FAM20B Gain-of-Function Blocks the Synthesis of Glycosaminoglycan Chains of Proteoglycans and Inhibits Proliferation and Migration of Glioblastoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Vector Constructions and Cell Transfection
2.3. FAM20B Knockdown
2.4. Site-Directed Mutagenesis
2.5. N-Glycosylation Analysis
2.6. Western Blotting
2.7. Metabolic Labelling of GAG Chains
2.8. Immunofluorescence Analysis
2.9. Data Analysis and Statistical Procedures
3. Results
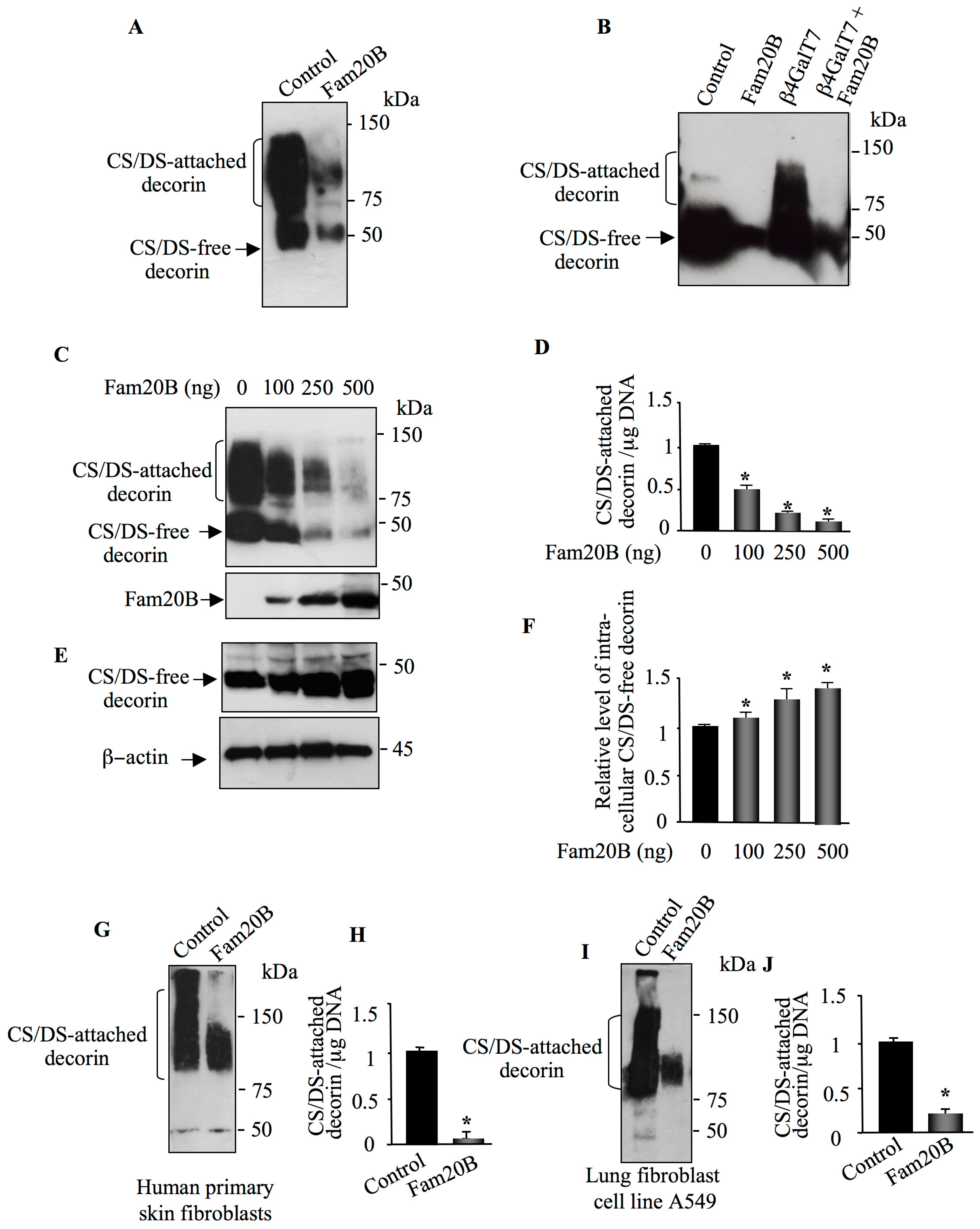
3.1. FAM20B Gain-of-Function Reduces the Synthesis of PG–GAG Chains
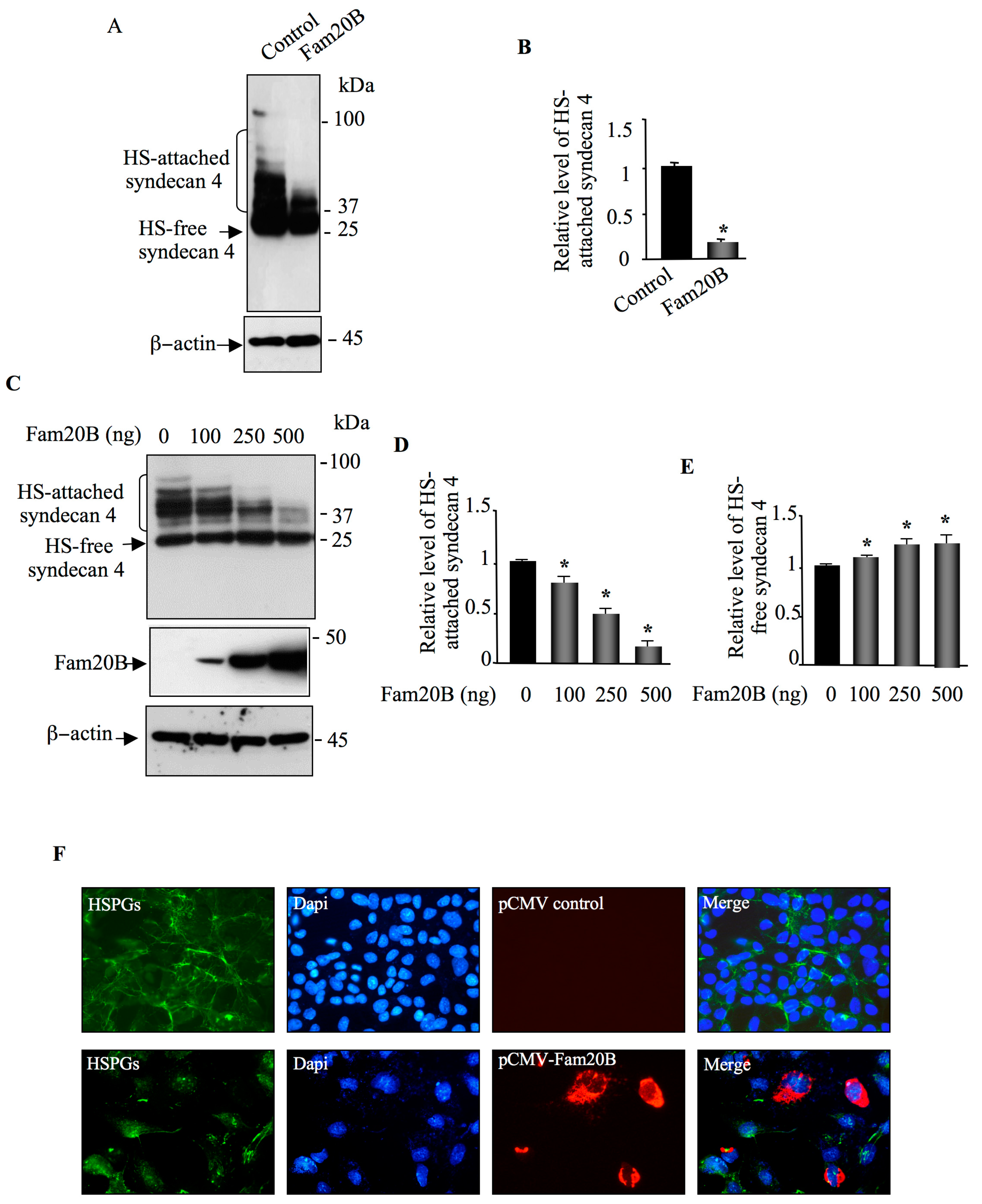
3.2. FAM20B Gain-of-Function Reduces the Synthesis of Both CS– and HS–GAG Chains
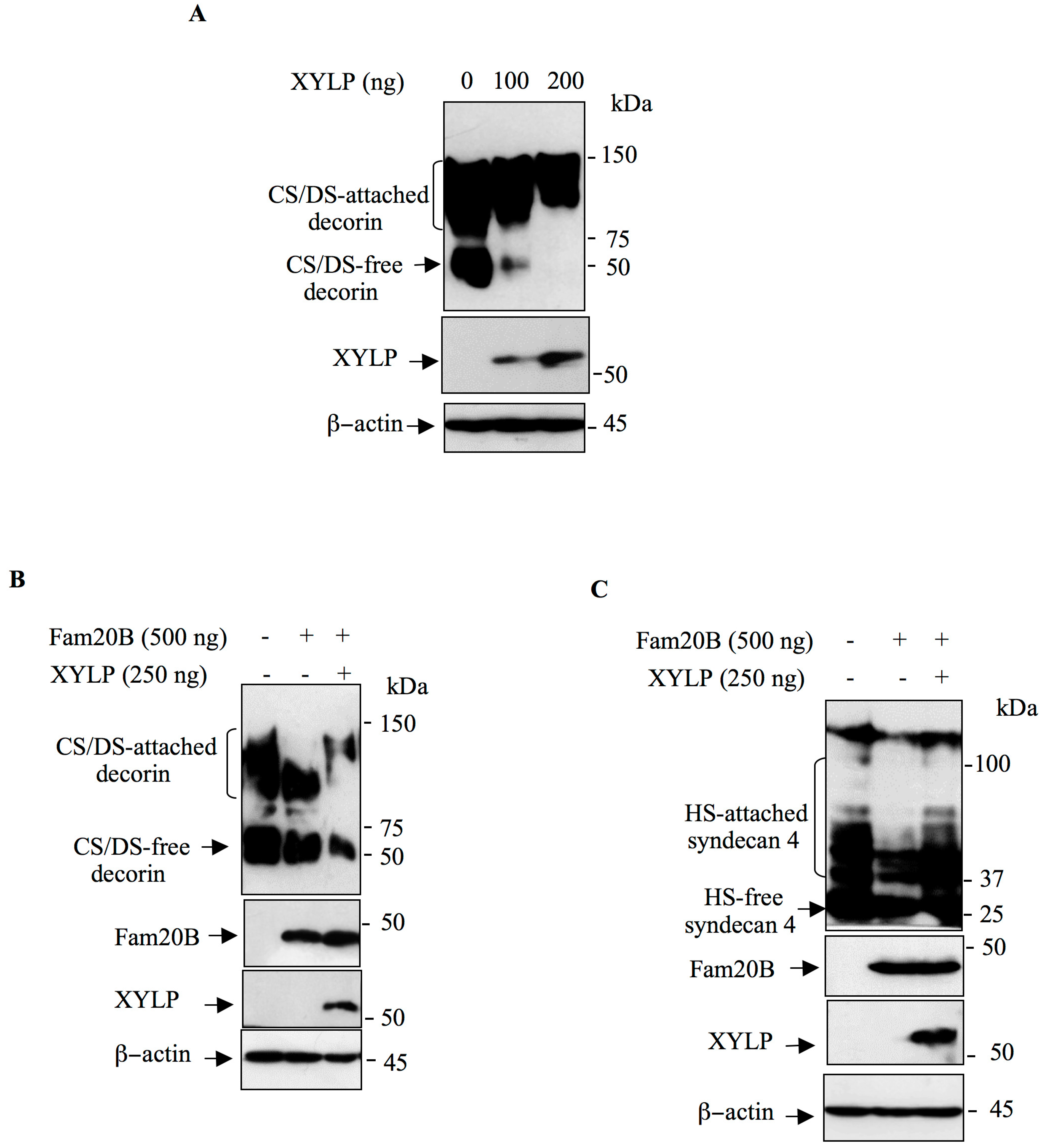
3.3. XYLP Rescues the Synthesis of GAG-Attached PG Blockage Induced by FAM20B
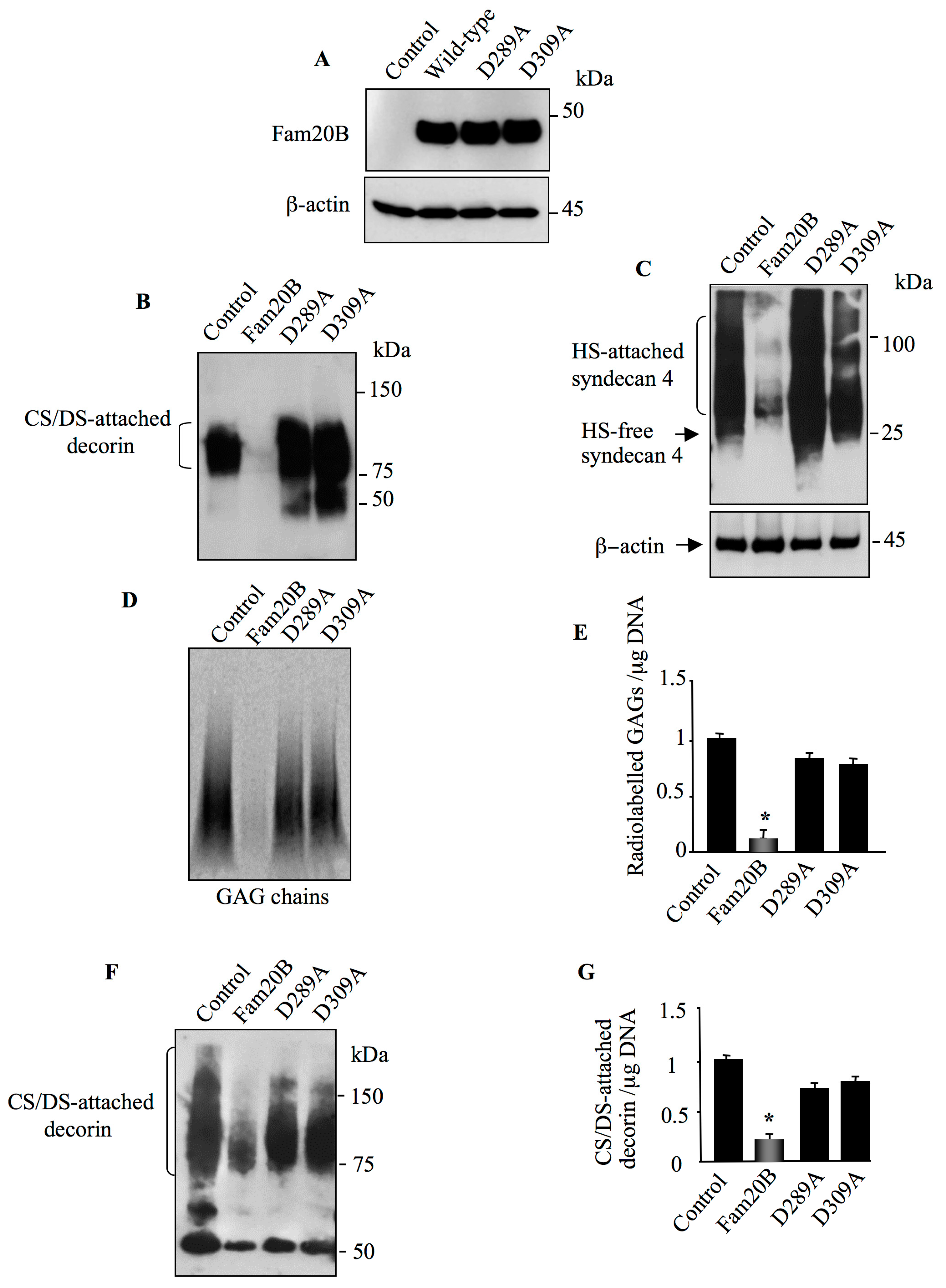
3.4. Aspartic Acid Residues in Catalytic Domain and DFG Motif Are Essential for FAM20B Activity
3.5. Knockout of FAM20B Reduced the Synthesis of Both CS and HSPGs
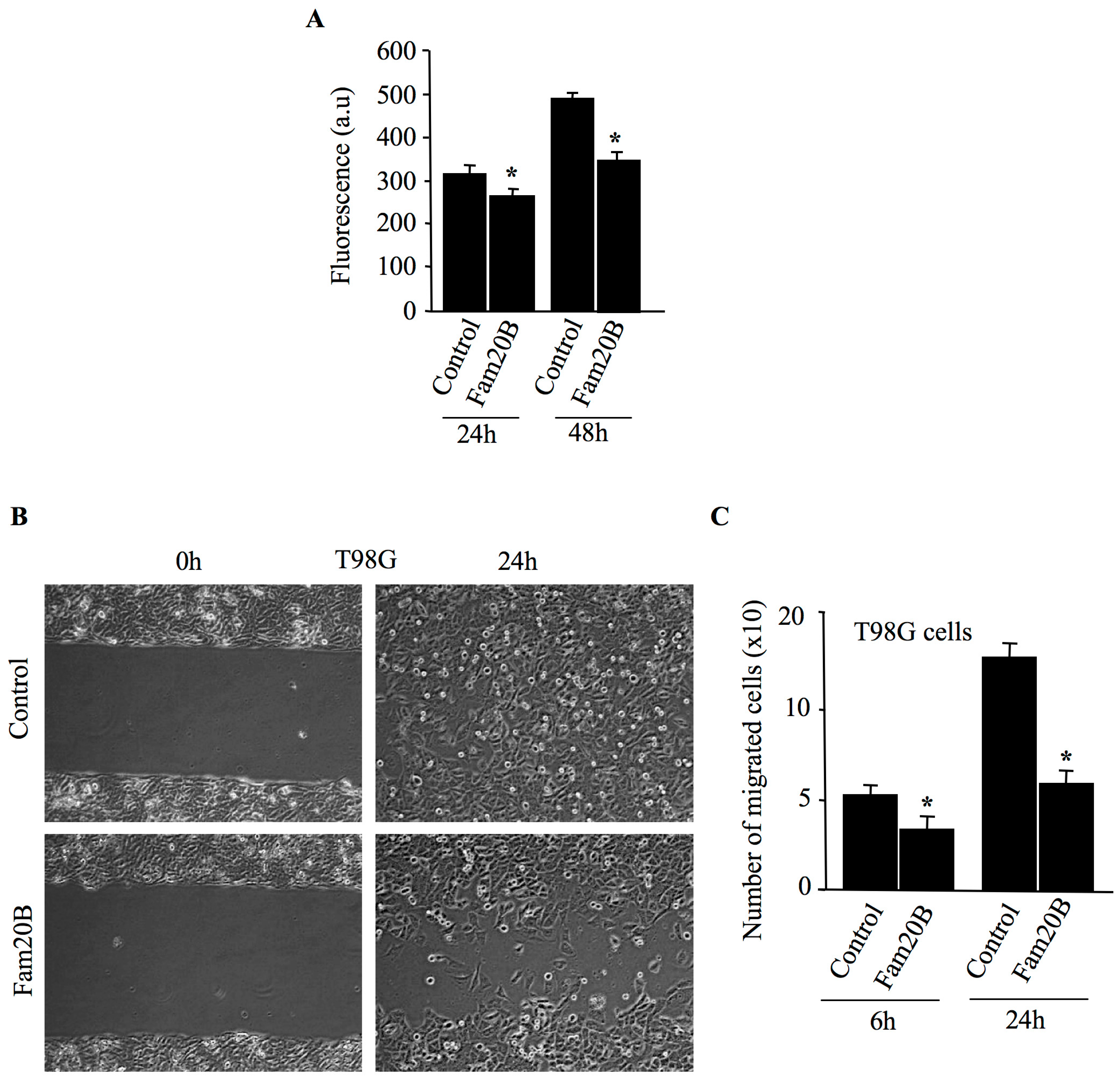
3.6. FAM20B Inhibits Proliferation and Migration of Glioblastoma Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perrimon, N.; Bernfield, M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature 2000, 404, 725–728. [Google Scholar] [CrossRef]
