The Diversity of Fibrillin Functions: Lessons from the Periodontal Ligament
Abstract
1. Introduction
2. The Periodontal Ligament
2.1. Overview
2.2. Means of Analysis
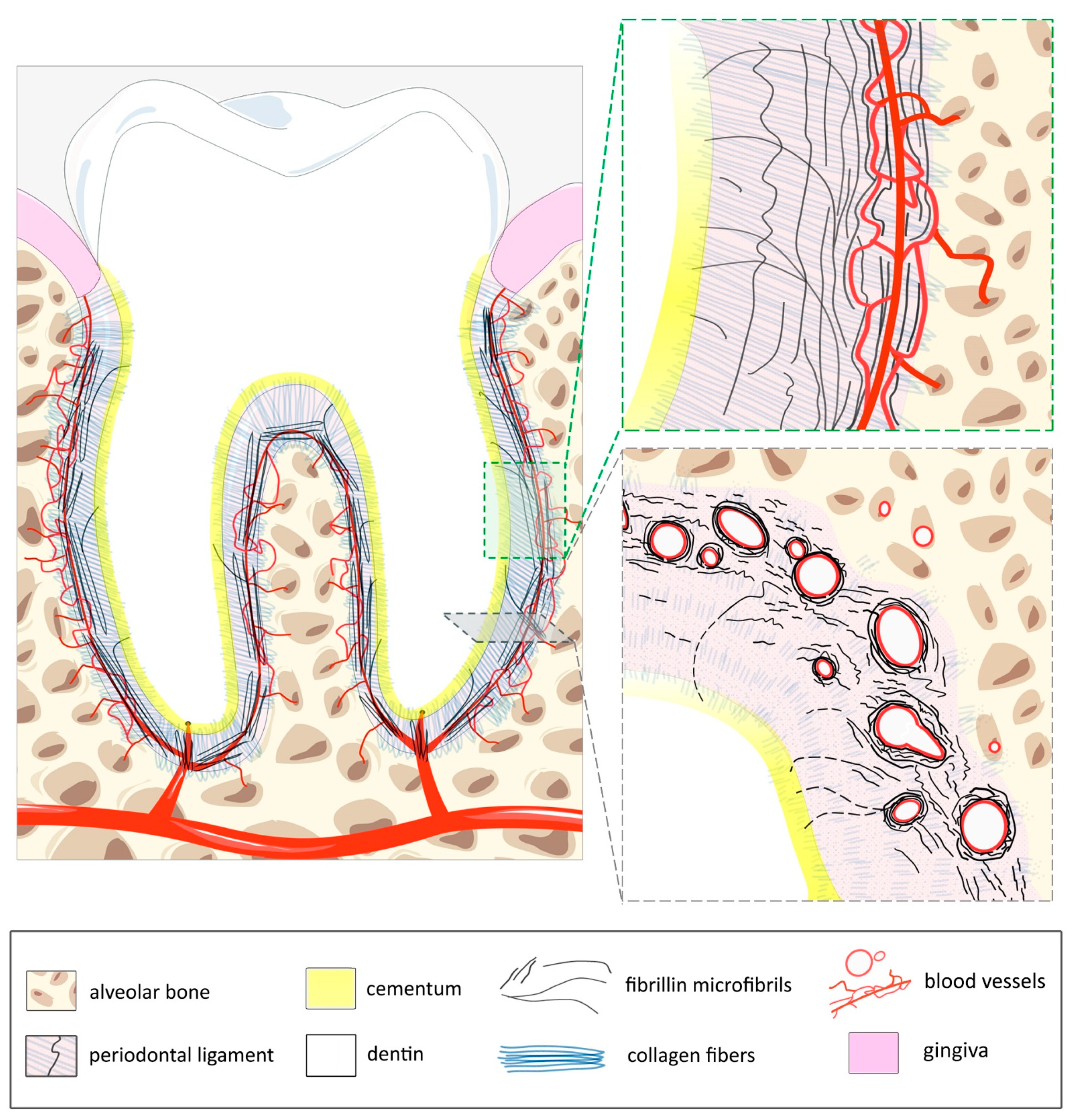
2.3. Anatomy of the PDL
2.4. Elastic System–Blood Vasculature Coupling in the PDL
3. Fibrillins at Work in the PDL
3.1. Fibrillin Isoforms and Functional Domains
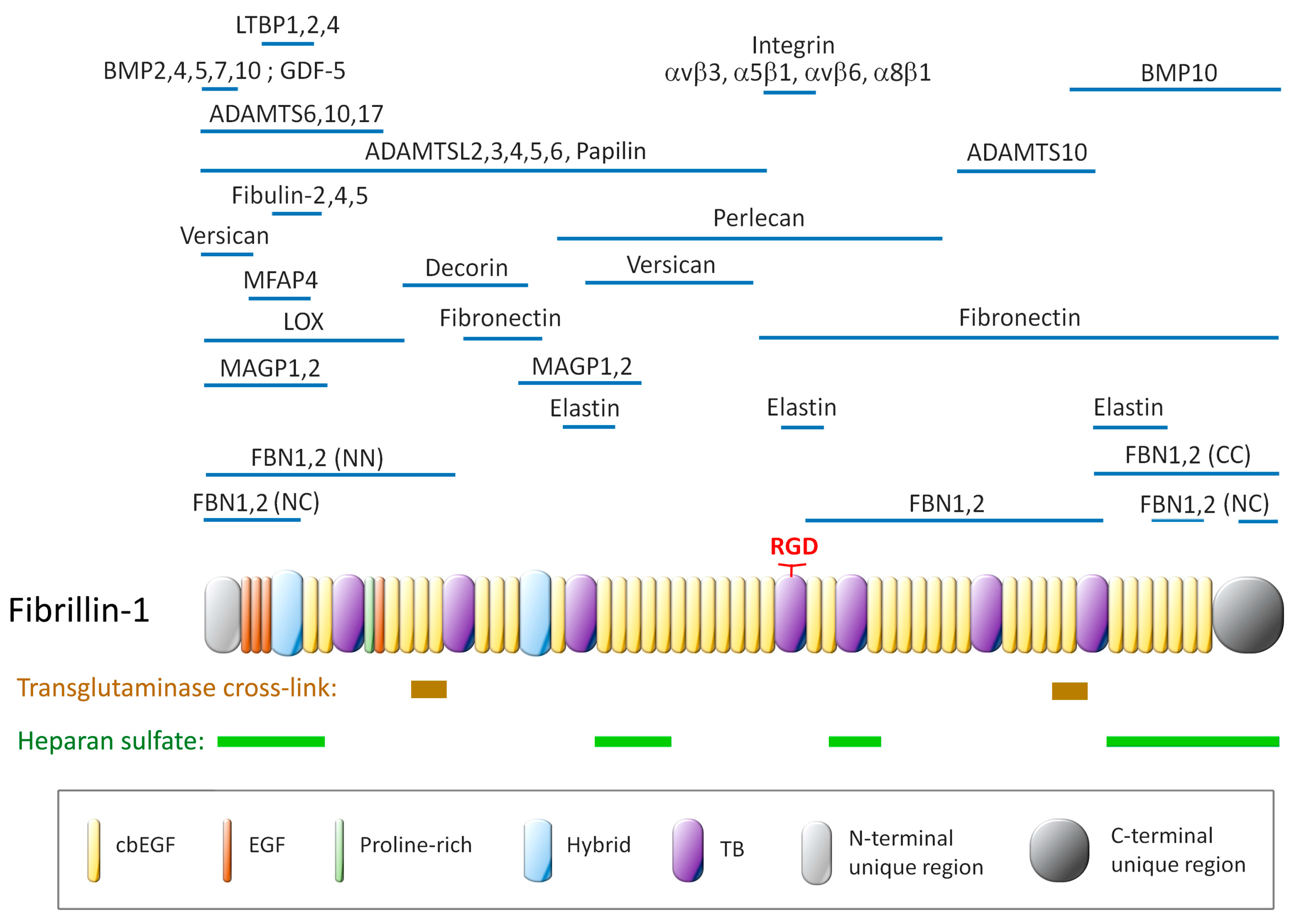
3.2. Fibrillin Assembly into Microfibrils
3.3. Fibrillin and Mechanosensing
3.4. Fibrillin Effector Protein-Mediated Functions and Homeostasis
4. The PDL in Marfan Patients
5. Therapeutic Advances as a Result of Improved Basic Knowledge of Fibrillin and Fibrillin Microfibrils
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDL | Periodontal Ligament |
| CZ | Ciliary Zonule |
| TGFβ | Transforming Growth Factor beta |
| BMP | Bone Morphogenetic Protein |
| MFS | Marfan Syndrome |
References
- Dietz, H.C.; Cutting, G.R.; Pyeritz, R.E.; Maslen, C.L.; Sakai, L.Y.; Corson, G.M.; Puffenberger, E.G.; Hamosh, A.; Nanthakumar, E.J.; Curristin, S.M.; et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991, 352, 337–339. [Google Scholar] [CrossRef]
- Kozel, B.A.; Mecham, R.P. Elastic fiber ultrastructure and assembly. Matrix Biol. 2019, 84, 31–40. [Google Scholar] [CrossRef]
- Godwin, A.R.F.; Starborg, T.; Smith, D.J.; Sherratt, M.J.; Roseman, A.M.; Baldock, C. Multiscale imaging reveals the hierarchical organization of fibrillin microfibrils. J. Mol. Biol. 2018, 430, 4142–4155. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.; Singh, M.; Eckersley, A.; Cain, S.A.; Sherratt, M.J.; Baldock, C. Fibrillin microfibrils and elastic fibre proteins: Functional interactions and extracellular regulation of growth factors. Semin. Cell Dev. Biol. 2019, 89, 109–117. [Google Scholar] [CrossRef]
- Alonso, F.; Li, L.; Fremaux, I.; Reinhardt, D.P.; Genot, E. Fibrillin-1 regulates arteriole integrity in the retina. Biomolecules 2022, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Rathaur; Rodriguez, J.; Kuchtey, J.; Insignares, S.; Jones, W.B.; Kuchtey, R.W.; Bassnett, S. The biomechanics of fibrillin microfibrils: Lessons from the ciliary zonule. Cells 2024, 13, 2097. [Google Scholar] [CrossRef]
- Adamo, C.S.; Zuk, A.V.; Sengle, G. The fibrillin microfibril/elastic fibre network: A critical extracellular supramolecular scaffold to balance skin homoeostasis. Exp. Dermatol. 2021, 30, 25–37. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Gerli, R.; Ibba, L.; Fruschelli, C. A fibrillar elastic apparatus around human lymph capillaries. Anat. Embryol. 1990, 181, 281–286. [Google Scholar] [CrossRef]
- Reinhardt, D.P.; Sasaki, T.; Dzamba, B.J.; Keene, D.R.; Chu, M.L.; Gohring, W.; Timpl, R.; Sakai, L.Y. Fibrillin-1 and fibulin-2 interact and are colocalized in some tissues. J. Biol. Chem. 1996, 271, 19489–19496. [Google Scholar] [CrossRef]
