CYR61 as a Potential Biomarker and Target in Cancer Prognosis and Therapies
Abstract
1. Introduction
2. Discovery
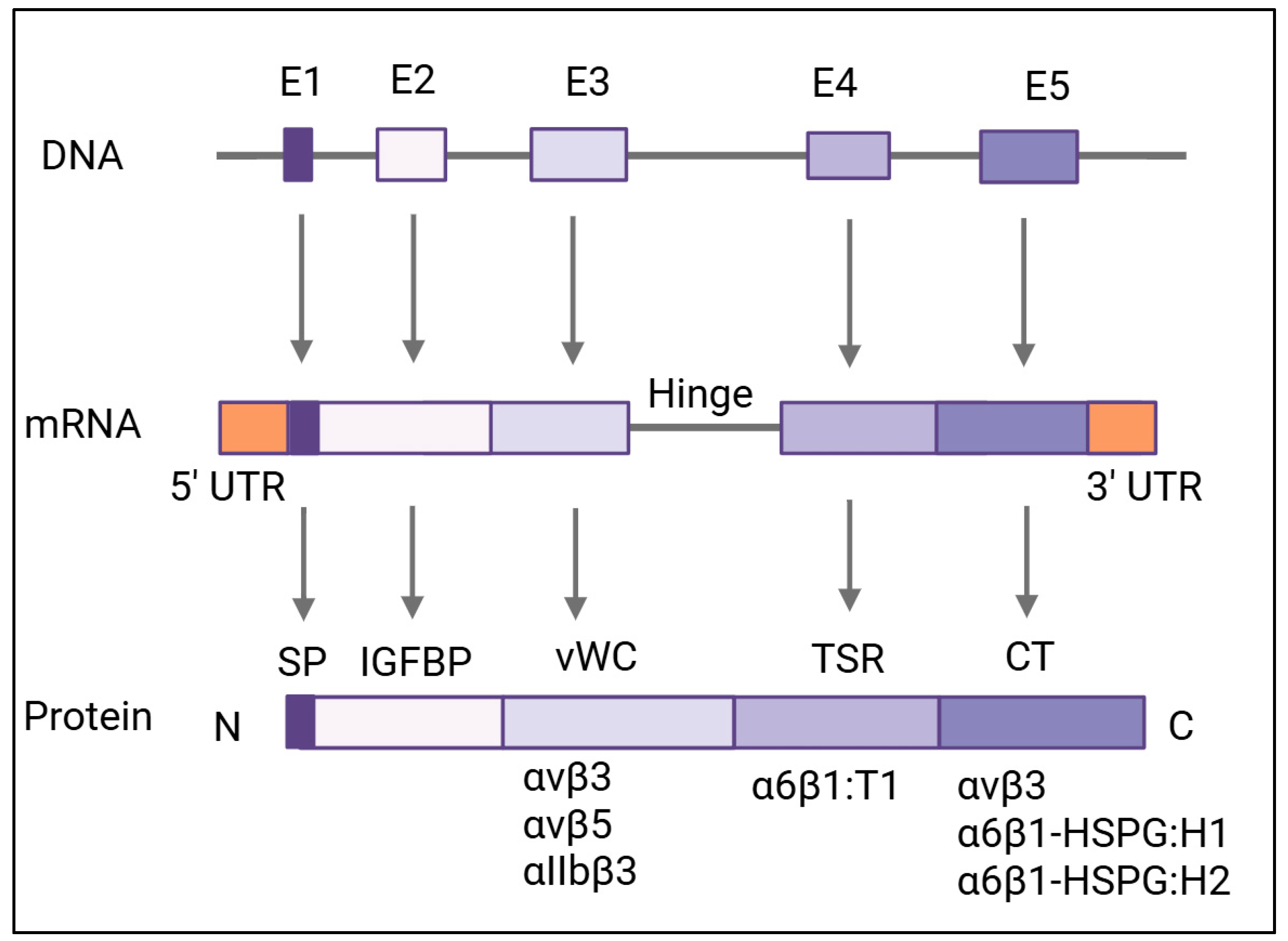
3. Structural Domain and Functions
4. CYR61 Interactome
4.1. Integrin α6β1
4.2. Integrin αvβ3
4.3. Integrin αvβ5
4.4. Integrin αIIbβ3
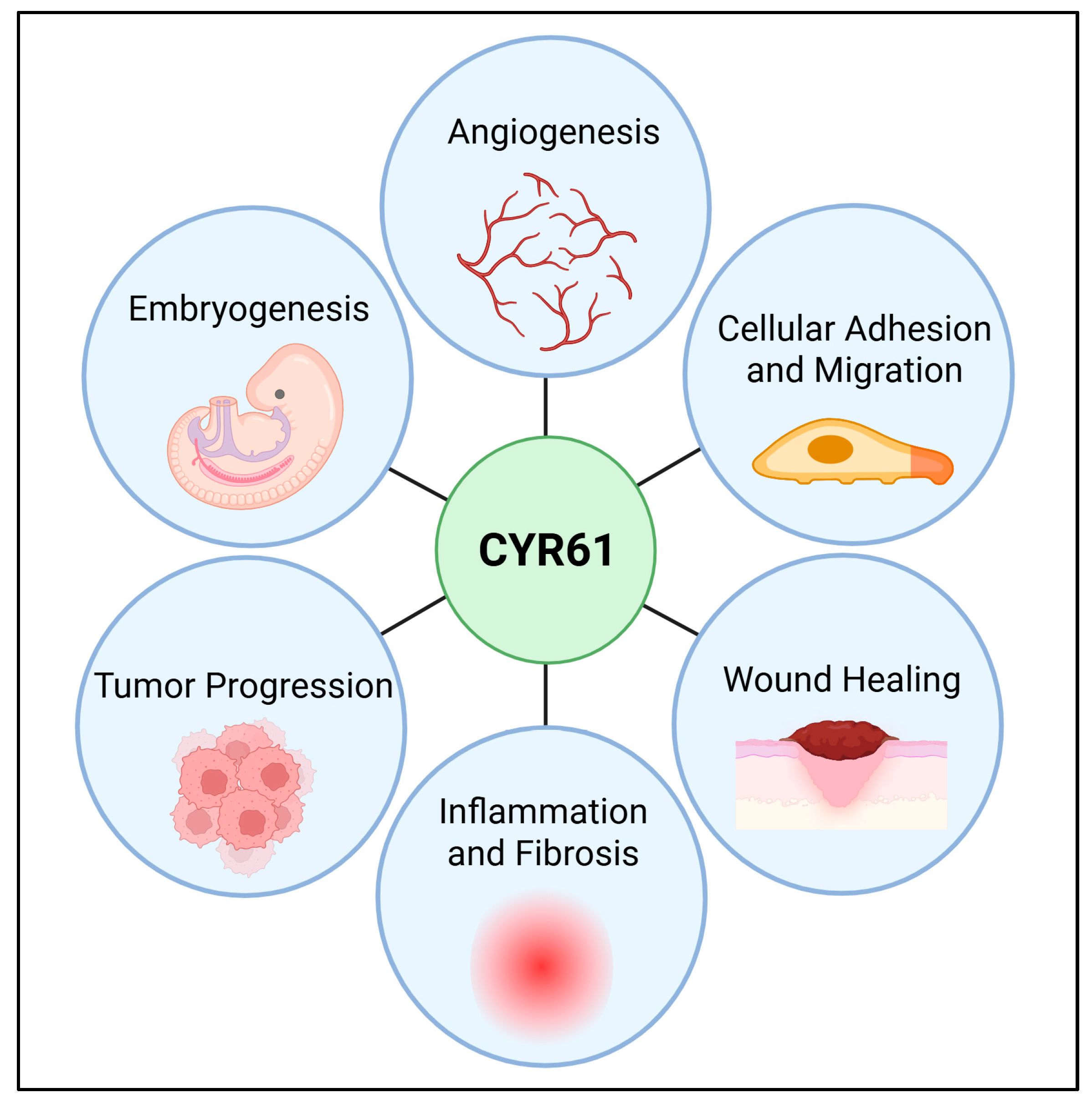
5. CYR61 Roles in Cancer
6. CYR61 in Liquid Biopsies
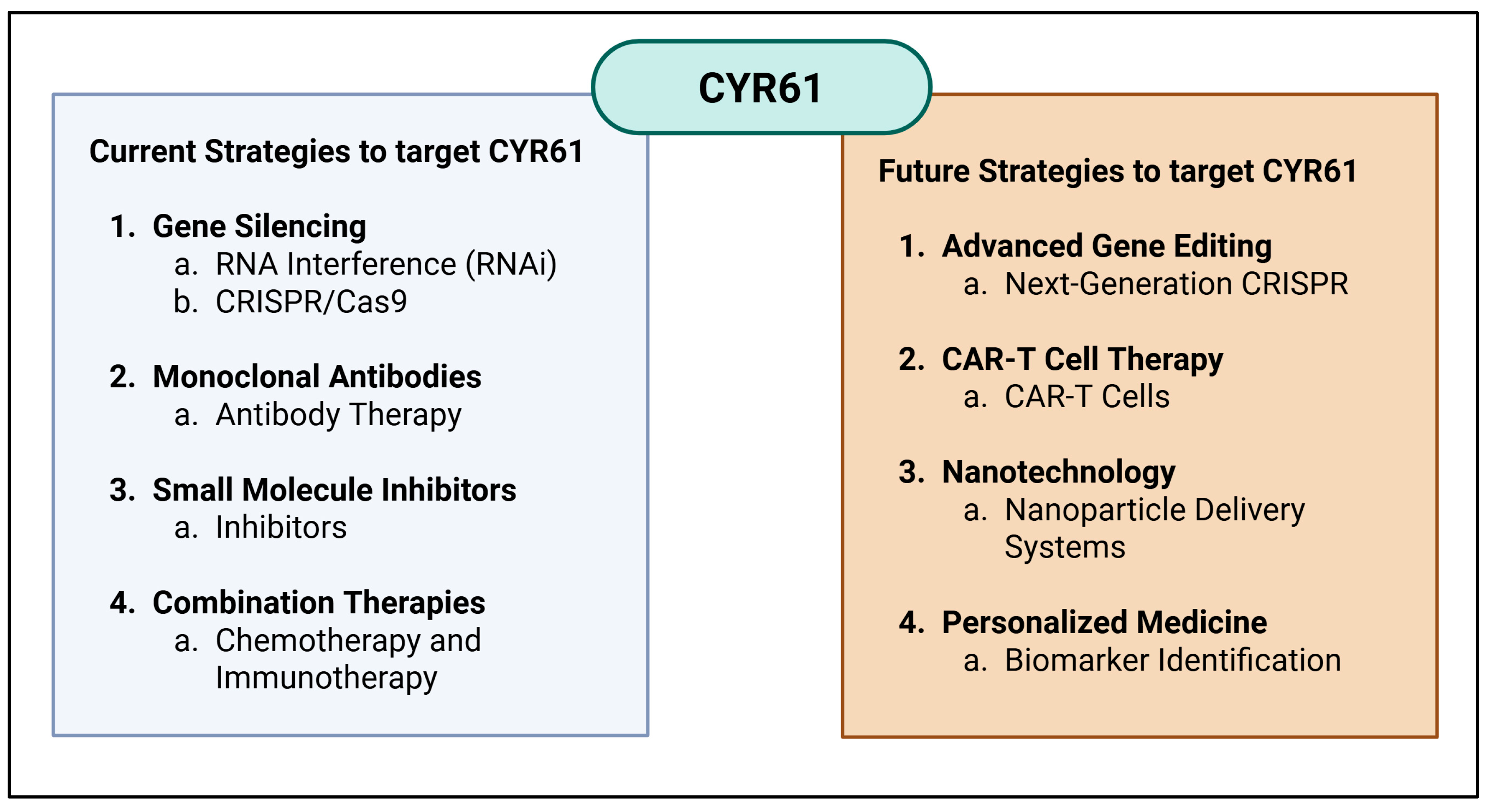
7. CYR61 as a Potential Target in Cancer
8. Challenges
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAR-T | Chimeric Antigen Receptor T cells |
| CCN | Cysteine-Rich Protein, Connective Tissue Growth Factor, Nephroblastoma |
| CT | C-Terminal |
| CTGF | Connective Tissue Growth Factor |
| CYR61 | Cysteine-Rich 61 |
| ECM | Extracellular Matrix |
| HRG | Heregulin |
| HSPG | Heparan Sulfate Proteoglycan |
| hUCMSC | Human Umbilical Mesenchymal Stromal Cells |
| IGFBP | Insulin-like Growth Factor-Binding Protein |
| mDTC | Mesenchymal Disseminated Tumor Cell |
| NOV | Nephroblastoma |
| RGD | Arginine, Glycine, Aspartic Acid |
| ROS | Reactive Oxygen Species |
| TSR | Thrombospondin Type-1 Repeat |
| VEGF | Vascular Endothelial Growth Factor |
| vWC | von Willebrand Factor Type-C Domain |
References
- Kireeva, M.L.; Mo, F.E.; Yang, G.P.; Lau, L.F. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol. Cell Biol. 1996, 16, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Bork, P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993, 327, 125–130. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.P.; Yang, G.P.; Sanders, L.; Lau, L.F. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol. Cell Biol. 1990, 10, 3569–3577. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Kireeva, M.L.; Kolesnikova, T.V.; Lau, L.F. Cyr61, product of a growth factor-inducible immediate-early gene, regulates chondrogenesis in mouse limb bud mesenchymal cells. Dev. Biol. 1997, 192, 492–508. [Google Scholar] [CrossRef]
- Grzeszkiewicz, T.M.; Lindner, V.; Chen, N.; Lam, S.C.; Lau, L.F. The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin alpha(6)beta(1) and cell surface heparan sulfate proteoglycans. Endocrinology 2002, 143, 1441–1450. [Google Scholar] [CrossRef]
- Yang, G.P.; Lau, L.F. Cyr61, product of a growth factor-inducible immediate early gene, is associated with the extracellular matrix and the cell surface. Cell Growth Differ. 1991, 2, 351–357. [Google Scholar]
- Babic, A.M.; Kireeva, M.L.; Kolesnikova, T.V.; Lau, L.F. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc. Natl. Acad. Sci. USA 1998, 95, 6355–6360. [Google Scholar] [CrossRef]
- Tsai, M.S.; Bogart, D.F.; Castaneda, J.M.; Li, P.; Lupu, R. Cyr61 promotes breast tumorigenesis and cancer progression. Oncogene 2002, 21, 8178–8185. [Google Scholar] [CrossRef]
- Chen, C.C.; Lau, L.F. Functions and mechanisms of action of CCN matricellular proteins. Int. J. Biochem. Cell Biol. 2009, 41, 771–783. [Google Scholar] [CrossRef]
- Huang, Y.T.; Lan, Q.; Lorusso, G.; Duffey, N.; Ruegg, C. The matricellular protein CYR61 promotes breast cancer lung metastasis by facilitating tumor cell extravasation and suppressing anoikis. Oncotarget 2017, 8, 9200–9215. [Google Scholar] [CrossRef]
