PDE3A as a Therapeutic Target for the Modulation of Compartmentalised Cyclic Nucleotide-Dependent Signalling
Abstract
1. Introduction
2. Structural and Functional Insights into PDE3A
2.1. PDE3A Structure and Isoforms
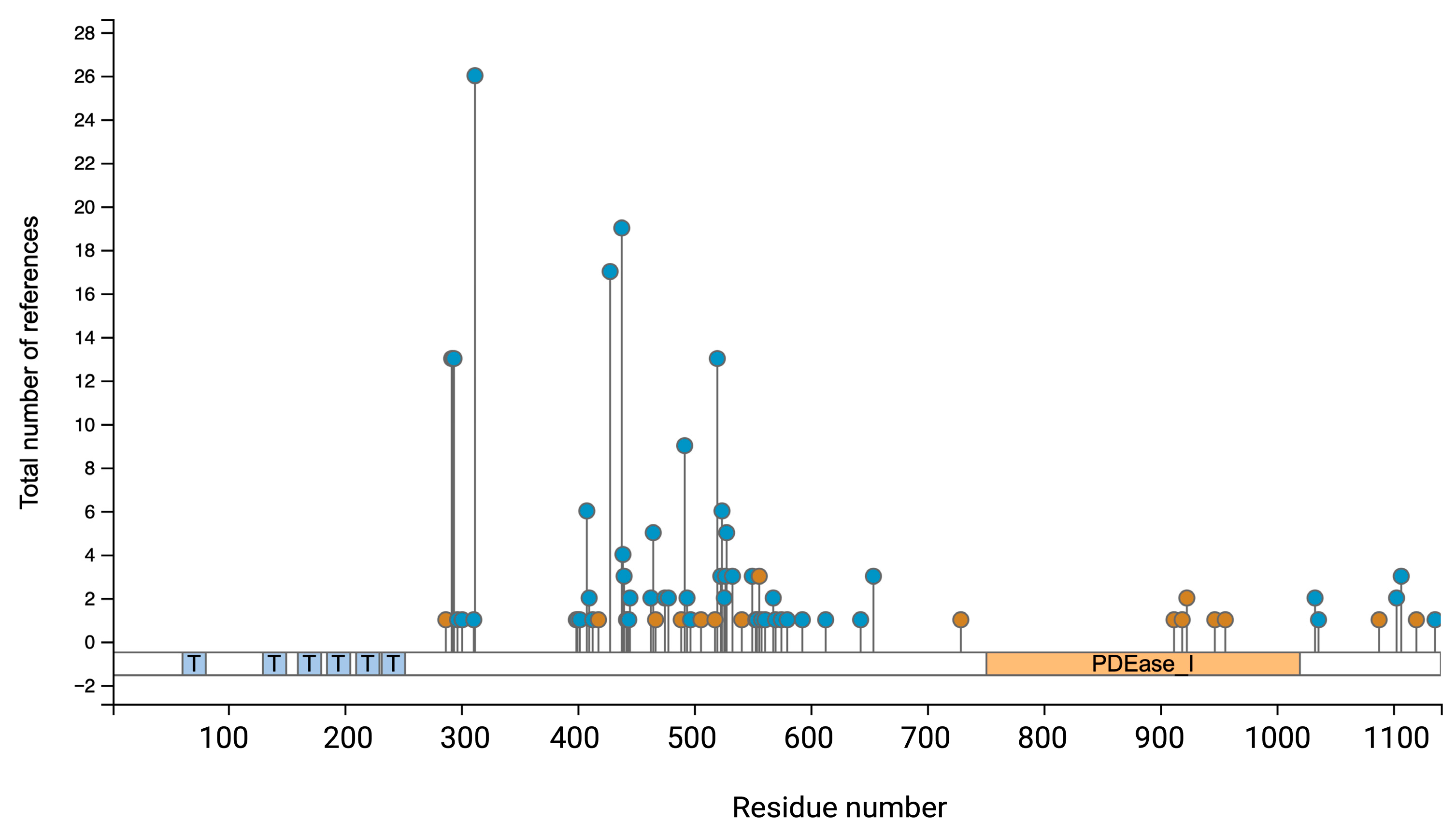
2.2. Phosphorylations Control PDE3A Function and Location
| Phosphorylation Site | Kinase/Mediator | Cellular Function/Outcome | Citation |
|---|---|---|---|
| Ser290–292 | Akt (PKB) | Phosphorylation increases PDE3A activity and regulates oocyte maturation in response to PI3K signalling. | [29] |
| Ser312 | PKA | Enhances catalytic activity of PDE3A; involved in feedback regulation of cAMP levels. Induces binding of 14-3-3 proteins. | [30,31,32] |
| Ser428 | PKC | Facilitates binding to 14-3-3 proteins; may influence PDE3A localization and stability. | [30,31,32] |
| Ser438 | PKC | Promotes 14-3-3 binding; associated with increased PDE3A activity in [30,31,32] platelets. | [30,31,32] |
| Ser465 | PKC | Associated with 14-3-3 interaction and activation during platelet activation. | [30,31,32] |
| Ser492 | PKC | Correlates with enhanced PDE3A activity; 14-3-3 binding during platelet activation. | [30,31,32] |
| Ser520 | Unknown | Unclear | www.phosphosite.org |
| Ser524 | Unknown | Unclear | www.phosphosite.org |
| Ser528 | Unknown | Unclear | www.phosphosite.org |
| Ser654 | PKG | Regulatory role and mediates proteasomal degradation of PDE3A. | [33] |
2.3. PDE3A Expression Pattern: Distinct PDE3A Compartments in the Same Cell
3. Pharmacologically Targeting PDE3A Activity and Its Protein–Protein Interactions
3.1. The PDE3 Family Is an Established Pharmacological Target
3.2. PDE3A as a Target
3.3. Targeting PDE3A with Pharmacological Agents
4. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PDE3A | Phosphodiesterase 3A |
| cAMP | Cyclic adenosine monophosphate |
| cNT | Cyclic nucleotides |
References
- Anton, S.E.; Kayser, C.; Maiellaro, I.; Nemec, K.; Moller, J.; Koschinski, A.; Zaccolo, M.; Annibale, P.; Falcke, M.; Lohse, M.J.; et al. Receptor-associated independent cAMP nanodomains mediate spatiotemporal specificity of GPCR signaling. Cell 2022, 185, 1130–1142.e11. [Google Scholar] [CrossRef] [PubMed]
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic targeting of 3′,5′-cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef] [PubMed]
- Bock, A.; Annibale, P.; Konrad, C.; Hannawacker, A.; Anton, S.E.; Maiellaro, I.; Zabel, U.; Sivaramakrishnan, S.; Falcke, M.; Lohse, M.J. Optical Mapping of cAMP Signaling at the Nanometer Scale. Cell 2020, 182, 1519–1530.e17. [Google Scholar] [CrossRef] [PubMed]
- Zaccolo, M.; Zerio, A.; Lobo, M.J. Subcellular Organization of the cAMP Signaling Pathway. Pharmacol. Rev. 2021, 73, 278–309. [Google Scholar] [CrossRef]
- Klussmann, E. Protein-protein interactions of PDE4 family members–Functions, interactions and therapeutic value. Cell Signal 2016, 28, 713–718. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, Y.; Yan, C.; Xiang, Y.K. Phosphodiesterase in heart and vessels: From physiology to diseases. Physiol. Rev. 2024, 104, 765–834. [Google Scholar] [CrossRef]
- Kelly, M.P.; Nikolaev, V.O.; Gobejishvili, L.; Lugnier, C.; Hesslinger, C.; Nickolaus, P.; Kass, D.A.; Pereira de Vasconcelos, W.; Fischmeister, R.; Brocke, S.; et al. Cyclic nucleotide phosphodiesterases as drug targets. Pharmacol. Rev. 2025, 77, 100042. [Google Scholar] [CrossRef]
- Dema, A.; Perets, E.; Schulz, M.S.; Deak, V.A.; Klussmann, E. Pharmacological targeting of AKAP-directed compartmentalized cAMP signalling. Cell Signal 2015, 27, 2474–2487. [Google Scholar] [CrossRef]
- Bucko, P.J.; Scott, J.D. Drugs that Regulate Local Cell Signaling: AKAP Targeting as a Therapeutic Option. Annu. Rev. Pharmacol. Toxicol. 2020, 61, 361–379. [Google Scholar] [CrossRef]
- Sholokh, A.; Klussmann, E. Local cyclic adenosine monophosphate signalling cascades-Roles and targets in chronic kidney disease. Acta Physiol. 2021, 232, e13641. [Google Scholar] [CrossRef]
- Subramanian, H.; Nikolaev, V.O. A-Kinase Anchoring Proteins in Cardiac Myocytes and Their Roles in Regulating Calcium Cycling. Cells 2023, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Beavo, J.A.; Conti, M.; Heaslip, R.J. Multiple cyclic nucleotide phosphodiesterases. Mol. Pharmacol. 1994, 46, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Beavo, J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: Essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 2007, 76, 481–511. [Google Scholar] [CrossRef]