- de Souza, R.B.; Lemes, R.B.; Foresto-Neto, O.; Cassiano, L.L.; Reinhardt, D.P.; Meek, K.M.; Koh, I.H.J.; Lewis, P.N.; Pereira, L.V. Extracellular matrix and vascular dynamics in the kidney of a murine model for marfan syndrome. PLoS ONE 2023, 18, e0285418. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.S.; Cain, S.A.; Morgan, A.; Dallas, S.L.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin-1 regulates the bioavailability of tgfbeta1. J. Cell Biol. 2007, 176, 355–367. [Google Scholar] [CrossRef]
- Sengle, G.; Charbonneau, N.L.; Ono, R.N.; Sasaki, T.; Alvarez, J.; Keene, D.R.; Bachinger, H.P.; Sakai, L.Y. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J. Biol. Chem. 2008, 283, 13874–13888. [Google Scholar] [CrossRef]
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of tgf-beta activation contributes to pathogenesis in marfan syndrome. Nat. Genet. 2003, 33, 407–411. [Google Scholar] [CrossRef]
- Habashi, J.P.; Judge, D.P.; Holm, T.M.; Cohn, R.D.; Loeys, B.L.; Cooper, T.K.; Myers, L.; Klein, E.C.; Liu, G.; Calvi, C.; et al. Losartan, an at1 antagonist, prevents aortic aneurysm in a mouse model of marfan syndrome. Science 2006, 312, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Spanou, C.E.S.; Yang, C.; Godwin, A.R.F.; Morosky, S.; Anbalagan, A.; Lutke, S.; Morgelin, M.; Marcous, F.; Aziz, U.; Wohl, A.P.; et al. Prodomain processing controls bmp-10 bioactivity and targeting to fibrillin-1 in latent conformation. FASEB J. 2025, 39, e70373. [Google Scholar] [CrossRef]
- Sengle, G.; Ono, R.N.; Lyons, K.M.; Bächinger, H.P.; Sakai, L.Y. A new model for growth factor activation: Type ii receptors compete with the prodomain for bmp-7. J. Mol. Biol. 2008, 381, 1025–1039. [Google Scholar] [CrossRef]
- Wohl, A.P.; Troilo, H.; Collins, R.F.; Baldock, C.; Sengle, G. Extracellular regulation of bone morphogenetic protein activity by the microfibril component fibrillin-1. J. Biol. Chem. 2016, 291, 12732–12746. [Google Scholar] [CrossRef]
- Jensen, S.A.; Handford, P.A. New insights into the structure, assembly and biological roles of 10–12 nm connective tissue microfibrils from fibrillin-1 studies. Biochem. J. 2016, 473, 827–838. [Google Scholar] [CrossRef]
- Munger, A.; Sheppard, D. Cross talk among tgf-β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb. Perspect. Biol. 2011, 3, a005017. [Google Scholar] [CrossRef]
- Sengle, G.; Sakai, L.Y. The fibrillin microfibril scaffold: A niche for growth factors and mechanosensation? Matrix Biol. 2015, 47, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.J.; Roitberg, A.E.; Dudley, A.T. Steered molecular dynamic simulations reveal marfan syndrome mutations disrupt fibrillin-1 cbegf domain mechanosensitive calcium binding. Sci. Rep. 2020, 10, 16844. [Google Scholar] [CrossRef]
- Shiga, M.; Saito, M.; Hattori, M.; Torii, C.; Kosaki, K.; Kiyono, T.; Suda, N. Characteristic phenotype of immortalized periodontal cells isolated from a marfan syndrome type i patient. Cell Tissue Res. 2008, 331, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Suda, N.; Shiga, M.; Ganburged, G.; Moriyama, K. Marfan syndrome and its disorder in periodontal tissues. J. Exp. Zool. Part B Mol. Dev. Evol. 2009, 312B, 503–509. [Google Scholar] [CrossRef]