- Jandova, J.; Beyer, T.E.; Meuillet, E.J.; Watts, G.S. The matrix protein CCN1/CYR61 is required for alpha(V)beta5-mediated cancer cell migration. Cell Biochem. Funct. 2012, 30, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Terada, N.; Kulkarni, P.; Getzenberg, R.H. Cyr61 is a potential prognostic marker for prostate cancer. Asian J. Androl. 2012, 14, 405–408. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Barnes, L.A.; Shields, D.J.; Huang, M.; Lau, S.K.; Prevost, N.; Tarin, D.; Shattil, S.J.; Cheresh, D.A. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat. Med. 2009, 15, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Xie, D.; O’Kelly, J.; Miller, C.W.; Muller-Tidow, C.; Koeffler, H.P. Cyr61, a member of CCN family, is a tumor suppressor in non-small cell lung cancer. J. Biol. Chem. 2001, 276, 47709–47714. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Dong, B.; He, X.; Qiu, Z.; Zhang, J.; Zhang, M.; Liu, H.; Pang, X.; Cui, Y. The challenges and opportunities of alphavbeta3-based therapeutics in cancer: From bench to clinical trials. Pharmacol. Res. 2023, 189, 106694. [Google Scholar] [CrossRef]
- Hooglugt, A.; van der Stoel, M.M.; Boon, R.A.; Huveneers, S. Endothelial YAP/TAZ Signaling in Angiogenesis and Tumor Vasculature. Front. Oncol. 2020, 10, 612802. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Q.; Xia, M.; Ru, Y.; Hu, W.; Yan, G.; Xiong, X.; Zhang, M.; Wang, J.; Li, Q.; et al. MIIP inhibits clear cell renal cell carcinoma proliferation and angiogenesis via negative modulation of the HIF-2alpha-CYR61 axis. Cancer Biol. Med. 2021, 19, 818–835. [Google Scholar] [CrossRef]
- Hou, C.H.; Lin, F.L.; Hou, S.M.; Liu, J.F. Cyr61 promotes epithelial-mesenchymal transition and tumor metastasis of osteosarcoma by Raf-1/MEK/ERK/Elk-1/TWIST-1 signaling pathway. Mol. Cancer 2014, 13, 236. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Y.; Chen, D. Biological functions and role of CCN1/Cyr61 in embryogenesis and tumorigenesis in the female reproductive system (Review). Mol. Med. Rep. 2018, 17, 3–10. [Google Scholar] [CrossRef]
- Maity, G.; Ghosh, A.; Gupta, V.; Haque, I.; Sarkar, S.; Das, A.; Dhar, K.; Bhavanasi, S.; Gunewardena, S.S.; Von Hoff, D.D.; et al. CYR61/CCN1 Regulates dCK and CTGF and Causes Gemcitabine-resistant Phenotype in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2019, 18, 788–800. [Google Scholar] [CrossRef]
- Lai, C.F.; Lin, S.L.; Chiang, W.C.; Chen, Y.M.; Wu, V.C.; Young, G.H.; Ko, W.J.; Kuo, M.L.; Tsai, T.J.; Wu, K.D. Blockade of cysteine-rich protein 61 attenuates renal inflammation and fibrosis after ischemic kidney injury. Am. J. Physiol. Renal Physiol. 2014, 307, F581–F592. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Kong, X.; Cui, X.; Wu, S.; Wang, Y.; Dai, X.; Chen, R.; Wang, C.; Jiang, L. CYR61/TGF-beta axis promotes adventitial fibrosis of Takayasu’s arteritis in the IL-17 mediated inflammatory microenvironment. Clin. Exp. Rheumatol. 2020, 38, 1102–1111. [Google Scholar] [PubMed]
- Kim, H.; Son, S.; Ko, Y.; Lim, H.; Lee, J.; Lee, K.M.; Shin, I. CYR61 confers chemoresistance by upregulating survivin expression in triple-negative breast cancer. Carcinogenesis 2024, 45, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.I.; Lau, L.F. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 945–963. [Google Scholar] [CrossRef]
- Lang, A.; Eastburn, E.A.; Younesi, M.; Nijsure, M.P.; Siciliano, C.; Pranatharthi Haran, A.; Panebianco, C.J.; Seidl, E.; Tang, R.; Alsberg, E.; et al. CYR61 delivery promotes angiogenesis during bone fracture repair. NPJ Regen. Med. 2025, 10, 20. [Google Scholar] [CrossRef]
- Chen, C.C.; Mo, F.E.; Lau, L.F. The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J. Biol. Chem. 2001, 276, 47329–47337. [Google Scholar] [CrossRef]
- Shome, D.; von Woedtke, T.; Riedel, K.; Masur, K. The HIPPO Transducer YAP and Its Targets CTGF and Cyr61 Drive a Paracrine Signalling in Cold Atmospheric Plasma-Mediated Wound Healing. Oxid. Med. Cell Longev. 2020, 2020, 4910280. [Google Scholar] [CrossRef]