- Francis, S.H.; Blount, M.A.; Corbin, J.D. Mammalian cyclic nucleotide phosphodiesterases: Molecular mechanisms and physiological functions. Physiol. Rev. 2011, 91, 651–690. [Google Scholar] [CrossRef]
- Ercu, M.; Marko, L.; Schachterle, C.; Tsvetkov, D.; Cui, Y.; Maghsodi, S.; Bartolomaeus, T.U.P.; Maass, P.G.; Zuhlke, K.; Gregersen, N.; et al. Phosphodiesterase 3A and Arterial Hypertension. Circulation 2020, 142, 133–149. [Google Scholar] [CrossRef]
- Ercu, M.; Mucke, M.B.; Pallien, T.; Marko, L.; Sholokh, A.; Schachterle, C.; Aydin, A.; Kidd, A.; Walter, S.; Esmati, Y.; et al. Mutant Phosphodiesterase 3A Protects From Hypertension-Induced Cardiac Damage. Circulation 2022, 146, 1758–1778. [Google Scholar] [CrossRef]
- Trawally, M. Beyond the heart—Exploring the therapeutic potential of PDE3 inhibitors. J. Res. Pharm. 2023, 27, 2218–2241. [Google Scholar] [CrossRef]
- Corpet, F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988, 16, 10881–10890. [Google Scholar] [CrossRef]
- Ercu, M.; Walter, S.; Klussmann, E. Mutations in Phosphodiesterase 3A (PDE3A) Cause Hypertension Without Cardiac Damage. Hypertension 2023, 80, 1171–1179. [Google Scholar] [CrossRef]
- Hambleton, R.; Krall, J.; Tikishvili, E.; Honeggar, M.; Ahmad, F.; Manganiello, V.C.; Movsesian, M.A. Isoforms of Cyclic Nucleotide Phosphodiesterase PDE3 and Their Contribution to cAMP Hydrolytic Activity in Subcellular Fractions of Human Myocardium. J. Biol. Chem. 2005, 280, 39168–39174. [Google Scholar] [CrossRef] [PubMed]
- Khalil, J.S.; Law, R.; Raslan, Z.; Cheah, L.T.; Hindle, M.S.; Aburima, A.A.; Kearney, M.T.; Naseem, K.M. Protein Kinase A Regulates Platelet Phosphodiesterase 3A through an A-Kinase Anchoring Protein Dependent Manner. Cells 2024, 13, 1104. [Google Scholar] [CrossRef] [PubMed]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.-H.; Lee, T.-Y.; Liang, H.-K.; Huang, C.-M.; Wang, T.-Y.; Yang, Y.-H.; Chu, C.-H.; Huang, H.-D.; Ko, M.-T.; Hwang, J.-K. KinasePhos 2.0: A web server for identifying protein kinase-specific phosphorylation sites based on sequences and coupling patterns. Nucleic Acids Res. 2007, 35, W588–W594. [Google Scholar] [CrossRef]
- Taylor, S.S.; Soberg, K.; Kobori, E.; Wu, J.; Pautz, S.; Herberg, F.W.; Skalhegg, B.S. The Tails of Protein Kinase A. Mol. Pharmacol. 2022, 101, 219–225. [Google Scholar] [CrossRef]
- Parnell, E.; Palmer, T.M.; Yarwood, S.J. The future of EPAC-targeted therapies: Agonism versus antagonism. Trends Pharmacol. Sci. 2015, 36, 203–214. [Google Scholar] [CrossRef]
- Gruscheski, L.; Brand, T. The Role of POPDC Proteins in Cardiac Pacemaking and Conduction. J. Cardiovasc. Dev. Dis. 2021, 8, 160. [Google Scholar] [CrossRef]
- Hennis, K.; Piantoni, C.; Biel, M.; Fenske, S.; Wahl-Schott, C. Pacemaker Channels and the Chronotropic Response in Health and Disease. Circ. Res. 2024, 134, 1348–1378. [Google Scholar] [CrossRef]
- Han, S.J.; Vaccari, S.; Nedachi, T.; Andersen, C.B.; Kovacina, K.S.; Roth, R.A.; Conti, M. Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J. 2006, 25, 5716–5725. [Google Scholar] [CrossRef]
- Pozuelo Rubio, M.; Campbell, D.G.; Morrice, N.A.; Mackintosh, C. Phosphodiesterase 3A binds to 14-3-3 proteins in response to PMA-induced phosphorylation of Ser428. Biochem. J. 2005, 392, 163–172. [Google Scholar] [CrossRef]
- Hunter, R.W.; MacKintosh, C.; Hers, I. Protein Kinase C-mediated Phosphorylation and Activation of PDE3A Regulate cAMP Levels in Human Platelets. J. Biol. Chem. 2009, 284, 12339–12348. [Google Scholar] [CrossRef] [PubMed]
- Vandeput, F.; Szabo-Fresnais, N.; Ahmad, F.; Kho, C.; Lee, A.; Krall, J.; Dunlop, A.; Hazel, M.W.; Wohlschlegel, J.A.; Hajjar, R.J.; et al. Selective regulation of cyclic nucleotide phosphodiesterase PDE3A isoforms. Proc. Natl. Acad. Sci. USA 2013, 110, 19778–19783. [Google Scholar] [CrossRef]
- Zemskov, E.A.; Zemskova, M.A.; Wu, X.; Moreno Caceres, S.; Caraballo Delgado, D.; Yegambaram, M.; Lu, Q.; Fu, P.; Wang, T.; Black, S.M. Novel mechanism of cyclic nucleotide crosstalk mediated by PKG-dependent proteasomal degradation of the Hsp90 client protein phosphodiesterase 3A. J. Biol. Chem. 2024, 300, 107723. [Google Scholar] [CrossRef] [PubMed]
- Maass, P.G.; Aydin, A.; Luft, F.C.; Schachterle, C.; Weise, A.; Stricker, S.; Lindschau, C.; Vaegler, M.; Qadri, F.; Toka, H.R.; et al. PDE3A mutations cause autosomal dominant hypertension with brachydactyly. Nat. Genet. 2015, 47, 647–653. [Google Scholar] [CrossRef]
