Exploring Risk and Protective Factors in Parkinson’s Disease
Abstract
1. Introduction
2. Molecular Mechanisms of PD
2.1. α-Synuclein Aggregation
- Mitochondrial dysfunction occurs through direct interaction with mitochondria, impairing their function and reducing ATP production.
- Oxidative stress disrupts cellular metabolism and mitochondrial function.
- Neuroinflammation is caused by the activation of microglia, the brain’s immune cells.
- Gut dysbiosis, like misfolded α-synuclein, can potentially propagate through the gut–brain axis.
2.2. Mitochondrial Dysfunction
- Oxidative stress is caused by increased ROS production.
- Energy deficits in neurons, particularly in dopaminergic neurons in the substantia nigra, are also observed.
- Ferroptosis by enhancing iron accumulation and lipid peroxidation.
2.3. Oxidative Stress
- Mitochondrial dysfunction is both a cause and a consequence of the oxidative stress.
- Oxidative damage activates microglia and induces neuroinflammation.
- Lipid peroxidation caused by oxidative stress contributes to ferroptosis of dopaminergic neurons.
2.4. Neuroinflammation
- Promoting further α-synuclein aggregation.
- Increasing oxidative stress through the release of pro-inflammatory cytokines and ROS.
- Contributing to ferroptosis by increasing the iron burden in neurons.
2.5. Ferroptosis
- Exacerbating oxidative stress through excessive ROS production and lipid peroxidation.
- Triggering neuroinflammation through the release of damage-associated molecular patterns (DAMPs).
- Further impairing mitochondrial function due to iron accumulation and oxidative stress.
2.6. Gut Dysbiosis
- Facilitating the propagation of α-synuclein aggregates from the gut to the brain.
- Activating systemic immune responses and promoting neuroinflammation.
- Altering the production of neurotransmitters and metabolites that influence brain function.
3. Risk Factors in PD
3.1. Demographic and Genetic Factors
3.1.1. Age
3.1.2. Sex
3.1.3. Racial and Ethnic Groups
3.1.4. Family History of PD
3.1.5. Genetic Contributions
3.1.6. Gene–Environment Interactions in PD
| Category | Subcategory | Key Observations | Mechanisms | References |
|---|---|---|---|---|
| Age | Median onset at 60 years; ~5% cases under 50 | Neuronal loss in substantia nigra; increased oxidative stress; mitochondrial dysfunction; iron accumulation | [7,23,24] | |
| Sex | Twice as prevalent in men compared to women | Protective effects of estrogen; sex-specific genetic mechanisms (e.g., LRRK2 variants); differences in immune response | [27,28] | |
| Racial/ Ethnic | Higher prevalence in Caucasians and Hispanics; lower in Black and Asian populations | Genetic predispositions; ancestry-linked gene expression; environmental and socioeconomic interactions | [30,32] | |
| Gene | SNCA | Associated with early-onset PD and cognitive decline | Alpha-synuclein aggregation; Lewy body formation | [5,39] |
| LRRK2 | Most common cause of familial PD; also linked to sporadic PD | Disrupted autophagy; synaptic dysfunction; dopamine toxicity | [40,41,42,43,44] | |
| GBA1 | Found in 7–10% of PD cases; linked to familial and sporadic forms | Impaired lysosomal function; glucosylceramide buildup | [44,45,46] | |
| PARK7 (DJ-1) | Associated with early-onset and idiopathic PD | Oxidative stress defense disruption; neuroinflammation | [47,48,49] | |
| PINK1 | Linked to hereditary early-onset PD | Mitochondrial dysfunction; impaired autophagy | [50,51,52,53,54] | |
| PRKN (Parkin) | Present in 10–20% of early-onset PD cases | Lysosomal dysfunction; toxic protein aggregation | [55,56,57,58,59] | |
| UCHL1 | Associated with protein aggregation and motor symptom severity | Disruption of ubiquitin-proteasome system; alpha-synuclein buildup | [60,61,62,63] |
3.2. Education and Intelligence
| Category | Key Observations | Mechanisms | References |
|---|---|---|---|
| Education & IQ | Higher education (≥9 years) and IQ associated with increased PD risk, particularly in men | Lower cholesterol levels; reduced smoking rates; less physical activity; higher cognitive reserve | [73,80,81] |
| Lifestyle Factors | Individuals with higher education/IQ exhibit healthier lifestyles (e.g., non-smoking, lower cholesterol) | Reduced smoking may negate protective effects; lower cholesterol linked to increased PD risk | [75,76] |
| Occupational Role | Higher education correlates with less physically demanding jobs, potentially increasing PD risk | Sedentary occupations may contribute to neurodegeneration | [73] |
| Cognitive Reserve | Greater cognitive reserve may modulate PD symptoms and delay progression | Enhanced cognitive performance may obscure early PD symptoms | [81] |
3.3. Environmental and Occupational Factors
3.3.1. Exposure to Herbicides and Pesticides
3.3.2. Heavy Metal Exposure
3.3.3. Industrial Chemicals
3.3.4. Urban and Rural Living
3.3.5. Occupational and Workplace Factors
3.3.6. Socioeconomic Status
| Factor | Key Observations | Mechanisms | References |
|---|---|---|---|
| Herbicides/Pesticides | Exposure linked to PD risk, particularly among agricultural workers and rural residents | Oxidative stress; mitochondrial dysfunction; genetic alterations | [84] |
| Heavy Metals | Exposure to mercury, manganese, lead increases PD risk | Disruption of redox homeostasis; alpha-synuclein aggregation; neuroinflammation; microbiome disruption | [86,87] |
| Industrial Chemicals | Solvents like trichloroethylene associated with PD | Impair autophagy, lysosomal function, and mitochondrial integrity | [101] |
| Urban Living | Air pollution contributes to PD risk | Neurotoxicity; systemic inflammation; oxidative stress | [92,93] |
| Rural Living | Increased risk through unwell-water consumption, soil pathogens, head injuries | Environmental exposures; infections; lifestyle factors | [59,60,61] |
| Occupational Roles | Agriculture, mining, and industries involving solvents and heavy metals linked to PD risk | Environmental toxins; genetic susceptibility; neurotoxic exposures | [73,88,99,100] |
| Socioeconomic Status | Lower SES correlates with higher PD prevalence | Limited healthcare access; higher exposure to environmental risk factors; protective lifestyle factors in higher SES groups | [103,104] |
3.4. Lifestyle Factors
3.4.1. Body Mass Index (BMI)
3.4.2. Alcohol Consumption
3.4.3. Dietary Habits and Nutrition
3.4.4. Sedentary Lifestyle
| Category | Key Observations | Mechanisms | References |
|---|---|---|---|
| Body Mass Index | Conflicting evidence on association; unintentional weight loss common in PD | Abdominal obesity may elevate risk; weight loss correlates with poor prognosis | [73,80,81,82,83] |
| Dairy Consumption | Linked to a higher risk of PD, particularly in men | Decreases serum urate levels; potential contaminants like pesticides | [70,71,72,73] |
| Alcohol Consumption | Mixed evidence: moderate consumption may reduce PD risk; heavy use may exacerbate progression | Dopamine release (acute use); prolonged use depletes dopamine; interferes with PD medication; impacts brain structure/connectivity | [75,76,77,78,79] |
| Sedentary Lifestyle | Associated with worse non-motor symptoms (e.g., cognitive decline, depression) | Dysregulated genes linked to cellular pathways; overlaps with molecular pathways influencing PD progression | [105,121] |
3.5. Metabolic and Systemic Factors
| Category | Key Observations | Mechanisms | References |
|---|---|---|---|
| Diabetes | T2DM linked to more severe motor/non-motor symptoms and accelerated PD progression | Neurovascular burden; white matter hyperintensities; cognitive decline | [84,85,86,87,88,89,90,91,92,93,94] |
| Hypertension | May elevate PD risk and worsen progression | Hypertensive vasculopathy; cerebral small vessel disease; white matter hyperintensities | [95,96,97,98,99,100] |
| Cholesterol/Triglycerides | Lower levels associated with higher PD risk; high LDL-C and total cholesterol linked to slower progression | Oxidative stress; alpha-synuclein aggregation; protective effects of LDL-C | [101,102,103,104,105,106,107,108,109,110] |
3.5.1. Diabetes
3.5.2. Hypertension
3.5.3. Cholesterol and Triglycerides
3.6. Inflammation, Immunity, and the Gut–Brain Axis
3.6.1. Viral Infections
3.6.2. Neuroinflammation and Chronic Inflammatory Diseases
3.6.3. Gut Dysbiosis
3.6.4. Chronic Constipation
| Category | Key Observations | Mechanisms | References |
|---|---|---|---|
| Viral Infections | Linked to PD through chronic neuroinflammation and immune dysregulation | Activation of microglia, TLRs; alpha-synuclein aggregation; immune responses to viral proteins | [146,149] |
| Neuroinflammation | Contributes to PD onset and progression | Elevated cytokines (e.g., TNF-α, IL-6); microglial activation; oxidative stress | [151,160] |
| Chronic Inflammatory Diseases | Associated with heightened PD risk and progression | Persistent cytokine production; gut–brain axis inflammation; genetic predispositions | [160,161,162,163] |
| Gut–Brain Axis | Bidirectional link between gastrointestinal inflammation and neurodegeneration in PD | Shared genetic factors; inflammatory markers in the gut | [153,154] |
| Gut Dysbiosis | Linked to neuroinflammation and alpha-synuclein misfolding; promotes PD progression. | Pro-inflammatory cytokines; toll-like receptor dysregulation; disrupted gut–brain communication. | [154,155,157] |
| Chronic Constipation | Common preclinical symptom of PD; linked to gut dysbiosis and intestinal permeability. | Alpha-synuclein misfolding in the gut propagating via the vagus nerve to the brain. | [156,158,164] |
3.7. Neurological and Psychological Factors
3.7.1. Traumatic Brain Injury
3.7.2. Sleep Disorders
3.7.3. Loss of Smell (Anosmia)
3.7.4. Chronic Stress
3.7.5. Depression and Anxiety
| Category | Key Observations | Mechanisms | References |
|---|---|---|---|
| Traumatic Brain Injury | Increases PD risk by 56%; severity dependent. | Inflammation, protein accumulation (APP, alpha-synuclein, etc.), microglial activation. | [165,166] |
| Sleep Disorders | REM sleep behavior disorder often precedes motor symptoms by years. | Degeneration of brainstem nuclei; alpha-synuclein accumulation forming Lewy bodies. | [167,168] |
| Loss of Smell (Anosmia) | Early non-motor symptom, precedes motor signs. | Alpha-synuclein accumulation in olfactory pathways. | [169,170] |
| Chronic Stress | Increases neuronal vulnerability; exacerbates motor and non-motor symptoms. | Disrupts dopaminergic pathways; increases pro-inflammatory cytokines; accelerates cell death. | [171,174] |
| Depression and Anxiety | Common non-motor symptoms; may precede motor symptom onset. | Activates HPA axis and SNS; increases glucocorticoids and catecholamines; induces neuroinflammation. | [172,178] |
3.8. Hormonal and Other Health Conditions
3.8.1. Estrogen and Its Neuroprotective Role
3.8.2. Postmenopausal Women and PD Risk
3.8.3. Hormone Replacement Therapy
3.8.4. Hyperuricemia
| Category | Key Observations | Mechanisms | References |
|---|---|---|---|
| Estrogen Levels | May protect dopaminergic neurons; reduced levels post-menopause linked to higher PD risk. | Modulates dopamine pathways; reduces inflammation and oxidative stress. | [179,180,181] |
| Postmenopausal Women | Higher PD risk linked to early menopause, shorter fertile life, and lack of HRT. | Reduced lifetime estrogen exposure affects neuroprotection. | [180,181] |
| HRT | Mixed effects: long-term combined estrogen-progesterone therapy may increase risk. | Duration- and regimen-specific impact on estrogen’s neuroprotective role. | [179,182] |
| Hyperuricemia | Paradoxical effects: antioxidant properties may protect but linked to cognitive decline and gout. | Reduces oxidative stress but compounds neurodegeneration with co-existing conditions. | [185,186] |
3.9. Medications
3.9.1. β2-Adrenoceptor Antagonists (e.g., Propranolol)
3.9.2. Diabetes Medications
3.9.3. Antibiotics
3.9.4. Psychological Medications
3.9.5. Cancer Medications
3.9.6. Epigenetic Modifications
| Factor | Key Observations | Mechanisms | References |
