Mouse SPAG6L, a Key Cytoskeleton Modulator Essential for Male Germ Cell Development, Is Not Required for Sertoli Cell Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sertoli Cell Culture
2.3. Animals and Genotyping
2.4. RT-PCR
2.5. Histological Examination
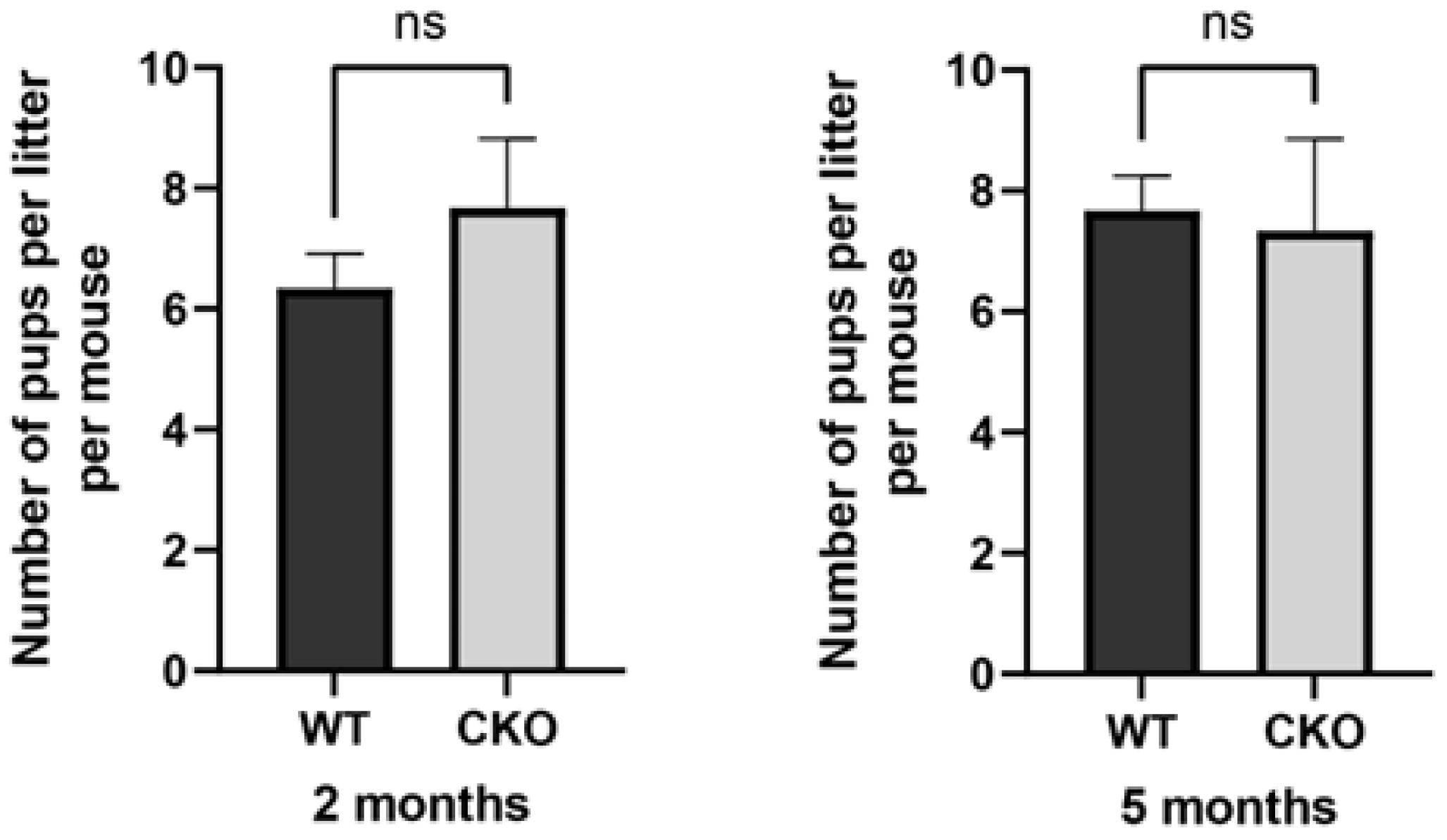
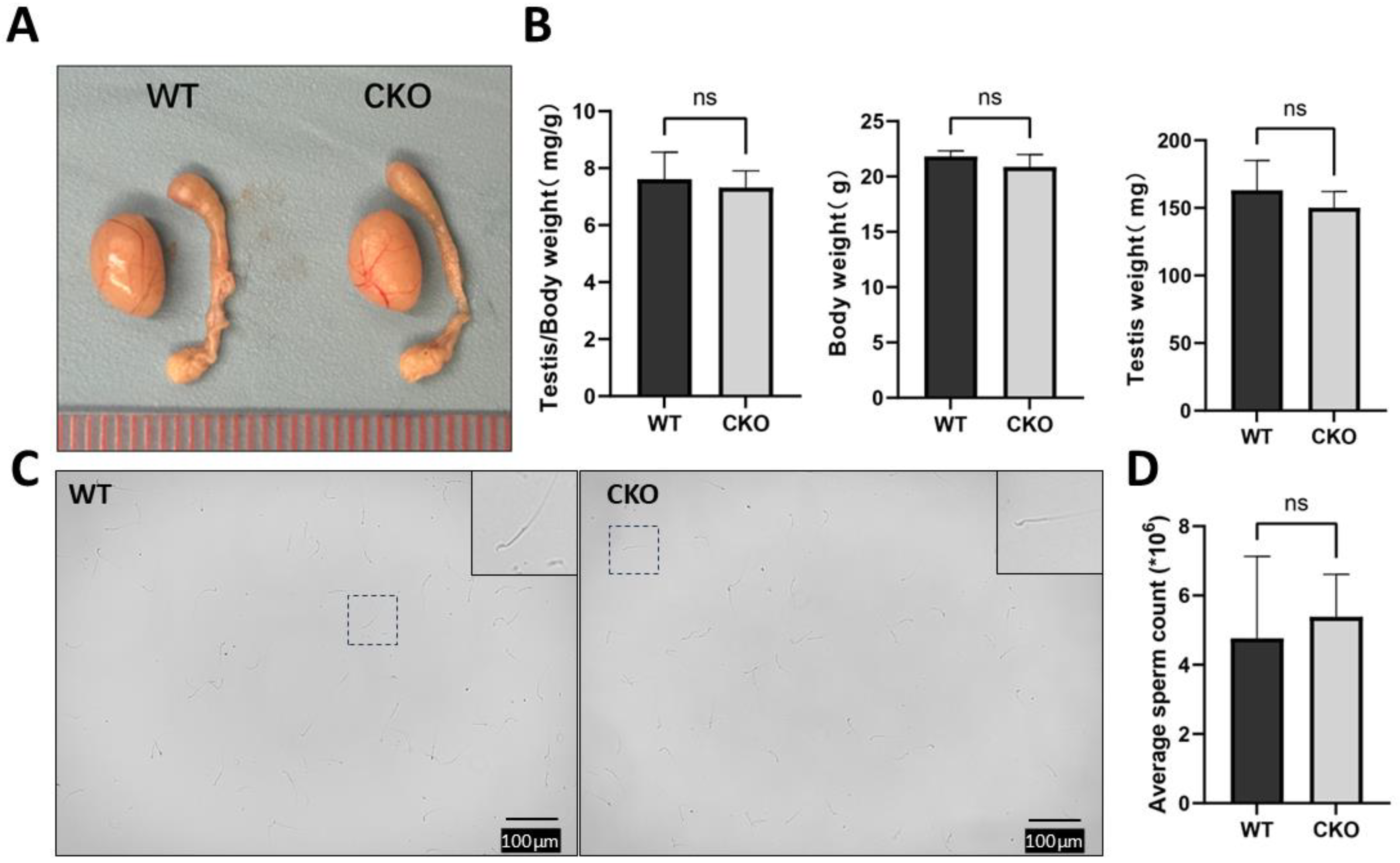
2.6. Male Fertility Test
2.7. Immunofluorescence Staining
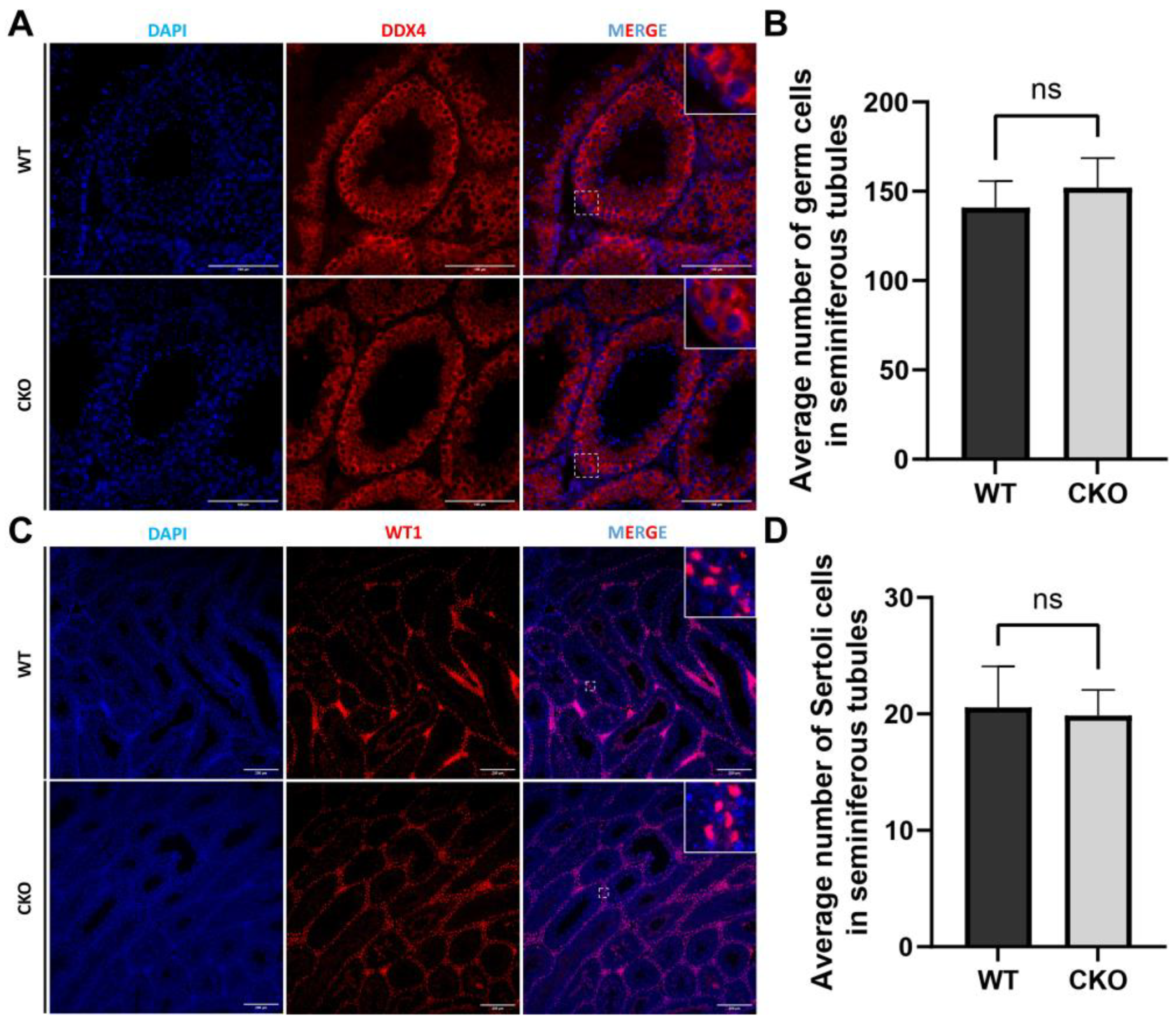
2.8. Cell Counts
2.9. Sperm Counting
2.10. Isolation of Sertoli Cells from Testis and Labeling of F-Actin with Phalloidin
2.11. Protein Extraction and Western Blot Analysis
2.12. Statistical Analysis
3. Results
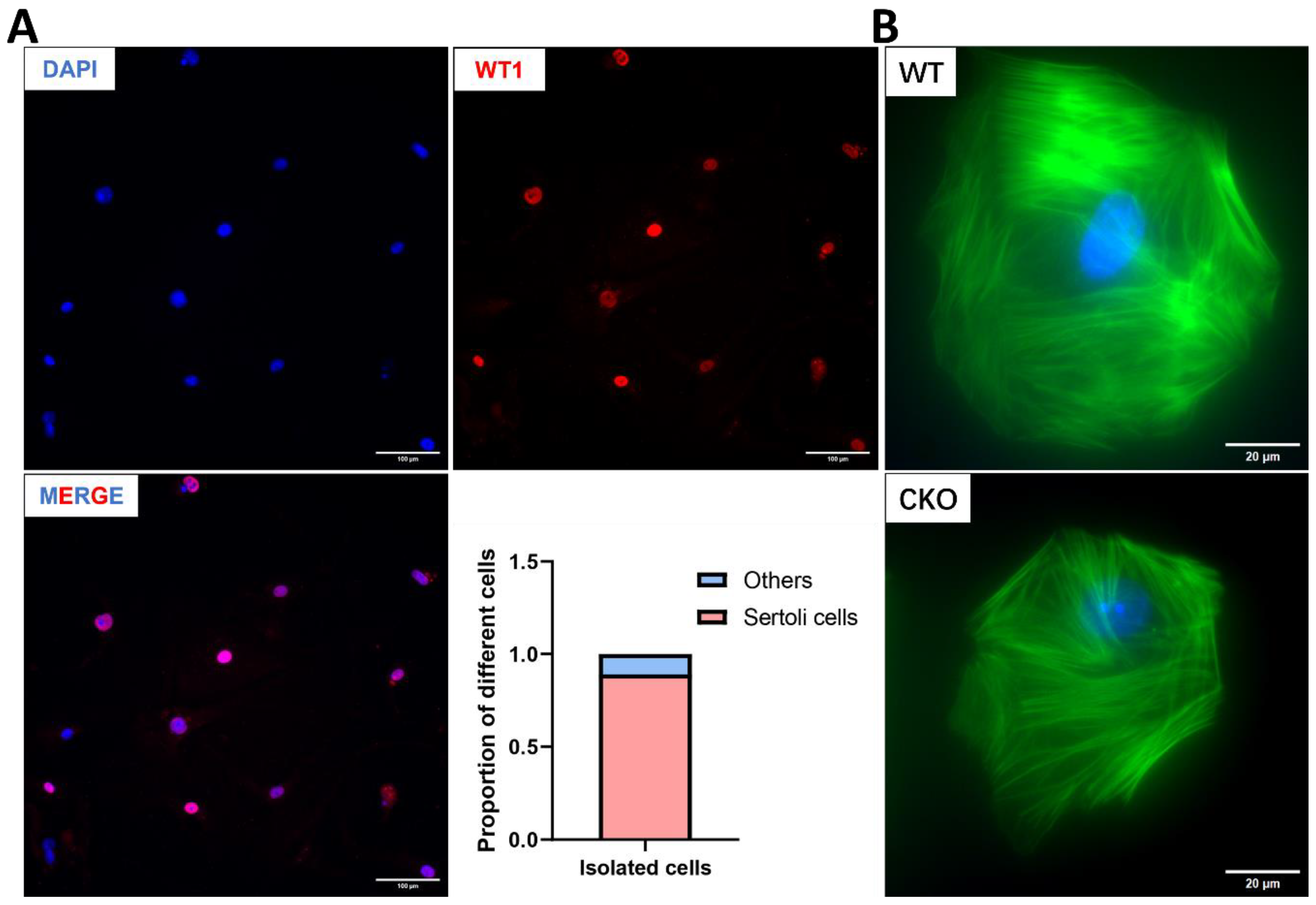
3.1. Spag6l mRNA Is Expressed in Sertoli Cells
3.2. Generation of Sertoli Cell-Specific Spag6l Knockout Mice
3.3. The cKO Male Mice Had Normal Fertility with Normal Testis Size and Sperm Number and Motility
3.4. Spermatogenesis Was Normal in the Spag6l cKO Mice
3.5. The Disruption of Spag6l in Sertoli Cells Did Not Change the Population of Germ Cells and Sertoli Cells in the Seminiferous Tubules
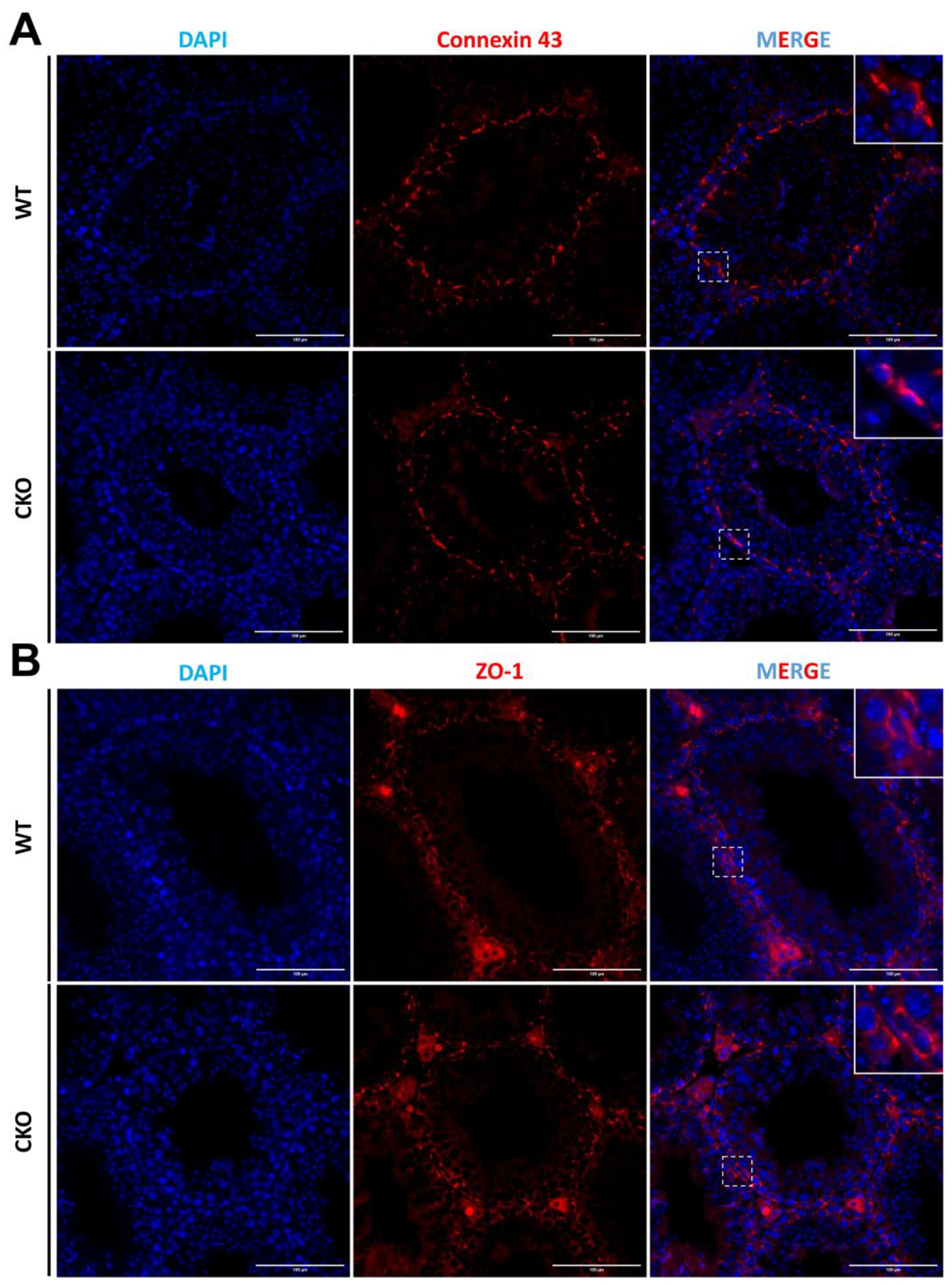
3.6. The Sertoli Cells Maintain a Normal Cellular Junction in the Absence of SPAG6L
3.7. The Cytoskeleton of Sertoli Cells Shows Normal Structure in the Absence of SPAG6L