- Dessauer, C.W. Adenylyl cyclase—A-kinase anchoring protein complexes: The next dimension in cAMP signaling. Mol. Pharmacol. 2009, 76, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Beca, S.; Ahmad, F.; Shen, W.; Liu, J.; Makary, S.; Polidovitch, N.; Sun, J.; Hockman, S.; Chung, Y.W.; Movsesian, M.; et al. Phosphodiesterase Type 3A Regulates Basal Myocardial Contractility Through Interacting With Sarcoplasmic Reticulum Calcium ATPase Type 2a Signaling Complexes in Mouse Heart. Circ. Res. 2013, 112, 289–297. [Google Scholar] [CrossRef]
- Ahmad, F.; Shen, W.; Vandeput, F.; Szabo-Fresnais, N.; Krall, J.; Degerman, E.; Goetz, F.; Klussmann, E.; Movsesian, M.; Manganiello, V. Regulation of sarcoplasmic reticulum Ca2+ ATPase 2 (SERCA2) activity by phosphodiesterase 3A (PDE3A) in human myocardium: Phosphorylation-dependent interaction of PDE3A1 with SERCA2. J. Biol. Chem. 2015, 290, 6763–6776. [Google Scholar] [CrossRef]
- Puxeddu, E.; Uhart, M.; Li, C.-C.; Ahmad, F.; Pacheco-Rodriguez, G.; Manganiello, V.C.; Moss, J.; Vaughan, M. Interaction of phosphodiesterase 3A with brefeldin A-inhibited guanine nucleotide-exchange proteins BIG1 and BIG2 and effect on ARF1 activity. Proc. Natl. Acad. Sci. USA 2009, 106, 6158–6163. [Google Scholar] [CrossRef]
- Penmatsa, H.; Zhang, W.; Yarlagadda, S.; Li, C.; Conoley, V.G.; Yue, J.; Bahouth, S.W.; Buddington, R.K.; Zhang, G.; Nelson, D.J.; et al. Compartmentalized Cyclic Adenosine 3′,5′-Monophosphate at the Plasma Membrane Clusters PDE3A and Cystic Fibrosis Transmembrane Conductance Regulator into Microdomains. MBoC 2010, 21, 1097–1110. [Google Scholar] [CrossRef]
- Mika, D.; Leroy, J.; Vandecasteele, G.; Fischmeister, R. PDEs create local domains of cAMP signaling. J. Mol. Cell. Cardiol. 2012, 52, 323–329. [Google Scholar] [CrossRef]
- Subramaniam, G.; Schleicher, K.; Kovanich, D.; Zerio, A.; Folkmanaite, M.; Chao, Y.C.; Surdo, N.C.; Koschinski, A.; Hu, J.; Scholten, A.; et al. Integrated Proteomics Unveils Nuclear PDE3A2 as a Regulator of Cardiac Myocyte Hypertrophy. Circ. Res. 2023, 132, 828–848. [Google Scholar] [CrossRef]
- Ahmad, F.; Lindh, R.; Tang, Y.; Ruishalme, I.; Öst, A.; Sahachartsiri, B.; Strålfors, P.; Degerman, E.; Manganiello, V.C. Differential regulation of adipocyte PDE3B in distinct membrane compartments by insulin and the β3-adrenergic receptor agonist CL316243: Effects of caveolin-1 knockdown on formation/maintenance of macromolecular signalling complexes. Biochem. J. 2009, 424, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Rondinone, C.M.; Carvalho, E.; Rahn, T.; Manganiello, V.C.; Degerman, E.; Smith, U.P. Phosphorylation of PDE3B by Phosphatidylinositol 3-Kinase Associated with the Insulin Receptor. J. Biol. Chem. 2000, 275, 10093–10098. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Lindh, R.; Wierup, N.; Zmuda-Trzebiatowska, E.; Lindqvist, A.; Manganiello, V.C.; Degerman, E. Phosphodiesterase 3B Is Localized in Caveolae and Smooth ER in Mouse Hepatocytes and Is Important in the Regulation of Glucose and Lipid Metabolism. PLoS ONE 2009, 4, e4671. [Google Scholar] [CrossRef] [PubMed]
- Degerman, E.; Ahmad, F.; Chung, Y.W.; Guirguis, E.; Omar, B.; Stenson, L.; Manganiello, V. From PDE3B to the regulation of energy homeostasis. Curr. Opin. Pharmacol. 2011, 11, 676–682. [Google Scholar] [CrossRef]
- Wilson, L.S.; Baillie, G.S.; Pritchard, L.M.; Umana, B.; Terrin, A.; Zaccolo, M.; Houslay, M.D.; Maurice, D.H. A Phosphodiesterase 3B-based Signaling Complex Integrates Exchange Protein Activated by cAMP 1 and Phosphatidylinositol 3-Kinase Signals in Human Arterial Endothelial Cells. J. Biol. Chem. 2011, 286, 16285–16296. [Google Scholar] [CrossRef]
- Maurice, D.H.; Ke, H.; Ahmad, F.; Wang, Y.; Chung, J.; Manganiello, V.C. Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug Discov. 2014, 13, 290–314. [Google Scholar] [CrossRef]
- Liu, H.; Maurice, D.H. Expression of cyclic GMP-inhibited phosphodiesterases 3A and 3B (PDE3A and PDE3B) in rat tissues: Differential subcellular localization and regulated expression by cyclic AMP. Br. J. Pharmacol. 1998, 125, 1501–1510. [Google Scholar] [CrossRef]
- Omori, K.; Kotera, J. Overview of PDEs and Their Regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef]
- Movsesian, M. Novel approaches to targeting PDE3 in cardiovascular disease. Pharmacol. Ther. 2016, 163, 74–81. [Google Scholar] [CrossRef]