- Knudson, C.B.; Nofal, G.A.; Pamintuan, L.; Aguiar, D.J. The chondrocyte pericellular matrix: A model for hyaluronan-mediated cell-matrix interactions. Biochem. Soc. Trans. 1999, 27, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Shu, C.; Whitelock, J.M.; Lord, M.S. The cartilage extracellular matrix as a transient developmental scaffold for growth plate maturation. Matrix Biol. 2016, 52–54, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Couchman, J.R. Transmembrane signaling proteoglycans. Annu. Rev. Cell Dev. Biol. 2010, 26, 89–114. [Google Scholar] [CrossRef]
- Manon-Jensen, T.; Itoh, Y.; Couchman, J.R. Proteoglycans in health and disease: The multiple roles of syndecan shedding. FEBS J. 2010, 277, 3876–3889. [Google Scholar] [CrossRef]
- Li, L.; Ly, M.; Linhardt, R.J. Proteoglycan sequence. Mol. BioSystems 2012, 8, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Gesslbauer, B.; Theuer, M.; Schweiger, D.; Adage, T.; Kungl, A.J. New targets for glycosaminoglycans and glycosaminoglycans as novel targets. Expert Rev. Proteom. 2013, 10, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Götting, C.; Kuhn, J.; Kleesiek, K. Human xylosyltransferases in health and disease. Cell. Mol. Life Sci. 2007, 64, 1498–1517. [Google Scholar] [CrossRef]
- Wilson, I.B.H. The never-ending story of peptide O-xylosyltransferase. Cell. Mol. Life Sci. CMLS 2004, 61, 794–809. [Google Scholar] [CrossRef]
- Venkatesan, N.; Barré, L.; Magdalou, J.; Mainard, D.; Netter, P.; Fournel-Gigleux, S.; Ouzzine, M. Modulation of xylosyltransferase i expression provides a mechanism regulating glycosaminoglycan chain synthesis during cartilage destruction and repair. FASEB J. 2009, 23, 813–822. [Google Scholar] [CrossRef]
