Abstract
Pulmonary hypertension (PH) is a progressive lung disease characterized by abnormal pulmonary vascular pressure and right ventricular (RV) dysfunction. Atrial arrhythmias, including atrial fibrillation (AF) and atrial flutter, are common in patients with PH and significantly contribute to disease progression and mortality. A bidirectional pathophysiological link exists between PH and AF, encompassing shared mechanisms such as endothelial dysfunction, DNA damage, autophagy, inflammation, and oxidative stress, as well as mutual risk factors, including diabetes, obesity, heart disease, and aging. Despite these shared pathways, limited research has been conducted to fully understand the intertwined relationship between PH and AF, hindering the development of effective treatments. In this review, we provide a comprehensive overview of the epidemiology of PH, the molecular mechanisms underlying the development of AF in PH, and the overlap in their pathophysiology. We also identify novel druggable targets and propose mechanism-based therapeutic approaches to treat this specific patient group. By shedding light on the molecular connection between PH and AF, this review aims to fuel the design and validation of innovative treatments to address this challenging comorbidity.
1. Introduction
Pulmonary hypertension (PH) is a complex and heterogeneous disease affecting the pulmonary vasculature, with an estimated prevalence of 1–3% in Western countries [1,2]. PH is clinically defined by an elevated mean pulmonary artery pressure (mPAP) greater than 20 mm Hg, increased pulmonary vascular resistance (PVR) due to vascular remodeling and obstruction, and dysfunction of the right ventricle (RV) dysfunction [1,2]. These hemodynamic and structural changes result in frequent episodes of decompensation in PH patients. Among PH subtypes, including pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH), 10–33% of patients develop atrial arrhythmias (AA), such as atrial flutter (AFL) and atrial fibrillation (AF) (Table 1). Mechanistically, these arrhythmias are driven by electrical conduction abnormalities, right atrial stretch, fibrosis, and sympathetic overactivity [3]. The resulting loss of atrial contraction during the diastole reduces RV filling, decreases cardiac output, and precipitates clinical deterioration with features of heart failure. Interventions aimed at restoring sinus rhythm, including electrical cardioversion, antiarrhythmic medications, and catheter ablation strategies, have demonstrated potential to reverse cardiac decompensation and improve clinical outcomes. Notably, AA in PAH and CTEPH are associated with an increased risk of mortality, emphasizing the importance of achieving and maintaining sinus rhythm as a key therapeutic goal (Table 1) [4]. In PH patients, AF is considered as a severe clinical complication that exacerbates hemodynamic compromise, increases the risk of stroke, worsens symptoms, and complicates disease management. Restoring sinus rhythm in these patients is crucial, as the continuation of AF can worsen hemodynamic compromise and increase the risk of complications [5,6,7,8]. Despite the clinical significance of both PH and AF, research studies addressing both PH and AF in parallel remain limited. Given the significant impact of AF in PH patients, there is a pressing need to develop mechanism-based treatment strategies specifically tailored for this patient population. These strategies should address both the underlying PH and the associated AF, ultimately improving the outcomes and overall management of PH patients.

Table 1.
Overview of studies showing prevalence of atrial arrhythmia in PH patients.
While the association between PH and AF has been recognized for several decades, predisposing risk factors, clinical manifestation, and corresponding molecular root causes for the development of AF in PH patients are unknown. Notably, atrial arrhythmias, including AF, are far more common in PH due to left heart disease (Group 2) and PH due to chronic lung diseases and hypoxia (Group 3) than in PAH (Group 1) [15,16]. A subset of Group 2 PH patients of heart failure with preserved ejection fraction (HFpEF), creating an additional substrate for AF development, making it difficult to determine cause or consequence [17,18]. Chronic elevation of left atrial pressure and volume overload in Group 2 PH promote atrial stretch, fibrosis, and electrical remodeling—factors known to drive AF [19].
Large-registry studies, such as the Swedish Pulmonary Arterial Hypertension Register (SPAHR), have demonstrated a relatively low AF prevalence in PAH, particularly in younger patients, challenging the generalized narrative of AF as a frequent complication in all PH subgroups [20]. The SPAHR analysis reported an AF prevalence of approximately 13% in the overall PAH population, with a markedly lower incidence in younger individuals [20].
Similarly, AF presents in various clinical forms, including paroxysmal (recurrent episodes of AF that resolve on their own within 7 days), persistent (last longer than 7 days or require intervention), and permanent AF (where sinus rhythm control can no longer be restored) [21]. These AF subtypes reflect different degrees of structural and electrical remodeling and may evolve over time in response to progressive cardiac stress [21,22]. In different PH cohort studies, the incidence and prevalence of subtypes of AF have been studied (Table 1), but to our knowledge, no clear relationship has been established yet. Furthermore, asymptomatic AF may go undiagnosed, yet still contribute to clinical deterioration and stroke risk [23]. Despite its importance, most studies do not stratify patients by AF subtype, making it difficult to determine whether molecular mechanisms differ between PH types.
Additionally, the longstanding pressure and volume overload in PH patients induce complex structural remodeling processes in the RV and right atrium (RA). However, while severe left atrial dilation is a well-established arrhythmogenic substrate for AF in left-sided heart diseases, including PH [24], the impact of RA dilation remains less definitive [25]. This paradigm raises critical questions about the relative contribution of right heart structural and electrophysiological changes to AF development in PH patients.
Moreover, little is known about the impact of AF on ventricular and lung function in PH. In patients with severe PH, the elevated pressure in the pulmonary arteries can impact the function of the right atrium. This may result in stretching and enlargement of the atrium, that may create a favorable environment for the onset of AF. Conversely, AF can impair atrial function and contribute to hemodynamic compromises, further worsening the condition of PH [4]. Despite the high clinical relevance, so far, little effort has been made to understand the molecular mechanism, how PH contributes to AF onset, and vice versa. These insights are important for developing patient-tailored treatment strategies. Interestingly, recent research findings disclose that several key molecular pathways, including proteostasis derailment by exhaustion of heat shock proteins, increase DNA damage, autophagy, endothelial dysfunction, inflammation, and oxidative stress underlie AF [21,26,27,28,29,30] as well as PH [31,32,33,34].
Finally, emerging evidence suggests that metabolic syndrome, such as obesity, insulin resistance, and dyslipidemia may contribute to both PH and AF development [35,36,37]. Adipose tissue, particularly epicardial fat, secretes pro-inflammatory cytokines and adipokines (e.g., activin A, p53, leptin, and caveolin-1) that can lead to endothelial dysfunction, fibrosis, and electrical remodeling in both atrial and pulmonary vascular tissues [35,36].
Over the past few decades, significant advancements have been made in both pharmaceutical and interventional therapies for PH and AF. Despite these advancements, there remains a critical need for the development of mechanism-based drugs to improve prognosis and reduce morbidity and mortality. This review discusses the epidemiology and prevalence of AF in PH, explores the molecular mechanisms underlying AF in the context of PH, identifies novel druggable targets, and discusses strategies for their validation. By elucidating the molecular interplay between PH and AF, this review aims to support the design and validation of innovative mechanism-based therapeutic approaches tailored to this specific patient population.
2. Clinical Classification of PH
PH is classified based on shared underlying disease mechanisms, clinical presentations, hemodynamic parameters, and therapeutic strategies. The latest guidelines from the European Society of Cardiology and the European Respiratory Society (ESC/ERS) categorize PH into five groups according to underlying causes and pathophysiological mechanisms (Figure 1). Given the strong clinical association between AF in specific subgroups of PH, namely pulmonary arterial hypertension (PAH, Group 1), PH associated with left heart disease (Group 2), and chronic thromboembolic pulmonary hypertension (CTEPH, Group 4), this review focuses particularly on these groups [4,5,15,38] (Table 1).
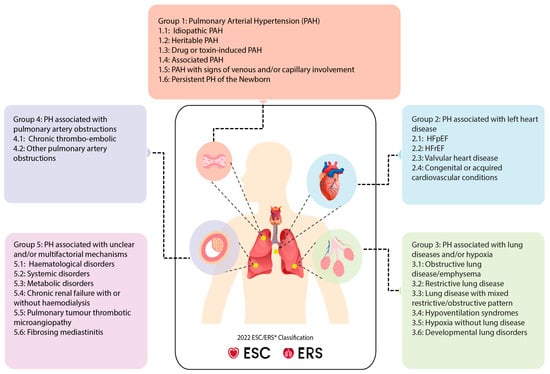
Figure 1.
Clinical classification of PH according to 2022 ESC/ERS guidelines. PH is divided into five clinical groups that are further subdivided into subgroups based upon underlying disease mechanisms, clinical presentations, hemodynamic parameters, and therapeutic interventions. ESC: European Society of Cardiology, ERS: European Respiratory Society, HFrEF: heart failure with reduced ejection fraction, HFpEF: heart failure with preserved ejection fraction.
3. Epidemiology and Prevalence of AF in PH
PH is a significant global health concern which is impacted in every age group. As mentioned, AF is frequently observed in PH patients within Group 1 (pulmonary arterial hypertension, (PAH)), Group 2 (PH associated with left heart disease), and Group 4 (chronic thromboembolic pulmonary hypertension (CTEPH)) due to distinct yet interconnected mechanisms. In PAH, excessive pulmonary vasoconstriction, smooth muscle hypertrophy, and fibrosis lead to progressive right ventricular (RV) pressure overload and dysfunction, causing right atrial stretch, fibrosis, and electrical conduction abnormalities that predispose to AF. In Group 2 PH, elevated left atrial pressures from left ventricular dysfunction, valvular disease, or abnormal filling increase pulmonary venous pressure, resulting in atrial dilation and fibrosis, key drivers of AF. CTEPH involves chronic fibrotic clots and thromboemboli that obstruct pulmonary arteries, leading to increased pulmonary vascular resistance and RV strain, which also induce atrial remodeling. Tongers et al. were the first to report major implications of atrial arrhythmias in group 1 and group 4 PH patients. They reported a cumulative 11.7% incidence of supraventricular tachyarrhythmias with AF (5.62%) and AFL (6.49%) among the selected cohort (Table 1) [6]. In a group of patients with PAH, 22% of the patients were reported with incidence of AA [9]. A study using Johns Hopkins PH registry data of 317 patients with different types of PH revealed that 13.2% of patients developed AA [13]. In a 5-year follow-up study, Olsson et al. observed a cumulative prevalence of 25.1% AF and AFL in patients with PAH (n = 157) and CTEPH (n = 82) [8]. In a relatively big study cohort (n = 755), patients with different types of PH with elevated pre and post-capillary pressure showed higher prevalence of supraventricular tachycardia (29%) with AF as the most prevalent type of arrhythmia (21%) (Table 1) [14]. Shared mechanisms across these groups include chronic atrial stretch, fibrosis, inflammation, and hemodynamic instability, all of which promote AF development. The presence of AF further exacerbates hemodynamic compromise, impairing ventricular filling, worsening symptoms, and increasing the risk of adverse outcomes, underscoring the clinical importance of managing AF in these PH subgroups.
