The stn1-sz2 Mutant Provides New Insight into the Impacts of Telomeric Cdc13-Stn1-Ten1 Dysfunction on Cell Cycle Progression
Abstract
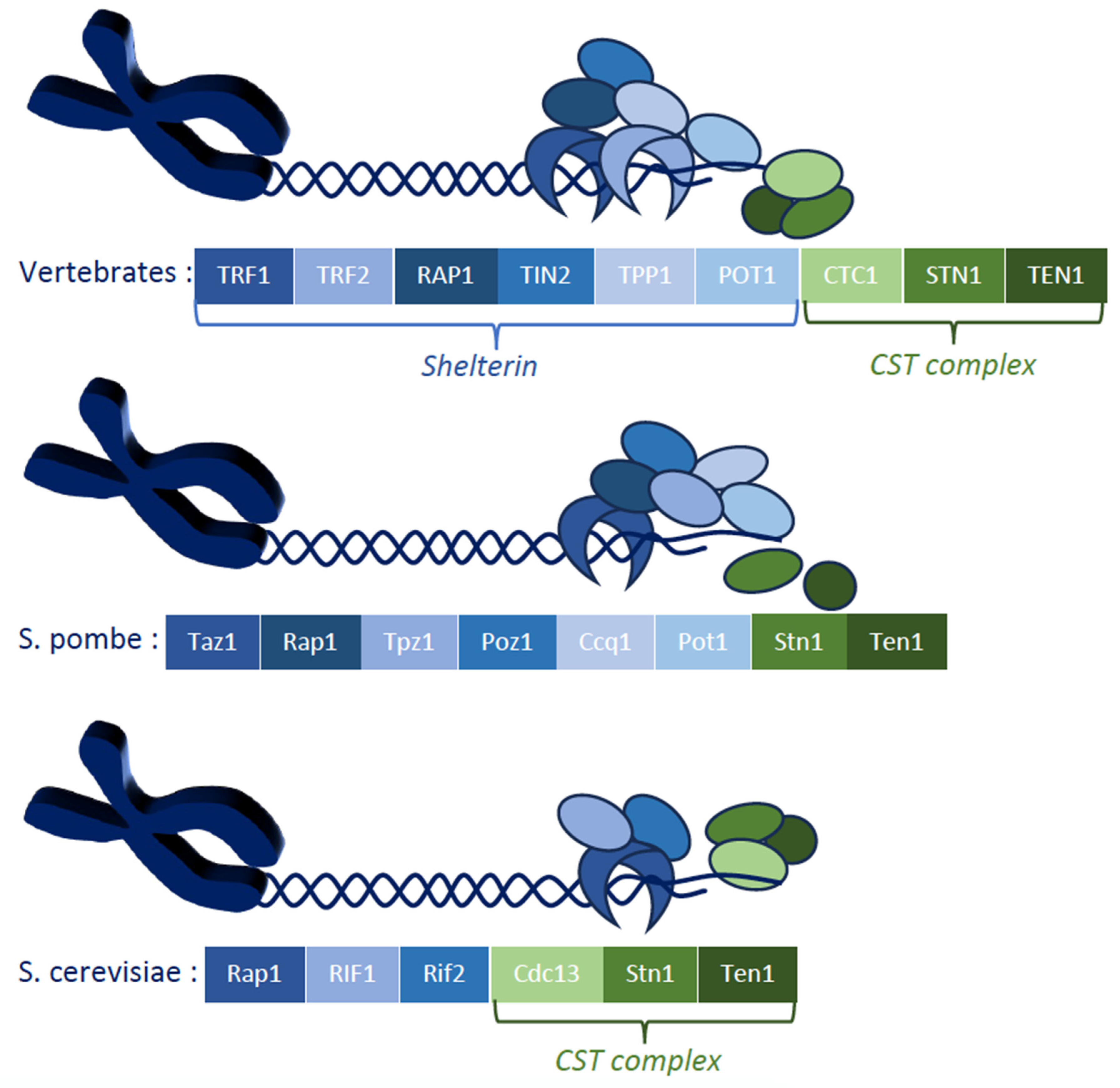
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Media
2.2. Fluorescence and Confocal Microscopy
2.3. Statistical Analyses
3. Results
3.1. Checkpoint Damage Sensors Activated in the stn1-sz2 Mutant
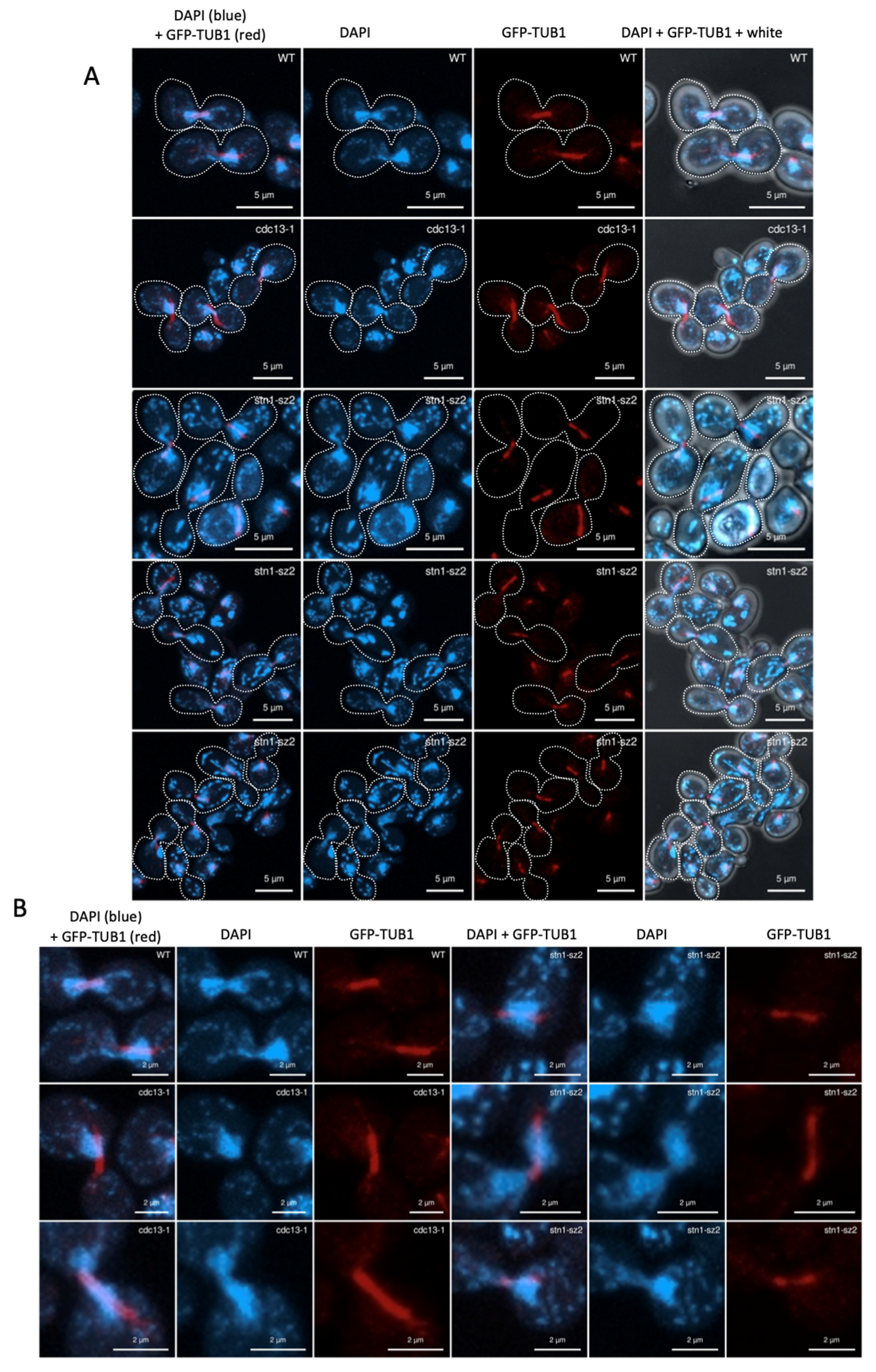
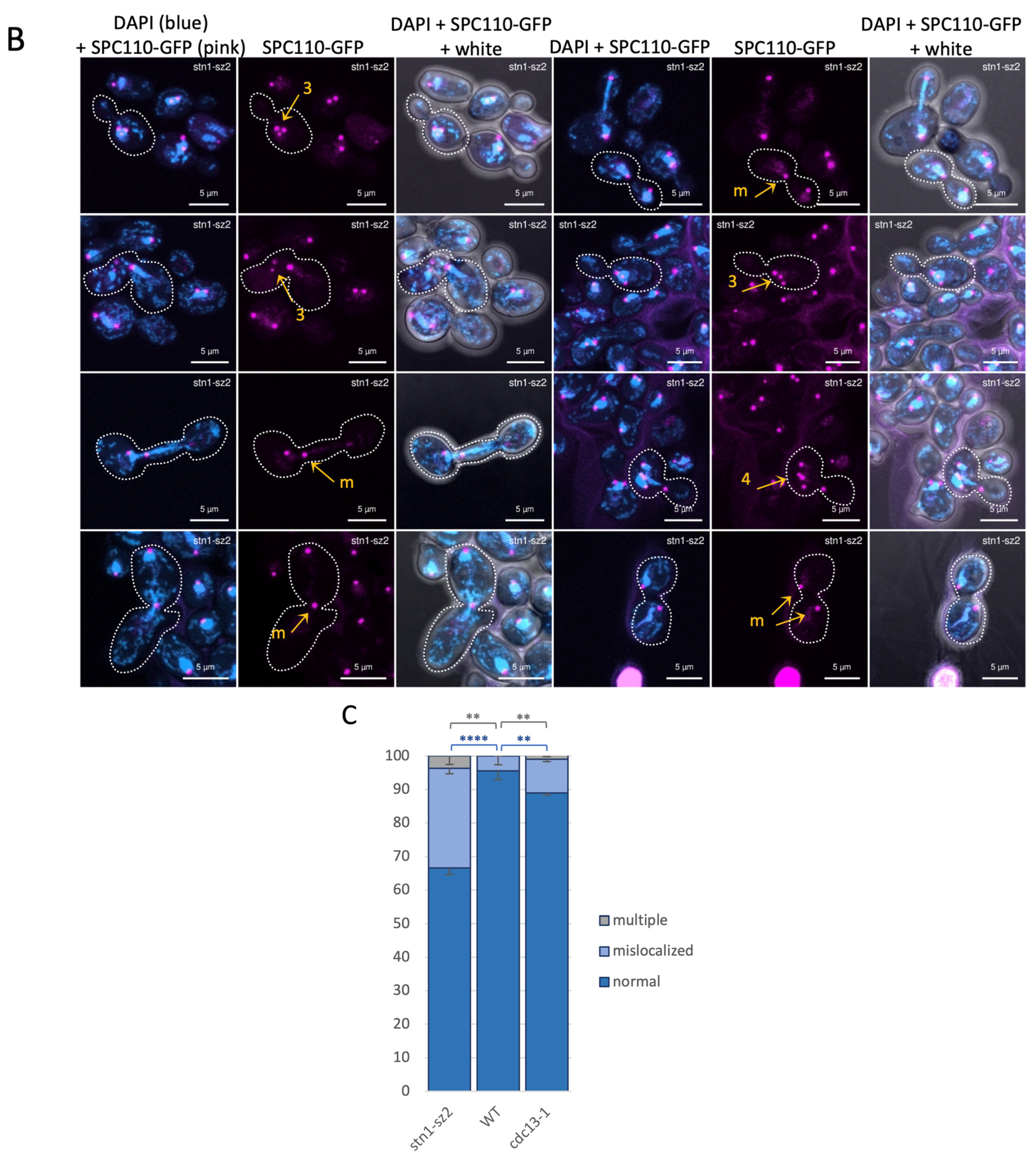
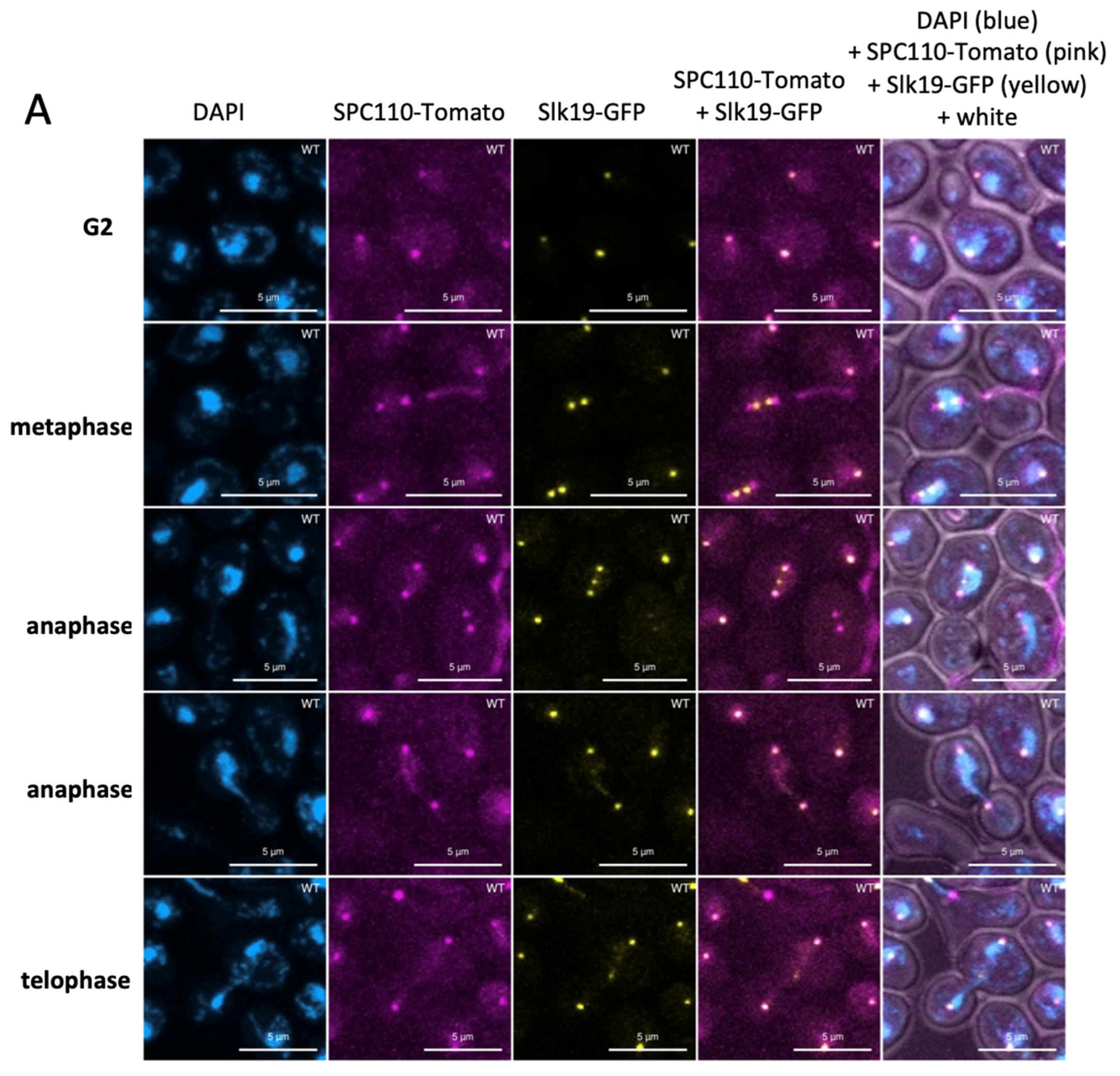
3.2. The Organization of the Mitotic Spindle Is Affected in the stn1-sz2 Mutant
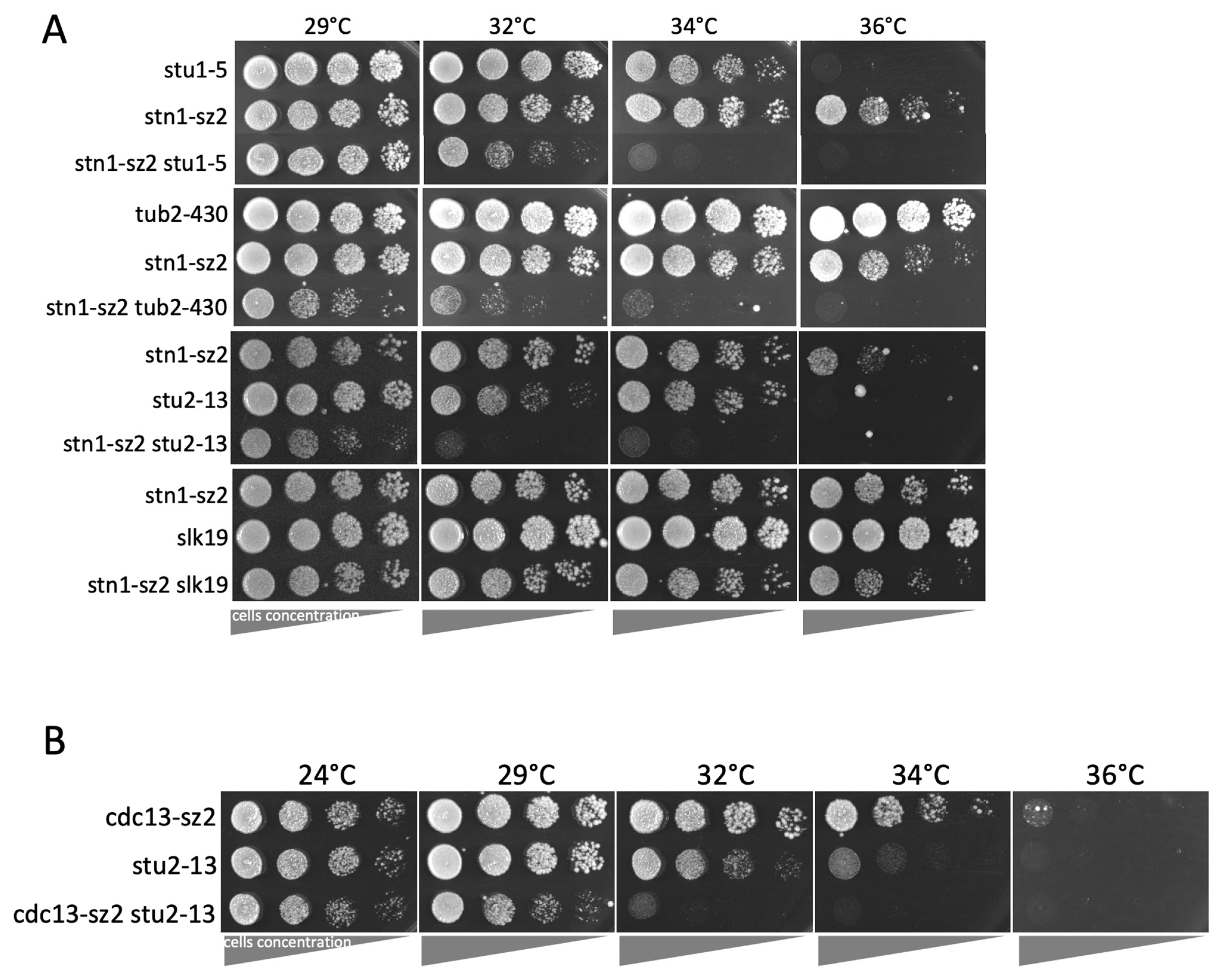
3.3. The stn1-sz2 Mutant Exhibits Genetic Interactions with Mutants of STU1, STU2, TUB2, and SLK19
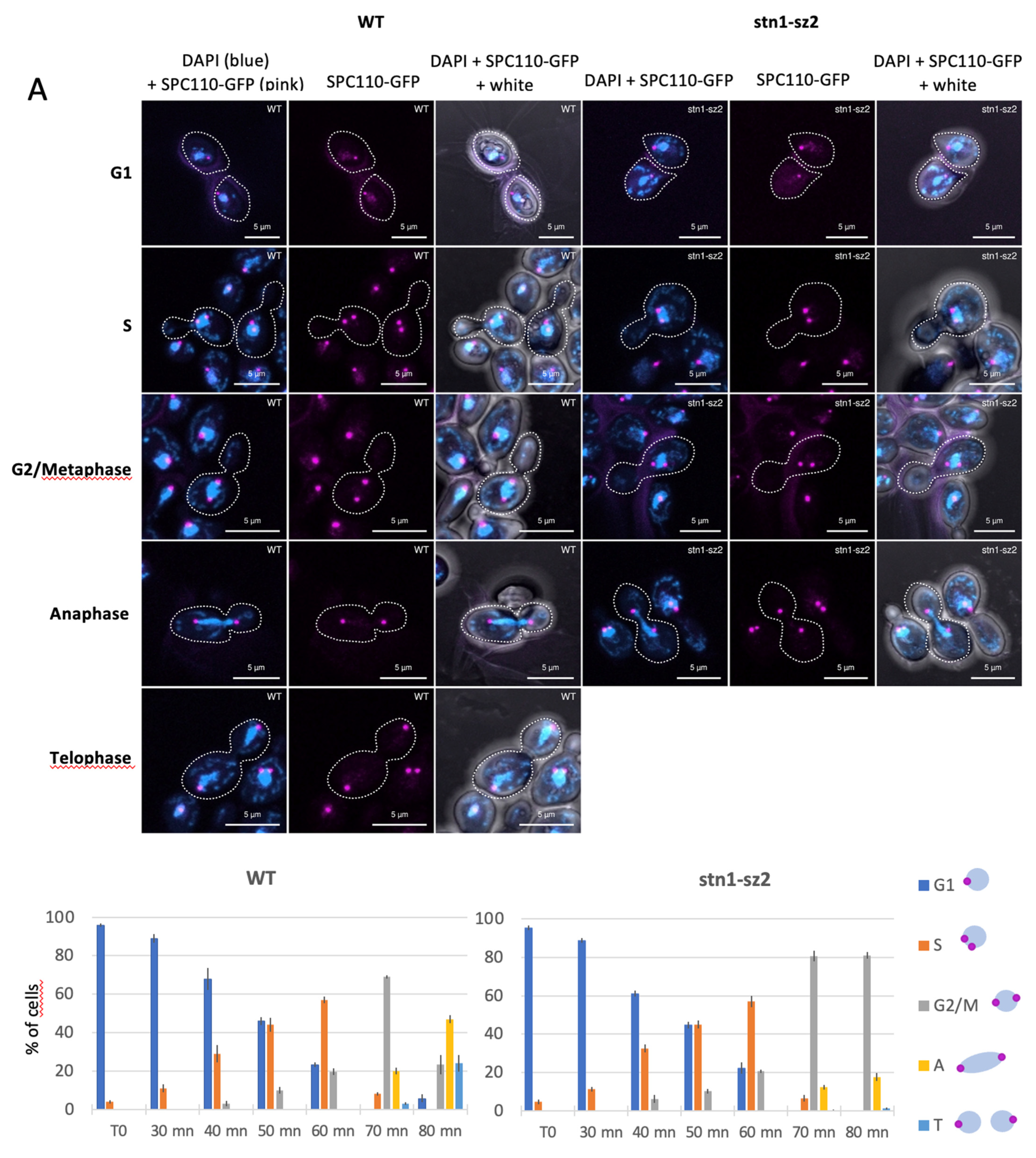
3.4. Dynamics of the Spindle Pole Bodies in the stn1-sz2 Mutant
3.5. Dynamics of the Kinetochores in the stn1-sz2 Mutant
4. Discussion
4.1. stn1-sz2 Is the Prototype for a Mitotic-Spindle-Damage-Characterized cst Mutant
4.2. A Major Defect of stn1-sz2 Is the Stability of the Mitotic Tubulin Spindle
4.3. The stn1-sz2 Mutant Exhibits “DNA Fragmentation”
4.4. Implication of the New stn1-sz2 Phenotypes for Cancer Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Lange, T. How telomeres solve the end-protection problem. Science 2009, 326, 948–952. [Google Scholar] [CrossRef]
- Garvik, B.; Carson, M.; Hartwell, L. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol. Cell. Biol. 1995, 15, 6128–6138. [Google Scholar] [CrossRef]
- Bonnell, E.; Pasquier, E.; Wellinger, R.J. Telomere Replication: Solving Multiple End Replication Problems. Front. Cell Dev. Biol. 2021, 9, 668171. [Google Scholar] [CrossRef]
- Nguyen, T.H.D.; Collins, K.; Nogales, E. Telomerase structures and regulation: Shedding light on the chromosome end. Curr. Opin. Struct. Biol. 2019, 55, 185–193. [Google Scholar] [CrossRef]