- Breton, C.; Fournel-Gigleux, S.; Palcic, M.M. Recent structures, evolution and mechanisms of glycosyltransferases. Curr. Opin. Struct. Biol. 2012, 22, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Habuchi, H.; Habuchi, O.; Kimata, K. Sulfation pattern in glycosaminoglycan: Does it have a code? Glycoconj. J. 2004, 21, 47–52. [Google Scholar] [CrossRef]
- Gulberti, S.; Lattard, V.; Fondeur, M.; Jacquinet, J.C.; Mulliert, G.; Netter, P.; Magdalou, J.; Ouzzine, M.; Fournel-Gigleux, S. Phosphorylation and sulfation of oligosaccharide substrates critically influence the activity of human β1,4-galactosyltransferase 7 (GalT-I) and β1,3-glucuronosyltransferase I (GlcAT-I) involved in the biosynthesis of the glycosaminoglycan-protein linkage region of proteoglycans. J. Biol. Chem. 2005, 280, 1417–1425. [Google Scholar]
- Gulberti, S.; Lattard, V.; Fondeur, M.; Jacquinet, J.C.; Mulliert, G.; Netter, P.; Magdalou, J.; Ouzzine, M.; Fournel-Gigleux, S. Modifications of the glycosaminoglycan-linkage region of proteoglycans: Phosphorylation and sulfation determine the activity of the human beta1,4-galactosyltransferase 7 and beta1,3-glucuronosyltransferase I. Sci. World J. Electron. Resour. 2005, 5, 510–514. [Google Scholar] [CrossRef]