4. Molecular Mechanisms Underlying AF Development in PH
Several hemodynamic parameters change during PH development. The elevated mean pulmonary artery pressure and increased right atrium pressure in PH can lead to frequent decompensation, atrial remodeling, electrical conduction abnormalities, and structural changes in the atrial tissue [39]. The increased stress on the atria can result in atrial enlargement, fibrosis, and conduction alterations, which create a substrate for the re-entry and onset and maintenance of AF [40]. Furthermore, in PH, there is often activation of neurohumoral systems, such as the sympathetic nervous system and the renin–angiotensin–aldosterone system. These neurohumoral factors can promote inflammation and oxidative stress, which are known to contribute to AF development [13,41] (Figure 2).
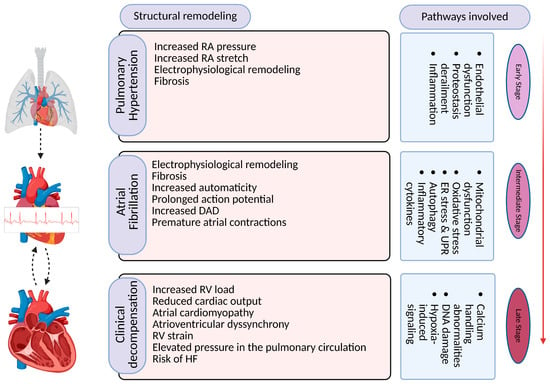
Figure 2.
Overview of pathways and mechanisms involved in pathogenesis of AF in PH and associated pathophysiological implications. RA: right atrium, RV: right ventricle, DAD: delayed after depolarization, HF: heart failure. Created with BioRender.com (BioRender, RU28BW4XQD).
The molecular mechanism of AF development in PH is multiplex and seems to share many common signaling pathways driven by multiple triggers. Emerging research identifies an overlap in various molecular mechanisms that are triggered during the disease progression of AF in PH. These include proteostasis derailment via the exhaustion of heat shock proteins (HSPs), induction of DNA damage via mitochondria dysfunction and reactive oxygen species (ROS) production, increase in autophagic protein degradation, thromboembolism, endothelial dysfunction, and inflammation as depicted below (Figure 3).
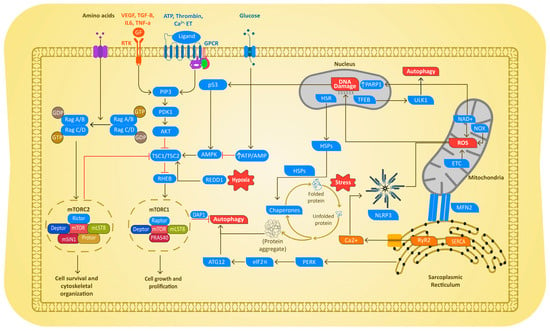
Figure 3.
Schematic diagram depicting the molecular mechanisms driving AF in PH. In both cases, disease-mediated cellular stress caused proteostasis derailment, resulting in exhaustion of the cardioprotective HSP. The exhaustion of HSPs results in the activation of endoplasmic reticulum (ER) stress, resulting in excessive activation of the autophagic protein degradation pathway. PH and AF also cause mitochondrial dysfunction, ROS production, and subsequent DNA damage and Ca2+ abnormalities, as well as mTOR-induced autophagy followed by activation of inflammation and endothelial dysfunction (ED) pathways. RTK = receptor tyrosine kinases; GPCR = G protein-coupled receptors; NOX = NADPH oxidases GF = growth factors, PARP1 = poly(ADP-ribose) polymerase 1; mTOR 1/2 = Mammalian target of rapamycin 1/2; PDK1= serine/threonine kinase-3′-phosphoinositide-dependent kinase 1; TSC1/2 = tuberous sclerosis complex 1/2; AMPK = adenosine monophosphate-activated protein kinase; Redd1 = DNA damage response 1; Rheb: GTP binding protein Ras homolog enriched in brain; PDGF: platelet-derived growth factor; EGF: epidermal growth factor; VEGF: vascular endothelial growth factor; IL-6: interleukin 6; TNF-α: tumor necrosis factor alpha; TGF-β: transforming growth factor beta; ALK: anaplastic lymphoma kinase; ET: endothelin; ATP = adenosine triphosphate; CaSR: calcium-sensing receptor;.
Proteins are complex macromolecules made up of a few to thousands of amino acid units and play a crucial role in the proper functioning of cells, including cardiomyocytes. Proteostasis plays an important role in preserving the integrity of proteins, which is maintained by protein quality control (PQC) and its derailment triggers specific stress-responsive and protein misfolding pathways. PQC prevents toxic aggregate formation by ensuring the correct folding, misfolding, and breakdown of malfunctioning proteins and it largely relies on chaperones, especially HSPs, protein degradation pathways, such as the ubiquitin–proteasome system (UPS) and macroautophagy pathways.
4.1. Role of Heat Shock Proteins
HSPs play a crucial role in the pathophysiology of PH and AF, particularly in modulating cellular stress responses and maintaining proteostasis [42,43,44]. In PAH, several studies have highlighted the involvement of HSP90 in vascular remodeling, a key factor influencing disease progression and outcomes in PAH patients [45]. Excessive proliferation of pulmonary artery smooth muscle cells (PASMCs), a hallmark of PAH, is closely linked to elevated HSP90 levels observed in both plasma and pulmonary arteriole membranes of PAH patients and experimental models [45]. HSP90 regulates proteins critical to PAH progression, and its mitochondrial accumulation in PASMCs contributes to abnormal cell proliferation and vascular remodeling—central features of PAH [45,46].
In contrast, the role of HSP70 in PH differs significantly. HSP70 is integral to the removal of damaged or misfolded proteins via the unfolded protein response (UPS), promoting cellular homeostasis [47]. However, in chronic thromboembolic pulmonary hypertension (CTEPH) patients and experimental models, HSP70 expression is notably downregulated, suggesting a diminished capacity to manage proteotoxic stress in this subtype of PH [48,49].
In AF, small heat shock proteins, particularly HSP27, are highly expressed in cardiomyocytes under normal conditions, where they stabilize cytoskeletal structures and support contractile and electrophysiological functions [50,51]. Experimental AF model systems, including HL-1 and canine atrial cardiomyocytes, have demonstrated a protective role of HSP27 in preserving cellular integrity and mitigating arrhythmogenic changes. However, during advanced stages of persistent AF, HSP27 levels in atrial tissue are significantly depleted, contributing to electrical conduction abnormalities, impaired calcium handling, altered action potential duration, and structural defects in cardiomyocytes [50]. However, in the advanced stages of persistent AF, levels of HSP27 in human atrial tissue become depleted, which is associated with structural damage and progression of the arrhythmia [52,53]. Similarly, HSP70, along with HSP27, has been linked to reduced incidences of post-operative AF, underscoring its protective role in AF [54,55,56]. Notably, experimental studies indicate that increasing HSP expression, either through genetic manipulation or pharmacological interventions, can mitigate pathological changes in both PH and AF. In particular, HSP27 has been shown to play a critical role in maintaining cardiac health by stabilizing cytoskeletal structures and counteracting stress-induced damage. These findings highlight the therapeutic potential of targeting HSPs to address the underlying mechanisms driving PH and AF progression [50,51].
4.2. Oxidative Stress and DNA Damage in PH and AF
Oxidative stress, marked by elevated ROS, has been widely implicated in DNA damage in both PH and AF [27,57,58]. NADPH oxidases (NOXs), particularly NOX1, NOX2, and NOX4, are major sources of ROS in PH, driving endothelial dysfunction, PASMC proliferation, and vascular remodeling, all hallmarks of PAH progression [59,60,61]. NOX-derived ROS activate growth factors, such as transforming growth factor beta (TGF-β1) and vascular endothelial growth factor (VEGF), further exacerbating oxidative damage and contributing to the pathophysiology of PH [62,63,64,65]. NOX-driven oxidative stress (NOX1, NOX2, and NOX4) also plays a key role in Group 2 Group 3, and Group 4 PH, contributing to endothelial dysfunction, myocardial fibrosis, and structural remodeling of the right ventricle [66,67]. The overexpression of NOX1 in AF results in increased Connexin 43 remodeling and subsequently microcirculatory dysfunction [68]. NOX2 overactivity triggers AF onset, leads to myofibril structural damage and abnormal sodium and potassium channel expressions, causing several electrical altercations [69,70]. Similarly, in human AF and in HL-1 derived cardiomyocyte model, NOX4 is associated with atrial fibrosis and mitochondrial dysfunction, leading to increased oxidative stress, abnormal calcium handling, and structural heart changes, all of which heighten susceptibility to AF [69,71,72]. This elevated oxidative stress led by the NOX supra family collectively shortens APD, promoting reentry and sustained AF [73,74].
Increased oxidative stress also activates DNA damage repair pathways, such as poly(ADP-ribose) polymerase 1 (PARP-1), in both PH and AF [26,33,75]. While PARP-1 facilitates DNA double-strand break repair, its overactivation depletes nicotinamide adenine dinucleotide (NAD+) stores, exacerbating mitochondrial dysfunction and inflammation [33]. Genetic studies in PH have identified mutations in BMPR2, SMAD9, and other DNA repair-related genes, which correlate with higher DNA damage and altered BMP signaling in PAH [33,76,77,78]. Studies on experimental models further suggest that inhibiting PARP-1 or other DNA repair proteins, such as PIM1, KU70, or EYA3, could provide therapeutic benefits by reducing cell proliferation and vascular remodeling [33,79,80,81].
Mitochondrial dysfunction is another key contributor to DNA damage in PAH and AF [82,83]. In PH, mitochondrial abnormalities, including reduced mitochondrial copy number and enhanced fission, correlate with increased glycolysis and ROS production [84,85,86]. PARP-1’s involvement in regulating mitochondrial energy metabolism suggests that it may contribute to these mitochondrial alterations [87]. In AF, mitochondrial DNA (mtDNA) damage, reflected in circulating markers such as 8-hydroxy-2′-deoxyguanosine (8-OHdG), escalates with disease progression [88]. PARP-1 overactivation in AF depletes NAD+, leading to impaired energy production and ROS-induced DNA damage, further worsening atrial remodeling and electrical dysfunction [89]. In addition, recent research findings revealed increased quantities of DNA lesions in atrial tissue and blood samples of patients with AF [90].
These findings highlight the central role of oxidative stress and DNA damage in driving structural and functional changes in PH and AF. Targeting ROS generation, DNA repair pathways, and mitochondrial dysfunction could present novel therapeutic avenues for both conditions.