- Movsesian, M.A.; Bristow, M.R. Alterations in cAMP-Mediated Signaling and Their Role in the Pathophysiology of Dilated Cardiomyopathy. In Current Topics in Developmental Biology; Academic Press: Cambridge, MA, USA, 2005; Volume 68, pp. 25–48. [Google Scholar]
- Belleville-Rolland, T.; Leuci, A.; Mansour, A.; Decouture, B.; Martin, F.; Poirault-Chassac, S.; Rouaud, M.; Guerineau, H.; Dizier, B.; Pidard, D.; et al. Role of Membrane Lipid Rafts in MRP4 (ABCC4) Dependent Regulation of the cAMP Pathway in Blood Platelets. Thromb. Haemost. 2021, 121, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Tanaka, M.; Takeda, S.; Ito, H.; Nagano, K. Cilostazol Induces PGI2 Production via Activation of the Downstream Epac-1/Rap1 Signaling Cascade to Increase Intracellular Calcium by PLCε and to Activate p44/42 MAPK in Human Aortic Endothelial Cells. PLoS ONE 2015, 10, e0132835. [Google Scholar] [CrossRef]
- Conti, M.; Andersen, C.B.; Richard, F.; Mehats, C.; Chun, S.-Y.; Horner, K.; Jin, C.; Tsafriri, A. Role of cyclic nucleotide signaling in oocyte maturation. Mol. Cell. Endocrinol. 2002, 187, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H. Phosphodiesterases as Drug Targets; Springer: Berlin/Heidelberg, Germany, 2011; p. 1. [Google Scholar]
- Fujiwara, T.; Ishii, S.; Minatsuki, S.; Hatano, M.; Takeda, N. Exploring Novel Therapeutics for Pulmonary Arterial Hypertension from the Bench to the Bedside. Int. Heart J. 2025, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ekholm, D.; Hemmer, B.; Gao, G.; Vergelli, M.; Martin, R.; Manganiello, V. Differential expression of cyclic nucleotide phosphodiesterase 3 and 4 activities in human T cell clones specific for myelin basic protein. J. Immunol. 1997, 159, 1520–1529. [Google Scholar] [CrossRef]
- Krause, P.N.; McGeorge, G.; McPeek, J.L.; Khalid, S.; Nelin, L.D.; Liu, Y.; Chen, B. Pde3a and Pde3b regulation of murine pulmonary artery smooth muscle cell growth and metabolism. Physiol. Rep. 2024, 12, e70089. [Google Scholar] [CrossRef]
- Greulich, H.; Kaplan, B.; Mertins, P.; Chen, T.-H.; Tanaka, K.E.; Yun, C.-H.; Zhang, X.; Lee, S.-H.; Cho, J.; Ambrogio, L.; et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc. Natl. Acad. Sci. USA 2012, 109, 14476–14481. [Google Scholar] [CrossRef]
- Hao, N.; Shen, W.; Du, R.; Jiang, S.; Zhu, J.; Chen, Y.; Huang, C.; Shi, Y.; Xiang, R.; Luo, Y. Phosphodiesterase 3A Represents a Therapeutic Target that Drives Stem Cell–like Property and Metastasis in Breast Cancer. Mol. Cancer Ther. 2020, 19, 868–881. [Google Scholar] [CrossRef]
- Vandenberghe, P.; Hagué, P.; Hockman, S.C.; Manganiello, V.C.; Demetter, P.; Erneux, C.; Vanderwinden, J.-M. Phosphodiesterase 3A: A new player in development of interstitial cells of Cajal and a prospective target in gastrointestinal stromal tumors (GIST). Oncotarget 2017, 8, 41026–41043. [Google Scholar] [CrossRef]
- Toivanen, K.; Kilpinen, S.; Ojala, K.; Merikoski, N.; Salmikangas, S.; Sampo, M.; Böhling, T.; Sihto, H. PDE3A Is a Highly Expressed Therapy Target in Myxoid Liposarcoma. Cancers 2023, 15, 5308. [Google Scholar] [CrossRef]
- Nazir, M.; Senkowski, W.; Nyberg, F.; Blom, K.; Edqvist, P.-H.; Jarvius, M.; Andersson, C.; Gustafsson, M.G.; Nygren, P.; Larsson, R.; et al. Targeting tumor cells based on Phosphodiesterase 3A expression. Exp. Cell Res. 2017, 361, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Argyrousi, E.K.; Heckman, P.R.A.; Prickaerts, J. Role of cyclic nucleotides and their downstream signaling cascades in memory function: Being at the right time at the right spot. Neurosci. Biobehav. Rev. 2020, 113, 12–38. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, R.R.; Bondy, C.A. Differential cellular pattern of gene expression for two distinct cGMP-inhibited cyclic nucleotide phosphodiesterases in developing and mature rat brain. Neuroscience 1996, 72, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Mitome-Mishima, Y.; Miyamoto, N.; Tanaka, R.; Oishi, H.; Arai, H.; Hattori, N.; Urabe, T. Differences in phosphodiesterase 3A and 3B expression after ischemic insult. Neurosci. Res. 2013, 75, 340–348. [Google Scholar] [CrossRef]
- Weiss, S.; Oz, S.; Benmocha, A.; Dascal, N. Regulation of cardiac L-type Ca2+ channel CaV1.2 via the beta-adrenergic-cAMP-protein kinase A pathway: Old dogmas, advances, and new uncertainties. Circ. Res. 2013, 113, 617–631. [Google Scholar] [CrossRef]