- Nalbant, D.; Youn, H.; Nalbant, S.I.; Sharma, S.; Cobos, E.; Beale, E.G.; Du, Y.; Williams, S.C. FAM20: An evolutionarily conserved family of secreted proteins expressed in hematopoietic cells. BMC Genom. 2005, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Tagliabracci, V.S.; Xiao, J.; Dixon, J.E. Phosphorylation of substrates destined for secretion by the Fam20 kinases. Biochem. Soc. Trans. 2013, 41, 1061–1065. [Google Scholar] [CrossRef]
- Vogel, P.; Hansen, G.; Read, R.; Vance, R.; Thiel, M.; Liu, J.; Wronski, T.; Smith, D.; Jeter-Jones, S.; Brommage, R. Amelogenesis imperfecta and other biomineralization defects in Fam20a and Fam20c null mice. Vet. Pathol. Online 2012, 49, 998–1017. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Seymen, F.; Lee, K.E.; Lee, S.K.; Kweon, Y.S.; Kim, K.J.; Jung, S.E.; Song, S.J.; Yildirim, M.; Bayram, M. Novel FAM20A mutations in hypoplastic amelogenesis imperfecta. Hum. Mutat. 2012, 33, 91–94. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Bitu, C.C.; Daly, S.B.; Urquhart, J.E.; Barron, M.J.; Bhaskar, S.S.; Martelli-Junior, H.; dos Santos Neto, P.E.; Mansilla, M.A.; Murray, J.C.; et al. Whole-Exome sequencing identifies FAM20A mutations as a cause of amelogenesis imperfecta and gingival hyperplasia syndrome. Am. J. Hum. Genet. 2011, 88, 616–620. [Google Scholar] [CrossRef]
- Jaureguiberry, G.; Parry, D.; Quentric, M.; Himmerkus, N.; Koike, T.; Poulter, J.; Klootwijk, E.; Robinette, S.; Howie, A.; Patel, V. Nephrocalcinosis (Enamel Renal Syndrome) Caused by Autosomal Recessive FAM20A Mutations. Nephron Physiol. 2013, 122, 1–6. [Google Scholar] [CrossRef]