4.3. Autophagic Protein Degradation
Autophagy, a highly conserved cellular process, plays a crucial role in cardiovascular diseases, particularly in PH and AF [31,42,91]. In various forms of PH, including idiopathic PAH (iPAH) and hypoxia-induced PH, autophagy markers such as light chain 3 (LC3)-B and its activated form LC3B-II are significantly elevated, along with decreased levels of p62, particularly in pulmonary arterial endothelial cells (PAECs) and PASMCs [92,93,94,95,96]. This suggests that autophagy is generally activated in response to PH, possibly contributing to disease progression by promoting vascular remodeling. The mechanistic target of rapamycin (mTOR) pathway plays a crucial role in regulating autophagy in PH, with pulmonary vascular remodeling associated with the downregulation of the mTOR pathway in lung tissues obtained from patients with PAH and in experimental PH animal models [93,97,98,99,100]. Furthermore, recent research has highlighted the role of other key players in autophagy regulation in PH, such as murine double minute 2 (MDM2), which is upregulated in both hypoxia-induced PH mouse models and kippah patients, leading to the degradation of angiotensin-converting enzyme 2 (ACE2), a key AMPK-dependent regulator in vasoconstriction [101]. In Group 1 PH patients, recent studies show that NLR-family pyrin domain containing 3 protein (NLRP3) activation exacerbates vascular remodeling, smooth muscle proliferation, and right ventricular fibrosis [102,103]. Additionally, hypoxia-inducible factor 1-alpha (HIF-1α) has been shown to induce pathological vascular remodeling in PAH by regulating autophagy-related genes [104,105].
In the context of AF, autophagy is involved in various aspects of disease progression, including atrial electrical, cellular, and energy metabolism remodeling [106]. Excessive autophagic protein degradation in AF is mainly regulated through endoplasmic reticulum (ER) stress, which is a critical factor in electrical and contractile dysfunction driving AF [106,107,108]. In a tachypaced mouse-derived HL-1 cardiomyocytes model, Wiersma et al. demonstrated that the overexpression of eukaryotic initiation factor 2α (eIF2α) prevents autophagy, which they also confirmed in a tachypaced Drosophila fly model by pharmacologically inhibiting ER stress with 4-phenyl butyrate (4PBA) [106]. Additionally, stress kinases, particularly JNK2, play a pivotal role in AF by orchestrating abnormal calcium handling [108]. JNK2 downregulates Cx43 and promotes diastolic calcium leak from the sarcoplasmic reticulum while simultaneously increasing the SR calcium content and creating a pro-arrhythmic environment [109,110] The AMPK pathway has also been implicated in AF [111], with the electrophysiological properties of atrial tissues in AMPK knockout mice showing an increase in atrial ectopic activity before the onset of AF, followed by delayed left atrial chamber enlargement [112,113]. Moreover, recent studies have identified a connection between AF and the mTOR signaling pathway [111,114], although further research is needed to understand the exact mechanism of mTOR driving autophagy in AF. The NLRP3 inflammasome has been identified as an important biomarker in many cardiovascular diseases, including AF [115,116], with the crosstalk between the PI3K/AKT/mTOR pathway and the NLRP3 inflammasome potentially influencing the autophagy response in AF [26]. HIF-1α has also been studied in AF in response to hypoxia in cardiomyocytes and in AF patients [117,118]. HIF-1α regulates VEGF which triggers AMPK-mediated transient autophagy [119]. Gramley et al. implicated a sustained increase in angiogenesis-related proteins, like HIF-1α and VEGF, in AF patients compared to SR patients, which suggests a direct role of HIF-1 in the regulation of autophagy in AF [120]. The intricate interplay between autophagy, mTOR/AMPK pathways, HIF-1α, and the NLRP3 inflammasome in PH and AF presents promising avenues for therapeutic interventions, with future research focusing on identifying specific autophagy markers, developing targeted therapies, and elucidating the complex interactions between autophagy, inflammation, and oxidative stress in cardiovascular diseases [115,117]. As research progresses, modulating autophagy may emerge as a promising strategy for managing PH and AF, ultimately improving patient outcomes in these challenging cardiovascular diseases.
4.4. Thromboembolism and Endothelial Dysfunction
Thromboembolism emerges as a significant complication in PH, particularly as the condition transitions from an acute to a chronic phase, characterized by endothelial dysfunction, platelet activation, inflammation, and coagulation abnormalities [121,122]. Numerous prospective studies on PAH have revealed enhanced regulation of adhesion molecules, including soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK), P-selectin, vascular cell adhesion molecule-1 (VCAM-1), and platelet-derived microparticles (PMPs), which facilitate platelet adhesion and activation, strongly correlated with RV dysfunction and disease progression [123,124,125,126]. Endothelial dysfunction is also associated with a decrease in thrombomodulin expression, which plays a critical role in inhibiting thrombin generation and promoting anticoagulant pathways [126]. In the context of PAH, activated platelets contribute to thrombus formation in response to heightened stress within the pulmonary circulation, further exacerbated by prothrombotic factors, like thromboxane A2 and adenosine diphosphate [127,128,129]. Additionally, endothelial dysfunction can intensify the activation cascade of various coagulation factors, including fibrinogen, factor VIII, and von Willebrand factor, which together elevate thromboembolic risk, although their precise contributions to PAH progression remain debated [130,131]. The activation of the fibrinolytic system is crucial in regulating clot formation; however, an imbalance has been observed in PAH, with increased levels of plasminogen activator inhibitor-1 (PAI-1) linked to impaired fibrinolysis [132]. This phenomenon parallels findings in AF, where elevated P-selectin and VCAM-1 levels indicate platelet activation and an inflammatory state, respectively, alongside increased thromboxane A2 and fibrinogen levels that may enhance thrombosis risk in patients with AF and chronic kidney disease [133,134,135,136].
4.5. Inflammation
Inflammation is a prominent feature in the progression of both PH and AF [137]. Histopathological analyses of pulmonary vascular tissues from patients with PAH and corresponding animal models reveal diverse inflammatory infiltrates, including macrophages, lymphocytes, and mast cells, which contribute to the inflammatory cascade [137]. In a rat model, the presence of a genetic mutation in BMPR2 was found to intensify the inflammatory response, indicating that altered immunity may be a causative factor in PH rather than a mere consequence [138]. Tamosiuniene et al. investigated the role of TGF-β as a key mediator in the inflammatory response, demonstrating that its activation through a SMAD-dependent pathway triggers vascular remodeling and inflammation in T-cell-deficient rats [139]. Supporting these findings, Price et al. observed increased nuclear p65 and activation of the NF-κB signaling pathway in isolated PAECs from patients with iPAH, which is instrumental in the activation of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) [140]. In response to endothelial dysfunction, various adhesion molecules (e.g., ICAM-1 and VCAM-1), chemokines (e.g., MCP-1), and pro-inflammatory cytokines (e.g., IL-1β) are upregulated, promoting leukocyte adhesion and infiltrating tissues, thereby exacerbating disease pathogenesis [141]. In AF, C-reactive protein (CRP), an acute-phase protein produced by the liver, has been implicated in the inflammatory process. Research by Dernellis et al. first demonstrated elevated CRP levels in AF patients, revealing a progressive increase from paroxysmal AF (ParAF) to persistent AF (PerAF), suggesting a correlation with disease risk and progression [142]. Subsequent studies have further clarified CRP’s role in inflammation and oxidative stress [143,144]. Additionally, the NLRP3 inflammasome has been implicated in AF-related inflammation, promoting the production of pro-inflammatory cytokines such as IL-1β and IL-18, ultimately leading to atrial remodeling and fibrosis [26,116]. Moreover, innate immunity plays a significant role in the inflammatory response in AF through Toll-like receptor (TLR) signaling. Studies in AF cohorts indicate that TLR signaling is upregulated, resulting in the production of pro-inflammatory and pro-fibrotic cytokines—including IL-6, IL-18, and IL-17A—which contribute to inflammation and subsequent structural and contractile remodeling processes in different stages of AF, such as ParAF and PerAF [145,146,147,148].
5. Novel Druggable Targets
Despite the significant clinical relevance, there have been no (pre)clinical trials addressing both PH and AF within a single model to date. In managing AF in patients with PH, rhythm control and rate control medications are commonly utilized [6,7,15]. Recent ESC/ERS guidelines [15] advocate for rhythm control agents, given their critical role in cardiac contractility under hemodynamic stress, despite substantial clinical challenges posed by both treatment strategies. While these guidelines emphasize tailored approaches, primarily utilizing class III antiarrhythmic drugs without negative inotropic effects (e.g., amiodarone), the advancement of the disease often renders these options unsuitable due to potential drug–drug interactions [149], underscoring the urgent need for developing more effective therapies for patients with concurrent PH and AF.
Several promising drug targets have emerged from preclinical research, aimed at restoring proteostasis, repairing DNA damage, mitigating mitochondrial dysfunction, and reversing inflammatory responses (Table 2). Notably, L-glutamine has been tested in clinical trials for both PH and AF, functioning as a potent inducer of HSP70, which helps to alleviate hemolysis and oxidative stress [133]. Additionally, targeting the proteostasis network can relieve ER stress; for instance, 4-phenylbutyrate (4PBA) has been recognized for its ability to decrease ER stress by reducing the burden of misfolded proteins, thereby preventing the activation of the UPR, critical in both AF and PH [106,150]. The inhibition of histone deacetylases (HDACs), particularly HDAC6, has also shown therapeutic promise by facilitating the degradation of misfolded proteins while mitigating PASMC proliferation in PH. Tubastatin A, an HDAC6-selective inhibitor, enhances microtubule stability and as such attenuates AF promotion and PAH [28,151]. Restoring NAD+ levels presents another strategy for reversing proteostasis disruption, as inhibiting PARP1 preserves NAD+ levels crucial for sirtuin function, with selective PARP1 inhibitors like ABT-888 showing potential benefits in both AF and PH [27,152]. Furthermore, nicotinamide, a precursor to NAD+, has demonstrated preventive effects on cardiomyocyte remodeling in AF models [27] and RV dysfunction in PH models [153]. Finally, while non-specific steroids and nonsteroidal anti-inflammatory drugs are frequently used to mitigate inflammation in both conditions, they often yield suboptimal patient responses [154,155,156], highlighting the need for targeted approaches, such as NLRP3 inflammasome inhibition. MCC950, a selective NLRP3 inhibitor, has been effective in reducing pro-inflammatory cytokine release in animal studies of both AF and PH, underscoring the importance of specifically targeting the NLRP3 pathway to address chronic inflammation [102,157,158].

Table 2.
Drugs with potential benefits that target key molecular modulators involved in PH and AF.
