Hypoxia Promotes the In Vitro Proliferation of Buffalo Spermatogonial Cells by Increasing Lactate and H3K18la Lactylation Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Buffalo Testicle Collection
2.2. Culture of bSCs at Multiple Oxygen Concentrations
2.3. Induction of bSCs Meiosis In Vitro
2.4. Regulation of Glycolysis in bSCs
2.5. Immunofluorescence Analysis
2.6. Western Blot Analysis
2.7. Quantitative Real-Time PCR (qPCR) Analysis
2.8. mRNA Library Construction and Next-Generation Sequencing (RNA-Seq) Analysis
2.9. Flow Cytometric Analysis
2.10. Immunohistochemistry (IHC) Analysis
2.11. Lactate Level Analysis
2.12. CUT&Tag-Seq Analysis
2.13. Statistical Analysis
3. Results
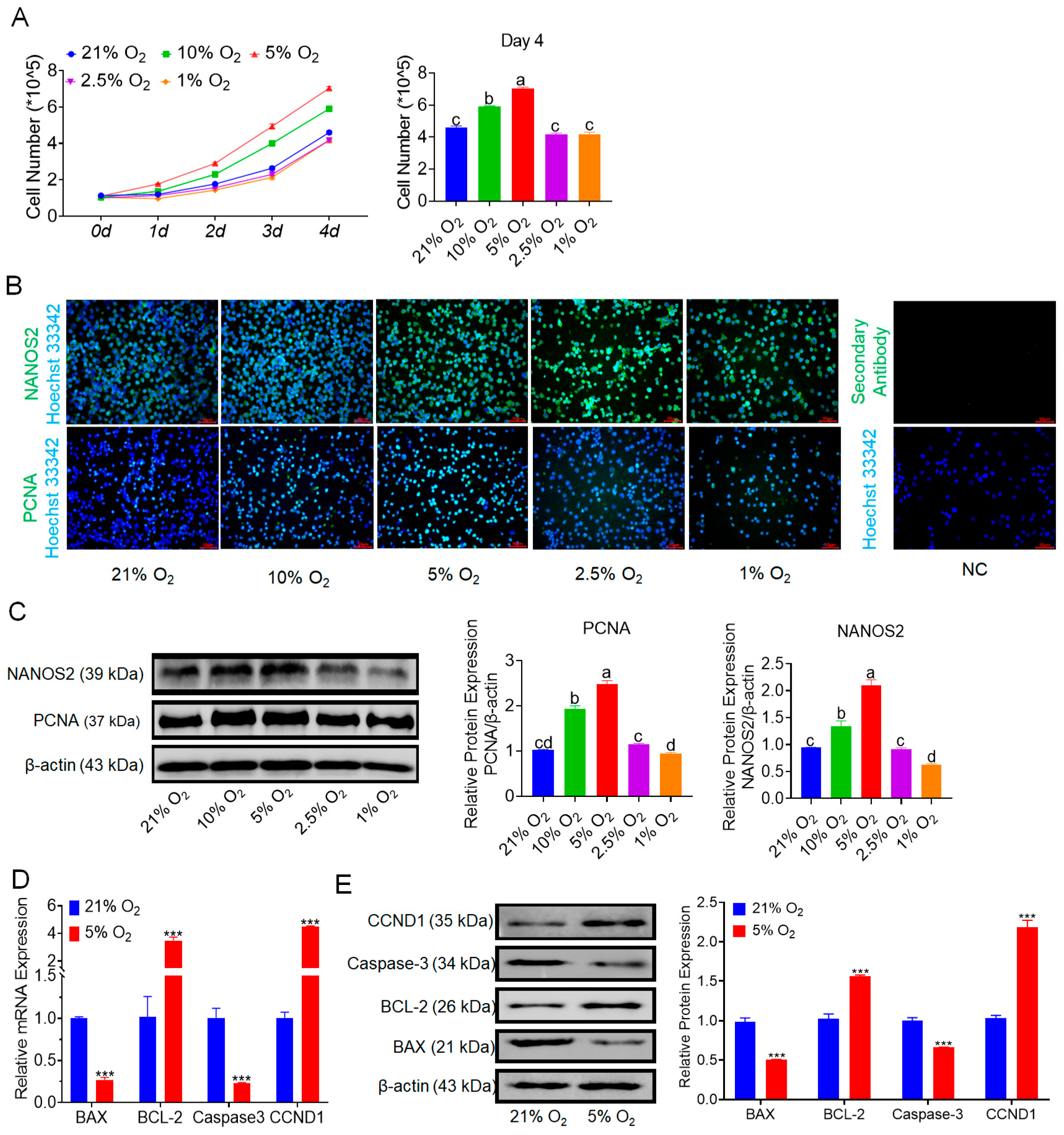
3.1. Hypoxia Promotes the Proliferation of bSCs
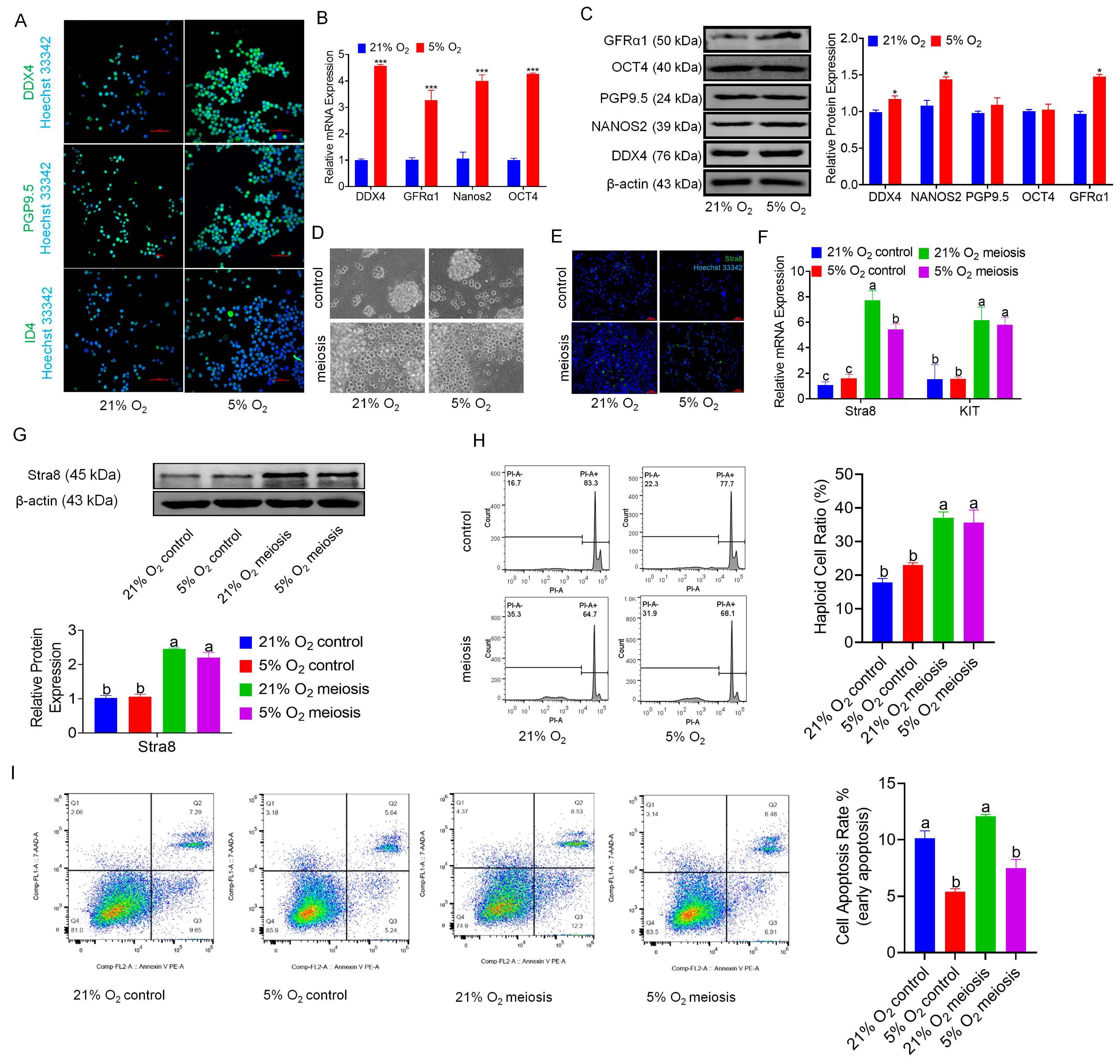
3.2. Hypoxia Benefits the Maintenance of bSCs
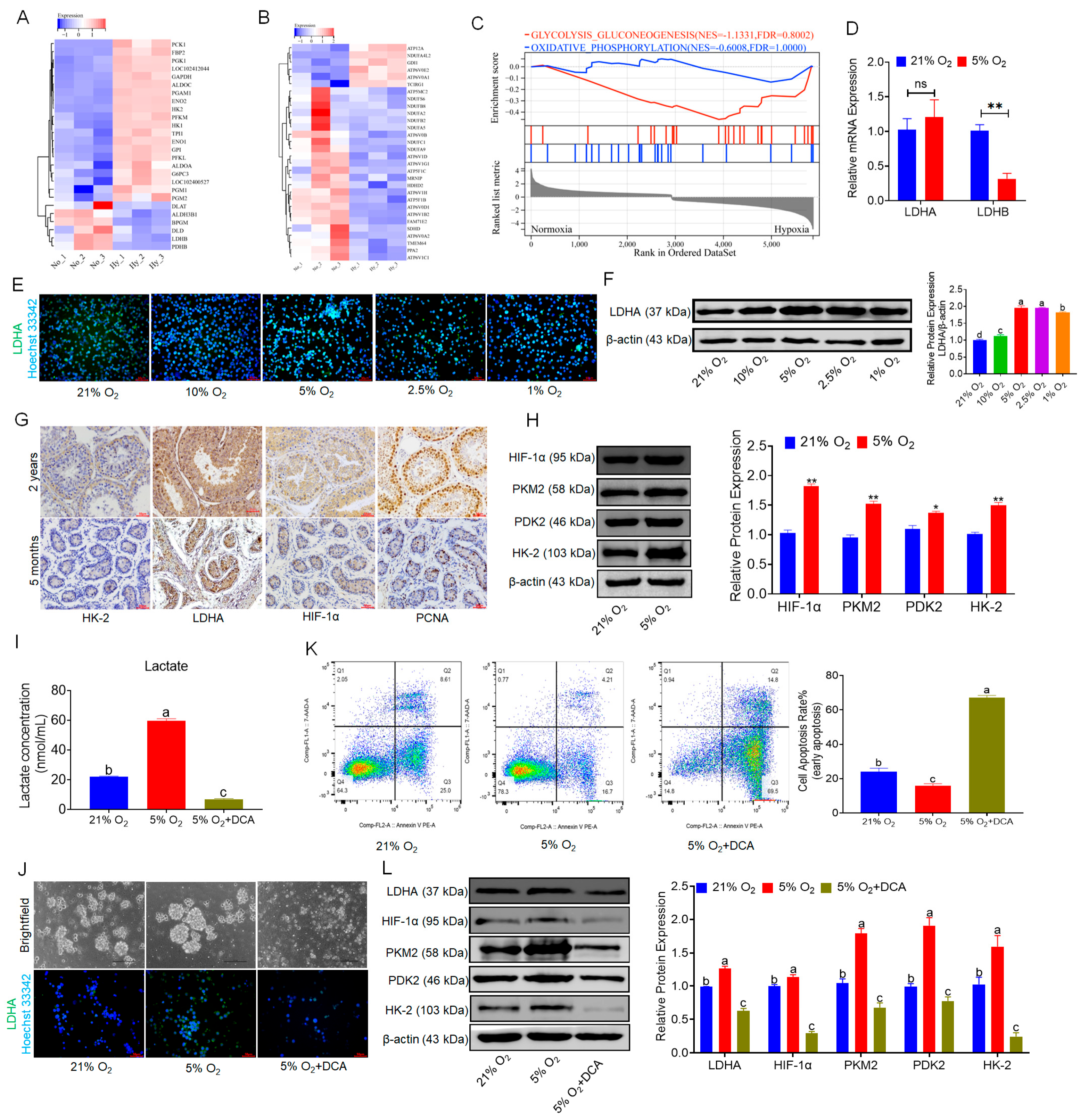
3.3. Hypoxia Promotes Glycolysis and Lactate Accumulation in bSCs
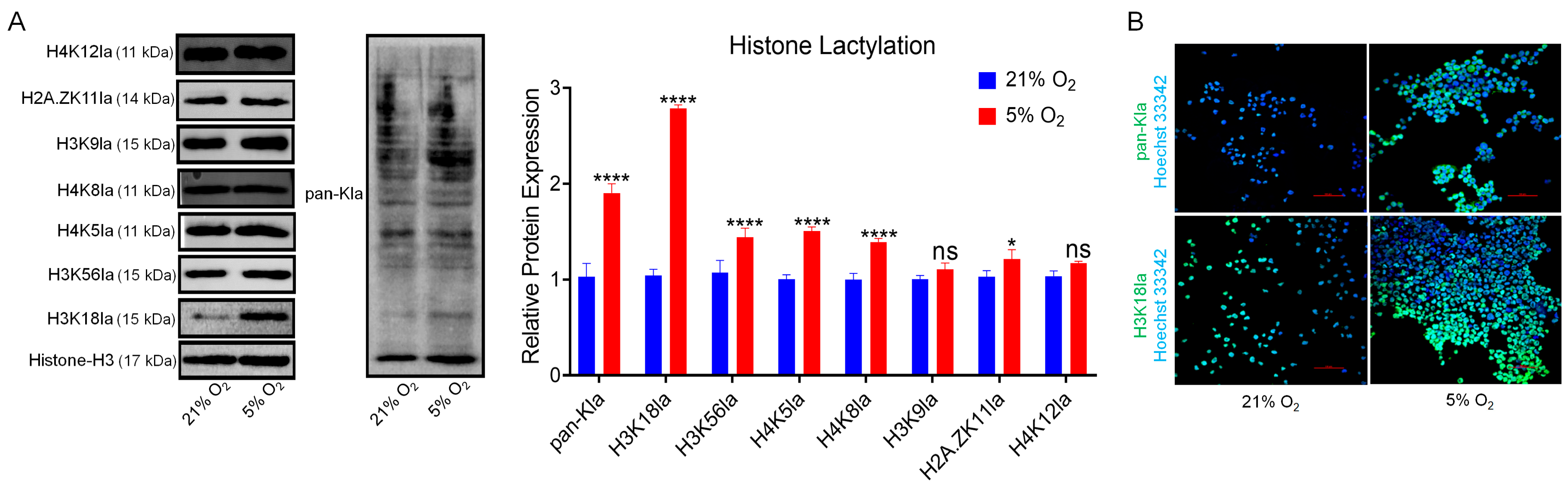
3.4. Hypoxia Elevates H3K18 Lactylation in bSCs
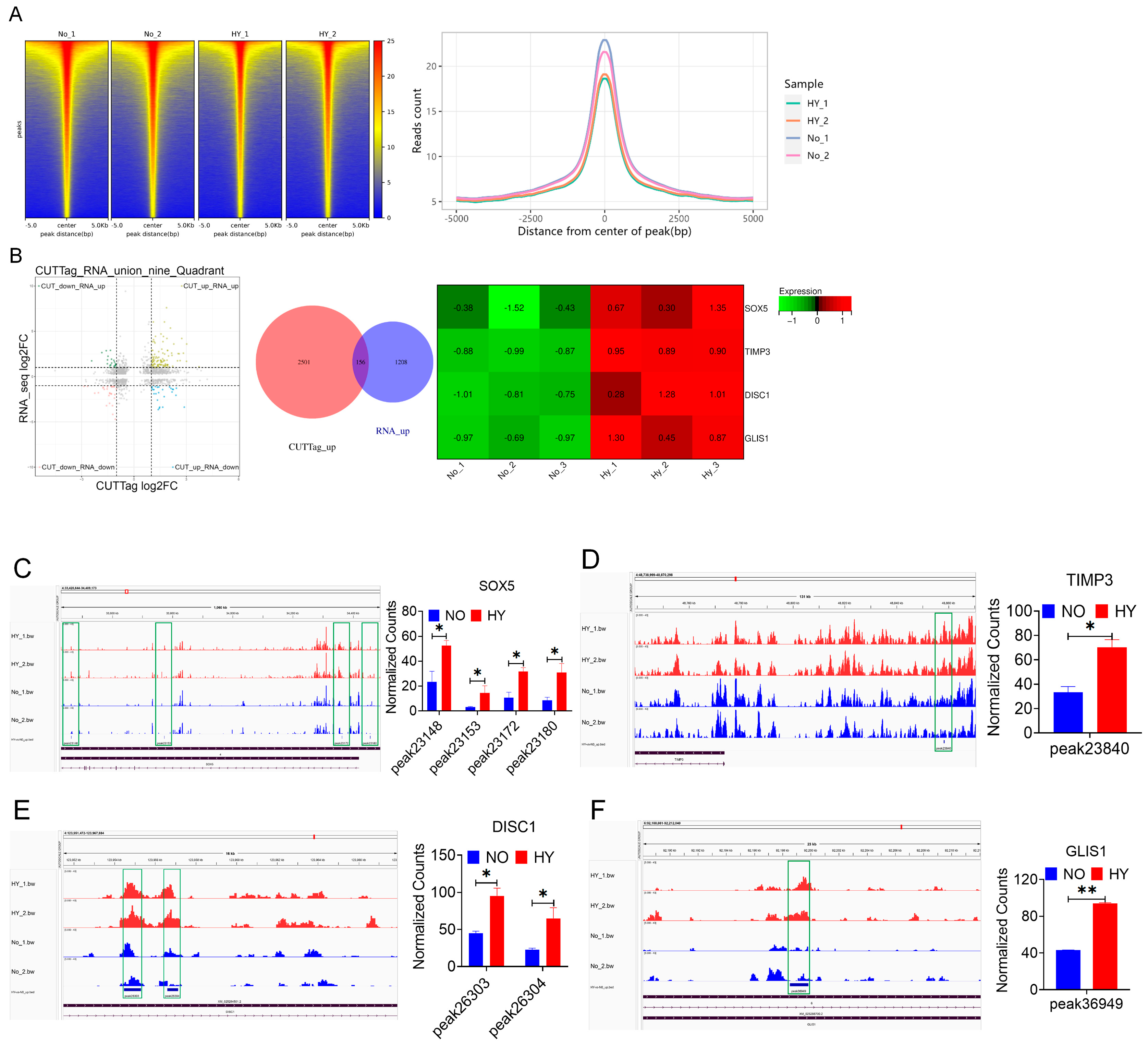
3.5. Combined CUT&Tag-Seq and RNA-Seq Identified the Target Genes That Promote bSCs Proliferation Through H3K18la
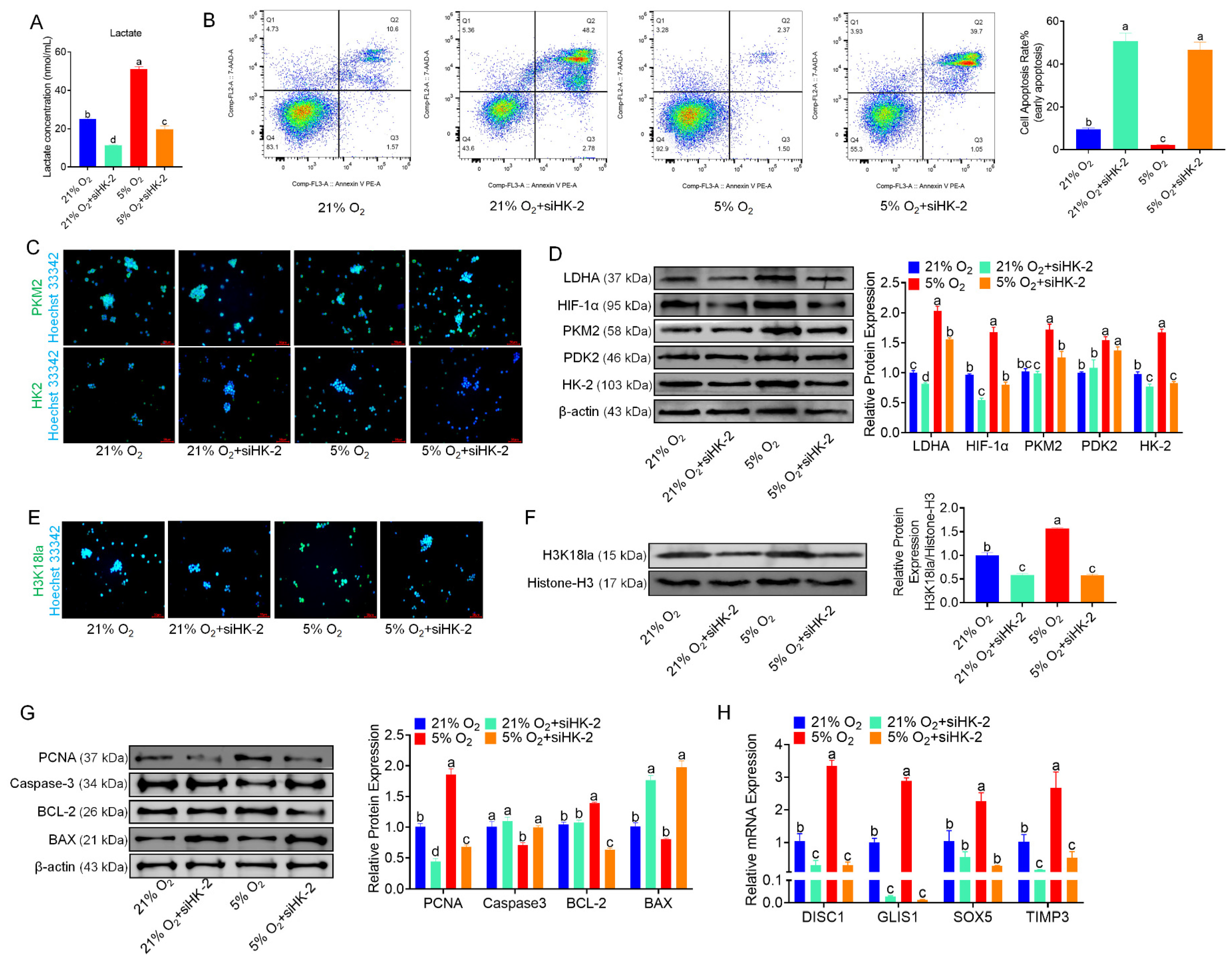
3.6. HK-2 Knockdown Inhibits Glycolysis and Proliferation in bSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSCs | Spermatogonial stem cells |
| ASC | Adult stem cell |
| iPSCs | Induced pluripotent stem cells |
| bSCs | Buffalo spermatogonial cells |
| FBS | Fetal bovine serum |
| DCA | Sodium dichloroacetate |
| WB | Western blot |
| SDS–PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| ECL | Enhanced chemiluminescence |
| qPCR | Quantitative real-time PCR |
| RNA-Seq | Next-generation sequencing |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PI | Propidium iodide |
| PBS | Phosphate-buffered saline |
| IHC | Immunohistochemistry |
| hMSCs | Human mesenchymal stem cells |
| ROS | Reactive oxygen species |
| hESCs | Human embryonic stem cells |
References
- Stowell, C.E.T.; Wang, Y. Quickening: Translational design of resorbable synthetic vascular grafts. Biomaterials 2018, 173, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Ermetin, O. Evaluation of the application opportunities of precision livestock farming (PLF) for water buffalo (Bubalus bubalis) breeding: SWOT analysis. Arch. Anim. Breed. 2023, 66, 41–50. [Google Scholar] [CrossRef]
- Pineda, P.S.; Flores, E.B.; Herrera, J.R.V.; Low, W.Y. Opportunities and Challenges for Improving the Productivity of Swamp Buffaloes in Southeastern Asia. Front. Genet. 2021, 12, 629861. [Google Scholar] [CrossRef]
- Sahare, M.G.; Suyatno; Imai, H. Recent advances of in vitro culture systems for spermatogonial stem cells in mammals. Reprod. Med. Biol. 2018, 17, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Brinster, R.L. Spermatogonial stem cells. Biol. Reprod. 2018, 99, 52–74. [Google Scholar] [CrossRef]
- Sanou, I.; van Maaren, J.; Eliveld, J.; Lei, Q.; Meißner, A.; de Melker, A.A.; Hamer, G.; van Pelt, A.M.M.; Mulder, C.L. Spermatogonial Stem Cell-Based Therapies: Taking Preclinical Research to the Next Level. Front. Endocrinol. 2022, 13, 850219. [Google Scholar] [CrossRef]