- Sun, Z.J.; Wang, Y.; Cai, Z.; Chen, P.P.; Tong, X.J.; Xie, D. Involvement of Cyr61 in growth, migration, and metastasis of prostate cancer cells. Br. J. Cancer 2008, 99, 1656–1667. [Google Scholar] [CrossRef]
- O’Brien, T.P.; Lau, L.F. Expression of the growth factor-inducible immediate early gene cyr61 correlates with chondrogenesis during mouse embryonic development. Cell Growth Differ. 1992, 3, 645–654. [Google Scholar]
- Mo, F.E.; Muntean, A.G.; Chen, C.C.; Stolz, D.B.; Watkins, S.C.; Lau, L.F. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol. Cell Biol. 2002, 22, 8709–8720. [Google Scholar] [CrossRef]
- Krupska, I.; Bruford, E.A.; Chaqour, B. Eyeing the Cyr61/CTGF/NOV (CCN) group of genes in development and diseases: Highlights of their structural likenesses and functional dissimilarities. Hum. Genom. 2015, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Rachfal, A.W.; Brigstock, D.R. Structural and functional properties of CCN proteins. Vitam. Horm. 2005, 70, 69–103. [Google Scholar] [CrossRef]
- Kireeva, M.L.; Latinkic, B.V.; Kolesnikova, T.V.; Chen, C.C.; Yang, G.P.; Abler, A.S.; Lau, L.F. Cyr61 and Fisp12 are both ECM-associated signaling molecules: Activities, metabolism, and localization during development. Exp. Cell Res. 1997, 233, 63–77. [Google Scholar] [CrossRef]
- Chen, N.; Chen, C.C.; Lau, L.F. Adhesion of human skin fibroblasts to Cyr61 is mediated through integrin alpha 6beta 1 and cell surface heparan sulfate proteoglycans. J. Biol. Chem. 2000, 275, 24953–24961. [Google Scholar] [CrossRef]
- Brigstock, D.R. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr. Rev. 1999, 20, 189–206. [Google Scholar] [CrossRef]
- Perbal, B. CCN proteins: Multifunctional signalling regulators. Lancet 2004, 363, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Emre, Y.; Imhof, B.A. Matricellular protein CCN1/CYR61: A new player in inflammation and leukocyte trafficking. Semin. Immunopathol. 2014, 36, 253–259. [Google Scholar] [CrossRef]
- Lau, L.F. CCN1/CYR61: The very model of a modern matricellular protein. Cell Mol. Life Sci. 2011, 68, 3149–3163. [Google Scholar] [CrossRef]
- Martinerie, C.; Viegas-Pequignot, E.; Nguyen, V.C.; Perbal, B. Chromosomal mapping and expression of the human cyr61 gene in tumour cells from the nervous system. Mol. Pathol. 1997, 50, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Jay, P.; Berge-Lefranc, J.L.; Marsollier, C.; Mejean, C.; Taviaux, S.; Berta, P. The human growth factor-inducible immediate early gene, CYR61, maps to chromosome 1p. Oncogene 1997, 14, 1753–1757. [Google Scholar] [CrossRef]
- Planque, N.; Perbal, B. A structural approach to the role of CCN (CYR61/CTGF/NOV) proteins in tumourigenesis. Cancer Cell Int. 2003, 3, 15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holbourn, K.P.; Acharya, K.R.; Perbal, B. The CCN family of proteins: Structure-function relationships. Trends Biochem. Sci. 2008, 33, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Huhtala, P.; Lee, H.M.; Adams, J.C.; Pihlajaniemi, T. Membrane-associated collagens with interrupted triple-helices (MACITs): Evolution from a bilaterian common ancestor and functional conservation in C. elegans. BMC Evol. Biol. 2015, 15, 281. [Google Scholar] [CrossRef] [PubMed]
- Grzeszkiewicz, T.M.; Kirschling, D.J.; Chen, N.; Lau, L.F. CYR61 stimulates human skin fibroblast migration through Integrin alpha vbeta 5 and enhances mitogenesis through integrin alpha vbeta 3, independent of its carboxyl-terminal domain. J. Biol. Chem. 2001, 276, 21943–21950. [Google Scholar] [CrossRef]