- Alonso, F.; Dong, Y.; Li, L.; Jahjah, T.; Dupuy, J.-W.; Fremaux, I.; Reinhardt, D.P.; Génot, E. Fibrillin-1 regulates endothelial sprouting during angiogenesis. Proc. Natl. Acad. Sci. USA 2023, 120, e2221742120. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. Vegf guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Dzamba, B.J.; Keene, D.R.; Isogai, Z.; Charbonneau, N.L.; Karaman-Jurukovska, N.; Simon, M.; Sakai, L.Y. Assembly of epithelial cell fibrillins. J. Investig. Dermatol. 2001, 117, 1612–1620. [Google Scholar] [CrossRef]
- Fullmer, H.M.; Lillie, R.D. The oxytalan fiber: A previously undescribed connective tissue fiber. J. Histochem. Cytochem. 1958, 6, 425–430. [Google Scholar] [CrossRef]
- Herrera, D.; Sanz, M.; Shapira, L.; Brotons, C.; Chapple, I.; Frese, T.; Graziani, F.; Hobbs, F.D.R.; Huck, O.; Hummers, E.; et al. Periodontal diseases and cardiovascular diseases, diabetes, and respiratory diseases: Summary of the consensus report by the european federation of periodontology and wonca europe. Eur. J. Gen. Pract. 2024, 30, 2320120. [Google Scholar] [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal disease: A risk factor for diabetes and cardiovascular disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- Ghanem, A.S.; Móré, M.; Nagy, A.C. Analysis of molecular aspects of periodontitis as a risk factor for neurodegenerative diseases: A single-center 10-year retrospective cohort study. Int. J. Mol. Sci. 2025, 26, 2382. [Google Scholar] [CrossRef] [PubMed]
- Mander, S.T.K.; Mander, L.N.; Carmichael, G.G. The staining mechanism of aldehyde-fuchsin, with reference to the oxytalan fiber in the mouse. J. Histochem. Cytochem. 1968, 16, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Sugawara, Y.; Asai, T.; Aida, N.; Yanagisawa, T.; Ohta, K.; Inoue, S. Immunohistochemical characterization of elastic system fibers in rat molar periodontal ligament. J. Histochem. Cytochem. 2006, 54, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Kira-Tatsuoka, M.; Oka, K.; Tsuruga, E.; Ozaki, M.; Sawa, Y. Immunohistochemical expression of fibrillin-1 and fibrillin-2 during tooth development. J. Periodontal Res. 2015, 50, 714–720. [Google Scholar] [CrossRef]
- Sims, M.R. Oxytalan-vascular relationships observed in histologic examination of the periodontal ligaments of man and mouse. Arch. Oral Biol. 1975, 20, 713–716. [Google Scholar] [CrossRef]
- Sims, M.R. Reconstitution of the human oxytalan system during orthodontic tooth movement. Am. J. Orthod. 1976, 70, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Godwin, A.R.F.; Dajani, R.; Zhang, X.; Thomson, J.; Holmes, D.F.; Adamo, C.S.; Sengle, G.; Sherratt, M.J.; Roseman, A.M.; Baldock, C. Fibrillin microfibril structure identifies long-range effects of inherited pathogenic mutations affecting a key regulatory latent tgfbeta-binding site. Nat. Struct. Mol. Biol. 2023, 30, 608–618. [Google Scholar] [CrossRef]