|---|---|---|---|
| β2-Adrenoceptor Antagonists | Linked to increased PD risk, particularly with chronic use (e.g., propranolol). | Disrupts dopaminergic activity and metabolic functions; accelerates Lewy body formation. | [189] |
| Diabetes Medications | Mixed effects: metformin may increase risk, while GLP1a treatments may be protective. | Insulin dysregulation; mitochondrial dysfunction; neuroinflammation. | [190,191] |
| Antibiotics | Macrolides and lincosamides associated with higher PD risk. | Disrupt gut microbiota; exacerbate neuroinflammation and α-synuclein aggregation. | [70,192] |
| Psychotropic Medications | Increased PD risk observed in older populations using antidepressants and anxiolytics. | Blocks dopamine receptors, affecting dopaminergic systems. | [193] |
| Cancer Medications | Potential increase in PD risk with certain chemotherapy agents. | Induces oxidative stress and mitochondrial dysfunction; affects the immune system. | [194] |
| Epigenetic Modifications | Long-term medication use may influence PD through altered DNA methylation. | Changes in epigenetic marks affecting gene expression. | [49,195] |
3.10. Comorbid Conditions
4. Prevent Factors in PD
4.1. Lifestyle Factors
4.1.1. Physical Activity
4.1.2. High-Quality Sleep
4.1.3. Social Support
4.1.4. Cognitive Engagement
4.1.5. Smoking
| Factor | Key Observations | Mechanisms | References |
|---|---|---|---|
| Physical Activity | Reduces PD incidence by up to 21%, delays onset, and improves symptoms. | Enhances neuroprotection, reduces inflammation, and improves gait and quality of life. | [196,198,199] |
| High-Quality Sleep | Reduces PD risk by promoting protein clearance and dopamine regulation. | Clears toxic proteins, reduces neuroinflammation, and supports brain health. | [201,202,203,219] |
| Social Support | Reduces PD risk, supports mental health, and encourages physical activity. | Reduces stress, improves motor/cognitive functions, and supports disease management. | [204,205,206] |
| Cognitive Engagement | Mental activities reduce PD risk and cognitive decline by enhancing neuroplasticity. | Boosts brain function, maintains neurotransmitters, and supports mitochondrial health. | [208,209,210,211] |
| Smoking | Smokers are less likely to develop PD, but it worsens non-motor symptoms and other health risks. | Nicotine may trigger dopamine release and reduce alpha-synuclein aggregation, but increases other health risks. | [79,214,215,217,218,220] |
4.2. Dietary and Nutritional Factors
4.2.1. Coffee
4.2.2. Tea
4.2.3. Diet Habits
4.2.4. Vitamins
4.2.5. Calcium
4.2.6. Gut–Brain Axis
4.2.7. Uric Acid (Urate Levels)
| Factor | Key Observations | Mechanisms | References |
|---|---|---|---|
| Coffee | Coffee may reduce PD risk by ~30% and slow progression. Inconsistent effects on motor/non-motor symptoms in PD patients. | Caffeine acts as an adenosine A2A receptor antagonist, reducing α-synuclein aggregation and inflammation. | [220,221,224,227] |
| Tea | Tea consumption is linked to a 26% reduction in PD risk. Black tea may be more protective than green tea. | Polyphenols (catechins, theaflavins) reduce oxidative stress, modulate signaling, and chelate metals. | [228,229,231,232] |
| Nutrition and Diet | A Mediterranean diet (fruits, vegetables, fish) is linked to a reduced PD risk and slower progression. | Anti-inflammatory and antioxidant effects help mitigate oxidative stress and neuroinflammation. | [234,235] |
| Vitamins | Vitamin D deficiency increases PD risk; vitamin E, B12, B6, and folate may help protect against PD and reduce disease progression. | Vitamins support neuronal function, reduce oxidative stress, and lower homocysteine, which is linked to neurodegeneration. | [186,234,241] |
| Calcium | The role of calcium in PD is unclear, but it is crucial for neuronal processes. Dysregulation may contribute to neuronal death. | Maintaining calcium homeostasis supports neurotransmitter release and neuronal health. | [241] |
| Gut–Brain Axis | A healthy gut microbiome may influence PD risk and progression. Diets rich in fiber and probiotics may promote brain health. | Improves gut health, reducing neuroinflammation and supporting brain health through the gut–brain connection. | [242] |
| Uric Acid (UA) | Higher uric acid levels may reduce PD risk by 6% for every 1 mg/dL increase. UA acts as an antioxidant, protecting against oxidative stress. | UA scavenges free radicals, protects mitochondrial integrity, and modulates inflammation, supporting neuronal function. | [185,244,246] |
4.3. Medications and Medical Factors
4.3.1. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
4.3.2. Calcium Channel Blockers (CCBs)
4.3.3. Statins
4.3.4. α1-Adrenergic Antagonists
| Factor | Key Observations | Mechanisms | References |
|---|---|---|---|
| Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) | Aspirin and ibuprofen may reduce PD risk and postpone the age of onset by as much as 5 years. Regular use is linked to a reduced risk of PD. | Inhibit COX-2 enzymes, reduce pro-inflammatory prostaglandins, decrease elevated cytokines like TNF-α and IL-1β, potentially mitigate neuroinflammation. Aspirin boosts dopamine production. | [247,248,251,252] |
| Calcium Channel Blockers (CCBs) | CCBs, like isradipine, may reduce PD risk but have mixed results in slowing progression. | Modulate calcium ion flows to regulate neurotransmitter release, reduce mitochondrial oxidant stress, and protect dopaminergic neurons. | [253,254,255,256] |
| Statins | Statins, particularly simvastatin, may slow PD progression by attenuating α-synuclein aggregation and reducing neuroinflammation. | Inhibit pro-inflammatory molecules, reduce oxidative stress, and regulate mitochondrial function via PPARα. | [257,258] |
| α1-Adrenergic Antagonists | Terazosin, doxazosin, and alfuzosin show reduced PD risk due to enhanced glycolysis and energy metabolism. | Activate PGK1 enzyme in glycolysis to increase ATP production, modulate the locus coeruleus to influence dopamine release and provide neuroprotection. | [259] |
4.4. Occupational Factors
| Factor | Key Observations | Mechanisms | References |
|---|---|---|---|
| Physical Activity in Occupations | Jobs involving physical exertion (e.g., engineering, production, construction, and metalworking) reduce PD risk. | Physical activity promotes neuroprotective benefits similar to structured exercise, improving mitochondrial function, reducing oxidative stress, and enhancing neuroplasticity. | [260] |
| Artistic Occupations | Engagement in artistic professions (e.g., visual arts, music, writing) correlates with lower PD risk. | Artistic occupations may preserve dopaminergic activity, as creativity requires dopaminergic function, offering neuroprotective effects through sustained brain function. | [261] |
| Professional/Technical Occupations | Occupations requiring cognitive engagement and technical skills (e.g., engineering, technical professions) show an inverse relationship with PD risk. | High cognitive engagement may promote neuroplasticity, maintaining neural networks and brain health, which protects against neurodegenerative diseases. | [262] |
| Farming Activities | Certain farming activities (e.g., gardening, landscaping, horse-related tasks) have been linked to reduced PD risk. | Physical activity in farming, along with cognitive engagement, may support neuronal resilience, reduce neuroinflammation, and promote neurotrophic factor production like BDNF, which supports neuronal survival. | [263] |
4.5. Therapeutic Implications
5. Challenges and Solutions in Identifying Risk and Protective Factors for PD
| Challenge | Details | Proposed Solutions | References |
|---|---|---|---|
| Complex Interplay of Genetic and Environmental Factors | Genetic predispositions interact with environmental exposures, complicating risk factor identification. Over 200 genes are implicated, with rare variants posing replication challenges. | -Integrate multi-omics data (genomics, proteomics, etc.) using AI to uncover patterns. -Identify specific biomarkers through advanced techniques. | [31,263,264,265] |
| Heterogeneity of PD | PD encompasses multiple subtypes with distinct molecular mechanisms. Variability in progression complicates universal findings. | Employ personalized approaches and population-specific studies. -Account for subtype-specific variability in genetic and environmental influences. | [31,265] |
| Methodological Challenges | Cross-sectional designs fail to capture temporal relationships. Confounding factors like lifestyle and recall bias obscure results. | Conduct longitudinal studies to establish causality. -Standardize protocols for clinical assessments and exposure reporting. Integrate digital tools and telehealth for continuous monitoring. | [15,266] |
| Inconsistencies Across Studies | Variations in study designs, sample sizes, and methodologies lead to contradictory findings. Examples include mixed results for smoking and coffee as protective factors. | Standardize methodologies across studies. -Use collaborative international frameworks for uniformity in research approaches and data collection. | [266] |
| Evolving Research Methodologies | Advanced techniques like Mendelian Randomization and polygenic risk scores require validation and standardization. | Validate emerging methods through large-scale studies. Develop global standards to ensure applicability across populations. | [265] |
| Lack of Comprehensive Data | Current studies focus on isolated factors, limiting holistic understanding. | Create large-scale collaborative initiatives and centers of excellence for data integration. -Leverage blockchain and digital twins for secure, holistic, and personalized research. | [266,267] |
| Technological Advancements | New tools like next-generation sequencing (NGS) and digital twins offer potential but need broader adoption. | Adopt NGS for genetic analysis and medical digital twins for personalized simulation [267]. Use blockchain for secure data management and sharing. |
6. Integrating Risk Factors into Biomarker Discovery
6.1. Age-Related Molecular Biomarkers
6.2. Sex-Specific Biological Signatures
6.3. Metabolic and Lifestyle-Derived Biomarkers
6.4. Environmental Exposure and Toxicological Biomarkers
6.5. Metabolic Dysregulation and Endocrine Markers
6.6. Neuroinflammation and the Gut–Brain Axis
6.7. Pharmacogenomics and Epigenetic Modifications
6.8. Population-Specific Genetic Risk Biomarkers
7. Raising Awareness in PD
7.1. Demographic and Genetic Factors
- Age: Aging is a major risk factor for PD, especially among individuals older than 60 years of age. Regular screenings (e.g., cognitive assessments and dopamine transporter scans) for neurodegenerative markers are crucial for early detection. Healthy individuals older than 60 years of age should consider these preventive measures. Patients with PD should undergo regular check-ups to monitor disease progression. Health systems should prioritize neurodegenerative screening in older adults for early intervention.
- Sex: Men are at a higher risk for developing PD. Public health campaigns should target men, focusing on both motor and non-motor symptoms. Healthy men should engage in regular screenings, while those diagnosed with PD require tailored care for gender-specific symptoms.
- Race and Ethnicity: Certain racial and ethnic groups have a higher PD risk. Targeted health programs and screenings can improve early diagnosis and access to treatment. High-risk populations should participate in community-based screenings, and health systems should implement targeted interventions.
- Family History and Genetics: A family history of PD increases the risk. Genetic counseling and predictive testing (e.g., LRRK2 mutations) can help monitor and personalize preventive care. Genetically predisposed individuals may benefit from targeted therapies like LRRK2 inhibitors. Health systems should provide genetic counseling and predictive testing for at-risk families.