- Pallien, T.; Klussmann, E. New aspects in cardiac L-type Ca2+ channel regulation. Biochem. Soc. Trans. 2020, 48, 39–49. [Google Scholar] [CrossRef]
- Oz, S.; Keren-Raifman, T.; Sharon, T.; Subramaniam, S.; Pallien, T.; Katz, M.; Tsemakhovich, V.; Sholokh, A.; Watad, B.; Tripathy, D.R.; et al. Tripartite interactions of PKA catalytic subunit and C-terminal domains of cardiac Ca2+ channel may modulate its beta-adrenergic regulation. BMC Biol. 2024, 22, 276. [Google Scholar] [CrossRef]
- Wu, H.; Lee, J.; Vincent, L.G.; Wang, Q.; Gu, M.; Lan, F.; Churko, J.M.; Sallam, K.I.; Matsa, E.; Sharma, A.; et al. Epigenetic Regulation of Phosphodiesterases 2A and 3A Underlies Compromised beta-Adrenergic Signaling in an iPSC Model of Dilated Cardiomyopathy. Cell Stem Cell 2015, 17, 89–100. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Greenstein, J.L.; Winslow, R.L. Interaction between phosphodiesterases in the regulation of the cardiac beta-adrenergic pathway. J. Mol. Cell. Cardiol. 2015, 88, 29–38. [Google Scholar] [CrossRef]
- Pavlaki, N.; De Jong, K.A.; Geertz, B.; Nikolaev, V.O.; Froese, A. Cardiac Hypertrophy Changes Compartmentation of cAMP in Non-Raft Membrane Microdomains. Cells 2021, 10, 535. [Google Scholar] [CrossRef]
- Lygren, B.; Carlson, C.R.; Santamaria, K.; Lissandron, V.; McSorley, T.; Litzenberg, J.; Lorenz, D.; Wiesner, B.; Rosenthal, W.; Zaccolo, M.; et al. AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep. 2007, 8, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Gorski, P.A.; Ceholski, D.K.; Young, H.S. Structure-Function Relationship of the SERCA Pump and Its Regulation by Phospholamban and Sarcolipin. In Membrane Dynamics and Calcium Signaling; Krebs, J., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 981, pp. 77–119. [Google Scholar]
- Hamm, N.C.; Stammers, A.N.; Susser, S.E.; Hlynsky, M.W.; Kimber, D.E.; Kehler, D.S.; Duhamel, T.A. Regulation of Cardiac Sarco(endo)plasmic Reticulum Calcium-ATPases (SERCA2a) in Response to Exercise. In Regulation of Ca2+-ATPases, V-ATPases and F-ATPases; Chakraborti, S., Dhalla, N.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 187–206. [Google Scholar]
- Kiess, T.-O.; Kockskämper, J. SERCA Activity Controls the Systolic Calcium Increase in the Nucleus of Cardiac Myocytes. Front. Physiol. 2019, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Lefkimmiatis, K.; Zaccolo, M. cAMP signaling in subcellular compartments. Pharmacol. Ther. 2014, 143, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Nemirovskaya, T.L.; Sharlo, K.A. Roles of ATP and SERCA in the Regulation of Calcium Turnover in Unloaded Skeletal Muscles: Current View and Future Directions. Int. J. Mol. Sci. 2022, 23, 6937. [Google Scholar] [CrossRef]
- Xu, H.; Van Remmen, H. The SarcoEndoplasmic Reticulum Calcium ATPase (SERCA) pump: A potential target for intervention in aging and skeletal muscle pathologies. Skelet. Muscle 2021, 11, 25. [Google Scholar] [CrossRef]
- Skogestad, J.; Albert, I.; Hougen, K.; Lothe, G.B.; Lunde, M.; Eken, O.S.; Veras, I.; Huynh, N.T.T.; Borstad, M.; Marshall, S.; et al. Disruption of Phosphodiesterase 3A Binding to SERCA2 Increases SERCA2 Activity and Reduces Mortality in Mice With Chronic Heart Failure. Circulation 2023, 147, 1221–1236. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Begum, N.; Hockman, S.; Manganiello, V.C. Phosphodiesterase 3A (PDE3A) Deletion Suppresses Proliferation of Cultured Murine Vascular Smooth Muscle Cells (VSMCs) via Inhibition of Mitogen-activated Protein Kinase (MAPK) Signaling and Alterations in Critical Cell Cycle Regulatory Proteins. J. Biol. Chem. 2011, 286, 26238–26249. [Google Scholar] [CrossRef]
- Kalantzi, K.; Tentolouris, N.; Melidonis, A.J.; Papadaki, S.; Peroulis, M.; Amantos, K.A.; Andreopoulos, G.; Bellos, G.I.; Boutel, D.; Bristianou, M.; et al. Efficacy and Safety of Adjunctive Cilostazol to Clopidogrel-Treated Diabetic Patients with Symptomatic Lower Extremity Artery Disease in the Prevention of Ischemic Vascular Events. J. Am. Heart Assoc. 2021, 10, e018184. [Google Scholar] [CrossRef]
- Suarez Ferreira, S.P.; Hall, R.; Majumdar, M.; Goudot, G.; Jessula, S.; Feldman, Z.M.; Bellomo, T.; Lee, I.; Owolabi, L.; Kirshkaln-Leahy, A.; et al. Effect of Cilostazol in Platelet Inhibition in Patients with Peripheral Artery Disease. J. Vasc. Surg. 2023, 77, e326–e327. [Google Scholar] [CrossRef]