6. Knowledge Gaps
Key knowledge gaps in PH and AF research include a lack of understanding of the molecular pathways that connect the two conditions, particularly specific signaling pathways and genetic factors. While several molecular pathways implicated in AF pathogenesis have been described in PH, these mechanisms are not unique to this condition and are shared across a spectrum of chronic cardiac and pulmonary diseases [162,163]. As such, the extent to which these pathways serve as specific contributors to AF in PH, rather than reflecting broader cardiovascular remodeling processes, requires further investigation. Additionally, there are insufficient data on the role of genetic predispositions and epigenetic modifications in their development and progression. Insights into the specific signaling and genetic pathways may fuel the identification of novel druggable mechanism-based targets [22]. The potential bidirectional relationship between PH and AF is also poorly understood, with unclear mechanisms regarding how each condition may exacerbate the other. These studies are challenging due to heterogeneous patient populations, multifactorial disease etiologies, and limited cardiovascular phenotyping, which hinder efforts to track PH development in patients with AF. Furthermore, research on the efficacy and tolerability of current treatment approaches, such as rate and rhythm control medications in PH patients with AF, is limited, and standardized protocols across studies are lacking, leading to variability in treatment strategies and outcomes. Moreover, there is a need for advanced in vivo and ex vivo models that accurately replicate the pathophysiological conditions of both PH and AF. As an example, the monocrotaline-induced PAH rat model (MCT) often develops RV dysfunction and can be used to study AF induction in PAH [164,165]. Moreover, longitudinal studies to track the progression of PH and AF together over time are also warranted. Finally, the impact of comorbidities commonly associated with PH and AF on clinical outcomes and treatment responses is not well understood, highlighting the need for further research in these areas to inform more effective management of these complex conditions.
7. Conclusions
The complex interplay between PH and AF represents a significant clinical challenge, necessitating a comprehensive understanding of their shared pathophysiological mechanisms. By illuminating the molecular intersections between these conditions, this review highlights the urgent need for multidisciplinary research efforts to develop targeted treatments. Future directions in PH-AF research should prioritize the identification of novel therapeutic targets and the validation of mechanism-based approaches through (pre)clinical trials. The integration of cutting-edge technologies, such as genomics and systems biology, will be essential for deciphering the intricate relationships between PH and AF. Moreover, the implementation of precision medicine principles will permit the development of tailored therapeutic strategies tailored to the unique characteristics of each patient. Ultimately, a deeper understanding of the PH-AF connection holds the promise of improving patient outcomes, quality of life, and reducing mortality, paving the way for a brighter future for patients affected by these devastating comorbidities.
Funding
This research was funded by the NWA-ORC project CIRCULAR NWO (NWA.1389.20.157) and NWO under the umbrella of the Partnership Fostering a European Research Area for Health (ERA4Health, GA No 101095426 SK4BLOCK-AFH) EU Horizon Europe Research and Innovation Program CARDINNOV. K.Kurakula also acknowledges funding from the ERC (European Research Council) starting grant FEMALE-PH (pulmonary hypertension) and Amsterdam University Medical Center fellowship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kurakula, K.; Smolders, V.; Tura-Ceide, O.; Jukema, J.W.; Quax, P.H.A.; Goumans, M.J. Endothelial Dysfunction in Pulmonary Hypertension: Cause or Consequence? Biomedicines 2021, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Corris, P.A.; Seeger, W. Call it by the correct name-pulmonary hypertension not pulmonary arterial hypertension: Growing recognition of the global health impact for a well-recognized condition and the role of the Pulmonary Vascular Research Institute. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L992–L994. [Google Scholar] [CrossRef]
- Wanamaker, B.; Cascino, T.; McLaughlin, V.; Oral, H.; Latchamsetty, R.; Siontis, K.C. Atrial Arrhythmias in Pulmonary Hypertension: Pathogenesis, Prognosis and Management. Arrhythm. Electrophysiol. Rev. 2018, 7, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Rottlaender, D.; Motloch, L.J.; Schmidt, D.; Reda, S.; Larbig, R.; Wolny, M.; Dumitrescu, D.; Rosenkranz, S.; Erdmann, E.; Hoppe, U.C. Clinical impact of atrial fibrillation in patients with pulmonary hypertension. PLoS ONE 2012, 7, e33902. [Google Scholar] [CrossRef]
- Sammut, M.A.; Condliffe, R.; Elliot, C.; Hameed, A.; Lewis, R.; Kiely, D.G.; Kyriacou, A.; Middleton, J.T.; Raithatha, A.; Rothman, A.; et al. Atrial flutter and fibrillation in patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension in the ASPIRE registry: Comparison of rate versus rhythm control approaches. Int. J. Cardiol. 2023, 371, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Tongers, J.; Schwerdtfeger, B.; Klein, G.; Kempf, T.; Schaefer, A.; Knapp, J.M.; Niehaus, M.; Korte, T.; Hoeper, M.M. Incidence and clinical relevance of supraventricular tachyarrhythmias in pulmonary hypertension. Am. Heart J. 2007, 153, 127–132. [Google Scholar] [CrossRef]
- Ruiz-Cano, M.J.; Gonzalez-Mansilla, A.; Escribano, P.; Delgado, J.; Arribas, F.; Torres, J.; Flox, A.; Riva, M.; Gomez, M.A.; Saenz, C. Clinical implications of supraventricular arrhythmias in patients with severe pulmonary arterial hypertension. Int. J. Cardiol. 2011, 146, 105–106. [Google Scholar] [CrossRef]
- Olsson, K.M.; Nickel, N.P.; Tongers, J.; Hoeper, M.M. Atrial flutter and fibrillation in patients with pulmonary hypertension. Int. J. Cardiol. 2013, 167, 2300–2305. [Google Scholar] [CrossRef]
- Cannillo, M.; Grosso Marra, W.; Gili, S.; D’Ascenzo, F.; Morello, M.; Mercante, L.; Mistretta, E.; Salera, D.; Zema, D.; Bissolino, A.; et al. Supraventricular Arrhythmias in Patients With Pulmonary Arterial Hypertension. Am. J. Cardiol. 2015, 116, 1883–1889. [Google Scholar] [CrossRef]
- Wen, L.; Sun, M.L.; An, P.; Jiang, X.; Sun, K.; Zheng, L.; Liu, Q.Q.; Wang, L.; Zhao, Q.H.; He, J.; et al. Frequency of supraventricular arrhythmias in patients with idiopathic pulmonary arterial hypertension. Am. J. Cardiol. 2014, 114, 1420–1425. [Google Scholar] [CrossRef]
- Witte, C.; Meyer Zur Heide Genannt Meyer-Arend, J.U.; Andrié, R.; Schrickel, J.W.; Hammerstingl, C.; Schwab, J.O.; Nickenig, G.; Skowasch, D.; Pizarro, C. Heart Rate Variability and Arrhythmic Burden in Pulmonary Hypertension. Adv. Exp. Med. Biol. 2016, 934, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Genuardi, M.V.; Koczo, A.; Zou, R.H.; Thoma, F.W.; Handen, A.; Craig, E.; Hogan, C.M.; Girard, T.; Althouse, A.D.; et al. Atrial arrhythmias are associated with increased mortality in pulmonary arterial hypertension. Pulm. Circ. 2018, 8, 2045894018790316. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, V.; Peloquin, G.; Bourji, K.I.; Diab, N.; Sato, T.; Enobun, B.; Housten-Harris, T.; Damico, R.; Kolb, T.M.; Mathai, S.C.; et al. Pulmonary arterial hypertension and atrial arrhythmias: Incidence, risk factors, and clinical impact. Pulm. Circ. 2018, 8, 2045894018769874. [Google Scholar] [CrossRef] [PubMed]
- Fingrova, Z.; Ambroz, D.; Jansa, P.; Kuchar, J.; Lindner, J.; Kunstyr, J.; Aschermann, M.; Linhart, A.; Havranek, S. The prevalence and clinical outcome of supraventricular tachycardia in different etiologies of pulmonary hypertension. PLoS ONE 2021, 16, e0245752. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Pausch, C.; Grünig, E.; Klose, H.; Staehler, G.; Huscher, D.; Pittrow, D.; Olsson, K.M.; Vizza, C.D.; Gall, H.; et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J. Heart Lung Transplant. 2020, 39, 1435–1444. [Google Scholar] [CrossRef]
- Hansdottir, S.; Groskreutz, D.J.; Gehlbach, B.K. WHO’s in second?: A practical review of World Health Organization group 2 pulmonary hypertension. Chest 2013, 144, 638–650. [Google Scholar] [CrossRef]
- Al-Omary, M.S.; Sugito, S.; Boyle, A.J.; Sverdlov, A.L.; Collins, N.J. Pulmonary Hypertension Due to Left Heart Disease: Diagnosis, Pathophysiology, and Therapy. Hypertension 2020, 75, 1397–1408. [Google Scholar] [CrossRef]
- Gopinathannair, R.; Chen, L.Y.; Chung, M.K.; Cornwell, W.K.; Furie, K.L.; Lakkireddy, D.R.; Marrouche, N.F.; Natale, A.; Olshansky, B.; Joglar, J.A. Managing Atrial Fibrillation in Patients With Heart Failure and Reduced Ejection Fraction: A Scientific Statement From the American Heart Association. Circ. Arrhythm. Electrophysiol. 2021, 14, e000078. [Google Scholar] [CrossRef]
- Rådegran, G.; Kjellström, B.; Ekmehag, B.; Larsen, F.; Rundqvist, B.; Blomquist, S.B.; Gustafsson, C.; Hesselstrand, R.; Karlsson, M.; Kornhall, B.; et al. Characteristics and survival of adult Swedish PAH and CTEPH patients 2000–2014. Scand. Cardiovasc. J. 2016, 50, 243–250. [Google Scholar] [CrossRef]