- Xi, H.M.; Ren, Y.J.; Ren, F.; Li, Y.; Feng, T.Y.; Wang, Z.; Du, Y.Q.; Zhang, L.K.; Hu, J.H. Recent advances in isolation, identification, and culture of mammalian spermatogonial stem cells. Asian J. Androl. 2022, 24, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.R.; Li, W.C.; Hu, J.H.; Li, Q.Q.; Zhang, Y.P.; Lu, K.H.; Xu, H.Y.; Liang, X.W.; Lu, Y.Q.; Yang, X.G. Comparison of the effects of buffalo LIF and mouse LIF on the in vitro culture of buffalo spermatogonia. Cell Biol. Int. 2023, 47, 981–989. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, Q.; Li, T.; Liu, R.; Cheng, Z.; Guo, M.; Xiao, J.; Wu, D.; Zeng, W. Sertoli cell and spermatogonial development in pigs. J. Anim. Sci. Biotechnol. 2022, 13, 45. [Google Scholar] [CrossRef]
- Damyanova, K.B.; Nixon, B.; Johnston, S.D.; Gambini, A.; Benitez, P.P.; Lord, T. Spermatogonial stem cell technologies: Applications from human medicine to wildlife conservation. Biol. Reprod. 2024, 111, 757–779. [Google Scholar] [CrossRef]
- Liu, W.; Du, L.; Li, J.; He, Y.; Tang, M. Microenvironment of spermatogonial stem cells: A key factor in the regulation of spermatogenesis. Stem Cell Res. Ther. 2024, 15, 294. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Miharada, K.; Karlsson, G.; Rehn, M.; Rörby, E.; Siva, K.; Cammenga, J.; Karlsson, S. Hematopoietic stem cells are regulated by Cripto, as an intermediary of HIF-1α in the hypoxic bone marrow niche. Ann. N. Y. Acad. Sci. 2012, 1266, 55–62. [Google Scholar] [CrossRef]
- Hsu, P.; Qu, C.K. Metabolic plasticity and hematopoietic stem cell biology. Curr. Opin. Hematol. 2013, 20, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.W.; Kawakatsu, M.; Idemitsu, M.; Urata, Y.; Goto, S.; Ono, Y.; Hamano, K.; Li, T.S. Culture under low physiological oxygen conditions improves the stemness and quality of induced pluripotent stem cells. J. Cell Physiol. 2013, 228, 2159–2166. [Google Scholar] [CrossRef]
- Bo, B.; Li, S.; Zhou, K.; Wei, J. The Regulatory Role of Oxygen Metabolism in Exercise-Induced Cardiomyocyte Regeneration. Front. Cell Dev. Biol. 2021, 9, 664527. [Google Scholar] [CrossRef]
- Turner, D.A.; Adamson, D.C. Neuronal-astrocyte metabolic interactions: Understanding the transition into abnormal astrocytoma metabolism. J. Neuropathol. Exp. Neurol. 2011, 70, 167–176. [Google Scholar] [CrossRef]
- Gille, A.S.; Givelet, M.; Pehlic, D.; Lapoujade, C.; Lassalle, B.; Barroca, V.; Bemelmans, A.P.; Borderie, D.; Moison, D.; Livera, G.; et al. Impact of the hypoxic microenvironment on spermatogonial stem cells in culture. Front. Cell Dev. Biol. 2024, 11, 1293068. [Google Scholar] [CrossRef]
- Reyes, J.G.; Farias, J.G.; Henríquez-Olavarrieta, S.; Madrid, E.; Parraga, M.; Zepeda, A.B.; Moreno, R.D. The hypoxic testicle: Physiology and pathophysiology. Oxid. Med. Cell Longev. 2012, 2012, 929285. [Google Scholar] [CrossRef]
- Ji, Y.-H.; Wang, L.-M.; Zhang, F.-X.; Hou, H.-Z.; Luo, Z.-R.; Xue, Q.; Shi, M.-M.; Jiao, Y.; Cui, D.; He, D.-L.; et al. Cascading effects of hypobaric hypoxia on the testis: Insights from a single-cell RNA sequencing analysis. Front. Cell Dev. Biol. 2023, 11, 1282119. [Google Scholar] [CrossRef]