- Cai, S.W.; de Lange, T. CST-Polα/Primase: The second telomere maintenance machine. Genes Dev. 2023, 37, 555–569. [Google Scholar] [CrossRef]
- Roake, C.M.; Artandi, S.E. Regulation of human telomerase in homeostasis and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 384–397. [Google Scholar] [CrossRef]
- Zhang, J.M.; Zou, L. Alternative lengthening of telomeres: From molecular mechanisms to therapeutic outlooks. Cell Biosci. 2020, 10, 30. [Google Scholar] [CrossRef]
- Grandin, N.; Reed, S.I.; Charbonneau, M. Stn1, a new Saccharomyces cerevisiae protein, is implicated in telomere size regulation in association with Cdc13. Genes Dev. 1997, 11, 512–527. [Google Scholar] [CrossRef]
- Grandin, N.; Damon, C.; Charbonneau, M. Ten1 functions in telomere end protection and length regulation in association with Stn1 and Cdc13. EMBO J. 2001, 20, 1173–1183. [Google Scholar] [CrossRef]
- Shore, D.; Bianchi, A. Telomere length regulation: Coupling DNA end processing to feedback regulation of telomerase. EMBO J. 2009, 28, 2309–2322. [Google Scholar] [CrossRef]
- de Lange, T. Shelterin-Mediated Telomere Protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef]
- Amir, M.; Khan, P.; Queen, A.; Dohare, R.; Alajmi, M.F.; Hussain, A.; Islam, A.; Ahmad, F.; Hassan, I. Structural Features of Nucleoprotein CST/Shelterin Complex Involved in the Telomere Maintenance and Its Association with Disease Mutations. Cells 2020, 9, 359. [Google Scholar] [CrossRef]
- Lim, C.J.; Cech, T.R. Shaping human telomeres: From shelterin and CST complexes to telomeric chromatin organization. Nat. Rev. Mol. Cell Biol. 2021, 22, 283–298. [Google Scholar] [CrossRef]
- Miyake, Y.; Nakamura, M.; Nabetani, A.; Shimamura, S.; Tamura, M.; Yonehara, S.; Saito, M.; Ishikawa, F. RPA-like mammalian Ctc1-Stn1-Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol. Cell 2009, 36, 193–206. [Google Scholar] [CrossRef]