3.8. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, E.F.; Lefebvre, P.A. PF16 encodes a protein with armadillo repeats and localizes to a single microtubule of the central apparatus in Chlamydomonas flagella. J. Cell Biol. 1996, 132, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Gołas, A.; Grzmil, P.; Wojnowski, L. Lineage-specific duplications of Muroidea Faim and Spag6 genes and atypical accelerated evolution of the parental Spag6 gene. J. Mol. Evol. 2013, 77, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.T.; Li, W.; Zhou, Q.; Haj-Diab, S.; Chowdhury, D.D.; Vaishnav, A.; Harding, P.; Williams, D.C., Jr.; Edwards, B.F.; Strauss, J.F., 3rd; et al. The Ancient and Evolved Mouse Sperm-Associated Antigen 6 Genes Have Different Biologic Functions In Vivo. Cells 2022, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Sapiro, R.; Kostetskii, I.; Olds-Clarke, P.; Gerton, G.L.; Radice, G.L.; Strauss, I.J. Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol. Cell Biol. 2002, 22, 6298–6305. [Google Scholar] [CrossRef]
- Teves, M.E.; Sears, P.R.; Li, W.; Zhang, Z.; Tang, W.; van Reesema, L.; Costanzo, R.M.; Davis, C.W.; Knowles, M.R.; Strauss, J.F., 3rd; et al. Sperm-associated antigen 6 (SPAG6) deficiency and defects in ciliogenesis and cilia function: Polarity, density, and beat. PLoS ONE 2014, 9, e107271. [Google Scholar] [CrossRef]