- Moirangthem, R.D.; Singh, S.; Adsul, A.; Jalnapurkar, S.; Limaye, L.; Kale, V.P. Hypoxic niche-mediated regeneration of hematopoiesis in the engraftment window is dominantly affected by oxygen tension in the milieu. Stem Cells Dev. 2015, 24, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhu, P.; Yu, C.; Wu, J. The Proliferation and Stemness of Peripheral Blood-Derived Mesenchymal Stromal Cells Were Enhanced by Hypoxia. Front. Endocrinol. 2022, 13, 873662. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Takazawa, Y.; Kagawa, T.; Taga, T. Organization of self-advantageous niche by neural stem/progenitor cells during development via autocrine VEGF-A under hypoxia. Inflamm. Regen. 2023, 43, 8. [Google Scholar] [CrossRef]
- Lord, T.; Nixon, B. Metabolic Changes Accompanying Spermatogonial Stem Cell Differentiation. Dev. Cell 2020, 52, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.P.; Minogue, E.; Krause, L.C.M.; Hess, R.M.; Bargiela, D.; Wadsworth, B.J.; Barbieri, L.; Brombach, C.; Foskolou, I.P.; Bogeski, I.; et al. Oxygen levels at the time of activation determine T cell persistence and immunotherapeutic efficacy. eLife 2023, 12, e84280. [Google Scholar] [CrossRef] [PubMed]
- Kierans, S.J.; Taylor, C.T. Glycolysis: A multifaceted metabolic pathway and signaling hub. J. Biol. Chem. 2024, 300, 107906. [Google Scholar] [CrossRef]
- Caballano-Infantes, E.; Díaz, I.; Hitos, A.B.; Cahuana, G.M.; Martínez-Ruiz, A.; Soria-Juan, B.; Rodríguez-Griñolo, R.; Hmadcha, A.; Martín, F.; Soria, B.; et al. Stemness of Human Pluripotent Cells: Hypoxia-Like Response Induced by Low Nitric Oxide. Antioxidants 2021, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Zhang, P.; Yuan, L.; Wang, L.; Guo, X.; Cui, G.; Zhang, Y.; Li, M.; Zhang, X.; Li, X.; et al. HIF-1α Affects the Neural Stem Cell Differentiation of Human Induced Pluripotent Stem Cells via MFN2-Mediated Wnt/β-Catenin Signaling. Front. Cell Dev. Biol. 2021, 9, 671704. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, J.; Guan, Y.; Ji, X. The role of hypoxia in stem cell regulation of the central nervous system: From embryonic development to adult proliferation. CNS Neurosci. Ther. 2021, 27, 1446–1457. [Google Scholar] [CrossRef]
- Podkalicka, P.; Stępniewski, J.; Mucha, O.; Kachamakova-Trojanowska, N.; Dulak, J.; Łoboda, A. Hypoxia as a Driving Force of Pluripotent Stem Cell Reprogramming and Differentiation to Endothelial Cells. Biomolecules 2020, 10, 1614. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, N.; Gong, Z.; Zhou, W.; Ku, Y.; Chen, Y. Lactate and lysine lactylation of histone regulate transcription in cancer. Heliyon 2024, 10, e38426. [Google Scholar] [CrossRef] [PubMed]
- Capatina, A.L.; Malcolm, J.R.; Stenning, J.; Moore, R.L.; Bridge, K.S.; Brackenbury, W.J.; Holding, A.N. Hypoxia-induced epigenetic regulation of breast cancer progression and the tumour microenvironment. Front. Cell Dev. Biol. 2024, 12, 1421629. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, K.; Wang, T.; Wu, Y.; Xing, G.; Chen, M.; Hao, Z.; Zhang, C.; Zhang, J.; Ma, B.; et al. Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat. Metab. 2020, 2, 882–892. [Google Scholar] [CrossRef]
- Hu, X.; Huang, X.; Yang, Y.; Sun, Y.; Zhao, Y.; Zhang, Z.; Qiu, D.; Wu, Y.; Wu, G.; Lei, L. Dux activates metabolism-lactylation-MET network during early iPSC reprogramming with Brg1 as the histone lactylation reader. Nucleic Acids Res. 2024, 52, 5529–5548. [Google Scholar] [CrossRef]