- Surovtseva, Y.V.; Churikov, D.; Boltz, K.A.; Song, X.; Lamb, J.C.; Warrington, R.; Leehy, K.; Heacock, M.; Price, C.M.; Shippen, D.E. Conserved telomere maintenance component 1 interacts with STN1 and maintains chromosome ends in higher eukaryotes. Mol. Cell 2009, 36, 207–218. [Google Scholar] [CrossRef]
- Martín, V.; Du, L.L.; Rozenzhak, S.; Russell, P. Protection of telomeres by a conserved Stn1-Ten1 complex. Proc. Natl. Acad. Sci. USA 2007, 104, 14038–14043. [Google Scholar] [CrossRef]
- Chen, L.Y.; Redon, S.; Lingner, J. The human CST complex is a terminator of telomerase activity. Nature 2012, 488, 540–544. [Google Scholar] [CrossRef]
- Mirman, Z.; Lottersberger, F.; Takai, H.; Kibe, T.; Gong, Y.; Takai, K.; Bianchi, A.; Zimmermann, M.; Durocher, D.; de Lange, T. 53BP1-RIF1-shieldin counteracts DSB resection through CST- and Polα-dependent fill-in. Nature 2018, 560, 112–116. [Google Scholar] [CrossRef]
- Cai, S.W.; Takai, H.; Zaug, A.J.; Dilgen, T.C.; Cech, T.R.; Walz, T.; de Lange, T. POT1 recruits and regulates CST-Polα/primase at human telomeres. Cell 2024, 187, 3638–3651.e3618. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef]
- Lee, J.W.; Ong, E.B.B. Genomic Instability and Cellular Senescence: Lessons From the Budding Yeast. Front. Cell Dev. Biol. 2020, 8, 619126. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, health, and hallmarks of aging. Cell 2021, 184, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Holohan, B.; Wright, W.E.; Shay, J.W. Cell biology of disease: Telomeropathies: An emerging spectrum disorder. J. Cell Biol. 2014, 205, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, T.; Liu, W.; Li, H.; Luo, Z.; Feng, X. Pan-Cancer Analyses Identify the CTC1-STN1-TEN1 Complex as a Protective Factor and Predictive Biomarker for Immune Checkpoint Blockade in Cancer. Front. Genet. 2022, 13, 859617. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Kim, E.; Le, N.T.; Ding, X.; Jaiswal, R.K.; Kostlan, R.J.; Nguyen, T.N.T.; Shiva, O.; Le, M.T.; Chai, W. Deficiency in mammalian STN1 promotes colon cancer development via inhibiting DNA repair. Sci. Adv. 2023, 9, eadd8023. [Google Scholar] [CrossRef]
- Lyu, X.; Sang, P.B.; Chai, W. CST in maintaining genome stability: Beyond telomeres. DNA Repair 2021, 102, 103104. [Google Scholar] [CrossRef]
- Weinert, T.A.; Hartwell, L.H. Cell cycle arrest of cdc mutants and specificity of the RAD9 checkpoint. Genetics 1993, 134, 63–80. [Google Scholar] [CrossRef]
- Grandin, N.; Charbonneau, M. Dysfunction of Telomeric Cdc13-Stn1-Ten1 Simultaneously Activates DNA Damage and Spindle Checkpoints. Cells 2024, 13, 1605. [Google Scholar] [CrossRef]
- Gietz, R.D.; Sugino, A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 1988, 74, 527–534. [Google Scholar] [CrossRef]
- Musacchio, A. The Molecular Biology of Spindle Assembly Checkpoint Signaling Dynamics. Curr. Biol. 2015, 25, R1002–R1018. [Google Scholar] [CrossRef]
- Scarfone, I.; Piatti, S. Coupling spindle position with mitotic exit in budding yeast: The multifaceted role of the small GTPase Tem1. Small GTPases 2015, 6, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Lanz, M.C.; Dibitetto, D.; Smolka, M.B. DNA damage kinase signaling: Checkpoint and repair at 30 years. EMBO J. 2019, 38, e101801. [Google Scholar] [CrossRef] [PubMed]
- Weinert, T.A.; Kiser, G.L.; Hartwell, L.H. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 1994, 8, 652–665. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, F.; Elledge, S.J. The Bfa1/Bub2 GAP complex comprises a universal checkpoint required to prevent mitotic exit. Curr. Biol. 2000, 10, 1379–1382. [Google Scholar] [CrossRef]
- Hoyt, M.A.; Totis, L.; Roberts, B.T.S. Cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 1991, 66, 507–517. [Google Scholar] [CrossRef]
