Vasohibins in Health and Disease: From Angiogenesis to Tumorigenesis, Multiorgan Dysfunction, and Brain–Heart Remodeling
Abstract
1. Introduction
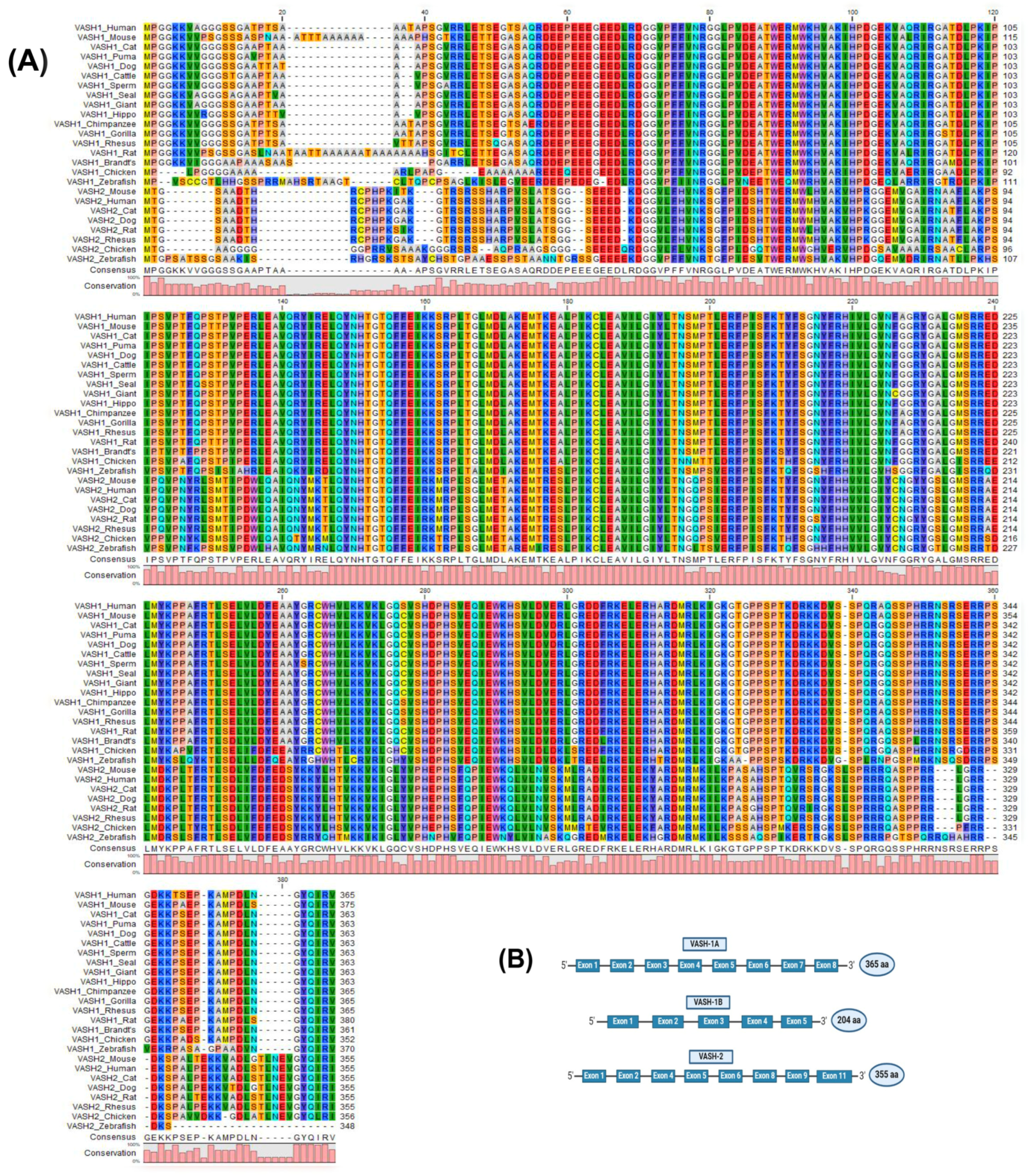
2. The Molecular Properties of VASHs
3. VASHs and Angiogenesis
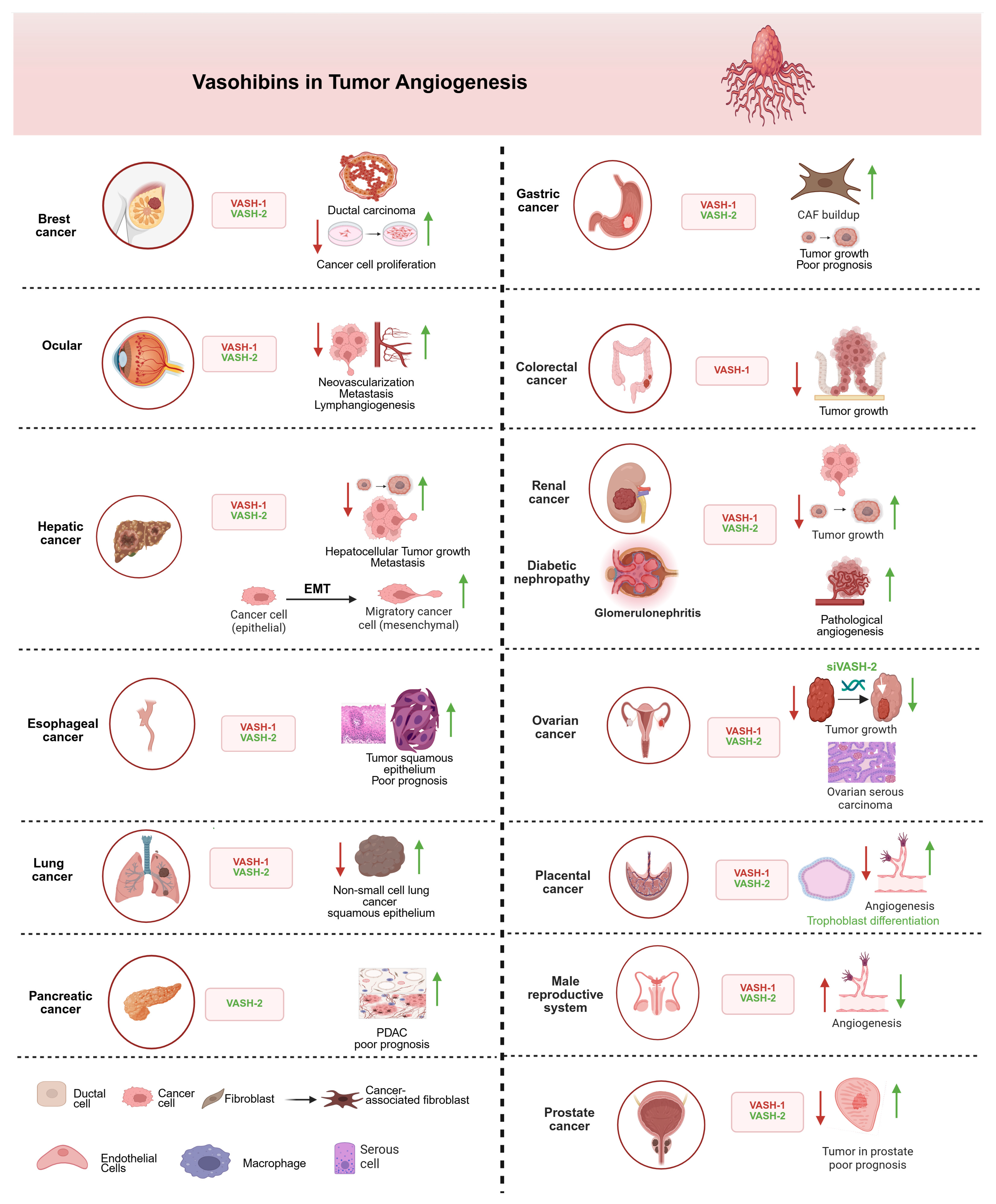
3.1. Angiogenic Role of VASHs in Tumorigenesis
3.2. Angiogenic Role of VASHs in Kidney Diseases
3.3. Angiogenic Role of VASHs in Pathophysiology of the Reproductive System
4. VASHs Serve as Tubulin Detyrosination Enzymes
4.1. Detyrosinizing Role of VASHs in Neuronal Disorders
4.2. Detyrosinizing Role of VASHs in Cardiac Diseases
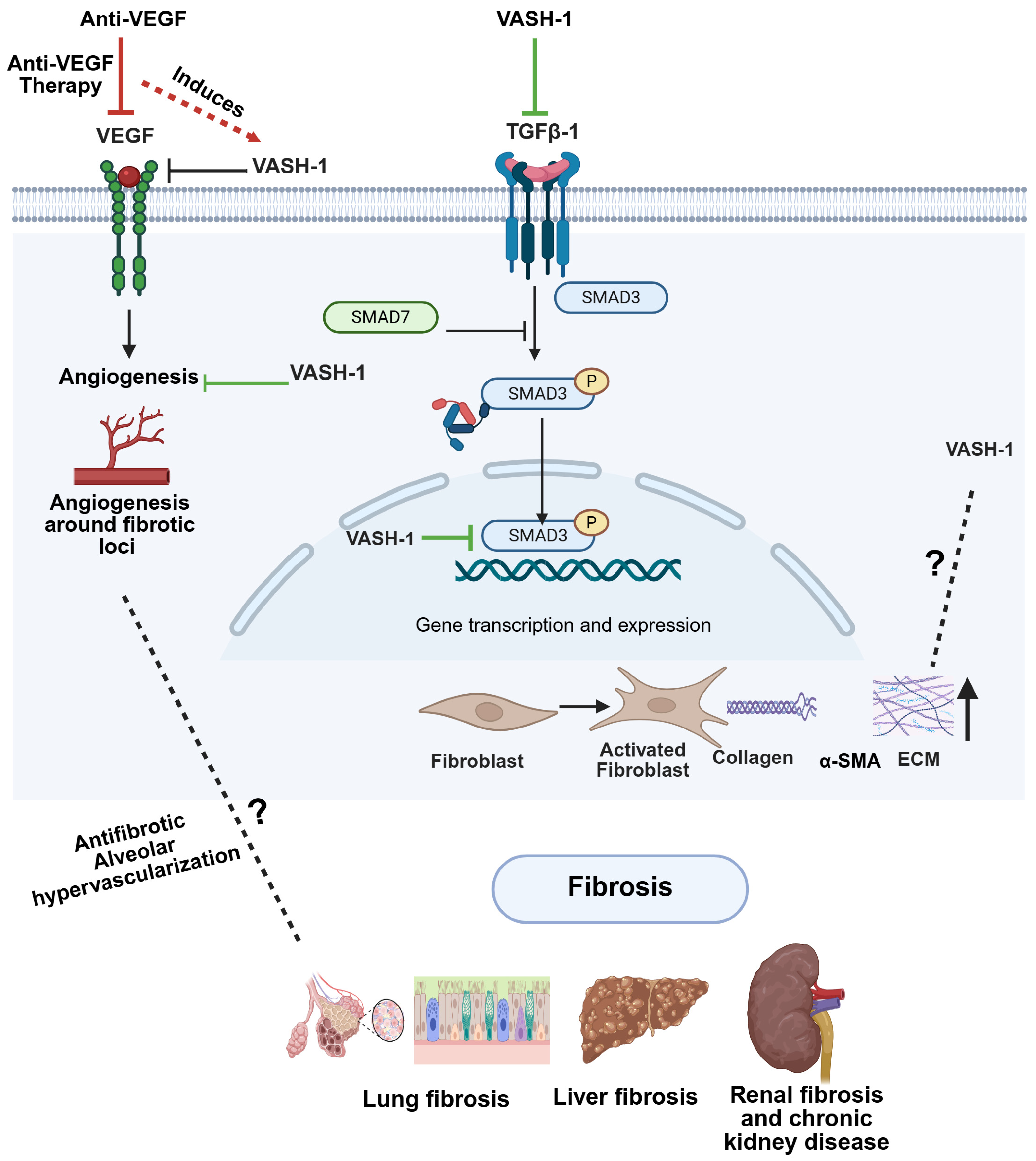
5. The Pathophysiological Role of VASHs in Fibrosis
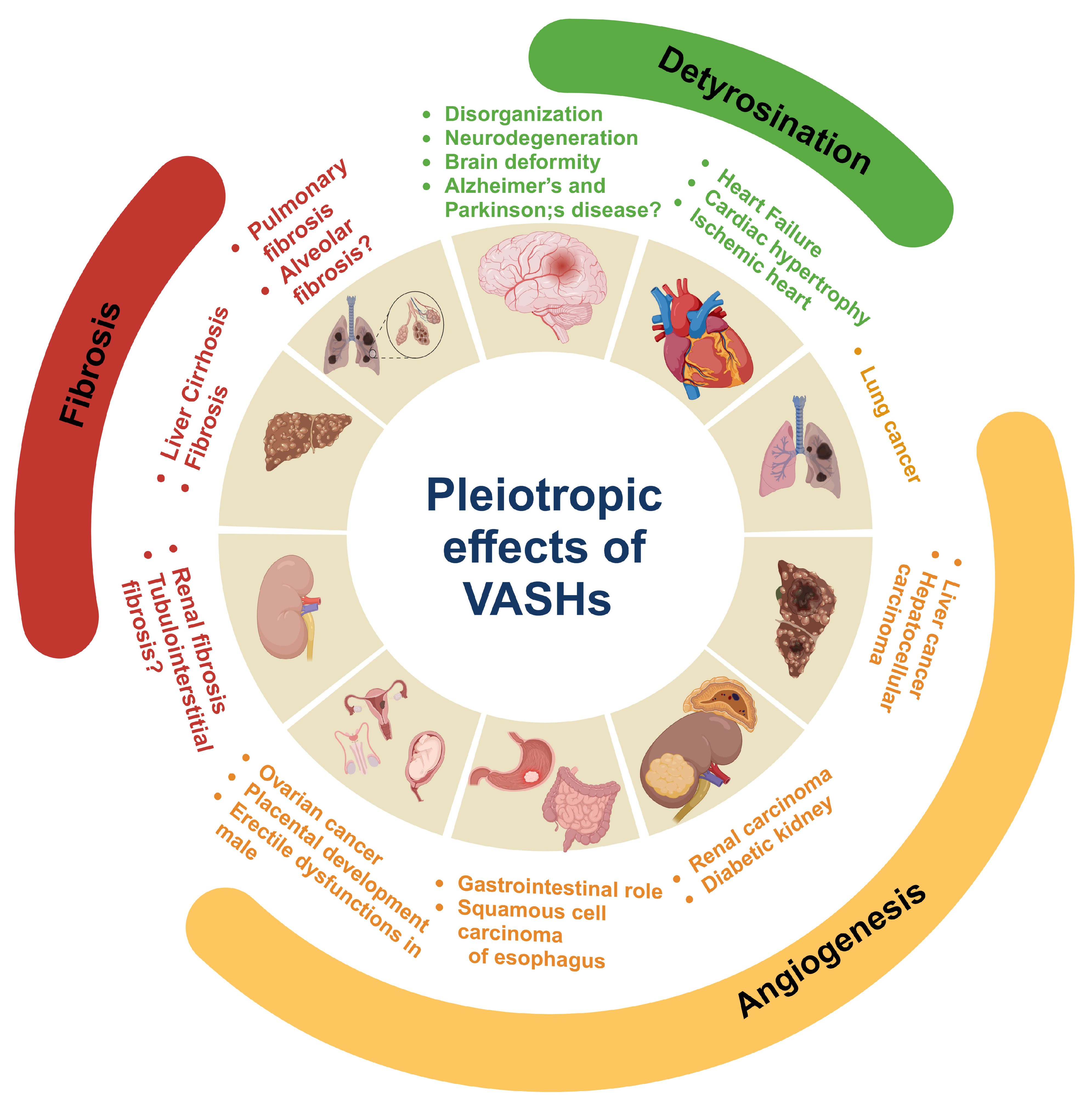
6. Conclusions and Future Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VASHs | Vasohibins |
| VEGF | Vascular endothelial growth factor |
| ECs | Endothelial cells |
| TCP | Tubulin carboxypeptidase |
| PTM | Post-translational modification |
| TTL | Tubulin tyrosine ligases |
| SVBP | Small vasohibin-binding protein |
| AA | Amino acids |
| FGF-2 | Fibroblast growth factor-2 |
| TGF | Transforming growth factor |
| ECM | Extracellular matrix |
| PKC-δ | Protein kinase C-delta |
| TNF-α | Tumor necrotic factor-alpha |
| IL-1β | Interleukin-1beta |
| IFN-γ | Interferon-gamma |
| HIF1-α | Hypoxia inducible factor 1 alpha |
| CKD | Chronic kidney disease |
| AKI | Acute kidney injury |
| DN | Diabetic nephropathy |
| I/R | Ischemia-reperfusion |
| CL | Corpus luteum |
| MTN | Microtubule network |
| MARK4 | Microtubule-affinity-regulating kinase 4 |
| MI | Myocardial infarction |
| DKD | Diabetic kidney disease |
| SMAD3 | Mothers against decapentaplegic homolog 3 |
References
- Watanabe, K.; Hasegawa, Y.; Yamashita, H.; Shimizu, K.; Ding, Y.; Abe, M.; Ohta, H.; Imagawa, K.; Hojo, K.; Maki, H. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J. Clin. Investig. 2004, 114, 898–907. [Google Scholar] [CrossRef]
- Nieuwenhuis, J.; Adamopoulos, A.; Bleijerveld, O.B.; Mazouzi, A.; Stickel, E.; Celie, P.; Altelaar, M.; Knipscheer, P.; Perrakis, A.; Blomen, V.A. Vasohibins encode tubulin detyrosinating activity. Science 2017, 358, 1453–1456. [Google Scholar] [CrossRef]
- Aillaud, C.; Bosc, C.; Peris, L.; Bosson, A.; Heemeryck, P.; Van Dijk, J.; Le Friec, J.; Boulan, B.; Vossier, F.; Sanman, L.E. Vasohibins/SVBP are tubulin carboxypeptidases (TCPs) that regulate neuron differentiation. Science 2017, 358, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Dogterom, M.; Koenderink, G.H. Actin-microtubule crosstalk in cell biology. Nat. Rev. Mol. Cell Biol. 2019, 20, 38–54. [Google Scholar] [CrossRef]
- Pimm, M.L.; Henty-Ridilla, J.L. New twists in actin-microtubule interactions. Mol. Biol. Cell 2021, 32, 211–310. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, T.; Dehghani, F. The Cytoskeleton—A complex interacting meshwork. Cells 2019, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal crosstalk in cell migration. Trends Cell Biol. 2020, 30, 720–735. [Google Scholar] [CrossRef]
- Janke, C.; Magiera, M.M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 307–326. [Google Scholar] [CrossRef]
- Roll-Mecak, A. The tubulin code in microtubule dynamics and information encoding. Dev. Cell 2020, 54, 7–20. [Google Scholar] [CrossRef]
- Caporizzo, M.A.; Chen, C.Y.; Prosser, B.L. Cardiac microtubules in health and heart disease. Exp. Biol. Med. 2019, 244, 1255–1272. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Xie, Y.; Xiang, M. Tubulin post-translational modifications: Potential therapeutic approaches to heart failure. Front. Cell Dev. Biol. 2022, 10, 872058. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-W.; Mahamdeh, M.; Tuna, Y.; Howard, J. The force required to remove tubulin from the microtubule lattice by pulling on its α-tubulin C-terminal tail. Nat. Commun. 2022, 13, 3651. [Google Scholar] [CrossRef]
- Nieuwenhuis, J.; Brummelkamp, T.R. The tubulin detyrosination cycle: Function and enzymes. Trends Cell Biol. 2019, 29, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, L.; Lou, G. Revealing the inhibitory effect of VASH1 on ovarian cancer from multiple perspectives. Cancer Biol. Ther. 2023, 24, 2285817. [Google Scholar] [CrossRef]
