function through Distinct Receptor Interactions. J. Biol. Chem. 2011, 286, 33466–33477. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, Q.; Qian, X.; Li, J.; Qi, Q.; Sun, R.; Han, J.; Zhu, X.; Xie, M.; Guo, X.; et al. Extracellular Ubiquitin Promotes Hepatoma Metastasis by Mediating M2 Macrophage Polarization via the Activation of the CXCR4/ERK Signaling Pathway. Ann. Transl. Med. 2020, 8, 929. [Google Scholar] [CrossRef]
- Layoun, A.; Samba, M.; Santos, M.M. Isolation of Murine Peritoneal Macrophages to Carry Out Gene Expression Analysis Upon Toll-like Receptors Stimulation. J. Vis. Exp. 2015, e52749. [Google Scholar] [CrossRef]
- Casteel, J.L.; Keever, K.R.; Ardell, C.L.; Williams, D.L.; Gao, D.; Podrez, E.A.; Byzova, T.V.; Yakubenko, V.P. Modification of Extracellular Matrix by the Product of DHA Oxidation Switches Macrophage Adhesion Patterns and Promotes Retention of Macrophages During Chronic Inflammation. Front. Immunol. 2022, 13, 867082. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Tian, P.X.; Han, F.; Zheng, J.; Xia, X.X.; Xue, W.J.; Ding, X.M.; Ding, C.G. Comparison of the Characteristics of Macrophages Derived from Murine Spleen, Peritoneal Cavity, and Bone Marrow. J. Zhejiang Univ. Sci. B. 2017, 18, 1055–1063. [Google Scholar] [CrossRef]
- Bailey, W.P.; Cui, K.; Ardell, C.L.; Keever, K.R.; Singh, S.; Rodriguez-Gil, D.J.; Ozment, T.R.; Williams, D.L.; Yakubenko, V.P. Frontline Science: The Expression of Integrin ADβ2 (CD11d/CD18) on Neutrophils Orchestrates the Defense Mechanism against Endotoxemia and Sepsis. J. Leukoc. Biol. 2021, 109, 877–890. [Google Scholar] [CrossRef]
- Scofield, S.L.C.; Daniels, C.R.; Dalal, S.; Millard, J.A.; Singh, M.; Singh, K. Extracellular Ubiquitin Modulates Cardiac Fibroblast Phenotype and Function via Its Interaction with CXCR4. Life Sci. 2018, 211, 8–16. [Google Scholar] [CrossRef]
- Vereyken, E.J.F.; Heijnen, P.D.A.M.; Baron, W.; de Vries, E.H.E.; Dijkstra, C.D.; Teunissen, C.E. Classically and Alternatively Activated Bone Marrow Derived Macrophages Differ in Cytoskeletal Functions and Migration towards Specific CNS Cell Types. J. Neuroinflammation 2011, 8, 1–16. [Google Scholar] [CrossRef]
- Jain, N.; Vogel, V. Spatial Confinement Downsizes the Inflammatory Response of Macrophages. Nat. Mater. 2018, 17, 1134–1144. [Google Scholar] [CrossRef]
- Kubota, A.; Frangogiannis, N.G. Macrophages in Myocardial Infarction. Am. J. Physiol. Cell Physiol. 2022, 323, C1304–C1324. [Google Scholar] [CrossRef] [PubMed]
- Shook, P.L.; Singh, M.; Singh, K. Macrophages in the Inflammatory Phase Following Myocardial Infarction: Role of Exogenous Ubiquitin. Biology 2023, 12, 1258. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Ardell, C.L.; Podolnikova, N.P.; Yakubenko, V.P. Distinct Migratory Properties of M1, M2, and Resident Macrophages Are Regulated by Adβ2and Amβ2integrin-Mediated Adhesion. Front. Immunol. 2018, 9, 422099. [Google Scholar] [CrossRef] [PubMed]
- Bellingan, G.J.; Caldwell, H.; Howie, S.E.M.; Dramfield, I.; Haslett, C. In Vivo Fate of the Inflammatory Macrophage during the Resolution of Inflammation: Inflammatory Macrophages Do Not Die Locally, but Emigrate to the Draining Lymph Nodes. J. Immunol. 1996, 157, 2577–2585. [Google Scholar] [CrossRef]
- Hu, X.; Ivashkiv, L.B. Cross-Regulation of Signaling Pathways by Interferon-Gamma: Implications for Immune Responses and Autoimmune Diseases. Immunity 2009, 31, 539–550. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. IFNγ: Signalling, Epigenetics and Roles in Immunity, Metabolism, Disease and Cancer Immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Samson, S.C.; Khan, A.M.; Mendoza, M.C. ERK Signaling for Cell Migration and Invasion. Front. Mol. Biosci. 2022, 9, 998475. [Google Scholar] [CrossRef]
- Goenka, S.; Kaplan, M.H. Transcriptional Regulation by STAT6. Immunol. Res. 2011, 50, 87–96. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of Macrophage Phenotype by Cell Shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef]