- Tagliabracci, V.S.; Engel, J.L.; Wen, J.; Wiley, S.E.; Worby, C.A.; Kinch, L.N.; Xiao, J.; Grishin, N.V.; Dixon, J.E. Secreted Kinase Phosphorylates Extracellular Proteins That Regulate Biomineralization. Science 2012, 336, 1150–1153. [Google Scholar] [CrossRef]
- Ishikawa, H.O.; Xu, A.; Ogura, E.; Manning, G.; Irvine, K.D. The Raine Syndrome Protein FAM20C Is a Golgi Kinase That Phosphorylates Bio-Mineralization Proteins. PLoS ONE 2012, 7, e42988. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Tagliabracci, V.S.; Wen, J.; Kim, S.-A.; Dixon, J.E. Crystal structure of the Golgi casein kinase. Proc. Natl. Acad. Sci. USA 2013, 110, 10574–10579. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Izumikawa, T.; Tamura, J.I.; Kitagawa, H. FAM20B is a kinase that phosphorylates xylose in the glycosaminoglycan–protein linkage region. Biochem. J. 2009, 421, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xiao, J.; Rahdar, M.; Choudhury, B.P.; Cui, J.; Taylor, G.S.; Esko, J.D.; Dixon, J.E. Xylose phosphorylation functions as a molecular switch to regulate proteoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 15723–15728. [Google Scholar] [CrossRef]
- Eames, B.F.; Yan, Y.-L.; Swartz, M.E.; Levic, D.S.; Knapik, E.W.; Postlethwait, J.H.; Kimmel, C.B. Mutations in fam20b and xylt1 Reveal That Cartilage Matrix Controls Timing of Endochondral Ossification by Inhibiting Chondrocyte Maturation. PLoS Genet. 2011, 7, e1002246. [Google Scholar] [CrossRef]