- Kobayashi, M.; Wakabayashi, I.; Suzuki, Y.; Fujiwara, K.; Nakayama, M.; Watabe, T.; Sato, Y. Tubulin carboxypeptidase activity of vasohibin-1 inhibits angiogenesis by interfering with endocytosis and trafficking of pro-angiogenic factor receptors. Angiogenesis 2021, 24, 159–176. [Google Scholar] [CrossRef]
- Hu, X.-N.; Ni, Y.; Luan, J.; Ding, Y.-Z. A review on vasohibin and ocular neovascularization. Int. J. Ophthalmol. 2020, 13, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Hinamoto, N.; Maeshima, Y.; Yamasaki, H.; Nasu, T.; Saito, D.; Watatani, H.; Ujike, H.; Tanabe, K.; Masuda, K.; Arata, Y.; et al. Exacerbation of diabetic renal alterations in mice lacking vasohibin-1. PLoS ONE 2014, 9, e107934. [Google Scholar] [CrossRef]
- Sato, H.; Abe, T.; Wakusawa, R.; Asai, N.; Kunikata, H.; Ohta, H.; Sonoda, H.; Sato, Y.; Nishida, K. Vitreous levels of vasohibin-1 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Diabetologia 2009, 52, 359–361. [Google Scholar] [CrossRef]
- Takeda, E.; Suzuki, Y.; Sato, Y. Age-associated downregulation of vasohibin-1 in vascular endothelial cells. Aging Cell 2016, 15, 885–892. [Google Scholar] [CrossRef]
- Sanchez-Pulido, L.; Ponting, C.P. Vasohibins: New transglutaminase-like cysteine proteases possessing a non-canonical Cys-His-Ser catalytic triad. Bioinformatics 2016, 32, 1441–1445. [Google Scholar] [CrossRef]
- Du, H.; Zhao, J.; Hai, L.; Wu, J.; Yi, H.; Shi, Y. The roles of vasohibin and its family members: Beyond angiogenesis modulators. Cancer Biol. Ther. 2017, 18, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, T.; Watanabe, K.; Yamashita, H.; Shimizu, K.; Miyashita, H.; Abe, M.; Moriya, T.; Ohta, H.; Sonoda, H.; Shimosegawa, T. Isolation and characterization of vasohibin-2 as a homologue of VEGF-inducible endothelium-derived angiogenesis inhibitor vasohibin. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1051–1057. [Google Scholar] [CrossRef]
- Sato, Y.; Sonoda, H. The vasohibin family: A negative regulatory system of angiogenesis genetically programmed in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, H.; Ohta, H.; Watanabe, K.; Yamashita, H.; Kimura, H.; Sato, Y. Multiple processing forms and their biological activities of a novel angiogenesis inhibitor vasohibin. Biochem. Biophys. Res. Commun. 2006, 342, 640–646. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kobayashi, M.; Miyashita, H.; Ohta, H.; Sonoda, H.; Sato, Y. Isolation of a small vasohibin-binding protein (SVBP) and its role in vasohibin secretion. J. Cell Sci. 2010, 123, 3094–3101. [Google Scholar] [CrossRef]
- Kadonosono, T.; Yimchuen, W.; Tsubaki, T.; Shiozawa, T.; Suzuki, Y.; Kuchimaru, T.; Sato, Y.; Kizaka-Kondoh, S. Domain architecture of vasohibins required for their chaperone-dependent unconventional extracellular release. Protein Sci. 2017, 26, 452–463. [Google Scholar] [CrossRef]
- Ramirez-Rios, S.; Choi, S.R.; Sanyal, C.; Blum, T.B.; Bosc, C.; Krichen, F.; Denarier, E.; Soleilhac, J.-M.; Blot, B.; Janke, C. VASH1–SVBP and VASH2–SVBP generate different detyrosination profiles on microtubules. J. Cell Biol. 2022, 222, e202205096. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, A.; Landskron, L.; Heidebrecht, T.; Tsakou, F.; Bleijerveld, O.B.; Altelaar, M.; Nieuwenhuis, J.; Celie, P.H.; Brummelkamp, T.R.; Perrakis, A. Crystal structure of the tubulin tyrosine carboxypeptidase complex VASH1–SVBP. Nat. Struct. Mol. Biol. 2019, 26, 567–570. [Google Scholar] [CrossRef]
- Wang, N.; Bosc, C.; Ryul Choi, S.; Boulan, B.; Peris, L.; Olieric, N.; Bao, H.; Krichen, F.; Chen, L.; Andrieux, A. Structural basis of tubulin detyrosination by the vasohibin–SVBP enzyme complex. Nat. Struct. Mol. Biol. 2019, 26, 571–582. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Zhu, J.; Xie, Y.; Liang, X.; Chen, Z.; Feng, Y.; Zhang, Y. Structural insights into tubulin detyrosination by vasohibins-SVBP complex. Cell Discov. 2019, 5, 65. [Google Scholar] [CrossRef]
- Dudley, A.C.; Griffioen, A.W. Pathological angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar] [CrossRef]
- Kretschmer, M.; Rüdiger, D.; Zahler, S. Mechanical aspects of angiogenesis. Cancers 2021, 13, 4987. [Google Scholar] [CrossRef]
- Sitohy, B.; Nagy, J.A.; Dvorak, H.F. Anti-VEGF/VEGFR therapy for cancer: Reassessing the target. Cancer Res. 2012, 72, 1909–1914. [Google Scholar] [CrossRef]