7.2. Cognitive and Educational Factors
7.3. Environmental and Occupational Risks
- Toxins: Exposure to pesticides, heavy metals, and industrial chemicals increases the risk of PD. Workers should wear protective gear and seek safer alternatives to reduce exposure. Regular health check-ups and screenings are vital for individuals exposed to toxins. Health systems should mandate safety regulations and offer regular screening for at-risk workers.
- Air Pollution: Living in high-pollution areas increases the risk. Urban residents should use air purifiers and wear masks. Public health initiatives should emphasize the connection between air pollution and PD risk and advocate cleaner air policies.
- Socioeconomic Disparities: Limited healthcare access increases the risk of PD in underserved populations. Expanding preventive screening and improving access to care in these communities are essential. Health systems should provide affordable screening and treatment for underserved populations.
7.4. Lifestyle and Health Habits
- Nutrition and Diet: A diet rich in antioxidants, healthy fats, and anti-inflammatory nutrients (e.g., the Mediterranean diet) supports brain health. Patients with PD should focus on neuroprotective foods (e.g., omega-3s and vitamin E). Health systems should encourage nutritional counseling for individuals at risk and those with PD.
- Physical Activity: Regular exercise, especially aerobic activities and balance-enhancing exercises, can reduce the risk of PD. Patients with PD should follow personalized exercise plans to manage their symptoms. Health systems should promote physical activity and integrate physical therapy into PD management.
- Alcohol Consumption: Excessive alcohol intake may contribute to neurotoxicity. Healthy individuals should limit their alcohol intake to protect their brain health. Patients with PD should monitor their alcohol consumption to avoid interactions with medications. Health systems should promote healthy drinking habits and provide resources for reducing alcohol consumption.
- Body Weight and Metabolic Health: Maintaining a healthy weight through diet and exercise reduces the risk of neurodegeneration. Patients with PD should monitor their BMI and metabolic health to prevent disease progression. Health systems should prioritize metabolic health screening in at-risk individuals and patients with PD.
7.5. Metabolic and Systemic Health Conditions
- Diabetes and Hypertension: Managing blood sugar and blood pressure can reduce the risk of PD. Patients with PD should closely monitor these conditions, as they may exacerbate neurological decline. Healthcare providers should emphasize the management of diabetes and hypertension in PD care.
- Cholesterol and Triglycerides: Maintaining balanced lipid levels through diet and medication can reduce inflammation and protect brain health. Patients with PD should regularly monitor their lipid levels. Health systems should incorporate lipid screening into PD management and preventive care.
- Metabolic Syndrome: Addressing metabolic syndrome through weight management and a healthy lifestyle reduces the risk of neurodegeneration. Patients with PD should focus on improving their diet and weight management to reduce systemic inflammation. Health systems should offer metabolic screening for at-risk populations.
7.6. Inflammatory and Immune-Related Factors
- Neuroinflammation and Chronic Diseases: Anti-inflammatory treatments (e.g., NSAIDs and curcumin) may help mitigate PD-related inflammation. Management of chronic inflammatory diseases (e.g., arthritis) can support neurological health. Vaccination can also reduce the risk of infection. Patients with PD should consider anti-inflammatory treatments and manage chronic conditions. Health systems should integrate care for chronic inflammatory diseases and promote vaccination programs.
7.7. Neurological and Psychological Factors
- Traumatic Brain Injury (TBI): High-risk occupations should enforce safety measures (e.g., helmet use and concussion monitoring) and provide annual neurocognitive assessments.
- Sleep Disorders: Screening for sleep disturbances (e.g., REM sleep behavior disorder) is important, as these are linked to increased PD risk. Treatment options like melatonin or CPAP therapy can improve sleep quality.
- Loss of Smell (Anosmia): Anosmia is an early symptom of PD. Routine smell tests during neurological assessments can help detect high-risk individuals.
- Stress, Depression, and Anxiety: Chronic stress and mental health issues can accelerate neurodegeneration in the brain. Psychological support (e.g., CBT) is essential. Early intervention for depression and anxiety can improve the quality of life and may slow PD progression.
7.8. Gut–Brain Axis and Microbiome Health
7.9. Hormonal Factors and Other Health Conditions
- Estrogen and Neuroprotection: Postmenopausal women may benefit from hormone replacement therapy (HRT) for neuroprotection. Regular hormonal evaluation should guide treatment.
- Uric Acid Levels: Managing uric acid levels through diet and hydration may help lower the risk of neurodegenerative diseases. Patients with PD should monitor their uric acid levels to reduce neurodegeneration. Health systems should offer regular evaluations of HRT and monitor uric acid levels in patients with PD.
7.10. Medications and Emerging Treatments
- Neuroprotective Drug Strategies: Emerging treatments like GLP-1 receptor agonists may reduce PD risk. Healthy individuals at risk should discuss these treatment options with their healthcare providers. Patients with PD should explore these drugs and monitor the long-term effects of medication. Health systems should support research on neuroprotective drugs and monitor the long-term effects of medication on PD care.
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Xu, G.; Liu, B.; Sun, Y.; Snetselaar, L.G.; Wallace, R.B.; Li, B.; Liao, J.; Bao, W.A.-O. Trends in Mortality from Parkinson Disease in the United States, 1999–2019. Neurology 2021, 97, e1986–e1993. [Google Scholar] [CrossRef]
- Lampropoulos, I.C.; Malli, F.; Sinani, O.; Gourgoulianis, K.I.; Xiromerisiou, G. Worldwide trends in mortality related to Parkinson’s disease in the period of 1994-2019: Analysis of vital registration data from the WHO Mortality Database. Front. Neurol. 2022, 13, 956440. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Q.; Wang, Z.; Wang, Y.; Lian, A.; Zhou, Q.; Zhao, G.; Xia, K.; Tang, B.; Li, B. Risk factors associated with age at onset of Parkinson’s disease in the UK Biobank. npj Park. Dis. 2024, 10, 3. [Google Scholar] [CrossRef]
- Pankratz, N.; Foroud, T. Genetics of Parkinson disease. Genet. Med. 2007, 9, 801–811. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Scelzo, E.; Beghi, E.; Rosa, M.; Angrisano, S.; Antonini, A.; Bagella, C.; Bianchi, E.; Caputo, E.; Lena, F.; Lopiano, L.; et al. Deep brain stimulation in Parkinson’s disease: A multicentric, long-term, observational pilot study. J. Neurol. Sci. 2019, 405, 116411. [Google Scholar] [CrossRef] [PubMed]
- Tsalenchuk, M.; Gentleman, S.M.; Marzi, S.J. Linking environmental risk factors with epigenetic mechanisms in Parkinson’s disease. npj Park. Dis. 2023, 9, 123. [Google Scholar] [CrossRef]
- Gabbert, C.; König, I.R.; Lüth, T.; Kasten, M.; Grünewald, A.; Klein, C.; Trinh, J. Lifestyle factors and clinical severity of Parkinson’s disease. Sci. Rep. 2023, 13, 9537. [Google Scholar] [CrossRef]
- Wu, L.; Chu, L.; Pang, Y.; Huo, J.; Cao, H.; Tian, Q.; Gao, Q. Effects of dietary supplements, foods, and dietary patterns in Parkinson’s disease: Meta-analysis and systematic review of randomized and crossover studies. Eur. J. Clin. Nutr. 2024, 78, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Shih, L.-C.; Lin, R.-J.; Chen, Y.-L.; Fu, S.-C. Unravelling the mechanisms of underweight in Parkinson’s disease by investigating into the role of gut microbiome. npj Park. Dis. 2024, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Zhang, K.; Paul, K.C.; Folle, A.D.; Del Rosario, I.; Jacobs, J.P.; Keener, A.M.; Bronstein, J.M.; Ritz, B. Diet and the gut microbiome in patients with Parkinson’s disease. npj Park. Dis. 2024, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Grotewold, N.; Albin, R.L. Update: Protective and risk factors for Parkinson disease. Park. Relat. Disord. 2024, 125, 107026. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The epidemiology of Parkinson’s disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef]
- Peña-Díaz, S.; García-Pardo, J.; Ventura, S. Development of Small Molecules Targeting α-Synuclein Aggregation: A Promising Strategy to Treat Parkinson’s Disease. Pharmaceutics 2023, 15, 839. [Google Scholar] [CrossRef]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef]
- Calabresi, P.; Di Lazzaro, G.; Marino, G.; Campanelli, F.; Ghiglieri, V. Advances in understanding the function of alpha-synuclein: Implications for Parkinson’s disease. Brain 2023, 146, 3587–3597. [Google Scholar] [CrossRef]
- Lee, J.; Sung, K.W.; Bae, E.J.; Yoon, D.; Kim, D.; Lee, J.S.; Park, D.H.; Park, D.Y.; Mun, S.R.; Kwon, S.C.; et al. Targeted degradation of α-synuclein aggregates in Parkinson’s disease using the AUTOTAC technology. Mol. Neurodegener. 2023, 18, 41. [Google Scholar] [CrossRef]
- Henrich, M.T.; Oertel, W.H.; Surmeier, D.J.; Geibl, F.F. Mitochondrial dysfunction in Parkinson’s disease—A key disease hallmark with therapeutic potential. Mol. Neurodegener. 2023, 18, 83. [Google Scholar] [CrossRef]
- Eriksen, J.L.; Wszolek, Z.; Petrucelli, L. Molecular pathogenesis of Parkinson disease. Arch. Neurol. 2005, 62, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Li, Y.; Zhou, Y.; Zhang, Y.; Shi, Y.; Zhang, C.; Bai, Y.; Wang, S. Neuroinflammation in Parkinson’s disease: Focus on the relationship between miRNAs and microglia. Front. Cell Neurosci. 2024, 18, 1429977. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Shulman, J.M.; Nag, S.; Leurgans, S.E.; Arnold, S.E.; Morris, M.C.; Schneider, J.A.; Bennett, D.A. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann. Neurol. 2012, 71, 258–266. [Google Scholar] [CrossRef]