- Li, R.; Murray, A.W. Feedback control of mitosis in budding yeast. Cell 1991, 66, 519–531. [Google Scholar] [CrossRef]
- Fraschini, R.; Formenti, E.; Lucchini, G.; Piatti, S. Budding yeast Bub2 is localized at spindle pole bodies and activates the mitotic checkpoint via a different pathway from Mad2. J. Cell Biol. 1999, 145, 979–991. [Google Scholar] [CrossRef]
- Johnston, G.C.; Pringle, J.R.; Hartwell, L.H. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp. Cell Res. 1977, 105, 79–98. [Google Scholar] [CrossRef]
- Lydall, D.; Weinert, T. Use of cdc13-1-induced DNA damage to study effects of checkpoint genes on DNA damage processing. Methods Enzym. 1997, 283, 410–424. [Google Scholar] [CrossRef]
- Clarke, D.J.; Segal, M.; Andrews, C.A.; Rudyak, S.G.; Jensen, S.; Smith, K.; Reed, S.I. S-phase checkpoint controls mitosis via an APC-independent Cdc20p function. Nat. Cell Biol. 2003, 5, 928–935. [Google Scholar] [CrossRef]
- Carmona-Gutierrez, D.; Eisenberg, T.; Büttner, S.; Meisinger, C.; Kroemer, G.; Madeo, F. Apoptosis in yeast: Triggers, pathways, subroutines. Cell Death Differ. 2010, 17, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.C.; Ismael, A.; Moore, J.K. Ase1 domains dynamically slow anaphase spindle elongation and recruit Bim1 to the midzone. Mol. Biol. Cell 2020, 31, 2733–2747. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, D.; Huffaker, T.C. STU1, a suppressor of a beta-tubulin mutation, encodes a novel and essential component of the yeast mitotic spindle. J. Cell Biol. 1994, 127, 1973–1984. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; You, L.; Pasqualone, D.; Kopski, K.M.; Huffaker, T.C. Stu1p is physically associated with beta-tubulin and is required for structural integrity of the mitotic spindle. Mol. Biol. Cell 2002, 13, 1881–1892. [Google Scholar] [CrossRef]