- Heng, E.C.; Huang, Y.; Black, S.A., Jr.; Trackman, P.C. CCN2, connective tissue growth factor, stimulates collagen deposition by gingival fibroblasts via module 3 and alpha6- and beta1 integrins. J. Cell Biochem. 2006, 98, 409–420. [Google Scholar] [CrossRef]
- Kireeva, M.L.; Lam, S.C.; Lau, L.F. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin alphavbeta3. J. Biol. Chem. 1998, 273, 3090–3096. [Google Scholar] [CrossRef]
- Schober, J.M.; Chen, N.; Grzeszkiewicz, T.M.; Jovanovic, I.; Emeson, E.E.; Ugarova, T.P.; Ye, R.D.; Lau, L.F.; Lam, S.C. Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): Immediate-early gene products expressed in atherosclerotic lesions. Blood 2002, 99, 4457–4465. [Google Scholar] [CrossRef]
- Jedsadayanmata, A.; Chen, C.C.; Kireeva, M.L.; Lau, L.F.; Lam, S.C. Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/mouse connective tissue growth factor is mediated through integrin alpha(IIb)beta(3). J. Biol. Chem. 1999, 274, 24321–24327. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Kalus, W.; Zweckstetter, M.; Renner, C.; Sanchez, Y.; Georgescu, J.; Grol, M.; Demuth, D.; Schumacher, R.; Dony, C.; Lang, K.; et al. Structure of the IGF-binding domain of the insulin-like growth factor-binding protein-5 (IGFBP-5): Implications for IGF and IGF-I receptor interactions. EMBO J. 1998, 17, 6558–6572. [Google Scholar] [CrossRef]
- Xu, E.R.; Blythe, E.E.; Fischer, G.; Hyvonen, M. Structural analyses of von Willebrand factor C domains of collagen 2A and CCN3 reveal an alternative mode of binding to bone morphogenetic protein-2. J. Biol. Chem. 2017, 292, 12516–12527. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Duquette, M.; Liu, J.H.; Dong, Y.; Zhang, R.; Joachimiak, A.; Lawler, J.; Wang, J.H. Crystal structure of the TSP-1 type 1 repeats: A novel layered fold and its biological implication. J. Cell Biol. 2002, 159, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.J.; Chen, N.; Chen, C.C.; Todorovic, V.; Bai, T.; Juric, V.; Liu, Y.; Yan, G.; Lam, S.C.; Lau, L.F. Targeted mutagenesis of the angiogenic protein CCN1 (CYR61). Selective inactivation of integrin alpha6beta1-heparan sulfate proteoglycan coreceptor-mediated cellular functions. J. Biol. Chem. 2004, 279, 44177–44187. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Fan, E.; Sun, S.; Ma, X.; Zhang, X.; Han, D.M.; Cong, Y.S. Cyr61 is up-regulated in prostate cancer and associated with the p53 gene status. J. Cell Biochem. 2009, 106, 738–744. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Zigrino, P.; Brucker, C.; Bourdon, C.; Freund, M.; De Arcangelis, A.; Abrams, S.I.; Orend, G.; Gachet, C.; Mangin, P.H. Platelet integrin alpha6beta1 controls lung metastasis through direct binding to cancer cell-derived ADAM9. JCI Insight 2016, 1, e88245. [Google Scholar] [CrossRef]
- Leu, S.J.; Lam, S.C.; Lau, L.F. Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbilical vein endothelial cells. J. Biol. Chem. 2002, 277, 46248–46255. [Google Scholar] [CrossRef]
- Chen, N.; Leu, S.J.; Todorovic, V.; Lam, S.C.; Lau, L.F. Identification of a novel integrin alphavbeta3 binding site in CCN1 (CYR61) critical for pro-angiogenic activities in vascular endothelial cells. J. Biol. Chem. 2004, 279, 44166–44176. [Google Scholar] [CrossRef]
- Gu, Y.; Du, Y.; Jiang, L.; Tang, X.; Li, A.; Zhao, Y.; Lang, Y.; Liu, X.; Liu, J. alphavbeta3 integrin-specific exosomes engineered with cyclopeptide for targeted delivery of triptolide against malignant melanoma. J. Nanobiotechnol. 2022, 20, 384. [Google Scholar] [CrossRef]