- Brundel, B.; Ai, X.; Hills, M.T.; Kuipers, M.F.; Lip, G.Y.H.; de Groot, N.M.S. Atrial fibrillation. Nat. Rev. Dis. Primers 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Heidbuchel, H.; Dagres, N.; Antz, M.; Kuck, K.H.; Lazure, P.; Murray, S.; Carrera, C.; Hindricks, G.; Vahanian, A. Major knowledge gaps and system barriers to guideline implementation among European physicians treating patients with atrial fibrillation: A European Society of Cardiology international educational needs assessment. Europace 2018, 20, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Deyell, M.W.; Bennett, R.; Macle, L. Assessment and management of asymptomatic atrial fibrillation. Heart 2024, 110, 675–682. [Google Scholar] [CrossRef]
- Kotecha, D.; Piccini, J.P. Atrial fibrillation in heart failure: What should we do? Eur. Heart J. 2015, 36, 3250–3257. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Borlaug, B.A. Pulmonary hypertension due to left heart disease. Circulation 2012, 126, 975–990. [Google Scholar] [CrossRef]
- Li, N.; Brundel, B. Inflammasomes and Proteostasis Novel Molecular Mechanisms Associated With Atrial Fibrillation. Circ. Res. 2020, 127, 73–90. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, X.; Li, J.; Liu, J.; Baks-Te Bulte, L.; Wiersma, M.; Malik, N.U.; van Marion, D.M.S.; Tolouee, M.; Hoogstra-Berends, F.; et al. DNA damage-induced PARP1 activation confers cardiomyocyte dysfunction through NAD+ depletion in experimental atrial fibrillation. Nat. Commun. 2019, 10, 1307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, C.T.; Qi, X.; Meijering, R.A.; Hoogstra-Berends, F.; Tadevosyan, A.; Cubukcuoglu Deniz, G.; Durdu, S.; Akar, A.R.; Sibon, O.C.; et al. Activation of histone deacetylase-6 induces contractile dysfunction through derailment of alpha-tubulin proteostasis in experimental and human atrial fibrillation. Circulation 2014, 129, 346–358. [Google Scholar] [CrossRef]
- van Wijk, S.W.; Ramos, K.S.; Brundel, B. Cardioprotective Role of Heat Shock Proteins in Atrial Fibrillation: From Mechanism of Action to Therapeutic and Diagnostic Target. Int. J. Mol. Sci. 2021, 22, 442. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Zhao, Q. Effects of Inflammatory Cell Death Caused by Catheter Ablation on Atrial Fibrillation. J. Inflamm. Res. 2023, 16, 3491–3508. [Google Scholar] [CrossRef]
- Gomez-Puerto, M.C.; van Zuijen, I.; Huang, C.J.; Szulcek, R.; Pan, X.; van Dinther, M.A.; Kurakula, K.; Wiesmeijer, C.C.; Goumans, M.J.; Bogaard, H.J.; et al. Autophagy contributes to BMP type 2 receptor degradation and development of pulmonary arterial hypertension. J. Pathol. 2019, 249, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Van der Feen, D.E.; Kurakula, K.; Tremblay, E.; Boucherat, O.; Bossers, G.P.L.; Szulcek, R.; Bourgeois, A.; Lampron, M.C.; Habbout, K.; Martineau, S.; et al. Multicenter Preclinical Validation of BET Inhibition for the Treatment of Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2019, 200, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Aldred, M.A. DNA Damage and Repair in Pulmonary Arterial Hypertension. Genes. 2020, 11, 1224. [Google Scholar] [CrossRef]
- Kurakula, K.; Hagdorn, Q.A.J.; van der Feen, D.E.; Vonk Noordegraaf, A.; Ten Dijke, P.; de Boer, R.A.; Bogaard, H.J.; Goumans, M.J.; Berger, R.M.F. Inhibition of the prolyl isomerase Pin1 improves endothelial function and attenuates vascular remodelling in pulmonary hypertension by inhibiting TGF-beta signalling. Angiogenesis 2022, 25, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Rubin, L.J. Metabolic dysfunction in pulmonary hypertension: From basic science to clinical practice. Eur. Respir. Rev. 2017, 26, 170094. [Google Scholar] [CrossRef]
- Zakynthinos, G.E.; Tsolaki, V.; Oikonomou, E.; Vavouranakis, M.; Siasos, G.; Zakynthinos, E. Metabolic Syndrome and Atrial Fibrillation: Different Entities or Combined Disorders. J. Pers. Med. 2023, 13, 1323. [Google Scholar] [CrossRef]
- Ranchoux, B.; Nadeau, V.; Bourgeois, A.; Provencher, S.; Tremblay, É.; Omura, J.; Coté, N.; Abu-Alhayja’a, R.; Dumais, V.; Nachbar, R.T.; et al. Metabolic Syndrome Exacerbates Pulmonary Hypertension due to Left Heart Disease. Circ. Res. 2019, 125, 449–466. [Google Scholar] [CrossRef]
- Havranek, S.; Fingrova, Z.; Skala, T.; Reichenbach, A.; Dusik, M.; Jansa, P.; Ambroz, D.; Dytrych, V.; Klimes, D.; Hutyra, M.; et al. Catheter ablation of atrial fibrillation and atrial tachycardia in patients with pulmonary hypertension: A randomized study. Europace 2023, 25, euad131. [Google Scholar] [CrossRef]
- Medi, C.; Kalman, J.M.; Ling, L.H.; Teh, A.W.; Lee, G.; Lee, G.; Spence, S.J.; Kaye, D.M.; Kistler, P.M. Atrial electrical and structural remodeling associated with longstanding pulmonary hypertension and right ventricular hypertrophy in humans. J. Cardiovasc. Electrophysiol. 2012, 23, 614–620. [Google Scholar] [CrossRef]
- Sanders, P.; Morton, J.B.; Davidson, N.C.; Spence, S.J.; Vohra, J.K.; Sparks, P.B.; Kalman, J.M. Electrical remodeling of the atria in congestive heart failure: Electrophysiological and electroanatomic mapping in humans. Circulation 2003, 108, 1461–1468. [Google Scholar] [CrossRef]
- Hiram, R.; Provencher, S. Pulmonary Disease, Pulmonary Hypertension and Atrial Fibrillation. Card. Electrophysiol. Clin. 2021, 13, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.H.; Brundel, B. Proteostasis in cardiac health and disease. Nat. Rev. Cardiol. 2017, 14, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Archer, S.L.; Dorfmüller, P.; Erzurum, S.C.; Guignabert, C.; Michelakis, E.; Rabinovitch, M.; Schermuly, R.; Stenmark, K.R.; Morrell, N.W. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D4–D12. [Google Scholar] [CrossRef] [PubMed]
- Sakao, S.; Tatsumi, K. Vascular remodeling in pulmonary arterial hypertension: Multiple cancer-like pathways and possible treatment modalities. Int. J. Cardiol. 2011, 147, 4–12. [Google Scholar] [CrossRef]
- Wang, G.K.; Li, S.H.; Zhao, Z.M.; Liu, S.X.; Zhang, G.X.; Yang, F.; Wang, Y.; Wu, F.; Zhao, X.X.; Xu, Z.Y. Inhibition of heat shock protein 90 improves pulmonary arteriole remodeling in pulmonary arterial hypertension. Oncotarget 2016, 7, 54263–54273. [Google Scholar] [CrossRef]
- Boucherat, O.; Peterlini, T.; Bourgeois, A.; Nadeau, V.; Breuils-Bonnet, S.; Boilet-Molez, S.; Potus, F.; Meloche, J.; Chabot, S.; Lambert, C.; et al. Mitochondrial HSP90 Accumulation Promotes Vascular Remodeling in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 90–103. [Google Scholar] [CrossRef]
- Rodriguez-Iturbe, B.; Johnson, R.J.; Sanchez-Lozada, L.G.; Pons, H. HSP70 and Primary Arterial Hypertension. Biomolecules 2023, 13, 272. [Google Scholar] [CrossRef]
- Salibe-Filho, W.; Araujo, T.L.; GMelo, E.; BCTCoimbra, L.; Lapa, M.S.; Acencio, M.M.; Freitas-Filho, O.; Capelozzi, V.L.; Teixeira, L.R.; Fernandes, C.J.; et al. Shear stress-exposed pulmonary artery endothelial cells fail to upregulate HSP70 in chronic thromboembolic pulmonary hypertension. PLoS ONE 2020, 15, e0242960. [Google Scholar] [CrossRef]
- Cao, J.; Yang, L.; Wang, L.; Zhao, Q.; Wu, D.; Li, M.; Mu, Y. Heat shock protein 70 attenuates hypoxia-induced apoptosis of pulmonary microvascular endothelial cells isolated from neonatal rats. Mol. Med. Rep. 2021, 24, 690. [Google Scholar] [CrossRef]
- Brundel, B.J.; Shiroshita-Takeshita, A.; Qi, X.; Yeh, Y.H.; Chartier, D.; van Gelder, I.C.; Henning, R.H.; Kampinga, H.H.; Nattel, S. Induction of heat shock response protects the heart against atrial fibrillation. Circ. Res. 2006, 99, 1394–1402. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; van Marion, D.M.S.; Zhang, D.; Brundel, B. Heat shock protein inducer GGA*-59 reverses contractile and structural remodeling via restoration of the microtubule network in experimental Atrial Fibrillation. J. Mol. Cell. Cardiol. 2019, 134, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Brundel, B.J.; Henning, R.H.; Ke, L.; van Gelder, I.C.; Crijns, H.J.; Kampinga, H.H. Heat shock protein upregulation protects against pacing-induced myolysis in HL-1 atrial myocytes and in human atrial fibrillation. J. Mol. Cell. Cardiol. 2006, 41, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Yeh, H.I.; Tsao, H.M.; Tai, C.T.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Tuan, T.C.; Suenari, K.; Li, C.H.; et al. Electrophysiological correlation and prognostic impact of heat shock protein 27 in atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2012, 5, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Mandal, K.; Torsney, E.; Poloniecki, J.; Camm, A.J.; Xu, Q.; Jahangiri, M. Association of high intracellular, but not serum, heat shock protein 70 with postoperative atrial fibrillation. Ann. Thorac. Surg. 2005, 79, 865–871; discussion 871. [Google Scholar] [CrossRef]
- St Rammos, K.; Koullias, G.J.; Hassan, M.O.; Argyrakis, N.P.; Voucharas, C.G.; Scarupa, S.J.; Cowte, T.G. Low preoperative HSP70 atrial myocardial levels correlate significantly with high incidence of postoperative atrial fibrillation after cardiac surgery. Cardiovasc. Surg. 2002, 10, 228–232. [Google Scholar] [CrossRef]