- Merkuri, F.; Rothstein, M.; Simoes-Costa, M. Histone lactylation couples cellular metabolism with developmental gene regulatory networks. Nat. Commun. 2024, 15, 90. [Google Scholar] [CrossRef]
- Hu, Y.; He, Z.; Li, Z.; Wang, Y.; Wu, N.; Sun, H.; Zhou, Z.; Hu, Q.; Cong, X. Lactylation: The novel histone modification influence on gene expression, protein function, and disease. Clin. Epigenetics 2024, 16, 72. [Google Scholar] [CrossRef] [PubMed]
- GB/T 5274.1-2018; Gas analysis—Preparation of calibration gas mixtures—Part 1: Gravimetric method for class I mixtures. State Administration for Market Regulation, Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- Lei, Q.; Lai, X.; Eliveld, J.; Chuva de Sousa Lopes, S.M.; van Pelt, A.M.M.; Hamer, G. In Vitro Meiosis of Male Germline Stem Cells. Stem Cell Rep. 2020, 15, 1140–1153. [Google Scholar] [CrossRef]
- Ibtisham, F.; Zhao, Y.; Nawab, A.; Wu, J.; Mei, X.; Honaramooz, A.; An, L. In vitro production of haploid germ cells from murine spermatogonial stem cells using a two-dimensional cell culture system. Theriogenology 2021, 162, 84–94. [Google Scholar] [CrossRef]
- He, Y.; Ji, Z.; Gong, Y.; Fan, L.; Xu, P.; Chen, X.; Miao, J.; Zhang, K.; Zhang, W.; Ma, P.; et al. Numb/Parkin-directed mitochondrial fitness governs cancer cell fate via metabolic regulation of histone lactylation. Cell Rep. 2023, 42, 112033. [Google Scholar] [CrossRef]
- Shen, W.; Song, Z.; Zhong, X.; Huang, M.; Shen, D.; Gao, P.; Qian, X.; Wang, M.; He, X.; Wang, T.; et al. Sangerbox: A comprehensive, interaction-friendly clinical bioinformatics analysis platform. iMeta 2022, 1, e36. [Google Scholar] [CrossRef]
- Rodríguez-Casuriaga, R.; Geisinger, A. Contributions of Flow Cytometry to the Molecular Study of Spermatogenesis in Mammals. Int. J. Mol. Sci. 2021, 22, 1151. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, T.T.; Liu, Y.R.; Geng, S.S.; Luo, A.L.; Jiang, M.S.; Liang, X.W.; Shang, J.H.; Lu, K.H.; Yang, X.G. Transcriptome analysis revealed differences in the microenvironment of spermatogonial stem cells in seminiferous tubules between pre-pubertal and adult buffaloes. Reprod. Domest. Anim. 2021, 56, 629–641. [Google Scholar] [CrossRef]
- Li, M.Q.; Luo, A.L.; Zhao, P.W.; Li, T.T.; Geng, S.S.; Liang, X.W.; Xu, H.Y.; Lu, Y.Q.; Lu, S.S.; Yang, X.G.; et al. Nanos2 is a molecular marker of inchoate buffalo spermatogonia. Anim. Reprod. Sci. 2017, 186, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Narazaki, A.; Shimizu, R.; Yoshihara, T.; Kikuta, J.; Sakaguchi, R.; Tobita, S.; Mori, Y.; Ishii, M.; Nishikawa, K. Determination of the physiological range of oxygen tension in bone marrow monocytes using two-photon phosphorescence lifetime imaging microscopy. Sci. Rep. 2022, 12, 3497. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Yoshihara, T.; Kamiya, M.; Mimura, I.; Fujikura, D.; Masuda, T.; Kikuchi, R.; Takahashi, I.; Urano, Y.; Tobita, S.; et al. Quantitating intracellular oxygen tension in vivo by phosphorescence lifetime measurement. Sci. Rep. 2015, 5, 17838. [Google Scholar] [CrossRef] [PubMed]
- Santilli, G.; Lamorte, G.; Carlessi, L.; Ferrari, D.; Rota Nodari, L.; Binda, E.; Delia, D.; Vescovi, A.L.; De Filippis, L. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS ONE 2010, 5, e8575. [Google Scholar] [CrossRef]