- Chen, C.C.; Young, J.L.; Monzon, R.I.; Chen, N.; Todorovic, V.; Lau, L.F. Cytotoxicity of TNFalpha is regulated by integrin-mediated matrix signaling. EMBO J. 2007, 26, 1257–1267. [Google Scholar] [CrossRef]
- Bougie, D.W.; Rasmussen, M.; Zhu, J.; Aster, R.H. Antibodies causing thrombocytopenia in patients treated with RGD-mimetic platelet inhibitors recognize ligand-specific conformers of alphaIIb/beta3 integrin. Blood 2012, 119, 6317–6325. [Google Scholar] [CrossRef]
- Adair, B.D.; Alonso, J.L.; van Agthoven, J.; Hayes, V.; Ahn, H.S.; Yu, I.S.; Lin, S.W.; Xiong, J.P.; Poncz, M.; Arnaout, M.A. Structure-guided design of pure orthosteric inhibitors of alphaIIbbeta3 that prevent thrombosis but preserve hemostasis. Nat. Commun. 2020, 11, 398. [Google Scholar] [CrossRef]
- Kardeh, S.; Ashkani-Esfahani, S.; Alizadeh, A.M. Paradoxical action of reactive oxygen species in creation and therapy of cancer. Eur. J. Pharmacol. 2014, 735, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Kim, K.H.; Lau, L.F. The matricellular protein CCN1 suppresses hepatocarcinogenesis by inhibiting compensatory proliferation. Oncogene 2016, 35, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, W.; Huang, P.; Lin, L.; Ye, H.; Lin, D.; Koeffler, H.P.; Wang, J.; Yin, D. Expression of CCN family members correlates with the clinical features of hepatocellular carcinoma. Oncol. Rep. 2015, 33, 1481–1492. [Google Scholar] [CrossRef]
- Kim, K.H.; Chen, C.C.; Monzon, R.I.; Lau, L.F. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol. Cell Biol. 2013, 33, 2078–2090. [Google Scholar] [CrossRef]
- Chien, W.; Kumagai, T.; Miller, C.W.; Desmond, J.C.; Frank, J.M.; Said, J.W.; Koeffler, H.P. Cyr61 suppresses growth of human endometrial cancer cells. J. Biol. Chem. 2004, 279, 53087–53096. [Google Scholar] [CrossRef]
- Lorusso, G.; Ruegg, C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem. Cell Biol. 2008, 130, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Juric, V.; Chen, C.C.; Lau, L.F. Fas-mediated apoptosis is regulated by the extracellular matrix protein CCN1 (CYR61) in vitro and in vivo. Mol. Cell Biol. 2009, 29, 3266–3279. [Google Scholar] [CrossRef]
- Huang, Y.T.; Lan, Q.; Ponsonnet, L.; Blanquet, M.; Christofori, G.; Zaric, J.; Ruegg, C. The matricellular protein CYR61 interferes with normal pancreatic islets architecture and promotes pancreatic neuroendocrine tumor progression. Oncotarget 2016, 7, 1663–1674. [Google Scholar] [CrossRef]
- Espinoza, I.; Kurapaty, C.; Park, C.H.; Vander Steen, T.; Kleer, C.G.; Wiley, E.; Rademaker, A.; Cuyas, E.; Verdura, S.; Buxo, M.; et al. Depletion of CCN1/CYR61 reduces triple-negative/basal-like breast cancer aggressiveness. Am. J. Cancer Res. 2022, 12, 839–851. [Google Scholar]
- Todorovic, V.; Chen, C.C.; Hay, N.; Lau, L.F. The matrix protein CCN1 (CYR61) induces apoptosis in fibroblasts. J. Cell Biol. 2005, 171, 559–568. [Google Scholar] [CrossRef]
- Lee, K.B.; Byun, H.J.; Park, S.H.; Park, C.Y.; Lee, S.H.; Rho, S.B. CYR61 controls p53 and NF-kappaB expression through PI3K/Akt/mTOR pathways in carboplatin-induced ovarian cancer cells. Cancer Lett. 2012, 315, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, G.; Brooks, P.C.; Biganzoli, E.; Vermeulen, P.B.; Bonoldi, E.; Dirix, L.Y.; Ranieri, G.; Miceli, R.; Cheresh, D.A. Vascular integrin alpha(v)beta3: A new prognostic indicator in breast cancer. Clin. Cancer Res. 1998, 4, 2625–2634. [Google Scholar]