- Sohn, M.; Lim, S. The Role of Cilostazol, a Phosphodiesterase-3 Inhibitor, in the Development of Atherosclerosis and Vascular Biology: A Review with Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 2593. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Shen, W.; Manganiello, V. Role of PDE3A in regulation of cell cycle progression in mouse vascular smooth muscle cells and oocytes: Implications in cardiovascular diseases and infertility. Curr. Opin. Pharmacol. 2011, 11, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Shitsukawa, K.; Andersen, C.B.; Richard, F.J.; Horner, A.K.; Wiersma, A.; van Duin, M.; Conti, M. Cloning and characterization of the cyclic guanosine monophosphate-inhibited phosphodiesterase PDE3A expressed in mouse oocyte. Biol. Reprod. 2001, 65, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Friis, U.G.; Madsen, K.; Stubbe, J.; Hansen, P.B.; Svenningsen, P.; Bie, P.; Skott, O.; Jensen, B.L. Regulation of renin secretion by renal juxtaglomerular cells. Pflug. Arch. 2013, 465, 25–37. [Google Scholar] [CrossRef]
- Dousa, T.P. Cyclic-3′,5′-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int. 1999, 55, 29–62. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, J.; Meng, Y.; Kasai, A.; Hiramatsu, N.; Hayakawa, K.; Miida, T.; Takeda, M.; Okada, M.; Kitamura, M. Profiling of functional phosphodiesterase in mesangial cells using a CRE-SEAP-based reporting system. Br. J. Pharmacol. 2006, 148, 833–844. [Google Scholar] [CrossRef]
- Torres, V.E.; Harris, P.C. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J. Am. Soc. Nephrol. 2014, 25, 18–32. [Google Scholar] [CrossRef]
- Stefan, E.; Wiesner, B.; Baillie, G.S.; Mollajew, R.; Henn, V.; Lorenz, D.; Furkert, J.; Santamaria, K.; Nedvetsky, P.; Hundsrucker, C.; et al. Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J. Am. Soc. Nephrol. 2007, 18, 199–212. [Google Scholar] [CrossRef]
- Wang, X.; Ward, C.J.; Harris, P.C.; Torres, V.E. Cyclic nucleotide signaling in polycystic kidney disease. Kidney Int. 2010, 77, 129–140. [Google Scholar] [CrossRef][Green Version]
- Ortiz-Capisano, M.C.; Liao, T.D.; Ortiz, P.A.; Beierwaltes, W.H. Calcium-dependent phosphodiesterase 1C inhibits renin release from isolated juxtaglomerular cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1469–R1476. [Google Scholar] [CrossRef]
- Shakur, Y.; Takeda, K.; Kenan, Y.; Yu, Z.X.; Rena, G.; Brandt, D.; Houslay, M.D.; Degerman, E.; Ferrans, V.J.; Manganiello, V.C. Membrane localization of cyclic nucleotide phosphodiesterase 3 (PDE3). Two N-terminal domains are required for the efficient targeting to, and association of, PDE3 with endoplasmic reticulum. J. Biol. Chem. 2000, 275, 38749–38761. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, K.; Xiao, C.; He, Z.; Liu, S.; Wu, X.; Shi, S.; Guo, Y. Phosphodiesterase inhibitor for heart failure with preserved ejection fraction: A systematic review and meta-analysis. Saudi. Pharm. J. 2022, 30, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Feneck, R. Phosphodiesterase inhibitors and the cardiovascular system. Contin. Educ. Anaesth. Crit. Care Pain. 2008, 8, 76. [Google Scholar] [CrossRef]
- Kamel, R.; Leroy, J.; Vandecasteele, G.; Fischmeister, R. Cyclic nucleotide phosphodiesterases as therapeutic targets in cardiac hypertrophy and heart failure. Nat. Rev. Cardiol. 2023, 20, 90–108. [Google Scholar] [CrossRef]
- Kherallah, R.Y.; Khawaja, M.; Olson, M.; Angiolillo, D.; Birnbaum, Y. Cilostazol: A Review of Basic Mechanisms and Clinical Uses. Cardiovasc. Drugs Ther. 2022, 36, 777–792. [Google Scholar] [CrossRef]
- Mokry, J.; Mokra, D. Immunological aspects of phosphodiesterase inhibition in the respiratory system. Respir. Physiol. Neurobiol. 2013, 187, 11–17. [Google Scholar] [CrossRef]
- Sala, V.; Margaria, J.P.; Murabito, A.; Morello, F.; Ghigo, A.; Hirsch, E. Therapeutic Targeting of PDEs and PI3K in Heart Failure with Preserved Ejection Fraction (HFpEF). Curr. Heart Fail. Rep. 2017, 14, 187–196. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; McKean, M.; Goldoni, S.; Genvresse, I.; Garrido, M.F.; Li, R.; Wilkinson, G.; Kneip, C.; Yap, T.A. First-in-Human Dose-Escalation Study of the First-in-Class PDE3A-SLFN12 Complex Inducer BAY 2666605 in Patients with Advanced Solid Tumors Coexpressing SLFN12 and PDE3A. Clin. Cancer Res. 2024, 30, 5568–5576. [Google Scholar] [CrossRef]
- Hoffman, T.M. Phosphodiesterase Inhibitors. In Heart Failure in the Child and Young Adult; Elsevier: Amsterdam, The Netherlands, 2018; pp. 517–522. [Google Scholar]