- Friedman, A.; Galazka-Friedman, J.; Bauminger, E.R. Iron as a trigger of neurodegeneration in Parkinson’s disease. Handb. Clin. Neurol. 2007, 83, 493–505. [Google Scholar]
- Rubinsztein, D.C.; Mariño, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Leimpeter, A.; Bloch, D.A.; Nelson, L.M. Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Nordengen, K.; Cappelletti, C.; Bahrami, S.; Frei, O.; Pihlstrøm, L.; Henriksen, S.P.; Geut, H.; Rozemuller, A.J.M.; van de Berg, W.D.J.; Andreassen, O.A.; et al. Pleiotropy with sex-specific traits reveals genetic aspects of sex differences in Parkinson’s disease. Brain 2024, 147, 858–870. [Google Scholar] [CrossRef]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Park. Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef]
- Wright Willis, A.; Evanoff, B.A.; Lian, M.; Criswell, S.R.; Racette, B.A. Geographic and ethnic variation in Parkinson disease: A population-based study of US Medicare beneficiaries. Neuroepidemiology 2010, 34, 143–151. [Google Scholar] [CrossRef]
- Harris, S.; Narayanan, N.S.; Tranel, D. Does Black vs. White race affect practitioners’ appraisal of Parkinson’s disease? npj Park. Dis. 2023, 9, 106. [Google Scholar] [CrossRef]
- Funayama, M.; Nishioka, K.; Li, Y.; Hattori, N. Molecular genetics of Parkinson’s disease: Contributions and global trends. J. Hum. Genet. 2023, 68, 125–130. [Google Scholar] [CrossRef]
- Benjamin, K.J.M.; Chen, Q.; Eagles, N.J.; Huuki-Myers, L.A.; Collado-Torres, L.; Stolz, J.M.; Pertea, G.; Shin, J.H.; Paquola, A.C.M.; Hyde, T.M.; et al. Analysis of gene expression in the postmortem brain of neurotypical Black Americans reveals contributions of genetic ancestry. Nat. Neurosci. 2024, 27, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.C.; Barnes, L.L.; Weissberger, G.H.; Lamar, M.; Nguyen, A.L.; Fenton, L.; Herrera, J.; Han, S.D. Quantification of race/ethnicity representation in Alzheimer’s disease neuroimaging research in the USA: A systematic review. Commun. Med. 2023, 3, 101. [Google Scholar] [CrossRef]
- Payami, H.; Cohen, G.; Murchison, C.F.; Sampson, T.R.; Standaert, D.G.; Wallen, Z.D. Population fraction of Parkinson disease attributable to preventable risk factors. npj Park. Dis. 2023, 9, 159. [Google Scholar] [CrossRef]
- Sellbach, A.N.; Boyle, R.S.; Silburn, P.A.; Mellick, G.D. Parkinson’s disease and family history. Park. Relat. Disord. 2006, 12, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Gokuladhas, S.; Fadason, T.; Farrow, S.; Cooper, A.; O’Sullivan, J.M. Discovering genetic mechanisms underlying the co-occurrence of Parkinson’s disease and non-motor traits. npj Park. Dis. 2024, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.; Anastacio, H.; Bardy, C. Genetic predispositions of Parkinson’s disease revealed in patient-derived brain cells. npj Park. Dis. 2020, 6, 8. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Diaw, S.H.; Borsche, M.; Streubel-Gallasch, L.; Dulovic-Mahlow, M.; Hermes, J.; Lenz, I.; Seibler, P.; Klein, C.; Brüggemann, N.; Vos, M. Characterization of the pathogenic α-Synuclein Variant V15A in Parkinson’ s disease. npj Park. Dis. 2023, 9, 148. [Google Scholar] [CrossRef]
- Zimprich, A.; Biskup, S.; Leitner, P.; Lichtner, P.; Farrer, M.; Lincoln, S.; Kachergus, J.; Hulihan, M.; Uitti, R.J.; Calne, D.B. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004, 44, 601–607. [Google Scholar] [CrossRef]
- Russo, I.; Bubacco, L.; Greggio, E. LRRK2 and neuroinflammation: Partners in crime in Parkinson’s disease? J. Neuroinflamm. 2014, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Krainc, D. LRRK2 phosphorylation of auxilin mediates synaptic defects in dopaminergic neurons from patients with Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, 5576–5581. [Google Scholar] [CrossRef]
- Lovegrove, C. The genetic and clinical phenotype of LRRK2-associated familial PD. Nat. Clin. Pract. Neurol. 2006, 2, 125. [Google Scholar] [CrossRef]
- Ysselstein, D.; Nguyen, M.; Young, T.J.; Severino, A.; Schwake, M.; Merchant, K.; Krainc, D. LRRK2 kinase activity regulates lysosomal glucocerebrosidase in neurons derived from Parkinson’s disease patients. Nat. Commun. 2019, 10, 5570. [Google Scholar] [CrossRef] [PubMed]
- Streubel-Gallasch, L.; Giusti, V.; Sandre, M.; Tessari, I.; Plotegher, N.; Giusto, E.; Masato, A.; Iovino, L.; Battisti, I.; Arrigoni, G. Parkinson’s disease–associated LRRK2 interferes with astrocyte-mediated alpha-synuclein clearance. Mol. Neurobiol. 2021, 58, 3119–3140. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, G.; deSouza, R.-M.; Balestrino, R.; Schapira, A.H. Glucocerebrosidase mutations in Parkinson disease. J. Park. Dis. 2017, 7, 411–422. [Google Scholar] [CrossRef]
- Bonifati, V.; Rizzu, P.; Van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef]
- Lind-Holm Mogensen, F.; Scafidi, A.; Poli, A.; Michelucci, A. PARK7/DJ-1 in microglia: Implications in Parkinson’s disease and relevance as a therapeutic target. J. Neuroinflamm. 2023, 20, 95. [Google Scholar] [CrossRef]
- Kochmanski, J.; Kuhn, N.C.; Bernstein, A.I. Parkinson’s disease-associated, sex-specific changes in DNA methylation at PARK7 (DJ-1), SLC17A6 (VGLUT2), PTPRN2 (IA-2β), and NR4A2 (NURR1) in cortical neurons. npj Park. Dis. 2022, 8, 120. [Google Scholar] [CrossRef]
- Vizziello, M.; Borellini, L.; Franco, G.; Ardolino, G. Disruption of mitochondrial homeostasis: The role of PINK1 in Parkinson’s disease. Cells 2021, 10, 3022. [Google Scholar] [CrossRef]
- Jones, N. PINK1 targets dysfunctional mitochondria for autophagy in Parkinson disease. Nat. Rev. Neurol. 2010, 6, 181. [Google Scholar] [CrossRef]
- Valente, E.M.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.; Harvey, K.; Gispert, S.; Ali, Z.; Del Turco, D.; Bentivoglio, A.R.; Healy, D.G. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 2004, 304, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Boussaad, I.; Jarazo, J.; Fitzgerald, J.C.; Antony, P.; Keatinge, M.; Blechman, J.; Schwamborn, J.C.; Krüger, R.; Placzek, M. PINK1 deficiency impairs adult neurogenesis of dopaminergic neurons. Sci. Rep. 2021, 11, 6617. [Google Scholar] [CrossRef] [PubMed]
- Tufi, R.; Gandhi, S.; De Castro, I.P.; Lehmann, S.; Angelova, P.R.; Dinsdale, D.; Deas, E.; Plun-Favreau, H.; Nicotera, P.; Abramov, A.Y. Enhancing nucleotide metabolism protects against mitochondrial dysfunction and neurodegeneration in a PINK1 model of Parkinson’s disease. Nat. Cell Biol. 2014, 16, 157–166. [Google Scholar] [CrossRef]
- Kano, M.; Takanashi, M.; Oyama, G.; Yoritaka, A.; Hatano, T.; Shiba-Fukushima, K.; Nagai, M.; Nishiyama, K.; Hasegawa, K.; Inoshita, T. Reduced astrocytic reactivity in human brains and midbrain organoids with PRKN mutations. npj Park. Dis. 2020, 6, 33. [Google Scholar] [CrossRef]
- Oczkowska, A.; Kozubski, W.; Lianeri, M.; Dorszewska, J. Mutations in PRKN and SNCA genes important for the progress of Parkinson’s disease. Curr. Genom. 2013, 14, 502–517. [Google Scholar] [CrossRef]
- Castelo Rueda, M.P.; Zanon, A.; Gilmozzi, V.; Lavdas, A.A.; Raftopoulou, A.; Delcambre, S.; Del Greco M, F.; Klein, C.; Grünewald, A.; Pramstaller, P.P. Molecular phenotypes of mitochondrial dysfunction in clinically non-manifesting heterozygous PRKN variant carriers. npj Park. Dis. 2023, 9, 65. [Google Scholar] [CrossRef]
- Kasten, M.; Hartmann, C.; Hampf, J.; Schaake, S.; Westenberger, A.; Vollstedt, E.J.; Balck, A.; Domingo, A.; Vulinovic, F.; Dulovic, M. Genotype-phenotype relations for the Parkinson’s disease genes Parkin, PINK1, DJ1: MDSGene systematic review. Mov. Disord. 2018, 33, 730–741. [Google Scholar] [CrossRef]
- Zhou, C.; Guan, X.J.; Guo, T.; Zeng, Q.L.; Gao, T.; Huang, P.Y.; Xuan, M.; Gu, Q.Q.; Xu, X.J.; Zhang, M.M. Progressive brain atrophy in Parkinson’s disease patients who convert to mild cognitive impairment. CNS Neurosci. Ther. 2020, 26, 117–125. [Google Scholar] [CrossRef]
- Youssef, P.; Hughes, L.; Kim, W.S.; Halliday, G.M.; Lewis, S.J.; Cooper, A.; Dzamko, N. Evaluation of plasma levels of NFL, GFAP, UCHL1 and tau as Parkinson’s disease biomarkers using multiplexed single molecule counting. Sci. Rep. 2023, 13, 5217. [Google Scholar] [CrossRef] [PubMed]
- Ragland, M.; Hutter, C.; Zabetian, C.; Edwards, K. Association between the ubiquitin carboxyl-terminal esterase L1 gene (UCHL1) S18Y variant and Parkinson’s Disease: A HuGE review and meta-analysis. Am. J. Epidemiol. 2009, 170, 1344–1357. [Google Scholar] [CrossRef]
- Mondello, S.; Constantinescu, R.; Zetterberg, H.; Andreasson, U.; Holmberg, B.; Jeromin, A. CSF α-synuclein and UCH-L1 levels in Parkinson’s disease and atypical parkinsonian disorders. Park. Relat. Disord. 2014, 20, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.S.L.; Tan, Y.J.; Lu, Z.; Ng, E.Y.; Ng, S.Y.E.; Chia, N.S.Y.; Setiawan, F.; Xu, Z.; Keong, N.C.H.; Tay, K.Y. Plasma ubiquitin C-terminal hydrolase L1 levels reflect disease stage and motor severity in Parkinson’s disease. Aging 2020, 12, 1488. [Google Scholar] [CrossRef]
- Spitz, M.; Pereira, J.S.; Nicareta, D.H.; de Medeiros Abreu, G.; Bastos, E.F.; Seixas, T.L.; Pimentel, M.M.G. Association of LRRK2 and GBA mutations in a Brazilian family with Parkinson’s disease. Park. Relat. Disord. 2015, 21, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Yahalom, G.; Greenbaum, L.; Israeli-Korn, S.; Fay-Karmon, T.; Livneh, V.; Ruskey, J.A.; Roncière, L.; Alam, A.; Gan-Or, Z.; Hassin-Baer, S. Carriers of both GBA and LRRK2 mutations, compared to carriers of either, in Parkinson’s disease: Risk estimates and genotype-phenotype correlations. Park. Relat. Disord. 2019, 62, 179–184. [Google Scholar] [CrossRef]
- da Silva, C.P.; Abreu, G.d.M.; Acero, P.H.C.; Júnior, M.C.; Pereira, J.S.; Ramos, S.R.d.A.; Nascimento, C.M.; Voigt, D.D.; Rosso, A.L.; Leite, M.A.A. Clinical profiles associated with LRRK2 and GBA mutations in Brazilians with Parkinson’s disease. J. Neurol. Sci. 2017, 381, 160–164. [Google Scholar] [CrossRef]