- Sampath, D.; Winneker, R.C.; Zhang, Z. The angiogenic factor Cyr61 is induced by the progestin R5020 and is necessary for mammary adenocarcinoma cell growth. Endocrine 2002, 18, 147–159. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, K.B.; Schultz, L.; Albadine, R.; Mondul, A.M.; Platz, E.A.; Netto, G.J.; Getzenberg, R.H. Decreased expression of Cyr61 is associated with prostate cancer recurrence after surgical treatment. Clin. Cancer Res. 2010, 16, 5908–5913. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Santini, D.; Meo, G.; Vincenzi, B.; Zappavigna, S.; Baldi, A.; Rosolowski, M.; Tonini, G.; Loeffler, M.; Lupu, R.; et al. Cyr61 downmodulation potentiates the anticancer effects of zoledronic acid in androgen-independent prostate cancer cells. Int. J. Cancer 2009, 125, 2004–2013. [Google Scholar] [CrossRef]
- Franzen, C.A.; Chen, C.C.; Todorovic, V.; Juric, V.; Monzon, R.I.; Lau, L.F. Matrix protein CCN1 is critical for prostate carcinoma cell proliferation and TRAIL-induced apoptosis. Mol. Cancer Res. 2009, 7, 1045–1055. [Google Scholar] [CrossRef]
- Lin, C.M.; Liang, C.Z. Cyr61: A potential therapeutic target for prostate cancer. Asian J. Androl. 2014, 16, 788–789. [Google Scholar] [CrossRef] [PubMed]
- Pilarsky, C.P.; Schmidt, U.; Eissrich, C.; Stade, J.; Froschermaier, S.E.; Haase, M.; Faller, G.; Kirchner, T.W.; Wirth, M.P. Expression of the extracellular matrix signaling molecule Cyr61 is downregulated in prostate cancer. Prostate 1998, 36, 85–91. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, T.; Tian, G.; Cui, M. Cysteine-rich, angiogenic inducer, 61 expression in patients with ovarian epithelial carcinoma. J. Int. Med. Res. 2014, 42, 300–306. [Google Scholar] [CrossRef]
- Xie, D.; Yin, D.; Tong, X.; O’Kelly, J.; Mori, A.; Miller, C.; Black, K.; Gui, D.; Said, J.W.; Koeffler, H.P. Cyr61 is overexpressed in gliomas and involved in integrin-linked kinase-mediated Akt and beta-catenin-TCF/Lef signaling pathways. Cancer Res. 2004, 64, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Lin, Z.; Song, Y.; Li, Z.; Zeng, M.; Luo, L.; Cao, Y.; Zhu, X. Chemotherapy-initiated cysteine-rich protein 61 decreases acute B-lymphoblastic leukemia chemosensitivity. J. Cancer Res. Clin. Oncol. 2024, 150, 159. [Google Scholar] [CrossRef]
- Han, S.; Bui, N.T.; Ho, M.T.; Kim, Y.M.; Cho, M.; Shin, D.B. Dexamethasone Inhibits TGF-beta1-Induced Cell Migration by Regulating the ERK and AKT Pathways in Human Colon Cancer Cells Via CYR61. Cancer Res. Treat. 2016, 48, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Heo, S.; Sung Ahn, T.; Lee, S.; Park, S.; Kim, H.; Park, D.; Byung Bae, S.; Lee, S.S.; Soo Lee, M.; et al. Cyr61 expression is associated with prognosis in patients with colorectal cancer. BMC Cancer 2014, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.F.; Xu, Z.B.; Zhu, X.J.; Tao, X.; Liu, J.L.; Gao, F.L.; Wu, C.L.; Song, B.; Lin, Q. Serum Cyr61 as a potential biomarker for diagnosis of colorectal cancer. Clin. Transl. Oncol. 2017, 19, 519–524. [Google Scholar] [CrossRef]
- Chen, P.C.; Cheng, H.C.; Yang, S.F.; Lin, C.W.; Tang, C.H. The CCN family proteins: Modulators of bone development and novel targets in bone-associated tumors. Biomed. Res. Int. 2014, 2014, 437096. [Google Scholar] [CrossRef]
- Lin, Z.; Song, Y.; Qiu, Y.; Shi, P.; Zeng, M.; Cao, Y.; Zhu, X. Serum CYR61 as a potential biomarker to improve breast cancer diagnostics. Biomark. Med. 2022, 16, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, K.; Heidrich, I.; Kwiatkowski, M.; Gorges, T.M.; Andreas, A.; Geffken, M.; Verpoort, K.; Muller, V.; Schluter, H.; Pantel, K. Cysteine-Rich Angiogenic Inducer 61: Pro-Survival Function and Role as a Biomarker for Disseminating Breast Cancer Cells. Cancers 2021, 13, 563. [Google Scholar] [CrossRef]