- Cao, H.; Xue, L.; Xu, X.; Wu, Y.; Zhu, J.; Chen, L.; Chen, D.; Chen, Y. Heat shock proteins in stabilization of spontaneously restored sinus rhythm in permanent atrial fibrillation patients after mitral valve surgery. Cell Stress Chaperones 2011, 16, 517–528. [Google Scholar] [CrossRef]
- Federici, C.; Drake, K.M.; Rigelsky, C.M.; McNelly, L.N.; Meade, S.L.; Comhair, S.A.; Erzurum, S.C.; Aldred, M.A. Increased Mutagen Sensitivity and DNA Damage in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2015, 192, 219–228. [Google Scholar] [CrossRef]
- Meloche, J.; Pflieger, A.; Vaillancourt, M.; Paulin, R.; Potus, F.; Zervopoulos, S.; Graydon, C.; Courboulin, A.; Breuils-Bonnet, S.; Tremblay, E.; et al. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation 2014, 129, 786–797. [Google Scholar] [CrossRef]
- Stam, K.; Cai, Z.; van der Velde, N.; van Duin, R.; Lam, E.; van der Velden, J.; Hirsch, A.; Duncker, D.J.; Merkus, D. Cardiac remodelling in a swine model of chronic thromboembolic pulmonary hypertension: Comparison of right vs. left ventricle. J. Physiol. 2019, 597, 4465–4480. [Google Scholar] [CrossRef]
- Wang, E.L.; Jia, M.M.; Luo, F.M.; Li, T.; Peng, J.J.; Luo, X.J.; Song, F.L.; Yang, J.F.; Peng, J.; Liu, B. Coordination between NADPH oxidase and vascular peroxidase 1 promotes dysfunctions of endothelial progenitor cells in hypoxia-induced pulmonary hypertensive rats. Eur. J. Pharmacol. 2019, 857, 172459. [Google Scholar] [CrossRef]
- Agarwal, S.; Sharma, H.; Chen, L.; Dhillon, N.K. NADPH oxidase-mediated endothelial injury in HIV- and opioid-induced pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1097–L1108. [Google Scholar] [CrossRef]
- Xu, D.; Hu, Y.H.; Gou, X.; Li, F.Y.; Yang, X.Y.; Li, Y.M.; Chen, F. Oxidative Stress and Antioxidative Therapy in Pulmonary Arterial Hypertension. Molecules 2022, 27, 3724. [Google Scholar] [CrossRef]
- Wu, J.; Pan, W.; Wang, C.; Dong, H.; Xing, L.; Hou, J.; Fang, S.; Li, H.; Yang, F.; Yu, B. H(2)S attenuates endoplasmic reticulum stress in hypoxia-induced pulmonary artery hypertension. Biosci. Rep. 2019, 39, 304. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Senchanthisai, S.; Sowden, M.; Pang, J.; White, R.J.; Berk, B.C. Endothelial-to-Mesenchymal Transition and Inflammation Play Key Roles in Cyclophilin A-Induced Pulmonary Arterial Hypertension. Hypertension 2020, 76, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R. Signaling Pathways Involved in the Development of Bronchopulmonary Dysplasia and Pulmonary Hypertension. Children 2020, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, P.; Gratacós, M.; Samuel, K.; Orriols, R.; Tura-Ceide, O. Oxidative Stress and Antioxidant Therapy in Pulmonary Hypertension. Antioxidants 2023, 12, 1006. [Google Scholar] [CrossRef]
- Huetsch, J.C.; Suresh, K.; Shimoda, L.A. Regulation of Smooth Muscle Cell Proliferation by NADPH Oxidases in Pulmonary Hypertension. Antioxidants 2019, 8, 56. [Google Scholar] [CrossRef]
- Dudley, S.C., Jr.; Hoch, N.E.; McCann, L.A.; Honeycutt, C.; Diamandopoulos, L.; Fukai, T.; Harrison, D.G.; Dikalov, S.I.; Langberg, J. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: Role of the NADPH and xanthine oxidases. Circulation 2005, 112, 1266–1273. [Google Scholar] [CrossRef]
- Nabeebaccus, A.A.; Reumiller, C.M.; Shen, J.; Zoccarato, A.; Santos, C.X.C.; Shah, A.M. The regulation of cardiac intermediary metabolism by NADPH oxidases. Cardiovasc. Res. 2023, 118, 3305–3319. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kattach, H.; Ratnatunga, C.; Pillai, R.; Channon, K.M.; Casadei, B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J. Am. Coll. Cardiol. 2008, 51, 68–74. [Google Scholar] [CrossRef]
- Chen, W.J.; Chang, S.H.; Chan, Y.H.; Lee, J.L.; Lai, Y.J.; Chang, G.J.; Tsai, F.C.; Yeh, Y.H. Tachycardia-induced CD44/NOX4 signaling is involved in the development of atrial remodeling. J. Mol. Cell. Cardiol. 2019, 135, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Youn, J.Y.; Kim, A.Y.; Ramirez, R.J.; Gao, L.; Ngo, D.; Chen, P.; Scovotti, J.; Mahajan, A.; Cai, H. NOX4-Dependent Hydrogen Peroxide Overproduction in Human Atrial Fibrillation and HL-1 Atrial Cells: Relationship to Hypertension. Front. Physiol. 2012, 3, 140. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.C.; Barca, E.; Subramanyam, P.; Komrowski, M.; Pajvani, U.; Colecraft, H.M.; Hirano, M.; Morrow, J.P. Inhibition of NAPDH Oxidase 2 (NOX2) Prevents Oxidative Stress and Mitochondrial Abnormalities Caused by Saturated Fat in Cardiomyocytes. PLoS ONE 2016, 11, e0145750. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Mondragón, R.; Lozhkin, A.; Vendrov, A.E.; Runge, M.S.; Isom, L.L.; Madamanchi, N.R. NADPH Oxidases and Oxidative Stress in the Pathogenesis of Atrial Fibrillation. Antioxidants 2023, 12, 1833. [Google Scholar] [CrossRef]
- Ramos, K.S.; Brundel, B. DNA Damage, an Innocent Bystander in Atrial Fibrillation and Other Cardiovascular Diseases? Front. Cardiovasc. Med. 2020, 7, 67. [Google Scholar] [CrossRef]
- Newman, J.H.; Wheeler, L.; Lane, K.B.; Loyd, E.; Gaddipati, R.; Phillips, J.A., 3rd; Loyd, J.E. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N. Engl. J. Med. 2001, 345, 319–324. [Google Scholar] [CrossRef]
- Ma, L.; Roman-Campos, D.; Austin, E.D.; Eyries, M.; Sampson, K.S.; Soubrier, F.; Germain, M.; Trégouët, D.A.; Borczuk, A.; Rosenzweig, E.B.; et al. A novel channelopathy in pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 351–361. [Google Scholar] [CrossRef]
- Drake, K.M.; Comhair, S.A.; Erzurum, S.C.; Tuder, R.M.; Aldred, M.A. Endothelial chromosome 13 deletion in congenital heart disease-associated pulmonary arterial hypertension dysregulates SMAD9 signaling. Am. J. Respir. Crit. Care Med. 2015, 191, 850–854. [Google Scholar] [CrossRef]
- Caron, M.C.; Sharma, A.K.; O’Sullivan, J.; Myler, L.R.; Ferreira, M.T.; Rodrigue, A.; Coulombe, Y.; Ethier, C.; Gagné, J.P.; Langelier, M.F.; et al. Poly(ADP-ribose) polymerase-1 antagonizes DNA resection at double-strand breaks. Nat. Commun. 2019, 10, 2954. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Lampron, M.C.; Vitry, G.; Nadeau, V.; Grobs, Y.; Paradis, R.; Samson, N.; Tremblay, È.; Boucherat, O.; Meloche, J.; Bonnet, S.; et al. PIM1 (Moloney Murine Leukemia Provirus Integration Site) Inhibition Decreases the Nonhomologous End-Joining DNA Damage Repair Signaling Pathway in Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J.P.; West, J.D. Redox biology in pulmonary arterial hypertension (2013 Grover Conference Series). Pulm. Circ. 2015, 5, 599–609. [Google Scholar] [CrossRef]
- Pool, L.; Wijdeveld, L.; de Groot, N.M.S.; Brundel, B. The Role of Mitochondrial Dysfunction in Atrial Fibrillation: Translation to Druggable Target and Biomarker Discovery. Int. J. Mol. Sci. 2021, 22, 8463. [Google Scholar] [CrossRef]
- Courboulin, A.; Paulin, R.; Giguère, N.J.; Saksouk, N.; Perreault, T.; Meloche, J.; Paquet, E.R.; Biardel, S.; Provencher, S.; Côté, J.; et al. Role for miR-204 in human pulmonary arterial hypertension. J. Exp. Med. 2011, 208, 535–548. [Google Scholar] [CrossRef]
- Marsboom, G.; Toth, P.T.; Ryan, J.J.; Hong, Z.; Wu, X.; Fang, Y.H.; Thenappan, T.; Piao, L.; Zhang, H.J.; Pogoriler, J.; et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ. Res. 2012, 110, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Paulin, R.; Meloche, J.; Jacob, M.H.; Bisserier, M.; Courboulin, A.; Bonnet, S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1798–H1809. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.M.; Kong, X.; Moncada, E.; Chen, Y.; Imamura, H.; Wang, P.; Berns, M.W.; Yokomori, K.; Digman, M.A. NAD+ consumption by PARP1 in response to DNA damage triggers metabolic shift critical for damaged cell survival. Mol. Biol. Cell 2019, 30, 2584–2597. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Ramos, K.S.; Baks, L.; Wiersma, M.; Lanters, E.A.H.; Bogers, A.; de Groot, N.M.S.; Brundel, B. Blood-based 8-hydroxy-2′-deoxyguanosine level: A potential diagnostic biomarker for atrial fibrillation. Heart Rhythm 2021, 18, 271–277. [Google Scholar] [CrossRef]
- Wiersma, M.; van Marion, D.M.S.; Bouman, E.J.; Li, J.; Zhang, D.; Ramos, K.S.; Lanters, E.A.H.; de Groot, N.M.S.; Brundel, B. Cell-Free Circulating Mitochondrial DNA: A Potential Blood-Based Marker for Atrial Fibrillation. Cells 2020, 9, 1159. [Google Scholar] [CrossRef]
- Pool, L.; van Wijk, S.W.; van Schie, M.S.; Taverne, Y.; de Groot, N.M.S.; Brundel, B. Quantifying DNA Lesions and Circulating Free DNA: Diagnostic Marker for Electropathology and Clinical Stage of AF. JACC Clin. Electrophysiol. 2024, 11, 321–332. [Google Scholar] [CrossRef]
- Zhang, C.F.; Zhao, F.Y.; Xu, S.L.; Liu, J.; Xing, X.Q.; Yang, J. Autophagy in pulmonary hypertension: Emerging roles and therapeutic implications. J. Cell. Physiol. 2019, 234, 16755–16767. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Smith, A.; Guo, L.; Alastalo, T.P.; Li, M.; Sawada, H.; Liu, X.; Chen, Z.H.; Ifedigbo, E.; Jin, Y.; et al. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2011, 183, 649–658. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Wang, L.; Qu, L.; Zhu, X.; Li, M.; Dang, X.; Li, P.; Gao, Y.; Peng, Z.; et al. Mammalian target of rapamycin overexpression antagonizes chronic hypoxia-triggered pulmonary arterial hypertension via the autophagic pathway. Int. J. Mol. Med. 2015, 36, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Teng, R.J.; Du, J.; Welak, S.; Guan, T.; Eis, A.; Shi, Y.; Konduri, G.G. Cross talk between NADPH oxidase and autophagy in pulmonary artery endothelial cells with intrauterine persistent pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L651–L663. [Google Scholar] [CrossRef]