- Li, X.; Xu, L.; Li, J.; Li, B.; Bai, X.; Strauss, J.F., 3rd; Zhang, Z.; Wang, H. Otitis media in sperm-associated antigen 6 (Spag6)-deficient mice. PLoS ONE 2014, 9, e112879. [Google Scholar] [CrossRef]
- Cooley, L.F.; El Shikh, M.E.; Li, W.; Keim, R.C.; Zhang, Z.; Strauss, J.F.; Zhang, Z.; Conrad, D.H. Impaired immunological synapse in sperm associated antigen 6 (SPAG6) deficient mice. Sci. Rep. 2016, 6, 25840. [Google Scholar] [CrossRef]
- Li, W.; Mukherjee, A.; Wu, J.; Zhang, L.; Teves, M.E.; Li, H.; Nambiar, S.; Henderson, S.C.; Horwitz, A.R.; Strauss, J.F., III; et al. Sperm Associated Antigen 6 (SPAG6) Regulates Fibroblast Cell Growth, Morphology, Migration and Ciliogenesis. Sci. Rep. 2015, 5, 16506. [Google Scholar] [CrossRef]
- Donker, L.; Godinho, S.A. Rethinking tubulin acetylation: From regulation to cellular adaptation. Curr. Opin. Cell Biol. 2025, 94, 102512. [Google Scholar] [CrossRef]
- McKenna, E.D.; Sarbanes, S.L.; Cummings, S.W.; Roll-Mecak, A. The Tubulin Code, from Molecules to Health and Disease. Annu. Rev. Cell Dev. Biol. 2023, 39, 331–361. [Google Scholar] [CrossRef]
- O’Donnell, L.; Smith, L.B.; Rebourcet, D. Sertoli cells as key drivers of testis function. Semin. Cell Dev. Biol. 2022, 121, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef]