- Cilia, R.; Tunesi, S.; Marotta, G.; Cereda, E.; Siri, C.; Tesei, S.; Zecchinelli, A.L.; Canesi, M.; Mariani, C.B.; Meucci, N. Survival and dementia in GBA-associated Parkinson’s disease: The mutation matters. Ann. Neurol. 2016, 80, 662–673. [Google Scholar] [CrossRef]
- Liu, G.; Locascio, J.J.; Corvol, J.-C.; Boot, B.; Liao, Z.; Page, K.; Franco, D.; Burke, K.; Jansen, I.E.; Trisini-Lipsanopoulos, A. Prediction of cognition in Parkinson’s disease with a clinical–genetic score: A longitudinal analysis of nine cohorts. Lancet Neurol. 2017, 16, 620–629. [Google Scholar] [CrossRef]
- Cannon, J.R.; Greenamyre, J.T. Gene-environment interactions in Parkinson’s disease: Specific evidence in humans and mammalian models. Neurobiol. Dis. 2013, 57, 38–46. [Google Scholar] [CrossRef]
- Dong-Chen, X.; Yong, C.; Yang, X.; Chen-Yu, S.; Li-Hua, P. Signaling pathways in Parkinson’s disease: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 73. [Google Scholar] [CrossRef]
- Flores-Dorantes, M.T.; Díaz-López, Y.E.; Gutiérrez-Aguilar, R. Environment and Gene Association with Obesity and Their Impact on Neurodegenerative and Neurodevelopmental Diseases. Front. Neurosci. 2020, 14, 863. [Google Scholar] [CrossRef]
- Ball, N.; Teo, W.-P.; Chandra, S.; Chapman, J. Parkinson’s Disease and the Environment. Front. Neurol. 2019, 10, 218. [Google Scholar] [CrossRef]
- Frigerio, R.; Elbaz, A.; Sanft, K.R.; Peterson, B.J.; Bower, J.H.; Ahlskog, J.E.; Grossardt, B.R.; de Andrade, M.; Maraganore, D.M.; Rocca, W.A. Education and occupations preceding Parkinson disease: A population-based case-control study. Neurology 2005, 65, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Fardell, C.; Torén, K.; Schiöler, L.; Nissbrandt, H.; Åberg, M. High IQ in early adulthood is associated with Parkinson’s disease. J. Park. Dis. 2020, 10, 1649–1656. [Google Scholar] [CrossRef]
- Shi, J.; Tian, J.; Fan, Y.; Hao, X.; Li, M.; Li, J.; Ma, D.; Guo, M.; Li, S.; Xu, Y. Intelligence, education level, and risk of Parkinson’s disease in European populations: A Mendelian randomization study. Front. Genet. 2022, 13, 963163. [Google Scholar] [CrossRef] [PubMed]
- Lara, M.; Amigo, H. Association between education and blood lipid levels as income increases over a decade: A cohort study. BMC Public Health 2018, 18, 286. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, B.; Zhang, X.; Chen, C.; Gao, Y.; Li, N. Association of serum cholesterol with Parkinson’s disease in a cohort of statin-free individuals. Brain Behav. 2022, 12, e2454. [Google Scholar] [CrossRef]
- García-Sanz, P.; MFG Aerts, J.; Moratalla, R. The role of cholesterol in α-synuclein and Lewy body pathology in GBA1 Parkinson’s disease. Mov. Disord. 2021, 36, 1070–1085. [Google Scholar] [CrossRef]
- Pärna, K.; Pürjer, M.-L.; Ringmets, I.; Tekkel, M. Educational differences in cigarette smoking among adult population in Estonia, 1990–2010: Does the trend fit the model of tobacco epidemic? BMC Public Health 2014, 14, 709. [Google Scholar] [CrossRef]
- Modig, K.; Silventoinen, K.; Tynelius, P.; Kaprio, J.; Rasmussen, F. Genetics of the association between intelligence and nicotine dependence: A study of male Swedish twins. Addiction 2011, 106, 995–1002. [Google Scholar] [CrossRef]
- Cohen, O.S.; Vakil, E.; Tanne, D.; Nitsan, Z.; Schwartz, R.; Hassin-Baer, S. Educational level as a modulator of cognitive performance and neuropsychyatric features in Parkinson disease. Cogn. Behav. Neurol. 2007, 20, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Gamache, P.-L.; Salem, I.H.; Roux-Dubois, N.; Le Bouthillier, J.; Gan-Or, Z.; Dupré, N. Exposure to pesticides and welding hastens the age-at-onset of Parkinson’s disease. Can. J. Neurol. Sci. 2019, 46, 711–716. [Google Scholar] [CrossRef]
- Caballero, M.; Amiri, S.; Denney, J.T.; Monsivais, P.; Hystad, P.; Amram, O. Estimated residential exposure to agricultural chemicals and premature mortality by Parkinson’s disease in Washington state. Int. J. Environ. Res. Public Health 2018, 15, 2885. [Google Scholar] [CrossRef] [PubMed]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef]
- Zeng, X.; DeBono, N.L.; Harris, A.M.; Arrandale, V.H.; Demers, P.A. Neurodegenerative diseases among miners in Ontario, Canada, using a linked cohort. Occup. Environ. Med. 2021, 78, 385–392. [Google Scholar] [CrossRef]
- Uversky, V.N.; Li, J.; Fink, A.L. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J. Biol. Chem. 2001, 276, 44284–44296. [Google Scholar] [CrossRef]
- Pyatha, S.; Kim, H.; Lee, D.; Kim, K. Association between Heavy Metal Exposure and Parkinson’s Disease: A Review of the Mechanisms Related to Oxidative Stress. Antioxidants 2022, 11, 2467. [Google Scholar] [CrossRef]
- Shvachiy, L.; Geraldes, V.; Outeiro, T.F. Uncovering the Molecular Link Between Lead Toxicity and Parkinson’s Disease. Antioxid. Redox Signal. 2023, 39, 321–335. [Google Scholar] [CrossRef]
- Forero-Rodríguez, L.J.; Josephs-Spaulding, J.; Flor, S.; Pinzón, A.; Kaleta, C. Parkinson’s Disease and the Metal-Microbiome-Gut-Brain Axis: A Systems Toxicology Approach. Antioxidants 2021, 11, 71. [Google Scholar] [CrossRef]
- Goldman, S.M.; Quinlan, P.J.; Ross, G.W.; Marras, C.; Meng, C.; Bhudhikanok, G.S.; Comyns, K.; Korell, M.; Chade, A.R.; Kasten, M. Solvent exposures and Parkinson disease risk in twins. Ann. Neurol. 2012, 71, 776–784. [Google Scholar] [CrossRef]
- Jo, S.; Kim, Y.-J.; Park, K.W.; Hwang, Y.S.; Lee, S.H.; Kim, B.J.; Chung, S.J. Association of NO2 and other air pollution exposures with the risk of Parkinson disease. JAMA Neurol. 2021, 78, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Jami, M.S.; Murata, H.; Barnhill, L.M.; Li, S.; Bronstein, J.M. Diesel exhaust exposure alters the expression of networks implicated in neurodegeneration in zebrafish brains. Cell Biol. Toxicol. 2023, 39, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Barnhill, L.M.; Bronstein, J.M. Air Pollution and the Risk of Parkinson’s Disease: A Review. Mov. Disord. 2022, 37, 894–904. [Google Scholar] [CrossRef]
- Stern, R.A.; Riley, D.O.; Daneshvar, D.H.; Nowinski, C.J.; Cantu, R.C.; McKee, A.C. Long-term consequences of repetitive brain trauma: Chronic traumatic encephalopathy. PmR 2011, 3, S460–S467. [Google Scholar] [CrossRef]
- Whitton, P.S. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br. J. Pharmacol. 2007, 150, 963–976. [Google Scholar] [CrossRef]
- Breckenridge, C.B.; Berry, C.; Chang, E.T.; Sielken Jr, R.L.; Mandel, J.S. Association between Parkinson’s disease and cigarette smoking, rural living, well-water consumption, farming and pesticide use: Systematic review and meta-analysis. PLoS ONE 2016, 11, e0151841. [Google Scholar] [CrossRef]
- Timpka, J.; Dahlström, Ö.; Spreco, A.; Nilsson, M.H.; Iwarsson, S.; Timpka, T.; Odin, P. Reduced workforce participation 5 years prior to first Parkinson’s disease sick-leave. npj Park. Dis. 2018, 4, 36. [Google Scholar] [CrossRef]
- Lan, A.; Chen, J.; Chai, Z.; Hu, Y. The neurotoxicity of iron, copper and cobalt in Parkinson’s disease through ROS-mediated mechanisms. Biometals 2016, 29, 665–678. [Google Scholar] [CrossRef]
- Park, J.; Yoo, C.-I.; Sim, C.S.; Kim, H.K.; Kim, J.W.; Jeon, B.S.; Kim, K.-R.; Bang, O.-Y.; Lee, W.-Y.; Yi, Y. Occupations and Parkinson’s disease: A multi-center case-control study in South Korea. Neurotoxicology 2005, 26, 99–105. [Google Scholar] [CrossRef]
- Tsui, J.K.; Calne, D.B.; Wang, Y.; Schulzer, M.; Marion, S.A. Occupational risk factors in Parkinson’s disease. Can. J. Public Health 1999, 90, 334–337. [Google Scholar] [CrossRef]
- Chang, D.; Nalls, M.A.; Hallgrímsdóttir, I.B.; Hunkapiller, J.; van der Brug, M.; Cai, F.; Kerchner, G.A.; Ayalon, G.; Bingol, B.; Sheng, M.; et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet. 2017, 49, 1511–1516. [Google Scholar] [CrossRef]
- Bruce, H.J.; Tripodis, Y.; McClean, M.; Korell, M.; Tanner, C.M.; Contreras, B.; Gottesman, J.; Kirsch, L.; Karim, Y.; Martin, B. American football play and Parkinson disease among men. JAMA Netw. Open 2023, 6, e2328644. [Google Scholar] [CrossRef]
- Yang, F.; Johansson, A.L.V.; Pedersen, N.L.; Fang, F.; Gatz, M.; Wirdefeldt, K. Socioeconomic status in relation to Parkinson’s disease risk and mortality: A population-based prospective study. Medicine 2016, 95, e4337. [Google Scholar] [CrossRef]
- Lix, L.M.; Hobson, D.E.; Azimaee, M.; Leslie, W.D.; Burchill, C.; Hobson, S. Socioeconomic variations in the prevalence and incidence of Parkinson’s disease: A population-based analysis. J. Epidemiol. Community Health 2010, 64, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Schwarzschild, M.A. Lifestyle and Parkinson’s disease progression. Mov. Disord. 2019, 34, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.; Grotta, A.; Yang, F.; Bellocco, R.; Ye, W.; Adami, H.-O.; Wirdefeldt, K.; Trolle Lagerros, Y. Body mass index, sitting time, and risk of Parkinson disease. Neurology 2018, 90, e1413–e1417. [Google Scholar] [CrossRef]
- Hu, G.; Jousilahti, P.; Nissinen, A.; Antikainen, R.; Kivipelto, M.; Tuomilehto, J. Body mass index and the risk of Parkinson disease. Neurology 2006, 67, 1955–1959. [Google Scholar] [CrossRef]
- Park, K.-Y.; Nam, G.E.; Han, K.; Park, H.-K.; Hwang, H.-S. Waist circumference and risk of Parkinson’s disease. npj Park. Dis. 2022, 8, 89. [Google Scholar] [CrossRef]
- Cumming, K.; Macleod, A.D.; Myint, P.K.; Counsell, C.E. Early weight loss in parkinsonism predicts poor outcomes: Evidence from an incident cohort study. Neurology 2017, 89, 2254–2261. [Google Scholar] [CrossRef]
- Peters, S.; Gallo, V.; Vineis, P.; Middleton, L.T.; Forsgren, L.; Sacerdote, C.; Sieri, S.; Kyrozis, A.; Chirlaque, M.D.; Zamora-Ros, R. Alcohol consumption and risk of Parkinson’s disease: Data from a large prospective European cohort. Mov. Disord. 2020, 35, 1258–1263. [Google Scholar] [CrossRef]