- Bernstein, I.R.; Nixon, B.; Lyons, J.M.; Damyanova, K.B.; De Oliveira, C.S.; Mabotuwana, N.S.; Stanger, S.J.; Kaiko, G.E.; Ying, T.H.; Oatley, J.M.; et al. The hypoxia-inducible factor EPAS1 is required for spermatogonial stem cell function in regenerative conditions. iScience 2023, 26, 108424. [Google Scholar] [CrossRef]
- Estrada, J.C.; Albo, C.; Benguría, A.; Dopazo, A.; López-Romero, P.; Carrera-Quintanar, L.; Roche, E.; Clemente, E.P.; Enríquez, J.A.; Bernad, A.; et al. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012, 19, 743–755. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Sanz-Ros, J.; Román-Domínguez, A.; Inglés, M.; Gimeno-Mallench, L.; El Alami, M.; Viña-Almunia, J.; Gambini, J.; Viña, J.; Borrás, C. Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine. Int. J. Mol. Sci. 2019, 20, 1195. [Google Scholar] [CrossRef]
- Morimoto, H.; Yamamoto, T.; Miyazaki, T.; Ogonuki, N.; Ogura, A.; Tanaka, T.; Kanatsu-Shinohara, M.; Yabe-Nishimura, C.; Zhang, H.; Pommier, Y.; et al. An interplay of NOX1-derived ROS and oxygen determines the spermatogonial stem cell self-renewal efficiency under hypoxia. Genes. Dev. 2021, 35, 250–260. [Google Scholar] [CrossRef]
- Li, C.T.; Liu, J.X.; Yu, B.; Liu, R.; Dong, C.; Li, S.J. Notch signaling represses hypoxia-inducible factor-1α-induced activation of Wnt/β-catenin signaling in osteoblasts under cobalt-mimicked hypoxia. Mol. Med. Rep. 2016, 14, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Goda, N.; Ryan, H.E.; Khadivi, B.; McNulty, W.; Rickert, R.C.; Johnson, R.S. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol. Cell Biol. 2003, 23, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Xia, H.; Meng, T.; Fan, Y.; Tang, X.; Li, Y.; Zhang, T.; Deng, J.; Yao, B.; Huang, Y.; et al. Construction of human pluripotent stem cell-derived testicular organoids and their use as humanized testis models for evaluating the effects of semaglutide. Theranostics 2025, 15, 2597–2623. [Google Scholar] [CrossRef]
- Sakib, S.; Lara, N.; Huynh, B.C.; Dobrinski, I. Organotypic Rat Testicular Organoids for the Study of Testicular Maturation and Toxicology. Front. Endocrinol. 2022, 13, 892342. [Google Scholar] [CrossRef]
- Matsumura, T.; Sato, T.; Abe, T.; Sanjo, H.; Katagiri, K.; Kimura, H.; Fujii, T.; Tanaka, H.; Hirabayashi, M.; Ogawa, T. Rat in vitro spermatogenesis promoted by chemical supplementations and oxygen-tension control. Sci. Rep. 2021, 11, 3458. [Google Scholar] [CrossRef]
- Huang, Y.H.; Lin, M.H.; Wang, P.C.; Wu, Y.C.; Chiang, H.L.; Wang, Y.L.; Chang, J.H.; Huang, Y.K.; Gu, S.Y.; Ho, H.N.; et al. Hypoxia inducible factor 2α/insulin-like growth factor receptor signal loop supports the proliferation and Oct-4 maintenance of mouse germline stem cells. Mol. Hum. Reprod. 2014, 20, 526–537. [Google Scholar] [CrossRef]
- Oatley, J.M.; Brinster, R.L. The germline stem cell niche unit in mammalian testes. Physiol. Rev. 2012, 92, 577–595. [Google Scholar] [CrossRef]
- Tan, J.; Virtue, S.; Norris, D.M.; Conway, O.J.; Yang, M.; Bidault, G.; Gribben, C.; Lugtu, F.; Kamzolas, I.; Krycer, J.R.; et al. Limited oxygen in standard cell culture alters metabolism and function of differentiated cells. EMBO J. 2024, 43, 2127–2165. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, X.; Cai, Y.; Lu, T.; Ding, M.; Wang, X. Lactate-Mediated Histone H3K9 Lactylation Facilitates Tumorigenesis of T-Cell Lymphoma Via Activation of SFXN1 Expression. Blood 2023, 142, 1652. [Google Scholar] [CrossRef]