- Ni, F.D.; Hao, S.L.; Yang, W.X. Multiple signaling pathways in Sertoli cells: Recent findings in spermatogenesis. Cell Death Dis. 2019, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Mruk, D.D.; Cheng, C.Y. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 2004, 25, 747–806. [Google Scholar] [CrossRef]
- Wu, S.; Yan, M.; Ge, R.; Cheng, C.Y. Crosstalk between Sertoli and Germ Cells in Male Fertility. Trends Mol. Med. 2020, 26, 215–231. [Google Scholar] [CrossRef]
- Tang, E.I.; Mruk, D.D.; Cheng, C.Y. Regulation of microtubule (MT)-based cytoskeleton in the seminiferous epithelium during spermatogenesis. Semin. Cell Dev. Biol. 2016, 59, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Qu, H.; Han, Y.; Cheng, C.Y.; Xiao, X. Kinesins in Mammalian Spermatogenesis and Germ Cell Transport. Front. Cell Dev. Biol. 2022, 10, 837542. [Google Scholar] [CrossRef]
- França, L.R.; Auharek, S.A.; Hess, R.A.; Dufour, J.M.; Hinton, B.T. Blood-tissue barriers: Morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv. Exp. Med. Biol. 2012, 763, 237–259. [Google Scholar]
- Pelletier, R.M. The blood-testis barrier: The junctional permeability, the proteins and the lipids. Prog. Histochem. Cytochem. 2011, 46, 49–127. [Google Scholar] [CrossRef]