- Șulea, C.M.; Mártonfalvi, Z.; Csányi, C.; Haluszka, D.; Pólos, M.; Ágg, B.; Stengl, R.; Benke, K.; Szabolcs, Z.; Kellermayer, M.S.Z. Nanoscale structural comparison of fibrillin-1 microfibrils isolated from marfan and non-marfan syndrome human aorta. Int. J. Mol. Sci. 2023, 24, 7561. [Google Scholar] [CrossRef]
- Kielty, C.M. Fell-muir lecture: Fibrillin microfibrils: Structural tensometers of elastic tissues? Int. J. Exp. Pathol. 2017, 98, 172–190. [Google Scholar] [CrossRef]
- Beertsen, W.; McCulloch, C.A.G.; Sodek, J. The periodontal ligament: A unique, multifunctional connective tissue. Periodontology 2000 1997, 13, 20–40. [Google Scholar] [CrossRef]
- Foong, K.; Sims, W.R. Blood volume in human bicuspid periodontal ligament determined by electron microscopy. Arch. Oral Biol. 1999, 44, 465–474. [Google Scholar] [CrossRef]
- Moxham, B.J.; Shore, R.C.; Berkovitz, B.K. Fenestrated capillaries in the connective tissues of the periodontal ligament. Microvasc. Res. 1985, 30, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Hara, Y.; Sato, T. Development of the oxytalan fiber system in the rat molar periodontal ligament evaluated by light- and electron-microscopic analyses. Ann. Anat. 2012, 194, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Kuroda, N.; Sato, T. Elastic fiber system evaluated in the digestive organ of rats. Microscopy 2019, 68, 434–440. [Google Scholar] [CrossRef]
- Chantawiboonchai, P.; Warita, H.; Ohya, K.; Soma, K. Confocal laser scanning-microscopic observations on the three-dimensional distribution of oxytalan fibres in mouse periodontal ligament. Arch. Oral Biol. 1998, 43, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Hara, Y.; Kuroda, N.; Sato, T. Development of the oxytalan fiber system in the periodontal space of rat incisors. Ann. Anat. 2013, 195, 475–483. [Google Scholar] [CrossRef]
- Sims, M.R. Oxytalan fiber system of molars in the mouse mandible. J. Dent. Res. 1973, 52, 797–802. [Google Scholar] [CrossRef]
- Sims, M.R. Ultrastructural analysis of the microfibrillar component of mouse and human periodontal oxytalan fibers. Connect. Tissue Res. 1984, 13, 59–67. [Google Scholar] [CrossRef]
- Freezer, S.R.; Sims, M.R. Statistical correlations between cells, blood vessels, oxytalan fibres and nerves in normal mouse molar periodontal ligament using transmission electron microscopy. Aust. Orthod. J. 1988, 10, 227–230. [Google Scholar] [CrossRef]
- Ganburged, G.; Suda, N.; Saito, M.; Yamazaki, Y.; Isokawa, K.; Moriyama, K. Dilated capillaries, disorganized collagen fibers and differential gene expression in periodontal ligaments of hypomorphic fibrillin-1 mice. Cell Tissue Res. 2010, 341, 381–395. [Google Scholar] [CrossRef]