- Mousa, L.; Salem, M.E.; Mikhail, S. Biomarkers of angiogenesis in colorectal cancer: Supplementary issue: Biomarkers for colon cancer. Biomark. Cancer 2015, 7, BIC-S25250. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Langer, R.; Ferrara, N. Targeting angiogenesis in oncology, ophthalmology and beyond. Nat. Rev. Drug Discov. 2023, 22, 476–495. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Lian, S.; Lu, Y.; Jia, L. Cancer metastasis chemoprevention prevents circulating tumour cells from germination. Signal Transduct. Target. Ther. 2022, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Suzuki, H.; Ohkuchi, A.; Sato, Y. Mutual balance between vasohibin-1 and soluble VEGFR-1 in endothelial cells. Pharmaceuticals 2011, 4, 782–793. [Google Scholar] [CrossRef]
- Shimizu, K.; Watanabe, K.; Yamashita, H.; Abe, M.; Yoshimatsu, H.; Ohta, H.; Sonoda, H.; Sato, Y. Gene regulation of a novel angiogenesis inhibitor, vasohibin, in endothelial cells. Biochem. Biophys. Res. Commun. 2005, 327, 700–706. [Google Scholar] [CrossRef]
- Kozako, T.; Matsumoto, N.; Kuramoto, Y.; Sakata, A.; Motonagare, R.; Aikawa, A.; Imoto, M.; Toda, A.; Honda, S.; Shimeno, H. Vasohibin induces prolyl hydroxylase-mediated degradation of hypoxia-inducible factor-1α in human umbilical vein endothelial cells. FEBS Lett. 2012, 586, 1067–1072. [Google Scholar] [CrossRef]
- Kern, J.; Bauer, M.; Rychli, K.; Wojta, J.; Ritsch, A.; Gastl, G.; Gunsilius, E.; Untergasser, G. Alternative Splicing of Vasohibin-1 Generates an Inhibitor of Endothelial Cell Proliferation, Migration, and Capillary Tube Formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 478–484. [Google Scholar] [CrossRef]
- Shen, J.; Yang, X.; Xiao, W.-H.; Hackett, S.F.; Sato, Y.; Campochiaro, P.A. Vasohibin is up-regulated by VEGF in the retina and suppresses VEGF receptor 2 and retinal neovascularization. FASEB J. 2006, 20, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y. The vasohibin family: A novel family for angiogenesis regulation. J. Biochem. 2013, 153, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, L.; Zhao, Q.; Zhu, L. Vasohibin 2 as a potential predictor of aggressive behavior of triple-negative breast cancer. Am. J. Transl. Res. 2017, 9, 2911–2919. [Google Scholar] [PubMed]
- Hosaka, T.; Kimura, H.; Heishi, T.; Suzuki, Y.; Miyashita, H.; Ohta, H.; Sonoda, H.; Moriya, T.; Suzuki, S.; Kondo, T. Vasohibin-1 expression in endothelium of tumor blood vessels regulates angiogenesis. Am. J. Pathol. 2009, 175, 430–439. [Google Scholar] [CrossRef]
- Li, D.; Zhou, K.; Wang, S.; Shi, Z.; Yang, Z. Recombinant adenovirus encoding vasohibin prevents tumor angiogenesis and inhibits tumor growth. Cancer Sci. 2010, 101, 448–452. [Google Scholar] [CrossRef]
- Tamaki, K.; Sasano, H.; Maruo, Y.; Takahashi, Y.; Miyashita, M.; Moriya, T.; Sato, Y.; Hirakawa, H.; Tamaki, N.; Watanabe, M. Vasohibin-1 as a potential predictor of aggressive behavior of ductal carcinoma in situ of the breast. Cancer Sci. 2010, 101, 1051–1058. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, T.-T.; Zhang, D.-M.; Hou, X.-M.; Liu, X.-J.; Zhao, D.; Shan, L. Vasohibin-1 expression detected by immunohistochemistry correlates with prognosis in non-small cell lung cancer. Med. Oncol. 2014, 31, 963. [Google Scholar] [CrossRef]
- Ninomiya, Y.; Ozawa, S.; Oguma, J.; Kazuno, A.; Nitta, M.; Kajiwara, H.; Sato, Y. Expression of vasohibin-1 and -2 predicts poor prognosis among patients with squamous cell carcinoma of the esophagus. Oncol. Lett. 2018, 16, 5265–5274. [Google Scholar] [CrossRef]
- Kim, J.-C.; Kim, K.-T.; Park, J.-T.; Kim, H.-J.; Sato, Y.; Kim, H.-S. Expression of vasohibin-2 in pancreatic ductal adenocarcinoma promotes tumor progression and is associated with a poor clinical outcome. Hepatogastroenterology 2015, 62, 251–256. [Google Scholar]
- Yamamoto, M.; Ozawa, S.; Koyanagi, K.; Ninomiya, Y.; Hara, H.; Kazuno, A.; Yatabe, K.; Higuchi, T.; Nakamura, K.; Nabeshima, K. Clinicopathological Role of Vasohibin in Gastroenterological Cancers: A Meta-Analysis. Tohoku J. Exp. Med. 2022, 256, 291–301. [Google Scholar] [CrossRef]