- Hu, J.; Yang, Y.; Ma, Y.; Ning, Y.; Chen, G.; Liu, Y. Proliferation Cycle Transcriptomic Signatures are Strongly associated With Gastric Cancer Patient Survival. Front. Cell Dev. Biol. 2021, 9, 770994. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Chang, C.; Yang, Z.; Wang, P.; Fu, H.; Wei, X.; Chen, E.; Tan, S.; Huang, W.; et al. A bioenergetic shift is required for spermatogonial differentiation. Cell Discov. 2020, 6, 56. [Google Scholar] [CrossRef]
- Silva, R.; Carrageta, D.F.; Alves, M.G.; Oliveira, P.F. Testicular Glycogen Metabolism: An Overlooked Source of Energy for Spermatogenesis? BioChem 2022, 2, 198–214. [Google Scholar] [CrossRef]
- Kim, D.H.; Chauhan, S. The role of dichloroacetate in improving acute hypoxic tolerance and cardiac function: Translation to failing hearts? J. Physiol. 2018, 596, 2967–2968. [Google Scholar] [CrossRef]
- Pavlacky, J.; Polak, J. Technical Feasibility and Physiological Relevance of Hypoxic Cell Culture Models. Front. Endocrinol. 2020, 11, 57. [Google Scholar] [CrossRef]
- Shangguan, X.; He, J.; Ma, Z.; Zhang, W.; Ji, Y.; Shen, K.; Yue, Z.; Li, W.; Xin, Z.; Zheng, Q.; et al. SUMOylation controls the binding of hexokinase 2 to mitochondria and protects against prostate cancer tumorigenesis. Nat. Commun. 2021, 12, 1812. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Yamamoto, T.; Toh, H.; Kazuki, Y.; Kazuki, K.; Imoto, J.; Ikeo, K.; Oshima, M.; Shirahige, K.; Iwama, A.; et al. Aging of spermatogonial stem cells by Jnk-mediated glycolysis activation. Proc. Natl. Acad. Sci. USA 2019, 116, 16404–16409. [Google Scholar] [CrossRef]
- Voigt, A.L.; Thiageswaran, S.; de Lima, E.M.L.N.; Dobrinski, I. Metabolic Requirements for Spermatogonial Stem Cell Establishment and Maintenance In Vivo and In Vitro. Int. J. Mol. Sci. 2021, 22, 1998. [Google Scholar] [CrossRef]
- Gao, J.; Liu, R.; Huang, K.; Li, Z.; Sheng, X.; Chakraborty, K.; Han, C.; Zhang, D.; Becker, L.; Zhao, Y. Dynamic investigation of hypoxia-induced L-lactylation. Proc. Natl. Acad. Sci. USA 2025, 122, e2404899122. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Dong, Y.; Sun, L.V.; Zheng, Y. Lactate promotes H3K18 lactylation in human neuroectoderm differentiation. Cell Mol. Life Sci. 2024, 81, 459. [Google Scholar] [CrossRef]
- Sarkar, A.; Hochedlinger, K. The sox family of transcription factors: Versatile regulators of stem and progenitor cell fate. Cell Stem Cell 2013, 12, 15–30. [Google Scholar] [CrossRef]
- Lee, W.T.; Wu, P.Y.; Cheng, Y.M.; Huang, Y.F. Tissue Inhibitor of Metalloproteinase 3: Unravelling Its Biological Function and Significance in Oncology. Int. J. Mol. Sci. 2024, 25, 3191. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, W.; Luo, Z.; Li, Y.; Shu, Y.; Yue, Z.; Xiao, B.; Feng, L. DISC1 Regulates the Proliferation and Migration of Mouse Neural Stem/Progenitor Cells through Pax5, Sox2, Dll1 and Neurog2. Front. Cell Neurosci. 2017, 11, 261. [Google Scholar] [CrossRef]