- Ackar, L.; Casjens, S.; Andreas, A.; Raiko, I.; Bruning, T.; Geffken, M.; Peine, S.; Kollmeier, J.; Johnen, G.; Bartkowiak, K.; et al. Blood-based detection of lung cancer using cysteine-rich angiogenic inducer 61 (CYR61) as a circulating protein biomarker: A pilot study. Mol. Oncol. 2021, 15, 2877–2890. [Google Scholar] [CrossRef]
- Bauerschmitz, G.; Huchel, S.; Gallwas, J.; Grundker, C. Inhibition of Increased Invasiveness of Breast Cancer Cells With Acquired Tamoxifen Resistance by Suppression of CYR61. Cancer Genom. Proteom. 2023, 20, 531–538. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Hu, K.; Ren, Y.; Zhang, H.; Zhao, Y.; Wei, W.; Tu, S.; Yan, X. The prognostic implications and tumor-suppressive functions of CYR61 in estrogen receptor-positive breast cancer. Front. Immunol. 2023, 14, 1308807. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ghosh, A.; VonHoff, D.D.; Banerjee, S.K. Cyr61/CCN1 targets for chemosensitization in pancreatic cancer. Oncotarget 2019, 10, 3579–3580. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, P.; Gerber, U.; Jungel, E.; Schutze, N.; Blaheta, R.; Bendas, G. Cyr61/CCN1 affects the integrin-mediated migration of prostate cancer cells (PC-3) in vitro. Int. J. Clin. Pharmacol. Ther. 2013, 51, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Van Agthoven, J.F.; Xiong, J.P.; Alonso, J.L.; Rui, X.; Adair, B.D.; Goodman, S.L.; Arnaout, M.A. Structural basis for pure antagonism of integrin alphaVbeta3 by a high-affinity form of fibronectin. Nat. Struct. Mol. Biol. 2014, 21, 383–388. [Google Scholar] [CrossRef]
- Wang, J.; Fu, D.; Senouthai, S.; Jiang, Y.; Hu, R.; You, Y. Identification of the Transcriptional Networks and the Involvement in Angiotensin II-Induced Injury after CRISPR/Cas9-Mediated Knockdown of Cyr61 in HEK293T Cells. Mediat. Inflamm. 2019, 2019, 8697257. [Google Scholar] [CrossRef]
- Ma, J.; Ma, R.; Zhao, X.; Wang, Y.; Liao, S.; Nong, C.; Lu, F.; Liang, Z.; Huang, J.; Huang, Y.; et al. Cyr61 Mediates Angiotensin II-Induced Podocyte Apoptosis via the Upregulation of TXNIP. J. Immunol. Res. 2023, 2023, 8643548. [Google Scholar] [CrossRef]
- Haque, I.; Mehta, S.; Majumder, M.; Dhar, K.; De, A.; McGregor, D.; Van Veldhuizen, P.J.; Banerjee, S.K.; Banerjee, S. Cyr61/CCN1 signaling is critical for epithelial-mesenchymal transition and stemness and promotes pancreatic carcinogenesis. Mol. Cancer 2011, 10, 8. [Google Scholar] [CrossRef]
- Campos, A.; Salomon, C.; Bustos, R.; Diaz, J.; Martinez, S.; Silva, V.; Reyes, C.; Diaz-Valdivia, N.; Varas-Godoy, M.; Lobos-Gonzalez, L.; et al. Caveolin-1-containing extracellular vesicles transport adhesion proteins and promote malignancy in breast cancer cell lines. Nanomedicine 2018, 13, 2597–2609. [Google Scholar] [CrossRef]
- Kan, T.; Zhang, S.; Zhou, S.; Zhang, Y.; Zhao, Y.; Gao, Y.; Zhang, T.; Gao, F.; Wang, X.; Zhao, L.; et al. Single-cell RNA-seq recognized the initiator of epithelial ovarian cancer recurrence. Oncogene 2022, 41, 895–906. [Google Scholar] [CrossRef]
- Menendez, J.A.; Mehmi, I.; Griggs, D.W.; Lupu, R. The angiogenic factor CYR61 in breast cancer: Molecular pathology and therapeutic perspectives. Endocr. Relat. Cancer 2003, 10, 141–152. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Sheldrake, H.M.; Patterson, L.H. Strategies to inhibit tumor associated integrin receptors: Rationale for dual and multi-antagonists. J. Med. Chem. 2014, 57, 6301–6315. [Google Scholar] [CrossRef]