- Shao, C.; Wang, X.; Wang, P.; Tang, H.; He, J.; Wu, N. Parkinson’s Disease Risk and Alcohol Intake: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Front. Nutr. 2021, 8, 709846. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A. Alcohol consumption and risk for Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. 2019, 266, 1821–1834. [Google Scholar] [CrossRef]
- Kelsey, R. Lifestyle factors and progression of PD. Nat. Rev. Neurol. 2019, 15, 126. [Google Scholar] [CrossRef]
- Daviet, R.; Aydogan, G.; Jagannathan, K.; Spilka, N.; Koellinger, P.D.; Kranzler, H.R.; Nave, G.; Wetherill, R.R. Associations between alcohol consumption and gray and white matter volumes in the UK Biobank. Nat. Commun. 2022, 13, 1175. [Google Scholar] [CrossRef] [PubMed]
- Domenighetti, C.; Sugier, P.E.; Ashok Kumar Sreelatha, A.; Schulte, C.; Grover, S.; Mohamed, O.; Portugal, B.; May, P.; Bobbili, D.R.; Radivojkov-Blagojevic, M. Dairy intake and Parkinson’s disease: A mendelian randomization study. Mov. Disord. 2022, 37, 857–864. [Google Scholar] [CrossRef]
- Hughes, K.C.; Gao, X.; Kim, I.Y.; Wang, M.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A. Intake of dairy foods and risk of Parkinson disease. Neurology 2017, 89, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; O’Reilly, E.; McCullough, M.L.; Rodriguez, C.; Schwarzschild, M.A.; Calle, E.E.; Thun, M.J.; Ascherio, A. Consumption of dairy products and risk of Parkinson’s disease. Am. J. Epidemiol. 2007, 165, 998–1006. [Google Scholar] [CrossRef]
- Abbott, R.D.; Ross, G.W.; Petrovitch, H.; Masaki, K.H.; Launer, L.J.; Nelson, J.S.; White, L.R.; Tanner, C.M. Midlife milk consumption and substantia nigra neuron density at death. Neurology 2016, 86, 512–519. [Google Scholar] [CrossRef]
- Torti, M.; Fossati, C.; Casali, M.; De Pandis, M.F.; Grassini, P.; Radicati, F.G.; Stirpe, P.; Vacca, L.; Iavicoli, I.; Leso, V. Effect of family history, occupation and diet on the risk of Parkinson disease: A case-control study. PLoS ONE 2020, 15, e0243612. [Google Scholar] [CrossRef]
- Lange, K.W.; Nakamura, Y.; Chen, N.; Guo, J.; Kanaya, S.; Lange, K.M.; Li, S. Diet and medical foods in Parkinson’s disease. Food Sci. Hum. Wellness 2019, 8, 83–95. [Google Scholar] [CrossRef]
- Khan, A.; Ezeugwa, J.; Ezeugwu, V.E. A systematic review of the associations between sedentary behavior, physical inactivity, and non-motor symptoms of Parkinson’s disease. PLoS ONE 2024, 19, e0293382. [Google Scholar] [CrossRef] [PubMed]
- Chohan, H.; Senkevich, K.; Patel, R.K.; Bestwick, J.P.; Jacobs, B.M.; Bandres Ciga, S.; Gan-Or, Z.; Noyce, A.J. Type 2 diabetes as a determinant of Parkinson’s disease risk and progression. Mov. Disord. 2021, 36, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- De Pablo-Fernandez, E.; Goldacre, R.; Pakpoor, J.; Noyce, A.J.; Warner, T.T. Association between diabetes and subsequent Parkinson disease: A record-linkage cohort study. Neurology 2018, 91, e139–e142. [Google Scholar] [CrossRef]
- Yu, H.; Sun, T.; He, X.; Wang, Z.; Zhao, K.; An, J.; Wen, L.; Li, J.-Y.; Li, W.; Feng, J. Association between Parkinson’s disease and diabetes mellitus: From epidemiology, pathophysiology and prevention to treatment. Aging Dis. 2022, 13, 1591. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Polychronis, S.; Wilson, H.; Giordano, B.; Ferrara, N.; Niccolini, F.; Politis, M. Diabetes mellitus and Parkinson disease. Neurology 2018, 90, e1654–e1662. [Google Scholar] [CrossRef]
- Ou, R.; Wei, Q.; Hou, Y.; Zhang, L.; Liu, K.; Lin, J.; Jiang, Z.; Song, W.; Cao, B.; Shang, H. Effect of diabetes control status on the progression of Parkinson’s disease: A prospective study. Ann. Clin. Transl. Neurol. 2021, 8, 887–897. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Zimmermann, J.; Sixel-Döring, F.; Focke, N.K.; Wicke, T.; Ebentheuer, J.; Schaumburg, M.; Lang, E.; Friede, T.; Trenkwalder, C. Baseline predictors for progression 4 years after Parkinson’s disease diagnosis in the De Novo Parkinson Cohort (DeNoPa). Mov. Disord. 2019, 34, 67–77. [Google Scholar] [CrossRef]
- Cereda, E.; Barichella, M.; Cassani, E.; Caccialanza, R.; Pezzoli, G. Clinical features of Parkinson disease when onset of diabetes came first: A case-control study. Neurology 2012, 78, 1507–1511. [Google Scholar] [CrossRef]
- Camargo Maluf, F.; Feder, D.; Alves de Siqueira Carvalho, A. Analysis of the relationship between type II diabetes mellitus and Parkinson’s disease: A systematic review. Park. Dis. 2019, 2019, 4951379. [Google Scholar] [CrossRef]
- Athauda, D.; Evans, J.; Wernick, A.; Virdi, G.; Choi, M.L.; Lawton, M.; Vijiaratnam, N.; Girges, C.; Ben-Shlomo, Y.; Ismail, K. The impact of type 2 diabetes in Parkinson’s disease. Mov. Disord. 2022, 37, 1612–1623. [Google Scholar] [CrossRef]
- Han, K.; Kim, B.; Lee, S.H.; Kim, M.K. A nationwide cohort study on diabetes severity and risk of Parkinson disease. npj Park. Dis. 2023, 9, 11. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Vernooij, M.W.; Cordonnier, C.; Viswanathan, A.; Al-Shahi Salman, R.; Warach, S.; Launer, L.J.; Van Buchem, M.A.; Breteler, M.M.; Group, M.S. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009, 8, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Lemprière, S. White matter hyperintensities—Location matters. Nat. Rev. Neurol. 2023, 19, 129. [Google Scholar] [CrossRef] [PubMed]
- Dufouil, C.; de Kersaint–Gilly, A.; Besancon, V.; Levy, C.; Auffray, E.; Brunnereau, L.; Alperovitch, A.; Tzourio, C. Longitudinal study of blood pressure and white matter hyperintensities: The EVA MRI Cohort. Neurology 2001, 56, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, Z.; Long, J.; Li, W.; Wang, X.; Hu, N.; Zhao, X.; Sun, T. White matter changes in Parkinson’s disease. npj Park. Dis. 2023, 9, 150. [Google Scholar] [CrossRef]
- Macías-García, D.; Periñán, M.T.; Muñoz-Delgado, L.; Jimenez-Jaraba, M.V.; Labrador-Espinosa, M.Á.; Jesús, S.; Buiza-Rueda, D.; Méndez-Del Barrio, C.; Adarmes-Gómez, A.; Gómez-Garre, P. Serum lipid profile among sporadic and familial forms of Parkinson’s disease. npj Park. Dis. 2021, 7, 59. [Google Scholar] [CrossRef]
- De Lau, L.M.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Serum cholesterol levels and the risk of Parkinson’s disease. Am. J. Epidemiol. 2006, 164, 998–1002. [Google Scholar] [CrossRef]
- Fang, F.; Zhan, Y.; Hammar, N.; Shen, X.; Wirdefeldt, K.; Walldius, G.; Mariosa, D. Lipids, Apolipoproteins, and the risk of Parkinson disease: A prospective cohort study and a Mendelian randomization analysis. Circ. Res. 2019, 125, 643–652. [Google Scholar] [CrossRef]
- Paul, R.; Choudhury, A.; Borah, A. Cholesterol–a putative endogenous contributor towards Parkinson’s disease. Neurochem. Int. 2015, 90, 125–133. [Google Scholar] [CrossRef]
- Huang, X.; Auinger, P.; Eberly, S.; Oakes, D.; Schwarzschild, M.; Ascherio, A.; Mailman, R.; Chen, H.; Investigators, P.S.G.D. Serum cholesterol and the progression of Parkinson’s disease: Results from DATATOP. PLoS ONE 2011, 6, e22854. [Google Scholar] [CrossRef]
- Hurh, K.; Park, M.; Jang, S.-i.; Park, E.-C.; Jang, S.-Y. Association between serum lipid levels over time and risk of Parkinson’s disease. Sci. Rep. 2022, 12, 21020. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, C.-w.; Nam, M.J.; Kim, H.; Kwon, D.-Y.; Yoo, J.W.; Lee, K.N.; Han, K.; Jung, J.-H.; Park, Y.-G. Association of high-density lipoprotein cholesterol variability and the risk of developing Parkinson disease. Neurology 2021, 96, e1391–e1401. [Google Scholar] [CrossRef]
- Benn, M.; Nordestgaard, B.G.; Frikke-Schmidt, R.; Tybjærg-Hansen, A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: Mendelian randomisation study. BMJ 2017, 357, j1648. [Google Scholar] [CrossRef]
- Nam, G.E.; Kim, S.M.; Han, K.; Kim, N.H.; Chung, H.S.; Kim, J.W.; Han, B.; Cho, S.J.; Yu, J.H.; Park, Y.G. Metabolic syndrome and risk of Parkinson disease: A nationwide cohort study. PLoS Med. 2018, 15, e1002640. [Google Scholar] [CrossRef]
- Hu, G.; Antikainen, R.; Jousilahti, P.; Kivipelto, M.; Tuomilehto, J. Total cholesterol and the risk of Parkinson disease. Neurology 2008, 70, 1972–1979. [Google Scholar] [CrossRef]
- Smeyne, R.J.; Noyce, A.J.; Byrne, M.; Savica, R.; Marras, C. Infection and Risk of Parkinson’s Disease. J. Park. Dis. 2021, 11, 31–43. [Google Scholar] [CrossRef]
- Anghelescu, A.; Onose, G.; Popescu, C.; Băilă, M.; Stoica, S.I.; Postoiu, R.; Brumă, E.; Petcu, I.R.; Ciobanu, V.; Munteanu, C. Parkinson’s Disease and SARS-CoV-2 Infection: Particularities of Molecular and Cellular Mechanisms Regarding Pathogenesis and Treatment. Biomedicines 2022, 10, 1000. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Chhetri, G.; Peng, X.; Wu, J.; Wang, Z.; Zhao, B.; Zhao, W.; Li, X. Parkinson’s Disease Causative Mutation in Vps35 Disturbs Tetherin Trafficking to Cell Surfaces and Facilitates Virus Spread. Cells 2021, 10, 746. [Google Scholar] [CrossRef]
- Conte, C. Possible Link between SARS-CoV-2 Infection and Parkinson’s Disease: The Role of Toll-Like Receptor 4. Int. J. Mol. Sci. 2021, 22, 7135. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Y.; Quan, H.; Liu, D.; Zhang, H.; Qi, Y.; Li, Q.; Liao, J.; Gao, H.M.; Zhou, H.; et al. Double-stranded RNA-induced dopaminergic neuronal loss in the substantia nigra in the presence of Mac1 receptor. Biochem. Biophys. Res. Commun. 2020, 533, 1148–1154. [Google Scholar] [CrossRef]
- Herrero, M.T.; Estrada, C.; Maatouk, L.; Vyas, S. Inflammation in Parkinson’s disease: Role of glucocorticoids. Front. Neuroanat. 2015, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation-An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef] [PubMed]
- Rolli-Derkinderen, M.; Leclair-Visonneau, L.; Bourreille, A.; Coron, E.; Neunlist, M.; Derkinderen, P. Is Parkinson’s disease a chronic low-grade inflammatory bowel disease? J. Neurol. 2020, 267, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lobbestael, E.; Vermeire, S.; Sabino, J.; Cleynen, I. Inflammatory bowel disease and Parkinson’s disease: Common pathophysiological links. Gut 2021, 70, 408–417. [Google Scholar] [CrossRef]