- Koike, T.; Izumikawa, T.; Sato, B.; Kitagawa, H. Identification of Phosphatase That Dephosphorylates Xylose in the Glycosaminoglycan-Protein Linkage Region of Proteoglycans. J. Biol. Chem. 2014, 289, 6695–6708. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- De Vries, B.; Van den Berg, W.; Vitters, E.; Van de Putte, L. Quantitation of glycosaminoglycan metabolism in anatomically intact articular cartilage of the mouse patella: In Vitro and in vivo studies with 35S-sulfate, 3H-glucosamine, and 3H-acetate. Rheumatol. Int. 1986, 6, 273–281. [Google Scholar] [CrossRef]
- Bronson, R.E.; Bertolami, C.N.; Siebert, E.P. Modulation of fibroblast growth and glycosaminoglycan synthesis by interleukin-1. Coll. Relat. Res. 1987, 7, 323–332. [Google Scholar] [CrossRef]
- Mani, K.; Cheng, F.; Sandgren, S.; Van Den Born, J.; Havsmark, B.; Ding, K.; Fransson, L.-Å. The heparan sulfate–specific epitope 10E4 is NO-sensitive and partly inaccessible in glypican-1. Glycobiology 2004, 14, 599–607. [Google Scholar] [CrossRef] [PubMed]
- David, G.; Bai, X.M.; Van Der Schueren, B.; Cassiman, J.-J.; Van Den Berghe, H. Developmental changes in heparan sulfate expression: In situ detection with mAbs. J. Cell Biol. 1992, 119, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Leteux, C.; Chai, W.; Nagai, K.; Herbert, C.G.; Lawson, A.M.; Feizi, T. 10E4 antigen of scrapie lesions contains an unusual nonsulfated heparan motif. J. Biol. Chem. 2001, 276, 12539–12545. [Google Scholar] [CrossRef]