- Strydom, H.; Maltha, J.C.; Kuijpers-Jagtman, A.M.; Von den Hoff, J.W. The oxytalan fibre network in the periodontium and its possible mechanical function. Arch. Oral Biol. 2012, 57, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, N.L.; Carlson, E.J.; Tufa, S.; Sengle, G.; Manalo, E.C.; Carlberg, V.M.; Ramirez, F.; Keene, D.R.; Sakai, L.Y. In vivo studies of mutant fibrillin-1 microfibrils. J. Biol. Chem. 2010, 285, 24943–24955. [Google Scholar] [CrossRef] [PubMed]
- Brinckmann, J.; Hunzelmann, N.; Kahle, B.; Rohwedel, J.; Kramer, J.; Gibson, M.A.; Hubmacher, D.; Reinhardt, D.P. Enhanced fibrillin-2 expression is a general feature of wound healing and sclerosis: Potential alteration of cell attachment and storage of tgf-beta. Lab. Investig. 2010, 90, 739–752. [Google Scholar] [CrossRef] [PubMed]
- McKnight, H.; Kelsey, W.P.; Hooper, D.A.; Hart, T.C.; Mariotti, A. Proteomic analyses of human gingival and periodontal ligament fibroblasts. J. Periodontol. 2014, 85, 810–818. [Google Scholar] [CrossRef]
- Rossi, A.; Gabbrielli, E.; Villano, M.; Messina, M.; Ferrara, F.; Weber, E. Human microvascular lymphatic and blood endothelial cells produce fibrillin: Deposition patterns and quantitative analysis. J. Anat. 2010, 217, 705–714. [Google Scholar] [CrossRef]
- Charbonneau, N.L.; Manalo, E.C.; Tufa, S.F.; Carlson, E.J.; Carlberg, V.M.; Keene, D.R.; Sakai, L.Y. Fibrillin-1 in the vasculature: In vivo accumulation of egfp-tagged fibrillin-1 in a knockin mouse model. Anat. Rec. 2020, 303, 1590–1603. [Google Scholar] [CrossRef]
- Kielty, C.M.; Sherratt, M.J.; Shuttleworth, C.A. Elastic fibres. J. Cell Sci. 2002, 115, 2817–2828. [Google Scholar] [CrossRef]
- Reinhardt, D.P.; Ono, R.N.; Sakai, L.Y. Calcium stabilizes fibrillin-1 against proteolytic degradation. J. Biol. Chem. 1997, 272, 1231–1236. [Google Scholar] [CrossRef]
- McGettrick, A.J.; Knott, V.; Willis, A.; Handford, P.A. Molecular effects of calcium binding mutations in marfan syndrome depend on domain context. Hum. Mol. Genet. 2000, 9, 1987–1994. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Z.; Chen, T.; Sun, X.; Jiang, Y. Cysteine substitution and calcium-binding mutations in fbn1 cbegf-like domains are associated with severe ocular involvement in patients with congenital ectopia lentis. Front. Cell Dev. Biol. 2021, 9, 816397. [Google Scholar] [CrossRef]
- Hubmacher, D.; Reinhardt, D.P. Microfibrils and fibrillin. In The Extracellular Matrix: An Overview; Mecham, R.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 233–265. [Google Scholar]
- Sengle, G.; Tsutsui, K.; Keene, D.R.; Tufa, S.F.; Carlson, E.J.; Charbonneau, N.L.; Ono, R.N.; Sasaki, T.; Wirtz, M.K.; Samples, J.R.; et al. Microenvironmental regulation by fibrillin-1. PLoS Genet. 2012, 8, e1002425. [Google Scholar] [CrossRef]
- Ono, R.N.; Sengle, G.; Charbonneau, N.L.; Carlberg, V.; Bächinger, H.P.; Sasaki, T.; Lee-Arteaga, S.; Zilberberg, L.; Rifkin, D.B.; Ramirez, F.; et al. Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. J. Biol. Chem. 2009, 284, 16872–16881. [Google Scholar] [CrossRef]