- Cai, J.; Qian, X.; Qi, Q.; Han, J.; Zhu, X.; Zhang, Q.; Xia, R. Extracellular Ubiquitin Inhibits the Apoptosis of Hepatoma Cells via the Involvement of Macrophages. Transl. Cancer Res. 2020, 9, 2855–2864. [Google Scholar] [CrossRef]
- Ramana, C.V.; Chatterjee-Kishore, M.; Nguyen, H.; Stark, G.R. Complex Roles of Stat1 in Regulating Gene Expression. Oncogene 2000, 19, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Wick, K.L.R.; Berton, M.T. IL-4 Induces Serine Phosphorylation of the STAT6 Transactivation Domain in B Lymphocytes. Mol. Immunol. 2000, 37, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gómez, A.; Perretti, M.; Soehnlein, O. Resolution of Inflammation: An Integrated View. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef]
- Greenlee-Wacker, M.C.; Nauseef, W.M. IFN-γ Targets Macrophage-Mediated Immune Responses toward Staphylococcus Aureus. J. Leukoc. Biol. 2017, 101, 751–758. [Google Scholar] [CrossRef]
- Fernandez-Boyanapalli, R.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Dinauer, M.C.; Riches, D.W.H.; Henson, P.M.; Byrne, A.; Bratton, D.L. Impaired Phagocytosis of Apoptotic Cells by Macrophages in Chronic Granulomatous Disease Is Reversed by IFN-γ in a Nitric Oxide-Dependent Manner. J. Immunol. 2010, 185, 4030–4041. [Google Scholar] [CrossRef]
- Daseke, M.J.; Tenkorang-Impraim, M.A.A.; Ma, Y.; Chalise, U.; Konfrst, S.R.; Garrett, M.R.; DeLeon-Pennell, K.Y.; Lindsey, M.L. Exogenous IL-4 Shuts off pro-Inflammation in Neutrophils While Stimulating Anti-Inflammation in Macrophages to Induce Neutrophil Phagocytosis Following Myocardial Infarction. J. Mol. Cell Cardiol. 2020, 145, 112–121. [Google Scholar] [CrossRef]
- Kagan, W.A.; O’Neill, G.J.; Incefy, G.S.; Goldstein, G.; Good, R.A. Induction of Human Granulocyte Differentiation In Vitro by Ubiquitin and Thymopoietin. Blood 1977, 50, 275–288. [Google Scholar] [CrossRef]
- Lin, D.; Kang, X.; Shen, L.; Tu, S.; Lenahan, C.; Chen, Y.; Wang, X.; Shao, A. Efferocytosis and Its Associated Cytokines: A Light on Non-Tumor and Tumor Diseases? Mol. Ther. Oncolytics 2020, 17, 394–407. [Google Scholar] [CrossRef]
- Wiesner, C.; Le-Cabec, V.; El Azzouzi, K.; Maridonneau-Parini, I.; Linder, S. Podosomes in Space: Macrophage Migration and Matrix Degradation in 2D and 3D Settings. Cell. Adh. Migr. 2014, 8, 179–191. [Google Scholar] [CrossRef]
- Ma, Y.; Mouton, A.J.; Lindsey, M.L. Cardiac Macrophage Biology in the Steady-State Heart, the Aging Heart, and Following Myocardial Infarction. Transl. Res. 2018, 191, 15–28. [Google Scholar] [CrossRef]
- Peet, C.; Ivetic, A.; Bromage, D.I.; Shah, A.M. Cardiac Monocytes and Macrophages after Myocardial Infarction. Cardiovasc. Res. 2020, 116, 1101–1112. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shook, P.L.; Wang-Heaton, H.; Casteel, J.L.; Dalal, S.; Singh, M.; Yakubenko, V.; Singh, K. Exogenous Ubiquitin Differentially Modulates the Phenotype and Function of M1 and M2 Macrophages. Cells 2025, 14, 879. https://doi.org/10.3390/cells14120879
Shook PL, Wang-Heaton H, Casteel JL, Dalal S, Singh M, Yakubenko V, Singh K. Exogenous Ubiquitin Differentially Modulates the Phenotype and Function of M1 and M2 Macrophages. Cells. 2025; 14(12):879. https://doi.org/10.3390/cells14120879
Chicago/Turabian StyleShook, Paige L., Hui Wang-Heaton, Jared L. Casteel, Suman Dalal, Mahipal Singh, Valentin Yakubenko, and Krishna Singh. 2025. "Exogenous Ubiquitin Differentially Modulates the Phenotype and Function of M1 and M2 Macrophages" Cells 14, no. 12: 879. https://doi.org/10.3390/cells14120879
APA StyleShook, P. L., Wang-Heaton, H., Casteel, J. L., Dalal, S., Singh, M., Yakubenko, V., & Singh, K. (2025). Exogenous Ubiquitin Differentially Modulates the Phenotype and Function of M1 and M2 Macrophages. Cells, 14(12), 879. https://doi.org/10.3390/cells14120879