- Qian, X.; Mruk, D.D.; Cheng, Y.H.; Tang, E.I.; Han, D.; Lee, W.M.; Wong, E.W.; Cheng, C.Y. Actin binding proteins, spermatid transport and spermiation. Semin. Cell Dev. Biol. 2014, 30, 75–85. [Google Scholar] [CrossRef]
- Man, Y.; Li, W.; Yap, Y.T.; Kearney, A.; Yee, S.P.; Strauss, J.F., 3rd; Harding, P.; Song, S.; Zhang, L.; Zhang, Z. Generation of floxed Spag6l mice and disruption of the gene by crossing to a Hprt-Cre line. Genesis 2023, 61, e23512. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, M.P.; Linder, C.C.; Morales, C.R.; Heckert, L.L.; Pikus, J.; Griswold, M.D. Relationship of a mouse Sertoli cell line (MSC-1) to normal Sertoli cells. Biol. Reprod. 1994, 51, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Lécureuil, C.; Fontaine, I.; Crepieux, P.; Guillou, F. Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis 2002, 33, 114–118. [Google Scholar] [CrossRef]
- Eskola, V.; Ryhänen, P.; Savisalo, M.; Rannikko, A.; Kananen, K.; Sprengel, R.; Huhtaniemi, I. Stable transfection of the rat follicle-stimulating hormone receptor complementary DNA into an immortalized murine Sertoli cell line. Mol. Cell Endocrinol. 1998, 139, 143–152. [Google Scholar] [CrossRef]