- Dalvi, P.; Sharma, H.; Chinnappan, M.; Sanderson, M.; Allen, J.; Zeng, R.; Choi, A.; O’Brien-Ladner, A.; Dhillon, N.K. Enhanced autophagy in pulmonary endothelial cells on exposure to HIV-Tat and morphine: Role in HIV-related pulmonary arterial hypertension. Autophagy 2016, 12, 2420–2438. [Google Scholar] [CrossRef]
- Long, L.; Yang, X.; Southwood, M.; Lu, J.; Marciniak, S.J.; Dunmore, B.J.; Morrell, N.W. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ. Res. 2013, 112, 1159–1170. [Google Scholar] [CrossRef]
- Kato, F.; Sakao, S.; Takeuchi, T.; Suzuki, T.; Nishimura, R.; Yasuda, T.; Tanabe, N.; Tatsumi, K. Endothelial cell-related autophagic pathways in Sugen/hypoxia-exposed pulmonary arterial hypertensive rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L899–L915. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Manhas, A.; Kaur, G.; Jagavelu, K.; Hanif, K. Inhibition of fatty acid synthase is protective in pulmonary hypertension. Br. J. Pharmacol. 2016, 173, 2030–2045. [Google Scholar] [CrossRef]
- Goncharov, D.A.; Kudryashova, T.V.; Ziai, H.; Ihida-Stansbury, K.; DeLisser, H.; Krymskaya, V.P.; Tuder, R.M.; Kawut, S.M.; Goncharova, E.A. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation 2014, 129, 864–874. [Google Scholar] [CrossRef]
- He, Y.; Zuo, C.; Jia, D.; Bai, P.; Kong, D.; Chen, D.; Liu, G.; Li, J.; Wang, Y.; Chen, G.; et al. Loss of DP1 Aggravates Vascular Remodeling in Pulmonary Arterial Hypertension via mTORC1 Signaling. Am. J. Respir. Crit. Care Med. 2020, 201, 1263–1276. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, J.; Wang, C.; Jain, P.P.; Xiong, M.; Shi, X.; Lei, Y.; Chen, S.; Yin, Q.; Thistlethwaite, P.A.; et al. MDM2-Mediated Ubiquitination of Angiotensin-Converting Enzyme 2 Contributes to the Development of Pulmonary Arterial Hypertension. Circulation 2020, 142, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Al-Qazazi, R.; Lima, P.D.A.; Prisco, S.Z.; Potus, F.; Dasgupta, A.; Chen, K.H.; Tian, L.; Bentley, R.E.T.; Mewburn, J.; Martin, A.Y.; et al. Macrophage-NLRP3 Activation Promotes Right Ventricle Failure in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2022, 206, 608–624. [Google Scholar] [CrossRef]
- Ren, X.; Johns, R.A.; Gao, W.D. EXPRESS: Right Heart in Pulmonary Hypertension: From Adaptation to Failure. Pulm. Circ. 2019, 9, 2045894019845611. [Google Scholar] [CrossRef] [PubMed]
- Cowburn, A.S.; Crosby, A.; Macias, D.; Branco, C.; Colaço, R.D.; Southwood, M.; Toshner, M.; Crotty Alexander, L.E.; Morrell, N.W.; Chilvers, E.R.; et al. HIF2α-arginase axis is essential for the development of pulmonary hypertension. Proc. Natl. Acad. Sci. USA 2016, 113, 8801–8806. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Shen, C.; Tan, J.; Wu, Z.; Wang, W.; Chen, Y.; Dai, Y.; Yang, X.; Ye, S.; Chen, J.; et al. Periostin: A Potential Therapeutic Target For Pulmonary Hypertension? Circ. Res. 2020, 127, 1138–1152. [Google Scholar] [CrossRef]
- Wiersma, M.; Meijering, R.A.M.; Qi, X.Y.; Zhang, D.; Liu, T.; Hoogstra-Berends, F.; Sibon, O.C.M.; Henning, R.H.; Nattel, S.; Brundel, B. Endoplasmic Reticulum Stress Is Associated With Autophagy and Cardiomyocyte Remodeling in Experimental and Human Atrial Fibrillation. J. Am. Heart Assoc. 2017, 6, e006458. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Brundel, B.; Wiersma, M. Imbalance of ER and Mitochondria Interactions: Prelude to Cardiac Ageing and Disease? Cells 2019, 8, 1617. [Google Scholar] [CrossRef]
- Yan, J.; Thomson, J.K.; Zhao, W.; Wu, X.; Gao, X.; DeMarco, D.; Kong, W.; Tong, M.; Sun, J.; Bakhos, M.; et al. The stress kinase JNK regulates gap junction Cx43 gene expression and promotes atrial fibrillation in the aged heart. J. Mol. Cell. Cardiol. 2018, 114, 105–115. [Google Scholar] [CrossRef]
- Yan, J.; Bare, D.J.; DeSantiago, J.; Zhao, W.; Mei, Y.; Chen, Z.; Ginsburg, K.; Solaro, R.J.; Wolska, B.M.; Bers, D.M.; et al. JNK2, a Newly-Identified SERCA2 Enhancer, Augments an Arrhythmic [Ca2+](SR) Leak-Load Relationship. Circ. Res. 2021, 128, 455–470. [Google Scholar] [CrossRef]
- Yan, J.; Kong, W.; Zhang, Q.; Beyer, E.C.; Walcott, G.; Fast, V.G.; Ai, X. c-Jun N-terminal kinase activation contributes to reduced connexin43 and development of atrial arrhythmias. Cardiovasc. Res. 2013, 97, 589–597. [Google Scholar] [CrossRef]
- Wei, Z.; Bing, Z.; Shaohuan, Q.; Yanran, W.; Shuo, S.; Bi, T.; Feiyu, Z.; Heng, Z.; Qin, G.; Pinfang, K. Expression of miRNAs in plasma exosomes derived from patients with atrial fibrillation. Clin. Cardiol. 2020, 43, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Su, K.N.; Ma, Y.; Cacheux, M.; Ilkan, Z.; Raad, N.; Muller, G.K.; Wu, X.; Guerrera, N.; Thorn, S.L.; Sinusas, A.J.; et al. Atrial AMP-activated protein kinase is critical for prevention of dysregulation of electrical excitability and atrial fibrillation. JCI Insight 2022, 7, e141213. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Schiattarella, G.G.; Jiang, N.; Daou, D.; Luo, Y.; Link, M.S.; Lavandero, S.; Gillette, T.G.; Hill, J.A. Impaired AMP-Activated Protein Kinase Signaling in Heart Failure With Preserved Ejection Fraction-Associated Atrial Fibrillation. Circulation 2022, 146, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Ebana, Y.; Sun, Y.; Yang, X.; Watanabe, T.; Makita, S.; Ozaki, K.; Tanaka, T.; Arai, H.; Furukawa, T. Pathway analysis with genome-wide association study (GWAS) data detected the association of atrial fibrillation with the mTOR signaling pathway. Int. J. Cardiol. Heart Vasc. 2019, 24, 100383. [Google Scholar] [CrossRef]
- Bullón, P.; Cano-García, F.J.; Alcocer-Gómez, E.; Varela-López, A.; Roman-Malo, L.; Ruiz-Salmerón, R.J.; Quiles, J.L.; Navarro-Pando, J.M.; Battino, M.; Ruiz-Cabello, J.; et al. Could NLRP3-Inflammasome Be a Cardiovascular Risk Biomarker in Acute Myocardial Infarction Patients? Antioxid. Redox Signal 2017, 27, 269–275. [Google Scholar] [CrossRef]
- Yao, C.; Veleva, T.; Scott, L., Jr.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.D.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factor 1 and cardiovascular disease. Annu. Rev. Physiol. 2014, 76, 39–56. [Google Scholar] [CrossRef]
- Babapoor-Farrokhran, S.; Gill, D.; Alzubi, J.; Mainigi, S.K. Atrial fibrillation: The role of hypoxia-inducible factor-1-regulated cytokines. Mol. Cell. Biochem. 2021, 476, 2283–2293. [Google Scholar] [CrossRef]
- Spengler, K.; Kryeziu, N.; Große, S.; Mosig, A.S.; Heller, R. VEGF Triggers Transient Induction of Autophagy in Endothelial Cells via AMPKα1. Cells 2020, 9, 687. [Google Scholar] [CrossRef]
- Gramley, F.; Lorenzen, J.; Jedamzik, B.; Gatter, K.; Koellensperger, E.; Munzel, T.; Pezzella, F. Atrial fibrillation is associated with cardiac hypoxia. Cardiovasc. Pathol. 2010, 19, 102–111. [Google Scholar] [CrossRef]
- Coons, J.C.; Pogue, K.; Kolodziej, A.R.; Hirsch, G.A.; George, M.P. Pulmonary Arterial Hypertension: A Pharmacotherapeutic Update. Curr. Cardiol. Rep. 2019, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Farber, H.W.; Ghofrani, H.A.; Benza, R.L.; Busse, D.; Meier, C.; Hoeper, M.M. Risk assessment in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1802004. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczyk, R.; Błaszczak, P.; Kowal, K.; Jasiewicz, M.; Knapp, M.; Szpakowicz, A.; Ptaszyńska-Kopczyńska, K.; Sobkowicz, B.; Waszkiewicz, E.; Grzywna, R.; et al. The significance of diminished sTWEAK and P-selectin content in platelets of patients with pulmonary arterial hypertension. Cytokine 2018, 107, 52–58. [Google Scholar] [CrossRef]
- Szulcek, R.; Happé, C.M.; Rol, N.; Fontijn, R.D.; Dickhoff, C.; Hartemink, K.J.; Grünberg, K.; Tu, L.; Timens, W.; Nossent, G.D.; et al. Delayed Microvascular Shear Adaptation in Pulmonary Arterial Hypertension. Role of Platelet Endothelial Cell Adhesion Molecule-1 Cleavage. Am. J. Respir. Crit. Care Med. 2016, 193, 1410–1420. [Google Scholar] [CrossRef]
- Ogawa, A.; Matsubara, H. Increased levels of platelet-derived microparticles in pulmonary hypertension. Thromb. Res. 2020, 195, 120–124. [Google Scholar] [CrossRef]
- Hickey, P.M.; Lawrie, A.; Condliffe, R. Circulating Protein Biomarkers in Systemic Sclerosis Related Pulmonary Arterial Hypertension: A Review of Published Data. Front. Med. 2018, 5, 175. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, L.; Jia, Y.; Balistrieri, A.; Fraidenburg, D.R.; Wang, J.; Tang, H.; Yuan, J.X. Pathogenic Mechanisms of Pulmonary Arterial Hypertension: Homeostasis Imbalance of Endothelium-Derived Relaxing and Contracting Factors. JACC Asia 2022, 2, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Handler, S.S.; Jin, J.; Ogawa, M.T.; Feinstein, J.A.; Lo, C. Abnormal platelet aggregation in pediatric pulmonary hypertension. Pulm. Circ. 2022, 12, e12104. [Google Scholar] [CrossRef]