- Maekawa, M.; Yamaguchi, K.; Nakamura, T.; Shibukawa, R.; Kodanaka, I.; Ichisaka, T.; Kawamura, Y.; Mochizuki, H.; Goshima, N.; Yamanaka, S. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature 2011, 474, 225–229. [Google Scholar] [CrossRef]
- Rho, H.; Terry, A.R.; Chronis, C.; Hay, N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab. 2023, 35, 1406–1423.e1408. [Google Scholar] [CrossRef]
- Ikeda, S.; Abe, F.; Matsuda, Y.; Kitadate, A.; Takahashi, N.; Tagawa, H. Hypoxia-inducible hexokinase-2 enhances anti-apoptotic function via activating autophagy in multiple myeloma. Cancer Sci. 2020, 111, 4088–4101. [Google Scholar] [CrossRef]
- Farooq, Z.; Ismail, H.; Bhat, S.A.; Layden, B.T.; Khan, M.W. Aiding Cancer’s “Sweet Tooth”: Role of Hexokinases in Metabolic Reprogramming. Life 2023, 13, 946. [Google Scholar] [CrossRef]
- Fan, W.; Wang, X.; Zeng, S.; Li, N.; Wang, G.; Li, R.; He, S.; Li, W.; Huang, J.; Li, X.; et al. Global lactylome reveals lactylation-dependent mechanisms underlying TH17 differentiation in experimental autoimmune uveitis. Sci. Adv. 2023, 9, eadh4655. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Wang, S.; Wang, M.; Chen, Y.; Zheng, L. Epigenetic Regulation of TET1-SP1 During Spermatogonia Self-Renewal and Proliferation. Front. Physiol. 2022, 13, 843825. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Gong, C.; Hu, Y.; Liu, J.; Wang, W.; Chen, Y.; Liao, Q.; He, B.; Huang, Y.; et al. Effects of Environmental and Pathological Hypoxia on Male Fertility. Front. Cell Dev. Biol. 2021, 9, 725933. [Google Scholar] [CrossRef]
| Protein Name | Manufacture | Catalog Number | IF Dilution | WB Dilution | IHC-P Dilution |
|---|---|---|---|---|---|
| NANOS2 | Santa Cruz (Dallas, Texas, USA) | sc-393794 | 1:100 | 1:1000 | - |
| PCNA | Proteintech (Wuhan, China) | 10205-2-AP | 1:500 | 1:5000 | 1:1000 |
| CCND1 | HUABIO (Hangzhou, China) | ET1601-31 | - | 1:1000 | - |
| Caspase-3 | Abcam (Cambridge, UK) | ab13847 | - | 1:1000 | - |
| BCL-2 | Proteintech | 68103-1-Ig | - | 1:1000 | - |
| BAX | Proteintech | 50599-2-Ig | - | 1:1000 | - |
| ID4 | Abcam | ab220881 | 1:200 | - | - |
| PGP9.5 | Abcam | ab108986 | 1:200 | 1:1000 | - |
| DDX4 | Abcam | ab13840 | 1:200 | 1:1000 | - |
| GFRα1 | Abcam | ab186855 | - | 1:1000 | - |
| OCT4 | Abcam | ab181557 | - | 1:1000 | - |
| Stra8 | Bioss (Beijing, China) | bs-1903R | 1:100 | 1: 1000 | - |
| LDHA | Bioss | bs-1810R | 1:100 | 1:1000 | 1:100 |
| HIF1a | ZenBio (Chengdu, China) | 340462 | 1:100 | 1: 500 | 1:100 |
| PKM2 | ZenBio | 321004 | 1:100 | 1: 500 | - |
| PDK2 | ZenBio | R382183 | 1:100 | 1: 500 | - |
| HK-2 | ZenBio | R24552 | 1:100 | 1: 500 | 1:100 |
| H4K12la | PTMbio (Hangzhou, China) | PTM-1411RM | - | 1:1000 | - |
| H2A.ZK11la | PTMbio | PTM-1422RM | - | 1:1000 | - |
| H3K9la | PTMbio | PTM-1419RM | - | 1:1000 | - |
| H4K8la | PTMbio | PTM-1415RM | - | 1:1000 | - |
| H4K5la | PTMbio | PTM-1407RM | - | 1:1000 | - |
| H3K56la | PTMbio | PTM-1421RM | - | 1:1000 | - |
| H3K18la | PTMbio | PTM-1427RM | 1:100 | 1:1000 | - |
| Pan-Kla | PTMbio | PTM-1401RM | 1:100 | 1:1000 | - |
| β-actin | Servicebio (Wuhan, China) | GB11001 | - | 1:4000 | - |
| Histone-H3 | Proteintech | 17168-1-AP | - | 1:2000 | - |
| HRP conjugated Goat Anti-Rabbit | Servicebio | GB23303 | - | 1:10,000 | 1:1000 |
| HRP conjugated Goat Anti-Mouse | Servicebio | GB23301 | - | 1:10,000 | 1:1000 |