- Kumar, N.; Srivastava, S.; Burek, M.; Förster, C.Y.; Roy, P. Assessment of estradiol-induced gene regulation and proliferation in an immortalized mouse immature Sertoli cell line. Life Sci. 2016, 148, 268–278. [Google Scholar] [CrossRef]
- Wang, Y.; Lui, W.Y. Opposite effects of interleukin-1alpha and transforming growth factor-beta2 induce stage-specific regulation of junctional adhesion molecule-B gene in Sertoli cells. Endocrinology 2009, 150, 2404–2412. [Google Scholar] [CrossRef]
- Takai, Y.; Ikeda, W.; Ogita, H.; Rikitake, Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu. Rev. Cell Dev. Biol. 2008, 24, 309–342. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mao, B.; Wu, S.; Lian, Q.; Ge, R.S.; Silvestrini, B.; Cheng, C.Y. Regulation of spermatid polarity by the actin- and microtubule (MT)-based cytoskeletons. Semin. Cell Dev. Biol. 2018, 81, 88–96. [Google Scholar] [CrossRef]
- Carmona, B.; Marinho, H.S.; Matos, C.L.; Nolasco, S.; Soares, H. Tubulin Post-Translational Modifications: The Elusive Roles of Acetylation. Biology 2023, 12, 561. [Google Scholar] [CrossRef]
- Ou, Y.; Dores, C.; Rodriguez-Sosa, J.R.; van der Hoorn, F.A.; Dobrinski, I. Primary cilia in the developing pig testis. Cell Tissue Res. 2014, 358, 597–605. [Google Scholar] [CrossRef]