- Hara, H.; Ozawa, S.; Ninomiya, Y.; Yamamoto, M.; Ogimi, M.; Nabeshima, K.; Nakamura, K.; Kajiwara, H.; Nakamura, N.; Sato, Y. Prognostic significance of vasohibin-1 and vasohibin-2 immunohistochemical expression in gastric cancer. Surg. Today 2020, 50, 1530–1543. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Han, B.; Zhang, Q.; Dou, J.; Wang, F.; Lin, W.; Sun, Y.; Peng, G. Vasohibin-1 suppresses colon cancer. Oncotarget 2015, 6, 7880–7898. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Na, R.; Li, L.; Xiao, H.; Ding, N.; Sun, Y.; Han, R. Vasohibin-1 inhibits angiogenesis and suppresses tumor growth in renal cell carcinoma. Oncol. Rep. 2017, 38, 1021–1028. [Google Scholar] [CrossRef]
- Sano, R.; Kanomata, N.; Suzuki, S.; Shimoya, K.; Sato, Y.; Moriya, T.; Shiota, M. Vasohibin-1 Is a Poor Prognostic Factor of Ovarian Carcinoma. Tohoku J. Exp. Med. 2017, 243, 107–114. [Google Scholar] [CrossRef]
- Suenaga, K.; Kitahara, S.; Suzuki, Y.; Kobayashi, M.; Horie, S.; Sugawara, J.; Yaegashi, N.; Sato, Y. Role of the vasohibin family in the regulation of fetoplacental vascularization and syncytiotrophoblast formation. PLoS ONE 2014, 9, e104728. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kosaka, T.; Mikami, S.; Kimura, T.; Hongo, H.; Kosugi, M.; Sato, Y.; Oya, M. Vasohibin-1 expression as a biomarker of aggressive nature in ductal adenocarcinoma of the prostate: A retrospective cohort study at two centres in Japan. BMJ J. 2021, 11, e056439. [Google Scholar] [CrossRef]
- Song, K.-M.; Kim, W.J.; Choi, M.-J.; Kwon, K.-D.; Limanjaya, A.; Ghatak, K.; Ock, J.; Yin, G.N.; Sato, Y.; Hong, S.-S.; et al. Vasohibin-1 rescues erectile function through up-regulation of angiogenic factors in the diabetic mice. Sci. Rep. 2021, 11, 1114. [Google Scholar] [CrossRef]
- Norita, R.; Suzuki, Y.; Furutani, Y.; Takahashi, K.; Yoshimatsu, Y.; Podyma-Inoue, K.A.; Watabe, T.; Sato, Y. Vasohibin-2 is required for epithelial-mesenchymal transition of ovarian cancer cells by modulating transforming growth factor-β signaling. Cancer Sci. 2017, 108, 419–426. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kitahara, S.; Suematsu, T.; Oshima, M.; Sato, Y. Requisite role of vasohibin-2 in spontaneous gastric cancer formation and accumulation of cancer-associated fibroblasts. Cancer Sci. 2017, 108, 2342–2351. [Google Scholar] [CrossRef]
- Tan, X.; Liao, Z.; Zou, S.; Ma, L.; Wang, A. VASH2 Promotes Cell Proliferation and Resistance to Doxorubicin in Non-Small Cell Lung Cancer via AKT Signaling. Oncol. Res. 2020, 28, 3–11. [Google Scholar] [CrossRef]
- Tu, M.; Li, Z.; Liu, X.; Lv, N.; Xi, C.; Lu, Z.; Wei, J.; Song, G.; Chen, J.; Guo, F.; et al. Vasohibin 2 promotes epithelial-mesenchymal transition in human breast cancer via activation of transforming growth factor β 1 and hypoxia dependent repression of GATA-binding factor 3. Cancer Lett. 2017, 388, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, A.A.; Viswanathan, S.; Ting, J.G.W.; Tan, J.; Shekeran, S.G.; Carling, D.; Lim, W.-W.; Cook, S.A. IL11 stimulates ERK/P90RSK to inhibit LKB1/AMPK and activate mTOR initiating a mesenchymal program in stromal, epithelial, and cancer cells. iScience 2022, 25, 104806. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.H.; Ng, B.; Lim, W.-W. Interleukin-11: A Potential Biomarker and Molecular Therapeutic Target in Non-Small Cell Lung Cancer. Cells 2022, 11, 2257. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, R.; Han, L.; Zhang, X.; Ye, Y.; Yu, W.; Ren, X.; Zhang, W.; Yu, J. Vasohibin 2 promotes lymphangiogenesis of lung squamous cell carcinoma through snail-dependent vascular endothelial growth factor-D (VEGF-D) signaling pathway. Ann. Transl. Med. 2022, 10, 39. [Google Scholar] [CrossRef]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.-J. Acute kidney injury. Nat. Rev. Dis. Primer 2021, 7, 52. [Google Scholar] [CrossRef]
- Tanimura, S.; Tanabe, K.; Miyake, H.; Masuda, K.; Tsushida, K.; Morioka, T.; Sugiyama, H.; Sato, Y.; Wada, J. Renal tubular injury exacerbated by vasohibin-1 deficiency in a murine cisplatin-induced acute kidney injury model. Am. J. Physiol. Ren. Physiol. 2019, 317, F264–F274. [Google Scholar] [CrossRef]
- Masuda, K.; Tanabe, K.; Ujike, H.; Hinamoto, N.; Miyake, H.; Tanimura, S.; Sugiyama, H.; Sato, Y.; Maeshima, Y.; Wada, J. Deletion of pro-angiogenic factor vasohibin-2 ameliorates glomerular alterations in a mouse diabetic nephropathy model. PLoS ONE 2018, 13, e0195779. [Google Scholar] [CrossRef]
- Liu, H.; Wang, D.; Tang, J.; Yu, L.; Su, S. Differences and Clinical Significance of Serum 25-Hydroxyvitamin D3 and Vasohibin-1 (VASH-1) Levels in Patients with Diabetic Nephropathy and Different Renal Injuries. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 1085–1091. [Google Scholar] [CrossRef]