- Moses, J.; Oldberg, A.; Cheng, F.; Fransson, L.A. Biosynthesis of the proteoglycan decorin--transient 2-phosphorylation of xylose during formation of the trisaccharide linkage region. Eur. J. Biochem. 1997, 248, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Moses, J.; Oldberg, A.; Fransson, L.A. Initiation of galactosaminoglycan biosynthesis. Separate galactosylation and dephosphorylation pathways for phosphoxylosylated decorin protein and exogenous xyloside. Eur. J. Biochem. 1999, 260, 879–884. [Google Scholar] [CrossRef]
- Siegbahn, A.; Manner, S.; Persson, A.; Tykesson, E.; Holmqvist, K.; Ochocinska, A.; Ronnols, J.; Sundin, A.; Mani, K.; Westergren-Thorsson, G.; et al. Rules for priming and inhibition of glycosaminoglycan biosynthesis; probing the [small beta]4GalT7 active site. Chem. Sci. 2014, 5, 3501–3508. [Google Scholar] [CrossRef]
- Siegbahn, A.; Aili, U.; Ochocinska, A.; Olofsson, M.; Ronnols, J.; Mani, K.; Widmalm, G.; Ellervik, U. Synthesis, conformation and biology of naphthoxylosides. Bioorg. Med. Chem. 2011, 19, 4114–4126. [Google Scholar] [CrossRef]
- Tsutsui, Y.; Ramakrishnan, B.; Qasba, P.K. Crystal Structures of β-1,4-Galactosyltransferase 7 Enzyme Reveal Conformational Changes and Substrate Binding. J. Biol. Chem. 2013, 288, 31963–31970. [Google Scholar] [CrossRef]