- Wallstabe, L.; Mades, A.; Frenz, S.; Einsele, H.; Rader, C.; Hudecek, M. CAR T cells targeting alpha(v)beta(3) integrin are effective against advanced cancer in preclinical models. Adv. Cell Gene Ther. 2018, 1, e11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, Y.; Tian, Y.; Chen, Y.; Ding, F.; Wu, H.; Ji, Y.; Shen, M. Cyr61 promotes Schwann cell proliferation and migration via alphavbeta3 integrin. BMC Mol. Cell Biol. 2021, 22, 21. [Google Scholar] [CrossRef]
- Cobb, D.A.; de Rossi, J.; Liu, L.; An, E.; Lee, D.W. Targeting of the alpha(v) beta(3) integrin complex by CAR-T cells leads to rapid regression of diffuse intrinsic pontine glioma and glioblastoma. J. Immunother. Cancer 2022, 10, e003816. [Google Scholar] [CrossRef]
- Harris, L.G.; Pannell, L.K.; Singh, S.; Samant, R.S.; Shevde, L.A. Increased vascularity and spontaneous metastasis of breast cancer by hedgehog signaling mediated upregulation of cyr61. Oncogene 2012, 31, 3370–3380. [Google Scholar] [CrossRef] [PubMed]
- Monnier, Y.; Farmer, P.; Bieler, G.; Imaizumi, N.; Sengstag, T.; Alghisi, G.C.; Stehle, J.C.; Ciarloni, L.; Andrejevic-Blant, S.; Moeckli, R.; et al. CYR61 and alphaVbeta5 integrin cooperate to promote invasion and metastasis of tumors growing in preirradiated stroma. Cancer Res. 2008, 68, 7323–7331. [Google Scholar] [CrossRef]
- Lin, J.; Huo, R.; Wang, L.; Zhou, Z.; Sun, Y.; Shen, B.; Wang, R.; Li, N. A novel anti-Cyr61 antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol. Immunother. 2012, 61, 677–687. [Google Scholar] [CrossRef]
| Domain | Conserved Sequence | Binding Sites | Ribbon Diagram |
|---|---|---|---|
| Insulin-like Growth Factor-Binding Protein (IGFBP) [40,50] | TCPAACHCPL EAPKCAPGVG LVRDGCGCCK VCAKQLNEDC SKTQPCDHTK GLECNFGASS TALKGICRAQ SEGRPCEYNS RIYQNGESFQ PNCKHQCTCI DGAVGCIPLC PQELSLPNLG CPNPRLVKVT GQCCEEWVCD EDSIKDPMED QDGLLGKELG FDASEVELTR NNELIAVGKG SSLKRLPVFG MEPRILYNPL | Insulin-like growth factor-binding proteins | 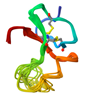 |
| von Willebrand Factor Type-C Repeat (vWC) [51] | GQKCIVQTTSWSQCSKS | αvβ3 αvβ5 αIIbβ3 | 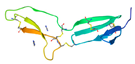 |
| Thrombospondin Type-1 Repeat (TSR) [52] | GQKCIVQTTSWSQCSKS | α6β1 | 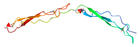 |
| C-Terminal (CT) [2,42] | KGKKCSKTKKSPEPVRFTYAGCSSVKKYRPKY | αvβ3, α6β1-HSPG:H1, α6β1-HSPG:H2 |  |
| Ligand | Binding Domain | Associated Cancers |
|---|---|---|
| α6β1 [38,53,54,55,71,72] | TSR, CT | Breast, ovarian, lung, lung metastasis, and prostate. |
| αvβ3 [13,15,38,44,56,58,70,73,74] | vWC | Bone metastasis, breast, cervical, colon, melanoma, non-small-cell lung, ovarian, glioblastoma, prostate, and pancreatic. |
| αvβ5 [11,44,59] | vWC | Breast, colorectal, gastric, liver metastasis, ovarian, glioblastoma, pancreatic, and prostate. |
| αIIbβ3 [7,34,60,61] | vWC | Breast, ovarian, and prostate. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schenker, A.J.; Ortiz-Hernández, G.L. CYR61 as a Potential Biomarker and Target in Cancer Prognosis and Therapies. Cells 2025, 14, 761. https://doi.org/10.3390/cells14110761
Schenker AJ, Ortiz-Hernández GL. CYR61 as a Potential Biomarker and Target in Cancer Prognosis and Therapies. Cells. 2025; 14(11):761. https://doi.org/10.3390/cells14110761
Chicago/Turabian StyleSchenker, Andrew J., and Greisha L. Ortiz-Hernández. 2025. "CYR61 as a Potential Biomarker and Target in Cancer Prognosis and Therapies" Cells 14, no. 11: 761. https://doi.org/10.3390/cells14110761
APA StyleSchenker, A. J., & Ortiz-Hernández, G. L. (2025). CYR61 as a Potential Biomarker and Target in Cancer Prognosis and Therapies. Cells, 14(11), 761. https://doi.org/10.3390/cells14110761