- Ahmad, T.; Miller, P.E.; McCullough, M.; Desai, N.R.; Riello, R.; Psotka, M.; Bohm, M.; Allen, L.A.; Teerlink, J.R.; Rosano, G.M.C.; et al. Why has positive inotropy failed in chronic heart failure? Lessons from prior inotrope trials. Eur. J. Heart Fail. 2019, 21, 1064–1078. [Google Scholar] [CrossRef]
- Greenberg, B.; Butler, J.; Felker, G.M.; Ponikowski, P.; Voors, A.A.; Desai, A.S.; Barnard, D.; Bouchard, A.; Jaski, B.; Lyon, A.R.; et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): A randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 2016, 387, 1178–1186. [Google Scholar] [CrossRef]
- McSorley, T.; Stefan, E.; Henn, V.; Wiesner, B.; Baillie, G.S.; Houslay, M.D.; Rosenthal, W.; Klussmann, E. Spatial organisation of AKAP18 and PDE4 isoforms in renal collecting duct principal cells. Eur. J. Cell Biol. 2006, 85, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Luft, F.C. Personal Genetic-Hypertension Odyssey from Phenotypes to Genotypes and Targets. Hypertension 2024, 81, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Schuster, H.; Wienker, T.E.; Bahring, S.; Bilginturan, N.; Toka, H.R.; Neitzel, H.; Jeschke, E.; Toka, O.; Gilbert, D.; Lowe, A.; et al. Severe autosomal dominant hypertension and brachydactyly in a unique Turkish kindred maps to human chromosome 12. Nat. Genet. 1996, 13, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Sholokh, A.; Walter, S.; Marko, L.; McMurray, B.J.; Sunaga-Franze, D.Y.; Xu, M.; Zuhlke, K.; Russwurm, M.; Bartolomaeus, T.U.P.; Langanki, R.; et al. Mutant phosphodiesterase 3A protects the kidney from hypertension-induced damage. Kidney Int. 2023, 104, 388–393. [Google Scholar] [CrossRef]
- Ai, Y.; He, H.; Chen, P.; Yan, B.; Zhang, W.; Ding, Z.; Li, D.; Chen, J.; Ma, Y.; Cao, Y.; et al. An alkaloid initiates phosphodiesterase 3A-schlafen 12 dependent apoptosis without affecting the phosphodiesterase activity. Nat. Commun. 2020, 11, 3236. [Google Scholar] [CrossRef]
- Aquilanti, E.; Goldoni, S.; Baker, A.; Kotynkova, K.; Andersen, S.; Bozinov, V.; Gao, G.F.; Cherniack, A.D.; Lange, M.; Lesche, R.; et al. Velcrin molecular glues induce apoptosis in glioblastomas with high PDE3A and SLFN12 expression. Neuro-Oncol. Adv. 2024, 6, vdae115. [Google Scholar] [CrossRef]
- Garvie, C.W.; Wu, X.; Papanastasiou, M.; Lee, S.; Fuller, J.; Schnitzler, G.R.; Horner, S.W.; Baker, A.; Zhang, T.; Mullahoo, J.P.; et al. Structure of PDE3A-SLFN12 complex reveals requirements for activation of SLFN12 RNase. Nat. Commun. 2021, 12, 4375. [Google Scholar] [CrossRef]
- Lee, S.; Hoyt, S.; Wu, X.; Garvie, C.; McGaunn, J.; Shekhar, M.; Tötzl, M.; Rees, M.G.; Cherniack, A.D.; Meyerson, M.; et al. Velcrin-induced selective cleavage of tRNALeu(TAA) by SLFN12 causes cancer cell death. Nat. Chem. Biol. 2023, 19, 301–310. [Google Scholar] [CrossRef]
- Wechsler, J.; Choi, Y.H.; Krall, J.; Ahmad, F.; Manganiello, V.C.; Movsesian, M.A. Isoforms of cyclic nucleotide phosphodiesterase PDE3A in cardiac myocytes. J. Biol. Chem. 2002, 277, 38072–38078. [Google Scholar] [CrossRef]
- Zhang, W.; Colman, R.W. Conserved amino acids in metal-binding motifs of PDE3A are involved in substrate and inhibitor binding. Blood 2000, 95, 3380–3386. [Google Scholar] [CrossRef]
- Klussmann, E.; Rosenthal, W. Protein-protein interactions as new drug targets. Preface. Handb. Exp. Pharmacol. 2008, 186, v–vi. [Google Scholar]
- Corradini, E.; Klaasse, G.; Leurs, U.; Heck, A.J.R.; Martin, N.I.; Scholten, A. Charting the interactome of PDE3A in human cells using an IBMX based chemical proteomics approach. Mol. BioSyst. 2015, 11, 2786–2797. [Google Scholar] [CrossRef] [PubMed]
- Otasek, D.; Morris, J.H.; Bouças, J.; Pico, A.R.; Demchak, B. Cytoscape Automation: Empowering workflow-based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef] [PubMed]
- Schambach, A.; Buchholz, C.J.; Torres-Ruiz, R.; Cichutek, K.; Morgan, M.; Trapani, I.; Büning, H. A new age of precision gene therapy. Lancet 2024, 403, 568–582. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Addendum: Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 636, E4. [Google Scholar] [CrossRef]
- Kenan, Y.; Murata, T.; Shakur, Y.; Degerman, E.; Manganiello, V.C. Functions of the N-terminal region of cyclic nucleotide phosphodiesterase 3 (PDE 3) isoforms. J. Biol. Chem. 2000, 275, 12331–12338. [Google Scholar] [CrossRef]
- Ercu, M.; Klussmann, E. Roles of A-Kinase Anchoring Proteins and Phosphodiesterases in the Cardiovascular System. J. Cardiovasc. Dev. Dis. 2018, 5, 14. [Google Scholar] [CrossRef]