- Kosco, K.A.; Pearson, C.G.; Maddox, P.S.; Wang, P.J.; Adams, I.R.; Salmon, E.D.; Bloom, K.; Huffaker, T.C. Control of microtubule dynamics by Stu2p is essential for spindle orientation and metaphase chromosome alignment in yeast. Mol. Biol. Cell 2001, 12, 2870–2880. [Google Scholar] [CrossRef]
- Aiken, J.; Sept, D.; Costanzo, M.; Boone, C.; Cooper, J.A.; Moore, J.K. Genome-wide analysis reveals novel and discrete functions for tubulin carboxy-terminal tails. Curr. Biol. 2014, 24, 1295–1303. [Google Scholar] [CrossRef]
- Fees, C.P.; Aiken, J.; O’Toole, E.T.; Giddings, T.H., Jr.; Moore, J.K. The negatively charged carboxy-terminal tail of β-tubulin promotes proper chromosome segregation. Mol. Biol. Cell 2016, 27, 1786–1796. [Google Scholar] [CrossRef]
- Winey, M.; Bloom, K. Mitotic spindle form and function. Genetics 2012, 190, 1197–1224. [Google Scholar] [CrossRef]
- Chen, R.; Wold, M.S. Replication protein A: Single-stranded DNA’s first responder: Dynamic DNA-interactions allow replication protein A to direct single-strand DNA intermediates into different pathways for synthesis or repair. BioEssays 2014, 36, 1156–1161. [Google Scholar] [CrossRef]
- Casari, E.; Gnugnoli, M.; Rinaldi, C.; Pizzul, P.; Colombo, C.V.; Bonetti, D.; Longhese, M.P. To Fix or Not to Fix: Maintenance of Chromosome Ends Versus Repair of DNA Double-Strand Breaks. Cells 2022, 11, 3224. [Google Scholar] [CrossRef]
- Amin, M.A.; Agarwal, S.; Varma, D. Mapping the kinetochore MAP functions required for stabilizing microtubule attachments to chromosomes during metaphase. Cytoskeleton 2019, 76, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Nsamba, E.T.; Gupta, M.L. Tubulin isotypes—Functional insights from model organisms. J. Cell Sci. 2022, 135, jcs259539. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.; Funk, C.; Schäfer, A.; Lechner, J. Stu1 inversely regulates kinetochore capture and spindle stability. Genes Dev. 2009, 23, 2778–2791. [Google Scholar] [CrossRef]