- Xiromerisiou, G.; Marogianni, C.; Androutsopoulou, A.; Ntavaroukas, P.; Mysiris, D.; Papoutsopoulou, S. Parkinson’s Disease, It Takes Guts: The Correlation between Intestinal Microbiome and Cytokine Network with Neurodegeneration. Biology 2023, 12, 93. [Google Scholar] [CrossRef]
- Chan, D.G.; Ventura, K.; Villeneuve, A.; Du Bois, P.; Holahan, M.R. Exploring the Connection Between the Gut Microbiome and Parkinson’s Disease Symptom Progression and Pathology: Implications for Supplementary Treatment Options. J. Park. Dis. 2022, 12, 2339–2352. [Google Scholar] [CrossRef]
- Caputi, V.; Giron, M.C. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 1689. [Google Scholar] [CrossRef]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef]
- Varesi, A.; Campagnoli, L.I.M.; Fahmideh, F.; Pierella, E.; Romeo, M.; Ricevuti, G.; Nicoletta, M.; Chirumbolo, S.; Pascale, A. The Interplay between Gut Microbiota and Parkinson’s Disease: Implications on Diagnosis and Treatment. Int. J. Mol. Sci. 2022, 23, 12289. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Sartori, A.C.; Vance, D.E.; Slater, L.Z.; Crowe, M. The impact of inflammation on cognitive function in older adults: Implications for health care practice and research. J. Neurosci. Nurs. 2012, 44, 206. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef]
- Allen Reish, H.E.; Standaert, D.G. Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. J. Park. Dis. 2015, 5, 1–19. [Google Scholar] [CrossRef]
- Klann, E.M.; Dissanayake, U.; Gurrala, A.; Farrer, M.; Shukla, A.W.; Ramirez-Zamora, A.; Mai, V.; Vedam-Mai, V. The Gut-Brain Axis and Its Relation to Parkinson’s Disease: A Review. Front. Aging Neurosci. 2021, 13, 782082. [Google Scholar] [CrossRef]
- Delic, V.; Beck, K.D.; Pang, K.C.H.; Citron, B.A. Biological links between traumatic brain injury and Parkinson’s disease. Acta Neuropathol. Commun. 2020, 8, 45. [Google Scholar] [CrossRef]
- Wee, I.C.; Arulsamy, A.; Corrigan, F.; Collins-Praino, L. Long-Term Impact of Diffuse Traumatic Brain Injury on Neuroinflammation and Catecholaminergic Signaling: Potential Relevance for Parkinson’s Disease Risk. Molecules 2024, 29, 1470. [Google Scholar] [CrossRef] [PubMed]
- Dodet, P.; Houot, M.; Leu-Semenescu, S.; Corvol, J.C.; Lehéricy, S.; Mangone, G.; Vidailhet, M.; Roze, E.; Arnulf, I. Sleep disorders in Parkinson’s disease, an early and multiple problem. npj Park. Dis. 2024, 10, 46. [Google Scholar] [CrossRef]
- Loddo, G.; Calandra-Buonaura, G.; Sambati, L.; Giannini, G.; Cecere, A.; Cortelli, P.; Provini, F. The Treatment of Sleep Disorders in Parkinson’s Disease: From Research to Clinical Practice. Front. Neurol. 2017, 8, 42. [Google Scholar] [CrossRef]
- Tarakad, A.; Jankovic, J. Anosmia and Ageusia in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 133, 541–556. [Google Scholar] [CrossRef]
- Doty, R.L. Olfaction in Parkinson’s disease and related disorders. Neurobiol. Dis. 2012, 46, 527–552. [Google Scholar] [CrossRef] [PubMed]
- Garlovsky, J.K.; Overton, P.G.; Simpson, J. Psychological Predictors of Anxiety and Depression in Parkinson’s Disease: A Systematic Review. J. Clin. Psychol. 2016, 72, 979–998. [Google Scholar] [CrossRef] [PubMed]
- Hemmerle, A.M.; Herman, J.P.; Seroogy, K.B. Stress, depression and Parkinson’s disease. Exp. Neurol. 2012, 233, 79–86. [Google Scholar] [CrossRef]
- Sagna, A.; Gallo, J.J.; Pontone, G.M. Systematic review of factors associated with depression and anxiety disorders among older adults with Parkinson’s disease. Park. Relat. Disord. 2014, 20, 708–715. [Google Scholar] [CrossRef]
- van der Heide, A.; Meinders, M.J.; Speckens, A.E.M.; Peerbolte, T.F.; Bloem, B.R.; Helmich, R.C. Stress and Mindfulness in Parkinson’s Disease: Clinical Effects and Potential Underlying Mechanisms. Mov. Disord. 2021, 36, 64–70. [Google Scholar] [CrossRef]
- Austin, K.W.; Ameringer, S.W.; Cloud, L.J. An Integrated Review of Psychological Stress in Parkinson’s Disease: Biological Mechanisms and Symptom and Health Outcomes. Park. Dis. 2016, 2016, 9869712. [Google Scholar] [CrossRef]
- Blundell, E.K.; Grover, L.E.; Stott, J.; Schrag, A. The experience of Anxiety for people with Parkinson’s disease. npj Park. Dis. 2023, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Lee, S.C.; Suh, J.H.; Yang, S.N.; Han, K.; Kim, Y.W. Different risks of early-onset and late-onset Parkinson disease in individuals with mental illness. npj Park. Dis. 2024, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Tian, R.; Li, J.; Yuan, T.F. Chronic stress and Parkinson’s disease. CNS Neurosci. Ther. 2014, 20, 1–2. [Google Scholar] [CrossRef]
- Yoo, J.E.; Shin, D.W.; Jang, W.; Han, K.; Kim, D.; Won, H.S.; Park, H.S. Female reproductive factors and the risk of Parkinson’s disease: A nationwide cohort study. Eur. J. Epidemiol. 2020, 35, 871–878. [Google Scholar] [CrossRef]
- Popat, R.A.; Van Den Eeden, S.K.; Tanner, C.M.; McGuire, V.; Bernstein, A.L.; Bloch, D.A.; Leimpeter, A.; Nelson, L.M. Effect of reproductive factors and postmenopausal hormone use on the risk of Parkinson disease. Neurology 2005, 65, 383–390. [Google Scholar] [CrossRef]
- Ragonese, P.; D’Amelio, M.; Savettieri, G. Implications for estrogens in Parkinson’s disease: An epidemiological approach. Ann. N. Y Acad. Sci. 2006, 1089, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.S.; Jeong, S.H. Association Between Menopausal Hormone Therapy and Risk for Parkinson’s Disease. J. Park. Dis. 2023, 13, 1357–1367. [Google Scholar] [CrossRef]
- Saunders-Pullman, R.; Gordon-Elliott, J.; Parides, M.; Fahn, S.; Saunders, H.R.; Bressman, S. The effect of estrogen replacement on early Parkinson’s disease. Neurology 1999, 52, 1417–1421. [Google Scholar] [CrossRef]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, Neuroinflammation, and Neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef] [PubMed]
- Alrouji, M.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alshammari, M.S.; Alexiou, A.; Papadakis, M.; Bahaa, M.M.; Batiha, G.E. Role of uric acid in neurodegenerative diseases, focusing on Alzheimer and Parkinson disease: A new perspective. Neuropsychopharmacol. Rep. 2024, 44, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Rizzi, L.; Somaa, F. The role of nutrition on Parkinson’s disease: A systematic review. Nutr. Neurosci. 2023, 26, 605–628. [Google Scholar] [CrossRef]
- Mertsalmi, T.H.; Pekkonen, E.; Scheperjans, F. Antibiotic exposure and risk of Parkinson’s disease in Finland: A nationwide case-control study. Mov. Disord. 2020, 35, 431–442. [Google Scholar] [CrossRef]
- Mittal, S.; Bjørnevik, K.; Im, D.S.; Flierl, A.; Dong, X.; Locascio, J.J.; Abo, K.M.; Long, E.; Jin, M.; Xu, B. β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease. Science 2017, 357, 891–898. [Google Scholar] [CrossRef]
- Chen, C.L.; Wang, S.Y.; Chen, T.C.; Chuang, C.S. Association between β2-Adrenoreceptor Medications and Risk of Parkinson’s Disease: A Meta-Analysis. Medicina 2021, 57, 1006. [Google Scholar] [CrossRef]
- Cheong, J.L.Y.; de Pablo-Fernandez, E.; Foltynie, T.; Noyce, A.J. The Association Between Type 2 Diabetes Mellitus and Parkinson’s Disease. J. Park. Dis. 2020, 10, 775–789. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, X.; Li, P.; Wang, M.; Yan, L.; Bao, Z.; Liu, Q. Association Between Diabetes Medications and the Risk of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 678649. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Monnet, A.; Reyes, A.; Ribba, B.; Svoboda, H.; Kustermann, T.; Simuni, T.; Postuma, R.B.; Pavese, N.; Stocchi, F.; et al. Sustained effect of prasinezumab on Parkinson’s disease motor progression in the open-label extension of the PASADENA trial. Nat. Med. 2024, 30, 3669–3675. [Google Scholar] [CrossRef]
- Beydoun, H.A.; Saquib, N.; Wallace, R.B.; Chen, J.C.; Coday, M.; Naughton, M.J.; Beydoun, M.A.; Shadyab, A.H.; Zonderman, A.B.; Brunner, R.L. Psychotropic medication use and Parkinson’s disease risk amongst older women. Ann. Clin. Transl. Neurol. 2022, 9, 1163–1176. [Google Scholar] [CrossRef]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chen, J.; Huang, J.; Sun, P.; Liu, Y.; Xu, L.; Wei, C.; Mu, X.; Lu, X.; Wang, W.; et al. Epigenetic modification in Parkinson’s disease. Front. Cell Dev. Biol. 2023, 11, 1123621. [Google Scholar] [CrossRef]
- Fang, X.; Han, D.; Cheng, Q.; Zhang, P.; Zhao, C.; Min, J.; Wang, F. Association of levels of physical activity with risk of Parkinson disease: A systematic review and meta-analysis. JAMA Netw. Open 2018, 1, e182421. [Google Scholar] [CrossRef]
- Portugal, B.; Artaud, F.; Degaey, I.; Roze, E.; Fournier, A.; Severi, G.; Canonico, M.; Proust-Lima, C.; Elbaz, A. Association of physical activity and Parkinson disease in women: Long-term follow-up of the E3N cohort study. Neurology 2023, 101, e386–e398. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.C.; Gao, X.; Molsberry, S.; Valeri, L.; Schwarzschild, M.A.; Ascherio, A. Physical activity and prodromal features of Parkinson disease. Neurology 2019, 93, e2157–e2169. [Google Scholar] [CrossRef]
- Tsukita, K.; Sakamaki-Tsukita, H.; Takahashi, R. Long-term effect of regular physical activity and exercise habits in patients with early Parkinson disease. Neurology 2022, 98, e859–e871. [Google Scholar] [CrossRef]
- Speelman, A.D.; Van De Warrenburg, B.P.; Van Nimwegen, M.; Petzinger, G.M.; Munneke, M.; Bloem, B.R. How might physical activity benefit patients with Parkinson disease? Nat. Rev. Neurol. 2011, 7, 528–534. [Google Scholar] [CrossRef]
- Morawska, M.M.; Moreira, C.G.; Ginde, V.R.; Valko, P.O.; Weiss, T.; Büchele, F.; Imbach, L.L.; Masneuf, S.; Kollarik, S.; Prymaczok, N.; et al. Slow-wave sleep affects synucleinopathy and regulates proteostatic processes in mouse models of Parkinson’s disease. Sci. Transl. Med. 2021, 13, eabe7099. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.; Coulson, E.J.; Rajnarayanan, R.; Oster, H.; Videnovic, A.; Rawashdeh, O. Sleep and circadian rhythms in Parkinson’s disease and preclinical models. Mol. Neurodegener. 2022, 17, 2. [Google Scholar] [CrossRef]