- Mythreye, K.; Blobe, G.C. Proteoglycan signaling co-receptors: Roles in cell adhesion, migration and invasion. Cell Signal. 2009, 21, 1548–1558. [Google Scholar] [CrossRef]
- Afratis, N.; Gialeli, C.; Nikitovic, D.; Tsegenidis, T.; Karousou, E.; Theocharis, A.D.; Pavao, M.S.; Tzanakakis, G.N.; Karamanos, N.K. Glycosaminoglycans: Key players in cancer cell biology and treatment. FEBS J. 2012, 279, 1177–1197. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barré, L.; Shaukat, I.; Ouzzine, M. FAM20B Gain-of-Function Blocks the Synthesis of Glycosaminoglycan Chains of Proteoglycans and Inhibits Proliferation and Migration of Glioblastoma Cells. Cells 2025, 14, 712. https://doi.org/10.3390/cells14100712
Barré L, Shaukat I, Ouzzine M. FAM20B Gain-of-Function Blocks the Synthesis of Glycosaminoglycan Chains of Proteoglycans and Inhibits Proliferation and Migration of Glioblastoma Cells. Cells. 2025; 14(10):712. https://doi.org/10.3390/cells14100712
Chicago/Turabian StyleBarré, Lydia, Irfan Shaukat, and Mohamed Ouzzine. 2025. "FAM20B Gain-of-Function Blocks the Synthesis of Glycosaminoglycan Chains of Proteoglycans and Inhibits Proliferation and Migration of Glioblastoma Cells" Cells 14, no. 10: 712. https://doi.org/10.3390/cells14100712
APA StyleBarré, L., Shaukat, I., & Ouzzine, M. (2025). FAM20B Gain-of-Function Blocks the Synthesis of Glycosaminoglycan Chains of Proteoglycans and Inhibits Proliferation and Migration of Glioblastoma Cells. Cells, 14(10), 712. https://doi.org/10.3390/cells14100712