- Hinamoto, N.; Maeshima, Y.; Saito, D.; Yamasaki, H.; Tanabe, K.; Nasu, T.; Watatani, H.; Ujike, H.; Kinomura, M.; Sugiyama, H.; et al. Urinary and plasma levels of vasohibin-1 can predict renal functional deterioration in patients with renal disorders. PLoS ONE 2014, 9, e96932. [Google Scholar] [CrossRef]
- Miyake, H.; Tanabe, K.; Tanimura, S.; Nakashima, Y.; Morioka, T.; Masuda, K.; Sugiyama, H.; Sato, Y.; Wada, J. Genetic Deletion of Vasohibin-2 Exacerbates Ischemia-Reperfusion-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2020, 21, 4545. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, H.; Lan, T.; Jia, R.; Cao, M.; Zhou, L.; Zhao, Z.; Pan, W. Physiological and pathological roles of Ang II and Ang-(1-7) in the female reproductive system. Front. Endocrinol. 2022, 13, 1080285. [Google Scholar] [CrossRef]
- Izadpanah, M.; Rahbarghazi, R.; Seghinsara, A.M.; Abedelahi, A. Novel Approaches used in ovarian tissue transplantation for fertility preservation: Focus on tissue engineering approaches and angiogenesis capacity. Reprod. Sci. 2023, 30, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Bertassoni, L.E.; Tayebi, L. Biological aspects in controlling angiogenesis: Current progress. Cell. Mol. Life Sci. 2022, 79, 349. [Google Scholar] [CrossRef]
- Umapathy, A.; Chamley, L.W.; James, J.L. Reconciling the distinct roles of angiogenic/anti-angiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies. Angiogenesis 2020, 23, 105–117. [Google Scholar] [CrossRef]
- Kumar, K.A.; Kavitha, S.; Sreekanth, K.S. Regulatory proteins in placental angiogenesis. Biomedicine 2021, 41, 694–700. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, F.; Chen, G.; Lu, W.; Zhang, Y. Vasohibin 1, a clinically relevant biomarker, contributes to pre-eclampsia. Int. J. Clin. Pract. 2021, 75, e14017. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Ito, K.; Moriya, T.; Nagase, S.; Takano, T.; Niikura, H.; Yaegashi, N.; Sato, Y. Expression of vasohibin as a novel endothelium-derived angiogenesis inhibitor in endometrial cancer. Cancer Sci. 2008, 99, 914–919. [Google Scholar] [CrossRef]
- Guo, X.; Yi, H.; Li, T.C.; Wang, Y.; Wang, H.; Chen, X. Role of vascular endothelial growth factor (VEGF) in human embryo implantation: Clinical implications. Biomolecules 2021, 11, 253. [Google Scholar] [CrossRef]
- Monaco, C.F.; Davis, J.S. Mechanisms of angioregression of the corpus luteum. Front. Physiol. 2023, 14, 1254943. [Google Scholar] [CrossRef]
- Shirasuna, K.; Sasahara, K.; Matsui, M.; Shimizu, T.; Miyamoto, A. Prostaglandin F2alpha differentially affects mRNA expression relating to angiogenesis, vasoactivation and prostaglandins in the early and mid corpus luteum in the cow. J. Reprod. Dev. 2010, 56, 428–436. [Google Scholar] [CrossRef]
- Shirasuna, K.; Kobayashi, A.; Nitta, A.; Nibuno, S.; Sasahara, K.; Shimizu, T.; Bollwein, H.; Miyamoto, A. Possible action of vasohibin-1 as an inhibitor in the regulation of vascularization of the bovine corpus luteum. Reprod. Camb. Engl. 2012, 143, 491–500. [Google Scholar] [CrossRef]
- Berto Gomes, L.A.; Smith, O.E.; Bollwein, H.; Kowalewski, M.P. Dynamic regulation of HIF1α and Oxygen-sensing factors in cyclic bovine corpus luteum and during LPS challenge. Animals 2025, 15, 595. [Google Scholar] [CrossRef] [PubMed]
- Berisha, B.; Schams, D.; Rodler, D.; Sinowatz, F.; Pfaffl, M.W. Expression pattern of HIF1alpha and vasohibins during follicle maturation and corpus luteum function in the bovine ovary. Reprod. Domest. Anim. Zuchthyg. 2017, 52, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Song, K.-M.; Park, J.-M.; Limanjaya, A.; Ghatak, K.; Minh, N.N.; Park, S.-H.; Park, W.-H.; Ock, J.; Yin, G.N.; et al. Mp89-01 vasohibin-1 is a novel therapeutic target for diabetic erectile dysfunction in mice. J. Urol. 2016, 195, e1137. [Google Scholar] [CrossRef]
- Song, K.-M.; Kim, W.J.; Yin, G.N.; Ryu, J.-K.; Suh, J.-K. AB035. Vasohibin-1 as a potential therapeutic target for erectile dysfunction. Transl. Androl. Urol. 2018, 7, AB035. [Google Scholar] [CrossRef]
- Suh, J.-K.; Kim, W.J.; Song, K.-M.; Limanjaya, A.; Ghatak, K.; Minh, N.N.; Park, S.-W.; Ock, J.; Ryu, J.-K. PS-04-009 Vasohibin-1 is a novel therapeutic target for diabetic erectile dysfunction. J. Sex. Med. 2017, 14, e118. [Google Scholar] [CrossRef]