- Aytekin, M.; Aulak, K.S.; Haserodt, S.; Chakravarti, R.; Cody, J.; Minai, O.A.; Dweik, R.A. Abnormal platelet aggregation in idiopathic pulmonary arterial hypertension: Role of nitric oxide. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L512–L520. [Google Scholar] [CrossRef]
- Myllylahti, L.; Ropponen, J.; Lax, M.; Lassila, R.; Nykänen, A.I. Upregulation of Coagulation Factor VIII and Fibrinogen After Pulmonary Endarterectomy in Patients with Chronic Thromboembolic Pulmonary Hypertension. Clin. Appl. Thromb. Hemost. 2023, 29, 10760296231158369. [Google Scholar] [CrossRef]
- Lu, M.; Blaine, K.P.; Cullinane, A.; Hall, C.; Dulau-Florea, A.; Sun, J.; Chenwi, H.F.; Graninger, G.M.; Harper, B.; Thompson, K.; et al. Pulmonary arterial hypertension patients display normal kinetics of clot formation using thrombelastography. Pulm. Circ. 2021, 11, 20458940211022204. [Google Scholar] [CrossRef] [PubMed]
- Katta, S.; Vadapalli, S.; Sastry, B.K.; Nallari, P. t-plasminogen activator inhibitor-1 polymorphism in idiopathic pulmonary arterial hypertension. Indian J. Hum. Genet. 2008, 14, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, M.F.; Ortiz-Quintero, J.; Garrido-Moreno, V.; Sanhueza-Olivares, F.; Guerrero-Moncayo, A.; Chiong, M.; Castro, P.F.; García, L.; Gabrielli, L.; Corbalán, R.; et al. VCAM-1 as a predictor biomarker in cardiovascular disease. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166170. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Pignatelli, P.; Perticone, F.; Sciacqua, A.; Carnevale, R.; Farcomeni, A.; Basili, S.; Corazza, G.R.; Davì, G.; Lip, G.Y.H.; et al. Aspirin and renal insufficiency progression in patients with atrial fibrillation and chronic kidney disease. Int. J. Cardiol. 2016, 223, 619–624. [Google Scholar] [CrossRef]
- Semczuk-Kaczmarek, K.; Płatek, A.E.; Ryś, A.; Adamowicz, J.; Legosz, P.; Kotkowski, M.; Dudzik-Płocica, A.; Gorko, D.; Szymański, F.M.; Filipiak, K.J. CHA2DS2-VASc score and fibrinogen concentration in patients with atrial fibrillation. Adv. Clin. Exp. Med. 2019, 28, 1451–1457. [Google Scholar] [CrossRef]
- Goette, A.; Ittenson, A.; Hoffmanns, P.; Reek, S.; Hartung, W.; Klein, H.; Ansorge, S.; Geller, J.C. Increased expression of P-selectin in patients with chronic atrial fibrillation. Pacing Clin. Electrophysiol. 2000, 23, 1872–1875. [Google Scholar] [CrossRef]
- Stacher, E.; Graham, B.B.; Hunt, J.M.; Gandjeva, A.; Groshong, S.D.; McLaughlin, V.V.; Jessup, M.; Grizzle, W.E.; Aldred, M.A.; Cool, C.D.; et al. Modern age pathology of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 261–272. [Google Scholar] [CrossRef]
- Tamosiuniene, R.; Tian, W.; Dhillon, G.; Wang, L.; Sung, Y.K.; Gera, L.; Patterson, A.J.; Agrawal, R.; Rabinovitch, M.; Ambler, K.; et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ. Res. 2011, 109, 867–879. [Google Scholar] [CrossRef]
- Guignabert, C.; Humbert, M. Targeting transforming growth factor-β receptors in pulmonary hypertension. Eur. Respir. J. 2021, 57, 2002341. [Google Scholar] [CrossRef]
- Price, L.C.; Caramori, G.; Perros, F.; Meng, C.; Gambaryan, N.; Dorfmuller, P.; Montani, D.; Casolari, P.; Zhu, J.; Dimopoulos, K.; et al. Nuclear factor κ-B is activated in the pulmonary vessels of patients with end-stage idiopathic pulmonary arterial hypertension. PLoS ONE 2013, 8, e75415. [Google Scholar] [CrossRef]
- Rabinovitch, M.; Guignabert, C.; Humbert, M.; Nicolls, M.R. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 2014, 115, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Dernellis, J.; Panaretou, M. C-reactive protein and paroxysmal atrial fibrillation: Evidence of the implication of an inflammatory process in paroxysmal atrial fibrillation. Acta Cardiol. 2001, 56, 375–380. [Google Scholar] [CrossRef]
- Li, J.; Solus, J.; Chen, Q.; Rho, Y.H.; Milne, G.; Stein, C.M.; Darbar, D. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm 2010, 7, 438–444. [Google Scholar] [CrossRef]
- Nortamo, S.; Ukkola, O.; Lepojärvi, S.; Kenttä, T.; Kiviniemi, A.; Junttila, J.; Huikuri, H.; Perkiömäki, J. Association of sST2 and hs-CRP levels with new-onset atrial fibrillation in coronary artery disease. Int. J. Cardiol. 2017, 248, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, A.E.; Vatasescu, R.G.; Stanciu, M.M.; Serdarevic, N.; Dorobantu, M. The role of pro-fibrotic biomarkers in paroxysmal and persistent atrial fibrillation. Cytokine 2018, 103, 63–68. [Google Scholar] [CrossRef]
- Gaudino, M.; Andreotti, F.; Zamparelli, R.; Di Castelnuovo, A.; Nasso, G.; Burzotta, F.; Iacoviello, L.; Donati, M.B.; Schiavello, R.; Maseri, A.; et al. The -174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation 2003, 108 (Suppl. S1), Ii195–Ii199. [Google Scholar] [CrossRef]
- Wu, N.; Xu, B.; Liu, Y.; Chen, X.; Tang, H.; Wu, L.; Xiang, Y.; Zhang, M.; Shu, M.; Song, Z.; et al. Elevated plasma levels of Th17-related cytokines are associated with increased risk of atrial fibrillation. Sci. Rep. 2016, 6, 26543. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Guo, Y.; Li, S.; Yu, B.; Zhu, S.; Li, S.; Li, N.; Tian, Z.; Peng, C.; Cheng, J.; et al. Interleukin-18 among atrial fibrillation patients in the absence of structural heart disease. Europace 2010, 12, 1713–1718. [Google Scholar] [CrossRef]
- Dwyer, N.; Kilpatrick, D. Bosentan for the treatment of adult pulmonary hypertension. Future Cardiol. 2011, 7, 19–37. [Google Scholar] [CrossRef]
- Wu, Y.; Adi, D.; Long, M.; Wang, J.; Liu, F.; Gai, M.T.; Aierken, A.; Li, M.Y.; Li, Q.; Wu, L.Q.; et al. 4-Phenylbutyric Acid Induces Protection against Pulmonary Arterial Hypertension in Rats. PLoS ONE 2016, 11, e0157538. [Google Scholar] [CrossRef]
- Boucherat, O.; Chabot, S.; Paulin, R.; Trinh, I.; Bourgeois, A.; Potus, F.; Lampron, M.C.; Lambert, C.; Breuils-Bonnet, S.; Nadeau, V.; et al. HDAC6: A Novel Histone Deacetylase Implicated in Pulmonary Arterial Hypertension. Sci. Rep. 2017, 7, 4546. [Google Scholar] [CrossRef] [PubMed]
- Shimauchi, T.; Boucherat, O.; Yokokawa, T.; Grobs, Y.; Wu, W.; Orcholski, M.; Martineau, S.; Omura, J.; Tremblay, E.; Shimauchi, K.; et al. PARP1-PKM2 Axis Mediates Right Ventricular Failure Associated With Pulmonary Arterial Hypertension. JACC Basic Transl. Sci. 2022, 7, 384–403. [Google Scholar] [CrossRef] [PubMed]
- Sztormowska-Achranowicz, K.; Jankowski, Z.; Kocić, I. Protective effect of nicotinamide and L-arginine against monocrotaline-induced pulmonary hypertension in rats: Gender dependence. Pharmacol. Rep. 2020, 72, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Yared, J.P.; Bakri, M.H.; Erzurum, S.C.; Moravec, C.S.; Laskowski, D.M.; Van Wagoner, D.R.; Mascha, E.; Thornton, J. Effect of dexamethasone on atrial fibrillation after cardiac surgery: Prospective, randomized, double-blind, placebo-controlled trial. J. Cardiothorac. Vasc. Anesth. 2007, 21, 68–75. [Google Scholar] [CrossRef]
- Iskandar, S.; Reddy, M.; Afzal, M.R.; Rajasingh, J.; Atoui, M.; Lavu, M.; Atkins, D.; Bommana, S.; Umbarger, L.; Jaeger, M.; et al. Use of Oral Steroid and its Effects on Atrial Fibrillation Recurrence and Inflammatory Cytokines Post Ablation—The Steroid AF Study. J. Atr. Fibrillation 2017, 9, 1604. [Google Scholar] [CrossRef]
- Price, L.C.; Shao, D.; Meng, C.; Perros, F.; Garfield, B.E.; Zhu, J.; Montani, D.; Dorfmuller, P.; Humbert, M.; Adcock, I.M.; et al. Dexamethasone induces apoptosis in pulmonary arterial smooth muscle cells. Respir. Res. 2015, 16, 114. [Google Scholar] [CrossRef]
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Muñoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A.; et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. [Google Scholar] [CrossRef]
- Pavillard, L.E.; Cañadas-Lozano, D.; Alcocer-Gómez, E.; Marín-Aguilar, F.; Pereira, S.; Robertson, A.A.B.; Muntané, J.; Ryffel, B.; Cooper, M.A.; Quiles, J.L.; et al. NLRP3-inflammasome inhibition prevents high fat and high sugar diets-induced heart damage through autophagy induction. Oncotarget 2017, 8, 99740–99756. [Google Scholar] [CrossRef]
- Starreveld, R.; Ramos, K.S.; Muskens, A.; Brundel, B.; de Groot, N.M.S. Daily Supplementation of L-Glutamine in Atrial Fibrillation Patients: The Effect on Heat Shock Proteins and Metabolites. Cells 2020, 9, 1729. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, J.; Gong, Y.; Wang, D.; Wang, X.; Yun, F.; Liu, Z.; Zhang, S.; Li, W.; Zhao, X.; et al. Autophagy exacerbates electrical remodeling in atrial fibrillation by ubiquitin-dependent degradation of L-type calcium channel. Cell Death Dis. 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.P.; Mullens, W.; Banaszewski, M.; Bauersachs, J.; Brunner-La Rocca, H.P.; Chioncel, O.; Collins, S.P.; Doehner, W.; Filippatos, G.S.; Flammer, A.J.; et al. Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2017, 19, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Ma, J.L.; Ding, D.; Ma, Y.J.; Wei, Y.P.; Jing, Z.C. Experimental animal models of pulmonary hypertension: Development and challenges. Animal Model. Exp. Med. 2022, 5, 207–216. [Google Scholar] [CrossRef]
- Bueno-Beti, C.; Sassi, Y.; Hajjar, R.J.; Hadri, L. Pulmonary Artery Hypertension Model in Rats by Monocrotaline Administration. Methods Mol. Biol. 2018, 1816, 233–241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).