- Hubmacher, D.; El-Hallous, E.I.; Nelea, V.; Kaartinen, M.T.; Lee, E.R.; Reinhardt, D.P. Biogenesis of extracellular microfibrils: Multimerization of the fibrillin-1 c terminus into bead-like structures enables self-assembly. Proc. Natl. Acad. Sci. USA 2008, 105, 6548–6553. [Google Scholar] [CrossRef]
- Wess, T.J.; Purslow, P.P.; Sherratt, M.J.; Ashworth, J.; Shuttleworth, C.A.; Kielty, C.M. Calcium determines the supramolecular organization of fibrillin-rich microfibrils. J. Cell Biol. 1998, 141, 829–837. [Google Scholar] [CrossRef]
- Zeyer, K.A.; Reinhardt, D.P. Fibrillin-containing microfibrils are key signal relay stations for cell function. J. Cell Commun. Signal. 2015, 9, 309–325. [Google Scholar] [CrossRef]
- Zheng, L.; Huang, Y.; Song, W.; Gong, X.; Liu, M.; Jia, X.; Zhou, G.; Chen, L.; Li, A.; Fan, Y. Fluid shear stress regulates metalloproteinase-1 and 2 in human periodontal ligament cells: Involvement of extracellular signal-regulated kinase (erk) and p38 signaling pathways. J. Biomech. 2012, 45, 2368–2375. [Google Scholar] [CrossRef]
- Ziegler, N.; Alonso, A.; Steinberg, T.; Woodnutt, D.; Kohl, A.; Müssig, E.; Schulz, S.; Tomakidi, P. Mechano-transduction in periodontal ligament cells identifies activated states of map-kinases p42/44 and p38-stress kinase as a mechanism for mmp-13 expression. BMC Cell Biol. 2010, 11, 10. [Google Scholar] [CrossRef]
- Jonas, I.E.; Riede, U.N. Reaction of oxytalan fibers in human periodontium to mechanical stress. A combined histochemical and morphometric analysis. J. Histochem. Cytochem. 1980, 28, 211–216. [Google Scholar] [CrossRef]
- Anastasi, G.; Cordasco, G.; Matarese, G.; Rizzo, G.; Nucera, R.; Mazza, M.; Militi, A.; Portelli, M.; Cutroneo, G.; Favaloro, A. An immunohistochemical, histological, and electron-microscopic study of the human periodontal ligament during orthodontic treatment. Int. J. Mol. Med. 2008, 21, 545–554. [Google Scholar] [CrossRef]
- Ren, Y.; Maltha, J.C.; Stokroos, I.; Liem, R.S.; Kuijpers-Jagtman, A.M. Effect of duration of force application on blood vessels in young and adult rats. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 752–757. [Google Scholar] [CrossRef]
- Tsuruga, E.; Nakashima, K.; Ishikawa, H.; Yajima, T.; Sawa, Y. Stretching modulates oxytalan fibers in human periodontal ligament cells. J. Periodontal Res. 2009, 44, 170–174. [Google Scholar] [CrossRef]
- Hisanaga, Y.; Nakashima, K.; Tsuruga, E.; Nakatomi, Y.; Hatakeyama, Y.; Ishikawa, H.; Sawa, Y. Fibulin-5 contributes to microfibril assembly in human periodontal ligament cells. Acta Histochem. Cytochem. 2009, 42, 151–157. [Google Scholar] [CrossRef]
- Nakashima, K.; Tsuruga, E.; Hisanaga, Y.; Ishikawa, H.; Sawa, Y. Stretching stimulates fibulin-5 expression and controls microfibril bundles in human periodontal ligament cells. J. Periodontal Res. 2009, 44, 622–627. [Google Scholar] [CrossRef]
- Redlich, M.; Roos, H.A.; Reichenberg, E.; Zaks, B.; Mussig, D.; Baumert, U.; Golan, I.; Palmon, A. Expression of tropoelastin in human periodontal ligament fibroblasts after simulation of orthodontic force. Arch. Oral Biol. 2004, 49, 119–124. [Google Scholar] [CrossRef]