- Kataoka, T.; Hotta, Y.; Kimura, K. A review of experimental techniques for erectile function researches and development of medical technology using animal erectile dysfunction models in sexual and reproductive medicine. Reprod. Med. Biol. 2023, 22, e12513. [Google Scholar] [CrossRef]
- van Der Laan, S.; Lévêque, M.F.; Marcellin, G.; Vezenkov, L.; Lannay, Y.; Dubra, G.; Bompard, G.; Ovejero, S.; Urbach, S.; Burgess, A. Evolutionary divergence of enzymatic mechanisms for tubulin detyrosination. Cell Rep. 2019, 29, 4159–4171. [Google Scholar] [CrossRef]
- Peris, L.; Parato, J.; Qu, X.; Soleilhac, J.M.; Lanté, F.; Kumar, A.; Pero, M.E.; Martínez-Hernández, J.; Corrao, C.; Falivelli, G. Tubulin tyrosination regulates synaptic function and is disrupted in Alzheimer’s disease. Brain 2022, 145, 2486–2506. [Google Scholar] [CrossRef]
- Ferreira, G.; Cardozo, R.; Chavarria, L.; Santander, A.; Sobrevia, L.; Chang, W.; Gundersen, G.; Nicolson, G.L. The LINC complex in blood vessels: From physiology to pathological implications in arterioles. J. Physiol. 2025. [Google Scholar] [CrossRef]
- Logan, C.M.; Menko, A.S. Microtubules: Evolving roles and critical cellular interactions. Exp. Biol. Med. 2019, 244, 1240–1254. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; van Ham, M.; Erck, C.; Korte, M.; Michaelsen-Preusse, K. The role of α-tubulin tyrosination in controlling the structure and function of hippocampal neurons. Front. Mol. Neurosci. 2022, 15, 931859. [Google Scholar] [CrossRef]
- Pellegrini, L.; Wetzel, A.; Grannó, S.; Heaton, G.; Harvey, K. Back to the tubule: Microtubule dynamics in Parkinson’s disease. Cell. Mol. Life Sci. 2017, 74, 409–434. [Google Scholar] [CrossRef]
- Peña-Ortega, F.; Robles-Gómez, Á.A.; Xolalpa-Cueva, L. Microtubules as regulators of neural network shape and function: Focus on excitability, plasticity and memory. Cells 2022, 11, 923. [Google Scholar] [CrossRef]
- Dent, E.W. Dynamic microtubules at the synapse. Curr. Opin. Neurobiol. 2020, 63, 9–14. [Google Scholar] [CrossRef]
- Parato, J.; Bartolini, F. The microtubule cytoskeleton at the synapse. Neurosci. Lett. 2021, 753, 135850. [Google Scholar] [CrossRef]
- Sanyal, C.; Pietsch, N.; Rios, S.R.; Peris, L.; Carrier, L.; Moutin, M.-J. The detyrosination/re-tyrosination cycle of tubulin and its role and dysfunction in neurons and cardiomyocytes. Semin. Cell Dev. Biol. 2023, 137, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Tawamie, H.; Ba, W.; Reis, A.; Halak, B.A.; Sticht, H.; Uebe, S.; Kasri, N.N.; Riazuddin, S.; van Bokhoven, H.; et al. Loss of function of SVBP leads to autosomal recessive intellectual disability, microcephaly, ataxia, and hypotonia. Genet. Med. Off. J. Am. Coll. Med. Genet. 2019, 21, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Pagnamenta, A.T.; Heemeryck, P.; Martin, H.C.; Bosc, C.; Peris, L.; Uszynski, I.; Gory-Fauré, S.; Couly, S.; Deshpande, C.; Siddiqui, A. Defective tubulin detyrosination causes structural brain abnormalities with cognitive deficiency in humans and mice. Hum. Mol. Genet. 2019, 28, 3391–3405. [Google Scholar] [CrossRef]
- Gobrecht, P.; Gebel, J.; Hilla, A.; Gisselmann, G.; Fischer, D. VASH1/2 inhibition accelerates functional recovery of injured nerves. bioRxiv 2022, 20, 5079. [Google Scholar]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The global burden of cardiovascular diseases and risk: A compass for future health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef]
- Warner, E.F.; Li, Y.; Li, X. Targeting Microtubules for the Treatment of Heart Disease. Circ. Res. 2022, 130, 1723–1741. [Google Scholar] [CrossRef] [PubMed]
- Loescher, C.M.; Freundt, J.K.; Unger, A.; Hessel, A.L.; Kühn, M.; Koser, F.; Linke, W.A. Titin governs myocardial passive stiffness with major support from microtubules and actin and the extracellular matrix. Nat. Cardiovasc. Res. 2023, 2, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, V.; Nijenkamp, L.L.A.M.; Regan, J.A.; van der Velden, J. The physiological role of cardiac cytoskeleton and its alterations in heart failure. Biochim. Biophys. Acta BBA—Biomembr. 2014, 1838, 700–722. [Google Scholar] [CrossRef]
- Lyra-Leite, D.M.; Petersen, A.P.; Ariyasinghe, N.R.; Cho, N.; McCain, M.L. Mitochondrial architecture in cardiac myocytes depends on cell shape and matrix rigidity. J. Mol. Cell. Cardiol. 2021, 150, 32–43. [Google Scholar] [CrossRef]