- Zheng, D.F.; Wang, Q.; Wang, J.P.; Bao, Z.Q.; Wu, S.W.; Ma, L.; Chai, D.M.; Wang, Z.P.; Tao, Y.S. The Emerging Role of Sperm-Associated Antigen 6 Gene in the Microtubule Function of Cells and Cancer. Mol. Ther. Oncolytics 2019, 15, 101–107. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Li, W.; Zheng, C.; Dufour, J.M.; Walker, W.H.; Yuan, S.; Zhang, Z. Mouse SPAG6L, a Key Cytoskeleton Modulator Essential for Male Germ Cell Development, Is Not Required for Sertoli Cell Function. Cells 2025, 14, 783. https://doi.org/10.3390/cells14110783
Li T, Li W, Zheng C, Dufour JM, Walker WH, Yuan S, Zhang Z. Mouse SPAG6L, a Key Cytoskeleton Modulator Essential for Male Germ Cell Development, Is Not Required for Sertoli Cell Function. Cells. 2025; 14(11):783. https://doi.org/10.3390/cells14110783
Chicago/Turabian StyleLi, Tao, Wei Li, Cheng Zheng, Jannette M. Dufour, William H. Walker, Shuiqiao Yuan, and Zhibing Zhang. 2025. "Mouse SPAG6L, a Key Cytoskeleton Modulator Essential for Male Germ Cell Development, Is Not Required for Sertoli Cell Function" Cells 14, no. 11: 783. https://doi.org/10.3390/cells14110783
APA StyleLi, T., Li, W., Zheng, C., Dufour, J. M., Walker, W. H., Yuan, S., & Zhang, Z. (2025). Mouse SPAG6L, a Key Cytoskeleton Modulator Essential for Male Germ Cell Development, Is Not Required for Sertoli Cell Function. Cells, 14(11), 783. https://doi.org/10.3390/cells14110783