- Reinhardt, D.P.; Keene, D.R.; Corson, G.M.; Pöschl, E.; Bächinger, H.P.; Gambee, J.E.; Sakai, L.Y. Fibrillin-1: Organization in microfibrils and structural properties. J. Mol. Biol. 1996, 258, 104–116. [Google Scholar] [CrossRef]
- Hubmacher, D.; Apte, S.S. Genetic and functional linkage between adamts superfamily proteins and fibrillin-1: A novel mechanism influencing microfibril assembly and function. Cell. Mol. Life Sci. 2011, 68, 3137–3148. [Google Scholar] [CrossRef]
- Nakatomi, Y.; Tsuruga, E.; Nakashima, K.; Sawa, Y.; Ishikawa, H. Emilin-1 regulates the amount of oxytalan fiber formation in periodontal ligaments in vitro. Connect. Tissue Res. 2011, 52, 30–35. [Google Scholar] [CrossRef]
- Schubert, A.; Schminke, B.; Miosge, N. Fibulins and matrilins are novel structural components of the periodontium in the mouse. Arch. Oral Biol. 2017, 82, 216–222. [Google Scholar] [CrossRef]
- Bax, D.V.; Bernard, S.E.; Lomas, A.; Morgan, A.; Humphries, J.; Shuttleworth, C.A.; Humphries, M.J.; Kielty, C.M. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha 5 beta 1 and alpha v beta 3 integrins. J. Biol. Chem. 2003, 278, 34605–34616. [Google Scholar] [CrossRef]
- Kimura, Y.; Komaki, M.; Iwasaki, K.; Sata, M.; Izumi, Y.; Morita, I. Recruitment of bone marrow-derived cells to periodontal tissue defects. Front. Cell Dev. Biol. 2014, 2, 19. [Google Scholar] [CrossRef]
- Komaki, M. Pericytes in the periodontal ligament. Adv. Exp. Med. Biol. 2019, 1122, 169–186. [Google Scholar] [CrossRef]
- Smaldone, S.; Clayton, N.P.; del Solar, M.; Pascual, G.; Cheng, S.H.; Wentworth, B.M.; Schaffler, M.B.; Ramirez, F. Fibrillin-1 regulates skeletal stem cell differentiation by modulating tgfbeta activity within the marrow niche. J. Bone Miner. Res. 2016, 31, 86–97. [Google Scholar] [CrossRef]
- Mourikis, P.; Sambasivan, R.; Castel, D.; Rocheteau, P.; Bizzarro, V.; Tajbakhsh, S. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells 2012, 30, 243–252. [Google Scholar] [CrossRef]
- Straub, A.M.; Grahame, R.; Scully, C.; Tonetti, M.S. Severe periodontitis in marfan’s syndrome: A case report. J. Periodontol. 2002, 73, 823–826. [Google Scholar] [CrossRef]
- Galletti, C.; Toledano-Serrabona, J.; Camps-Font, O.; Teixido-Tura, G.; Llobet-Poal, I.; Subirà-Pifarré, C.; Fiorillo, L.; Gay-Escoda, C. Prevalence of periodontitis among patients diagnosed with marfan syndrome: A cross-sectional study comparing samples of healthy patients. Biomed. Res. Int. 2022, 2022, 6238099. [Google Scholar] [CrossRef]
- Staufenbiel, I.; Hauschild, C.; Kahl-Nieke, B.; Vahle-Hinz, E.; von Kodolitsch, Y.; Berner, M.; Bauss, O.; Geurtsen, W.; Rahman, A. Periodontal conditions in patients with marfan syndrome—A multicenter case control study. BMC Oral Health 2013, 13, 59. [Google Scholar] [CrossRef]