- Liao, S.; Rajendraprasad, G.; Wang, N.; Eibes, S.; Gao, J.; Yu, H.; Wu, G.; Tu, X.; Huang, H.; Barisic, M.; et al. Molecular basis of vasohibins-mediated detyrosination and its impact on spindle function and mitosis. Cell Res. 2019, 29, 533–547. [Google Scholar] [CrossRef]
- Caturano, A.; Vetrano, E.; Galiero, R.; Salvatore, T.; Docimo, G.; Epifani, R.; Alfano, M.; Sardu, C.; Marfella, R.; Rinaldi, L.; et al. Cardiac Hypertrophy: From Pathophysiological Mechanisms to Heart Failure Development. Rev. Cardiovasc. Med. 2022, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, E.A.; Uchida, K.; Vogel, M.; Erlitzki, N.; Iyer, M.; Phyo, S.A.; Bogush, A.; Kehat, I.; Prosser, B.L. Microtubules orchestrate local translation to enable cardiac growth. Nat. Commun. 2021, 12, 1547. [Google Scholar] [CrossRef]
- Hantelys, F.; Godet, A.-C.; David, F.; Tatin, F.; Renaud-Gabardos, E.; Pujol, F.; Diallo, L.H.; Ader, I.; Ligat, L.; Henras, A.K.; et al. Vasohibin1, a new mouse cardiomyocyte IRES trans-acting factor that regulates translation in early hypoxia. eLife 2019, 8, e50094. [Google Scholar] [CrossRef]
- Robison, P.; Caporizzo, M.A.; Ahmadzadeh, H.; Bogush, A.I.; Chen, C.Y.; Margulies, K.B.; Shenoy, V.B.; Prosser, B.L. Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science 2016, 352, aaf0659. [Google Scholar] [CrossRef]
- Robison, P.; Prosser, B.L. Microtubule mechanics in the working myocyte. J. Physiol. 2017, 595, 3931–3937. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Caporizzo, M.A.; Bedi, K.; Vite, A.; Bogush, A.I.; Robison, P.; Heffler, J.G.; Salomon, A.K.; Kelly, N.A.; Babu, A.; et al. Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat. Med. 2018, 24, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.P.; Robison, P.; Shi, G.; Bogush, A.I.; Kempema, A.M.; Hexum, J.K.; Becerra, N.; Harki, D.A.; Martin, S.S.; Raiteri, R.; et al. Detyrosinated microtubules modulate mechanotransduction in heart and skeletal muscle. Nat. Commun. 2015, 6, 8526. [Google Scholar] [CrossRef] [PubMed]
- Schuldt, M.; Pei, J.; Harakalova, M.; Dorsch, L.M.; Schlossarek, S.; Mokry, M.; Knol, J.C.; Pham, T.V.; Schelfhorst, T.; Piersma, S.R.; et al. Proteomic and Functional Studies Reveal Detyrosinated Tubulin as Treatment Target in Sarcomere Mutation-Induced Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2021, 14, e007022. [Google Scholar] [CrossRef]
- Chen, C.Y.; Salomon, A.K.; Caporizzo, M.A.; Curry, S.; Kelly, N.A.; Bedi, K.; Bogush, A.I.; Krämer, E.; Schlossarek, S.; Janiak, P. Depletion of vasohibin 1 speeds contraction and relaxation in failing human cardiomyocytes. Circ. Res. 2020, 127, e14–e27. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Amrute-Nayak, M.; Allgeyer, E.; Zhao, A.; Chenoweth, H.; Clement, M.; Harrison, J.; Doreth, C.; Sirinakis, G. MARK4 controls ischaemic heart failure through microtubule detyrosination. Nature 2021, 594, 560–565. [Google Scholar] [CrossRef]
- Ren, H.; Shao, Y.; Wu, C.; Lv, C.; Zhou, Y.; Wang, Q. VASH-1 Regulates oxidative stress and fibrosis in diabetic kidney disease via SIRT1/HIF1α and TGFβ1/Smad3 signaling pathways. Front. Mol. Biosci. 2020, 7, 137. [Google Scholar] [CrossRef]
- Sa, A.; Na, A.; Me, M.; Aa, A.-K. Fibrosis: Types, effects, markers, mechanisms for disease progression, and its relation with oxidative stress, immunity, and inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef]
- Watatani, H.; Maeshima, Y.; Hinamoto, N.; Yamasaki, H.; Ujike, H.; Tanabe, K.; Sugiyama, H.; Otsuka, F.; Sato, Y.; Makino, H. Vasohibin-1 deficiency enhances renal fibrosis and inflammation after unilateral ureteral obstruction. Physiol. Rep. 2014, 2, e12054. [Google Scholar] [CrossRef]