| CoraLite488-conjugated Goat Anti-Mouse | Proteintech | SA00013-1 | 1:1000 | - | - |
| CoraLite488-conjugated Goat Anti-Rabbit | Proteintech | SA00013-2 | 1:1000 | - | - |
| Genes | Primer Sequences (5′-3′) | NCBI Reference Sequence | |
|---|---|---|---|
| β-actin | Forward | GATGATGATATTGCCGCGCTC | NM_001290932.1 |
| Reverse | CCGTGCTCAATGGGGTACTT | ||
| BAX | Forward | CCAGAGGCGGGGTTTCAT | XM_025269476.3 |
| Reverse | CAGCTGCGATCATCCTCTGT | ||
| BCL-2 | Forward | CTTCCGTTTGCTCGTGCTCT | XM_025273635.3 |
| Reverse | ACCTCCTCCGTGATGTTGTA | ||
| Caspase-3 | Forward | TGGAACCAATGGACCTGTCG | XM_025280224.2 |
| Reverse | CCAGGATCCGTACTTTGCGT | ||
| CCND1 | Forward | GCCGAGGAGAACAAGCAGAT | XM_006072357.4 |
| Reverse | TGTCAGGCGGTGATAGGAGA | ||
| DDX4 | Forward | AAGCCGCTGAGAGGTACAAC | NM_001319794.1 |
| Reverse | TTCTGACGATGAAGCCGGAG | ||
| GFRα1 | Forward | GTGGGCGTCCCTGAACTTT | XM_044934905.2 |
| Reverse | CGGTTCCGCTTTTAGGGGTT | ||
| Nanos2 | Forward | CCACCTACCACCAACACTCC | XM_025269353.3 |
| Reverse | TGCAAAAGTTGCACAGGGTG | ||
| OCT4 | Forward | TGGGAAACCTTTGACCCACC | XM_006052299.4 |
| Reverse | TTCCGGCTATGGTGAGGGTA | ||
| STRA8 | Forward | CAGAGTTCGTGAGCAGAGGC | XM_044946902.1 |
| Reverse | CCCTGCCACAGCCATATTTC | ||
| KIT | Forward | AGGTGTTCTGTTCCCGTTGG | NM_001290952.1 |
| Reverse | GACATGGATTGTTCTTTAAATGC | ||
| HK-2 | Forward | CAGGAGATCGACATGGGCTC | XM_006067902.4 |
| Reverse | GATCTGGCAGACCCGATGAG | ||
| DISC1 | Forward | TCAACTGCACATGGCAATCC | XM_025284501.2 |
| Reverse | CACTCCTCCCTCCATCCTGT | ||
| GLIS1 | Forward | TCCCCTATGGTGAGTGCTAC | XM_025288700.2 |
| Reverse | AAGGTGCACTAAGGCCATGA | ||
| SOX5 | Forward | CCAGGTGATGCTTCCAGGAC | XM_045165614.1 |
| Reverse | GCCAACAACACAAAGGCTCA | ||
| TIMP3 | Forward | GGACCCGCGCTATATCACTC | XM_006069554.4 |
| Reverse | TGCTGCTCGAGTCTCCAAAG | ||
| LDHA | Forward | CTCGCGGTTCCATTTAAGGC | XM_025286309.3 |
| Reverse | AGCTGATCCTTGAGAGTTGCC | ||
| LDHB | Forward | AGTGGATTACCCAAGCACCG | XM_006067641.4 |
| Reverse | TCCCCCAAAATCCATCCGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Ma, Y.; Wang, S.; Zheng, H.; Yang, C.; Duan, A.; Zhou, B.; Shang, J.; Liang, X.; Yang, X. Hypoxia Promotes the In Vitro Proliferation of Buffalo Spermatogonial Cells by Increasing Lactate and H3K18la Lactylation Levels. Cells 2025, 14, 832. https://doi.org/10.3390/cells14110832
Li M, Ma Y, Wang S, Zheng H, Yang C, Duan A, Zhou B, Shang J, Liang X, Yang X. Hypoxia Promotes the In Vitro Proliferation of Buffalo Spermatogonial Cells by Increasing Lactate and H3K18la Lactylation Levels. Cells. 2025; 14(11):832. https://doi.org/10.3390/cells14110832
Chicago/Turabian StyleLi, Mengqi, Yanyu Ma, Shenzhi Wang, Haiying Zheng, Chunyan Yang, Anqin Duan, Benliang Zhou, Jianghua Shang, Xingwei Liang, and Xiaogan Yang. 2025. "Hypoxia Promotes the In Vitro Proliferation of Buffalo Spermatogonial Cells by Increasing Lactate and H3K18la Lactylation Levels" Cells 14, no. 11: 832. https://doi.org/10.3390/cells14110832
APA StyleLi, M., Ma, Y., Wang, S., Zheng, H., Yang, C., Duan, A., Zhou, B., Shang, J., Liang, X., & Yang, X. (2025). Hypoxia Promotes the In Vitro Proliferation of Buffalo Spermatogonial Cells by Increasing Lactate and H3K18la Lactylation Levels. Cells, 14(11), 832. https://doi.org/10.3390/cells14110832