- Suda, N.; Moriyama, K.; Ganburged, G. Effect of angiotensin ii receptor blocker on experimental periodontitis in a mouse model of marfan syndrome. Infect. Immun. 2013, 81, 182–188. [Google Scholar] [CrossRef]
- Pereira, L.; Andrikopoulos, K.; Tian, J.; Lee, S.Y.; Keene, D.R.; Ono, R.; Reinhardt, D.P.; Sakai, L.Y.; Biery, N.J.; Bunton, T.; et al. Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of marfan syndrome. Nat. Genet. 1997, 17, 218–222. [Google Scholar] [CrossRef]
- Manabe, Y.; Shiga, M.; Kometani-Gunjigake, K.; Nakao-Kuroishi, K.; Mizuhara, M.; Toyono, T.; Seta, Y.; Kawamoto, T. Fibrillin-1 regulates periostin expression during maintenance of periodontal homeostasis. J. Dent. Sci. 2022, 17, 1714–1721. [Google Scholar] [CrossRef]
- Judge, D.P.; Biery, N.J.; Keene, D.R.; Geubtner, J.; Myers, L.; Huso, D.L.; Sakai, L.Y.; Dietz, H.C. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of marfan syndrome. J. Clin. Investig. 2004, 114, 172–181. [Google Scholar] [CrossRef]
- Handa, K.; Abe, S.; Suresh, V.V.; Fujieda, Y.; Ishikawa, M.; Orimoto, A.; Kobayashi, Y.; Yamada, S.; Yamaba, S.; Murakami, S.; et al. Fibrillin-1 insufficiency alters periodontal wound healing failure in a mouse model of marfan syndrome. Arch. Oral. Biol. 2018, 90, 53–60. [Google Scholar] [CrossRef]
- Pereira, L.; Lee, S.Y.; Gayraud, B.; Andrikopoulos, K.; Shapiro, S.D.; Bunton, T.; Biery, N.J.; Dietz, H.C.; Sakai, L.Y.; Ramirez, F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc. Natl. Acad. Sci. USA 1999, 96, 3819–3823. [Google Scholar] [CrossRef]
- Hubmacher, D.; Bergeron, E.; Fagotto-Kaufmann, C.; Sakai, L.Y.; Reinhardt, D.P. Early fibrillin-1 assembly monitored through a modifiable recombinant cell approach. Biomacromolecules 2014, 15, 1456–1468. [Google Scholar] [CrossRef]
- Oka, K. Fibrillin protein, a candidate for creating a suitable scaffold in pdl regeneration while avoiding ankylosis. Genesis 2022, 60, e23486. [Google Scholar] [CrossRef]
- Saito, M.; Tsuji, T. Extracellular matrix administration as a potential therapeutic strategy for periodontal ligament regeneration. Expert Opin. Biol. Ther. 2012, 12, 299–309. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genot, E.; Al Tabosh, T.; Catros, S.; Alonso, F.; Le Nihouannen, D. The Diversity of Fibrillin Functions: Lessons from the Periodontal Ligament. Cells 2025, 14, 764. https://doi.org/10.3390/cells14110764
Genot E, Al Tabosh T, Catros S, Alonso F, Le Nihouannen D. The Diversity of Fibrillin Functions: Lessons from the Periodontal Ligament. Cells. 2025; 14(11):764. https://doi.org/10.3390/cells14110764
Chicago/Turabian StyleGenot, Elisabeth, Tala Al Tabosh, Sylvain Catros, Florian Alonso, and Damien Le Nihouannen. 2025. "The Diversity of Fibrillin Functions: Lessons from the Periodontal Ligament" Cells 14, no. 11: 764. https://doi.org/10.3390/cells14110764
APA StyleGenot, E., Al Tabosh, T., Catros, S., Alonso, F., & Le Nihouannen, D. (2025). The Diversity of Fibrillin Functions: Lessons from the Periodontal Ligament. Cells, 14(11), 764. https://doi.org/10.3390/cells14110764