- Afkarian, M.; Zelnick, L.R.; Hall, Y.N.; Heagerty, P.J.; Tuttle, K.; Weiss, N.S.; de Boer, I.H. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA 2016, 316, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Affara, M.; Sanders, D.; Araki, H.; Tamada, Y.; Dunmore, B.J.; Humphreys, S.; Imoto, S.; Savoie, C.; Miyano, S.; Kuhara, S.; et al. Vasohibin-1 is identified as a master-regulator of endothelial cell apoptosis using gene network analysis. BMC Genom. 2013, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.J.; Gupta, S.; Dhawan, A. Cell therapy for liver disease: From liver transplantation to cell factory. J. Hepatol. 2015, 62, S157–S169. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dong, C.; Yang, J.; Yang, L.; Chang, N.; Qi, C.; Li, L. MicroRNA-26b-5p Inhibits Mouse Liver Fibrogenesis and Angiogenesis by Targeting PDGF Receptor-Beta. Mol. Ther. Nucleic Acids 2019, 16, 206–217. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Jiang, R.; He, J.; Zhao, T.; Peng, Y.; Zheng, Y. Research progress in the prevention and treatment of liver fibrosis in Chinese medicine based on miRNAs molecular regulation of angiogenesis. Pharmacol. Res. Mod. Chin. Med. 2022, 4, 100151. [Google Scholar] [CrossRef]
- Mejias, M.; Balvey, A.; Fernandez, M. Crosstalk between angiogenesis and fibrogenesis in liver disease. Curr. Tissue Microenviron. Rep. 2020, 1, 121–129. [Google Scholar] [CrossRef]
- Zadorozhna, M.; Di Gioia, S.; Conese, M.; Mangieri, D. Neovascularization is a key feature of liver fibrosis progression: Anti-angiogenesis as an innovative way of liver fibrosis treatment. Mol. Biol. Rep. 2020, 47, 2279–2288. [Google Scholar] [CrossRef]
- Chatterjee, S. Reversal of vasohibin-driven negative feedback loop of vascular endothelial growth factor/angiogenesis axis promises a novel antifibrotic therapeutic strategy for liver diseases. Hepatology 2014, 60, 458–460. [Google Scholar] [CrossRef]
- Zhou, Y.; Ling, T.; Shi, W. Current state of signaling pathways associated with the pathogenesis of idiopathic pulmonary fibrosis. Respir. Res. 2024, 25, 245. [Google Scholar] [CrossRef]
- Biasin, V.; Crnkovic, S.; Sahu-Osen, A.; Birnhuber, A.; El Agha, E.; Sinn, K.; Klepetko, W.; Olschewski, A.; Bellusci, S.; Marsh, L.M.; et al. PDGFRα and αSMA mark two distinct mesenchymal cell populations involved in parenchymal and vascular remodeling in pulmonary fibrosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L684–L697. [Google Scholar] [CrossRef]
- Kaner, R.J.; Ladetto, J.V.; Singh, R.; Fukuda, N.; Matthay, M.A.; Crystal, R.G. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am. J. Respir. Cell Mol. Biol. 2000, 22, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Mastui, Y.; Ito, Y.; Shibata, Y.; Betto, T.; Eshima, K.; Ogawa, F.; Satoh, Y.; Shibuya, M.; Majima, M. The role of vascular endothelial growth factor receptor 1 tyrosine kinase signaling in bleomycin-induced pulmonary fibrosis. Biomed. Pharmacother. 2019, 117, 109067. [Google Scholar] [CrossRef]
- Kulkarni, Y.M.; Dutta, S.; Iyer, A.K.V.; Venkatadri, R.; Kaushik, V.; Ramesh, V.; Wright, C.A.; Semmes, O.J.; Yakisich, J.S.; Azad, N. A proteomics approach to identifying key protein targets involved in VEGF inhibitor mediated attenuation of bleomycin-induced pulmonary fibrosis. Proteomics 2016, 16, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, H.; Yang, X.; Bi, Y.; Cui, S. Vasohibin attenuates bleomycin induced pulmonary fibrosis via inhibition of angiogenesis in mice. Pathology 2010, 42, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Barth, E.; Stämmler, G.; Speiser, B.; Schaper, J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J. Mol. Cell. Cardiol. 1992, 24, 669–681. [Google Scholar] [CrossRef]
- Cao, Y.-P.; Zheng, M. Mitochondrial dynamics and inter-mitochondrial communication in the heart. Arch. Biochem. Biophys. 2019, 663, 214–219. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, G.; Kirabo, A.; Ahmed, U.; Liu, J.; Chen, J. Vasohibins in Health and Disease: From Angiogenesis to Tumorigenesis, Multiorgan Dysfunction, and Brain–Heart Remodeling. Cells 2025, 14, 767. https://doi.org/10.3390/cells14110767
Abbas G, Kirabo A, Ahmed U, Liu J, Chen J. Vasohibins in Health and Disease: From Angiogenesis to Tumorigenesis, Multiorgan Dysfunction, and Brain–Heart Remodeling. Cells. 2025; 14(11):767. https://doi.org/10.3390/cells14110767
Chicago/Turabian StyleAbbas, Ghulam, Annet Kirabo, Usama Ahmed, Jie Liu, and Jidong Chen. 2025. "Vasohibins in Health and Disease: From Angiogenesis to Tumorigenesis, Multiorgan Dysfunction, and Brain–Heart Remodeling" Cells 14, no. 11: 767. https://doi.org/10.3390/cells14110767
APA StyleAbbas, G., Kirabo, A., Ahmed, U., Liu, J., & Chen, J. (2025). Vasohibins in Health and Disease: From Angiogenesis to Tumorigenesis, Multiorgan Dysfunction, and Brain–Heart Remodeling. Cells, 14(11), 767. https://doi.org/10.3390/cells14110767