- Kolenda, C.; Ortiz, J.; Pelzl, M.; Norell, S.; Schmeiser, V.; Lechner, J. Unattached kinetochores drive their own capturing by sequestering a CLASP. Nat. Commun. 2018, 9, 886. [Google Scholar] [CrossRef]
- Zeng, X.; Kahana, J.A.; Silver, P.A.; Morphew, M.K.; McIntosh, J.R.; Fitch, I.T.; Carbon, J.; Saunders, W.S. Slk19p is a centromere protein that functions to stabilize mitotic spindles. J. Cell Biol. 1999, 146, 415–425. [Google Scholar] [CrossRef]
- Sullivan, M.; Lehane, C.; Uhlmann, F. Orchestrating anaphase and mitotic exit: Separase cleavage and localization of Slk19. Nat. Cell Biol. 2001, 3, 771–777. [Google Scholar] [CrossRef]
- Norell, S.; Ortiz, J.; Lechner, J. Slk19 enhances cross-linking of microtubules by Ase1 and Stu1. Mol. Biol. Cell 2021, 32, ar22. [Google Scholar] [CrossRef]
- Al-Bassam, J.; Chang, F. Regulation of microtubule dynamics by TOG-domain proteins XMAP215/Dis1 and CLASP. Trends Cell Biol. 2011, 21, 604–614. [Google Scholar] [CrossRef]
- Severin, F.; Habermann, B.; Huffaker, T.; Hyman, T. Stu2 promotes mitotic spindle elongation in anaphase. J. Cell Biol. 2001, 153, 435–442. [Google Scholar] [CrossRef]
- Aravamudhan, P.; Felzer-Kim, I.; Gurunathan, K.; Joglekar, A.P. Assembling the protein architecture of the budding yeast kinetochore-microtubule attachment using FRET. Curr. Biol. 2014, 24, 1437–1446. [Google Scholar] [CrossRef]
- Prime, G.; Markie, D. The telomere repeat binding protein Trf1 interacts with the spindle checkpoint protein Mad1 and Nek2 mitotic kinase. Cell Cycle 2005, 4, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Blanco, R.; de Carcer, G.; Schoeftner, S.; Benetti, R.; Flores, J.M.; Malumbres, M.; Blasco, M.A. TRF1 controls telomere length and mitotic fidelity in epithelial homeostasis. Mol. Cell Biol. 2009, 29, 1608–1625. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Muramatsu, Y.; Yoshida, H.; Seimiya, H. TRF1 ensures the centromeric function of Aurora-B and proper chromosome segregation. Mol. Cell Biol. 2014, 34, 2464–2478. [Google Scholar] [CrossRef]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2011, 26, 125–132. [Google Scholar] [CrossRef]
- Fenech, M.; Knasmueller, S.; Bolognesi, C.; Bonassi, S.; Holland, N.; Migliore, L.; Palitti, F.; Natarajan, A.T.; Kirsch-Volders, M. Molecular mechanisms by which in vivo exposure to exogenous chemical genotoxic agents can lead to micronucleus formation in lymphocytes in vivo and ex vivo in humans. Mutat. Res. Rev. Mutat. Res. 2016, 770, 12–25. [Google Scholar] [CrossRef]
- Gu, P.; Jia, S.; Takasugi, T.; Smith, E.; Nandakumar, J.; Hendrickson, E.; Chang, S. CTC1-STN1 coordinates G- and C-strand synthesis to regulate telomere length. Aging Cell 2018, 17, e12783. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grandin, N.; Charbonneau, M. The stn1-sz2 Mutant Provides New Insight into the Impacts of Telomeric Cdc13-Stn1-Ten1 Dysfunction on Cell Cycle Progression. Cells 2025, 14, 784. https://doi.org/10.3390/cells14110784
Grandin N, Charbonneau M. The stn1-sz2 Mutant Provides New Insight into the Impacts of Telomeric Cdc13-Stn1-Ten1 Dysfunction on Cell Cycle Progression. Cells. 2025; 14(11):784. https://doi.org/10.3390/cells14110784
Chicago/Turabian StyleGrandin, Nathalie, and Michel Charbonneau. 2025. "The stn1-sz2 Mutant Provides New Insight into the Impacts of Telomeric Cdc13-Stn1-Ten1 Dysfunction on Cell Cycle Progression" Cells 14, no. 11: 784. https://doi.org/10.3390/cells14110784
APA StyleGrandin, N., & Charbonneau, M. (2025). The stn1-sz2 Mutant Provides New Insight into the Impacts of Telomeric Cdc13-Stn1-Ten1 Dysfunction on Cell Cycle Progression. Cells, 14(11), 784. https://doi.org/10.3390/cells14110784