- Ocana, A.; Pandiella, A.; Privat, C.; Bravo, I.; Luengo-Oroz, M.; Amir, E.; Gyorffy, B. Integrating artificial intelligence in drug discovery and early drug development: A transformative approach. Biomark. Res. 2025, 13, 45. [Google Scholar] [CrossRef]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef]
- Bordukova, M.; Makarov, N.; Rodriguez-Esteban, R.; Schmich, F.; Menden, M.P. Generative artificial intelligence empowers digital twins in drug discovery and clinical trials. Expert Opin. Drug Discov. 2024, 19, 33–42. [Google Scholar] [CrossRef]
- Alogna, A.; Berboth, L.; Faragli, A.; Otvos, J.; Lo Muzio, F.P.; di Mauro, V.; Modica, J.; Quarta, E.; Semmler, L.; Deissler, P.M.; et al. Lung-to-Heart Nano-in-Micro Peptide Promotes Cardiac Recovery in a Pig Model of Chronic Heart Failure. J. Am. Coll. Cardiol. 2024, 83, 47–59. [Google Scholar] [CrossRef]
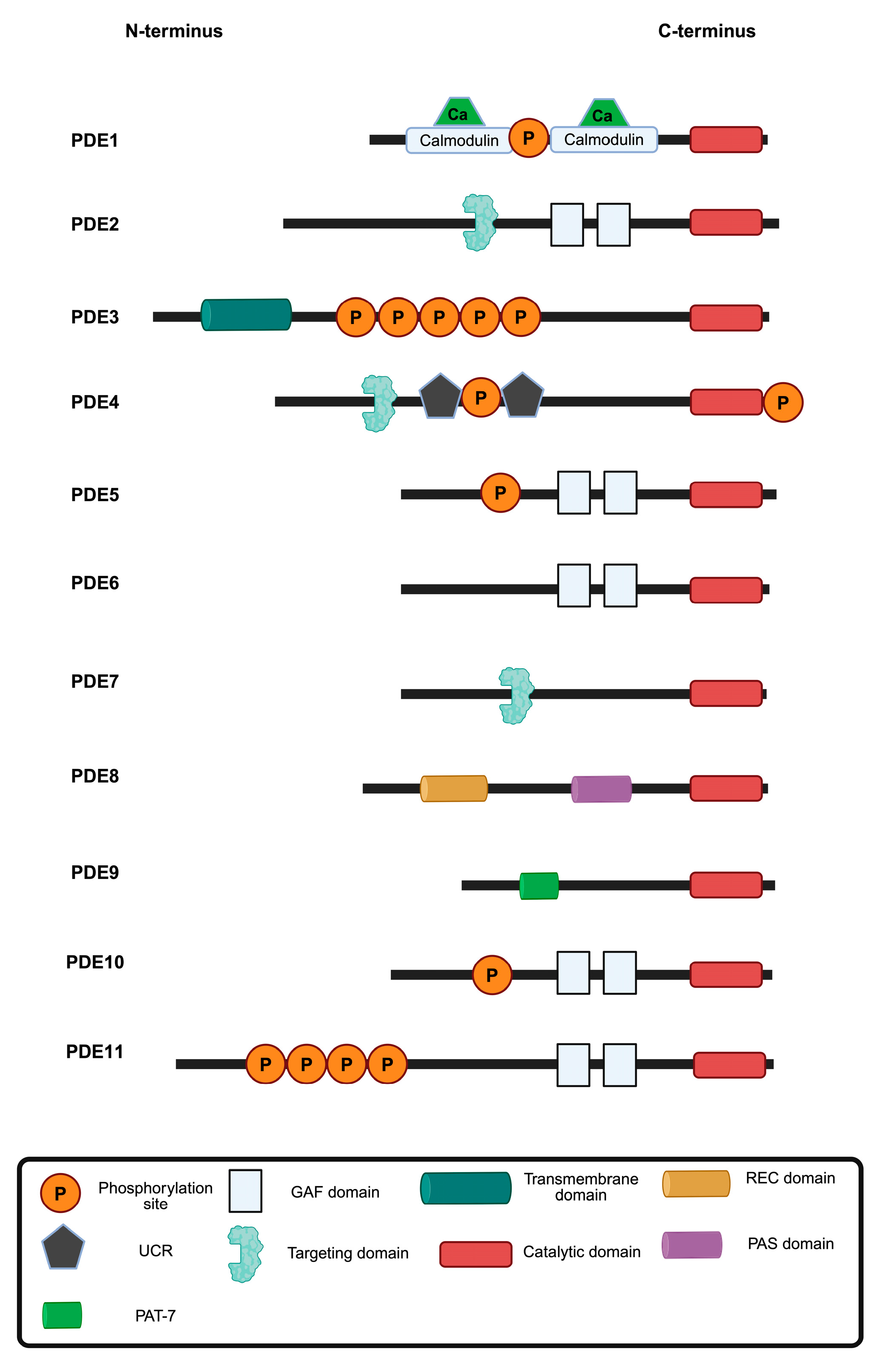




| PDE Family | Hydrolyses cAMP | Hydrolyses cGMP |
|---|---|---|
| PDE1 | √ | √ |
| PDE2 | √ | √ |
| PDE3 | √ | √ |
| PDE4 | √ | × |
| PDE5 | × | √ |
| PDE6 | × | √ |
| PDE7 | √ | × |
| PDE8 | √ | × |
| PDE9 | × | √ |
| PDE10 | √ | √ |
| PDE11 | √ | √ |
| Cell Type | Expression | Key Functions | Subcellular Localisation | Reference |
|---|---|---|---|---|
| Adipocytes | High expression in white and brown adipose tissue | Regulates lipolysis by hydrolysing cAMP, affecting hormone-sensitive lipase activity | Caveolae, endoplasmic reticulum | [42,43] |
| Hepatocytes | Expressed in liver cells | Regulates glucose and lipid metabolism; deficiency leads to gluconeogenesis and lipid accumulation | Caveolae, smooth ER | [44] |
| Pancreatic β-cells | Moderate | Modulates insulin secretion by regulating cAMP involved in granule exocytosis | Plasma membrane, insulin granules | [45] |
| Atrial endothelial cells (AECs) | Highly expressed | Regulating AECs adhesion, spreading and tubular formation; vital for angiogenesis | Plasma membrane | [46] |
| Cell Type | Expression | Key Functions | Subcellular Localisation | References |
|---|---|---|---|---|
| Vascular smooth muscle cells | High | Regulates vascular tone; inhibition causes vasodilation and lowers blood pressure | Plasma membrane, cytosol | [47,48,49] |
| Cardiomyocytes | High | Modulates cardiac contractility and cAMP signalling | SR, cytosol, nucleus | [47,50,51] |
| Platelets | Moderate | Inhibits aggregation via cAMP signalling | Plasma membrane, cytosol | [52] |
| Human aortic endothelial cells (HAECs) | Low to Moderate | Regulates endothelial barrier and angiogenesis | Plasma membrane, cytosol | [53] |
| Oocytes | High | Maintains meiotic arrest; inhibition resumes meiosis | Cytoplasm near plasma membrane, perinuclear | [54] |
| Pulmonary arterial cells | Moderate to High | Involved in vasodilation; target in pulmonary hypertension | Cytoplasm, membrane-associated | [48,55,56] |
| T-lymphocytes | Low | Possible modulation of cAMP-mediated immune responses | Not well defined, likely cytosolic | [47,57] |
| Cancer stem cells (breast cancer), intestinal cancer cells, myxoid liposarccoma (SA4, GOT3), cervical cancer (HeLa cells) | Aberrant/High | Promotes proliferation and survival | Nuclear and cytoplasmic (context-dependent) | [2,7,58,59,60,61,62,63] |
| Brain (cerebelum, cortex, callosum) | Moderately | Neuronal signalling, protection against neural damage | Plasma membrane, cytosol | [64,65,66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pati, S.R.; Sholokh, A.; Klussmann, E. PDE3A as a Therapeutic Target for the Modulation of Compartmentalised Cyclic Nucleotide-Dependent Signalling. Cells 2025, 14, 771. https://doi.org/10.3390/cells14110771
Pati SR, Sholokh A, Klussmann E. PDE3A as a Therapeutic Target for the Modulation of Compartmentalised Cyclic Nucleotide-Dependent Signalling. Cells. 2025; 14(11):771. https://doi.org/10.3390/cells14110771
Chicago/Turabian StylePati, Swaroop Ranjan, Anastasiia Sholokh, and Enno Klussmann. 2025. "PDE3A as a Therapeutic Target for the Modulation of Compartmentalised Cyclic Nucleotide-Dependent Signalling" Cells 14, no. 11: 771. https://doi.org/10.3390/cells14110771
APA StylePati, S. R., Sholokh, A., & Klussmann, E. (2025). PDE3A as a Therapeutic Target for the Modulation of Compartmentalised Cyclic Nucleotide-Dependent Signalling. Cells, 14(11), 771. https://doi.org/10.3390/cells14110771