- Mao, C.J.; Peng, H.; Zhuang, S.; Zhang, Y.C.; Xie, W.Y.; Yan, J.H.; Liu, H.H.; Chen, J.; Liu, J.Y.; Zhang, J.; et al. Association Between Sleep Characteristics and Likelihood of Prodromal Parkinson’s Disease: A Cross-Sectional Analysis in the HABIT Study. Nat. Sci. Sleep. 2024, 16, 1355–1364. [Google Scholar] [CrossRef]
- Simpson, J.; Haines, K.; Lekwuwa, G.; Wardle, J.; Crawford, T. Social support and psychological outcome in people with Parkinson’s disease: Evidence for a specific pattern of associations. Br. J. Clin. Psychol. 2006, 45, 585–590. [Google Scholar] [CrossRef]
- Ravenek, M.J.; Schneider, M.A. Social support for physical activity and perceptions of control in early Parkinson’s disease. Disabil. Rehabil. 2009, 31, 1925–1936. [Google Scholar] [CrossRef]
- Ahlskog, J.E. Aerobic Exercise: Evidence for a Direct Brain Effect to Slow Parkinson Disease Progression. Mayo Clin. Proc. 2018, 93, 360–372. [Google Scholar] [CrossRef]
- Jackson, A.; Forsyth, C.B.; Shaikh, M.; Voigt, R.M.; Engen, P.A.; Ramirez, V.; Keshavarzian, A. Diet in Parkinson’s Disease: Critical Role for the Microbiome. Front. Neurol. 2019, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M.; Leverenz, J.B.; Schneider, J.S.; Adler, C.H. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord. 2014, 29, 634–650. [Google Scholar] [CrossRef]
- Liu, G.; Ni, C.; Zhan, J.; Li, W.; Luo, J.; Liao, Z.; Locascio, J.J.; Xian, W.; Chen, L.; Pei, Z.; et al. Mitochondrial haplogroups and cognitive progression in Parkinson’s disease. Brain 2023, 146, 42–49. [Google Scholar] [CrossRef]
- Liu, G.; Peng, J.; Liao, Z.; Locascio, J.J.; Corvol, J.C.; Zhu, F.; Dong, X.; Maple-Grødem, J.; Campbell, M.C.; Elbaz, A.; et al. Genome-wide survival study identifies a novel synaptic locus and polygenic score for cognitive progression in Parkinson’s disease. Nat. Genet. 2021, 53, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.R.; Segerstrom, S.C.; van Horne, C.G.; Schmitt, F.A.; Koehl, L.M. Meta-Analysis of Cognition in Parkinson’s Disease Mild Cognitive Impairment and Dementia Progression. Neuropsychol. Rev. 2022, 32, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Timblin, H.; Rahmani, E.; Garrett, S.; Bunch, J.; Beaver, H.; Hill, C.R. Physical activity as a mediator of anxiety and cognitive functioning in Parkinson’s disease. Ment. Health Phys. Act. 2021, 20, 100382. [Google Scholar] [CrossRef]
- Mappin-Kasirer, B.; Pan, H.; Lewington, S.; Kizza, J.; Gray, R.; Clarke, R.; Peto, R. Tobacco smoking and the risk of Parkinson disease: A 65-year follow-up of 30,000 male British doctors. Neurology 2020, 94, e2132–e2138. [Google Scholar] [CrossRef] [PubMed]
- Ritz, B.; Rhodes, S.L. After half a century of research on smoking and PD, where do we go now? Neurology 2010, 74, 870–871. [Google Scholar] [CrossRef] [PubMed]
- Piao, W.-H.; Campagnolo, D.; Dayao, C.; Lukas, R.J.; Wu, J.; Shi, F.-D. Nicotine and inflammatory neurological disorders. Acta Pharmacol. Sin. 2009, 30, 715–722. [Google Scholar] [CrossRef]
- Hong, D.-P.; Fink, A.L.; Uversky, V.N. Smoking and Parkinson’s disease: Does nicotine affect α-synuclein fibrillation? Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2009, 1794, 282–290. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Park, Y.H.; Lee, S.C.; Suh, J.H.; Yang, S.N.; Kang, D.R.; Kim, Y.W. Association between smoking and all-cause mortality in Parkinson’s disease. npj Park. Dis. 2023, 9, 59. [Google Scholar] [CrossRef]
- Lysen, T.S.; Darweesh, S.K.L.; Ikram, M.K.; Luik, A.I.; Ikram, M.A. Sleep and risk of parkinsonism and Parkinson’s disease: A population-based study. Brain 2019, 142, 2013–2022. [Google Scholar] [CrossRef]
- Gabbert, C.; König, I.R.; Lüth, T.; Kolms, B.; Kasten, M.; Vollstedt, E.-J.; Balck, A.; Grünewald, A.; Klein, C.; Trinh, J. Coffee, smoking and aspirin are associated with age at onset in idiopathic Parkinson’s disease. J. Neurol. 2022, 269, 4195–4203. [Google Scholar] [CrossRef]
- Sääksjärvi, K.; Knekt, P.; Rissanen, H.; Laaksonen, M.; Reunanen, A.; Männistö, S. Prospective study of coffee consumption and risk of Parkinson’s disease. Eur. J. Clin. Nutr. 2008, 62, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Wirdefeldt, K.; Adami, H.-O.; Cole, P.; Trichopoulos, D.; Mandel, J. Epidemiology and etiology of Parkinson’s disease: A review of the evidence. Eur. J. Epidemiol. 2011, 26, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.T.; Chan, L.; Bai, C.-H. The effect of caffeine on the risk and progression of Parkinson’s disease: A meta-analysis. Nutrients 2020, 12, 1860. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.-H.; Choi, S.-M.; Kim, J.-T.; Kim, B.C. Association of coffee consumption and non-motor symptoms in drug-naïve, early-stage Parkinson’s disease. Park. Relat. Disord. 2018, 50, 42–47. [Google Scholar] [CrossRef]
- Cho, B.-H.; Choi, S.-M.; Kim, B.C. Gender-dependent effect of coffee consumption on tremor severity in de novo Parkinson’s disease. BMC Neurol. 2019, 19, 194. [Google Scholar] [CrossRef]
- Chen, J.-F.; Schwarzschild, M.A. Do caffeine and more selective adenosine A2A receptor antagonists protect against dopaminergic neurodegeneration in Parkinson’s disease? Park. Relat. Disord. 2020, 80, S45–S53. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, caffeine, and health outcomes: An umbrella review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef]
- Li, F.J.; Ji, H.F.; Shen, L. A meta-analysis of tea drinking and risk of Parkinson’s disease. Sci. World J. 2012, 2012, 923464. [Google Scholar] [CrossRef]
- Chen, S.Q.; Wang, Z.S.; Ma, Y.X.; Zhang, W.; Lu, J.L.; Liang, Y.R.; Zheng, X.Q. Neuroprotective Effects and Mechanisms of Tea Bioactive Components in Neurodegenerative Diseases. Molecules 2018, 23, 512. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Y.; Zhang, H.; Zang, Y.; Liu, X.; Hou, Y.; Zhan, S.; Cai, Y.; Mao, W.; Chan, P. Factors associated with phenoconversion of idiopathic rapid eye movement sleep behavior disorder: A prospective study. npj Park. Dis. 2025, 11, 10. [Google Scholar] [CrossRef]
- Tan, L.C.; Koh, W.P.; Yuan, J.M.; Wang, R.; Au, W.L.; Tan, J.H.; Tan, E.K.; Yu, M.C. Differential effects of black versus green tea on risk of Parkinson’s disease in the Singapore Chinese Health Study. Am. J. Epidemiol. 2008, 167, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Miyake, Y.; Fukushima, W.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Intake of Japanese and Chinese teas reduces risk of Parkinson’s disease. Park. Relat. Disord. 2011, 17, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, H.; Fung, T.T.; Logroscino, G.; Schwarzschild, M.A.; Hu, F.B.; Ascherio, A. Prospective study of dietary pattern and risk of Parkinson disease. Am. J. Clin. Nutr. 2007, 86, 1486–1494. [Google Scholar] [CrossRef]
- Tosefsky, K.N.; Zhu, J.; Wang, Y.N.; Lam, J.S.T.; Cammalleri, A.; Appel-Cresswell, S. The Role of Diet in Parkinson’s Disease. J. Park. Dis. 2024, 14, S21–S34. [Google Scholar] [CrossRef]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of Diet and Nutritional Supplements in Parkinson’s Disease Progression. Oxid. Med. Cell. Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kilkkinen, A.; Rissanen, H.; Marniemi, J.; Sääksjärvi, K.; Heliövaara, M. Serum vitamin D and the risk of Parkinson disease. Arch. Neurol. 2010, 67, 808–811. [Google Scholar] [CrossRef]
- Booij, J.; Tellier, S.P.; Seibyl, J.; Vriend, C. Dopamine Transporter Availability in Early Parkinson’s Disease is Dependent on Sunlight Exposure. Mov. Disord. 2023, 38, 2131–2135. [Google Scholar] [CrossRef]
- Schirinzi, T.; Martella, G.; Imbriani, P.; Di Lazzaro, G.; Franco, D.; Colona, V.L.; Alwardat, M.; Sinibaldi Salimei, P.; Mercuri, N.B.; Pierantozzi, M. Dietary vitamin E as a protective factor for Parkinson’s disease: Clinical and experimental evidence. Front. Neurol. 2019, 10, 148. [Google Scholar] [CrossRef]
- Dietiker, C.; Kim, S.; Zhang, Y.; Christine, C.W.; Investigators, N.N.-P. Characterization of vitamin b12 supplementation and correlation with clinical outcomes in a large longitudinal study of early Parkinson’s disease. J. Mov. Disord. 2019, 12, 91. [Google Scholar] [CrossRef]
- McCarter, S.J.; Stang, C.; Turcano, P.; Mielke, M.M.; Ali, F.; Bower, J.H.; Savica, R. Higher vitamin B12 level at Parkinson’s disease diagnosis is associated with lower risk of future dementia. Park. Relat. Disord. 2020, 73, 19–22. [Google Scholar] [CrossRef]
- Alizadeh, M.; Kheirouri, S.; Keramati, M. What Dietary Vitamins and Minerals Might Be Protective against Parkinson’s Disease? Brain Sci. 2023, 13, 1119. [Google Scholar] [CrossRef]
- Boulos, C.; Yaghi, N.; El Hayeck, R.; Heraoui, G.N.; Fakhoury-Sayegh, N. Nutritional Risk Factors, Microbiota and Parkinson’s Disease: What Is the Current Evidence? Nutrients 2019, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wang, B.; Shi, Y.; Zhu, R. Dose-response meta-analysis on urate, gout, and the risk for Parkinson’s disease. npj Park. Dis. 2022, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Leira, E.C.; Planas, A.M.; Chauhan, A.K.; Chamorro, A. Uric Acid: A Translational Journey in Cerebroprotection That Spanned Preclinical and Human Data. Neurology 2023, 101, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Crotty, G.F.; Ascherio, A.; Schwarzschild, M.A. Targeting urate to reduce oxidative stress in Parkinson disease. Exp. Neurol. 2017, 298, 210–224. [Google Scholar] [CrossRef]
- Shima, S.; Mizutani, Y.; Yoshimoto, J.; Maeda, Y.; Ohdake, R.; Nagao, R.; Maeda, T.; Higashi, A.; Ueda, A.; Ito, M.; et al. Uric acid and alterations of purine recycling disorders in Parkinson’s disease: A cross-sectional study. npj Park. Dis. 2024, 10, 170. [Google Scholar] [CrossRef]
- Esposito, E.; Di Matteo, V.; Benigno, A.; Pierucci, M.; Crescimanno, G.; Di Giovanni, G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp. Neurol. 2007, 205, 295–312. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef]
- Gao, X.; Chen, H.; Schwarzschild, M.A.; Ascherio, A. Use of ibuprofen and risk of Parkinson disease. Neurology 2011, 76, 863–869. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.M.; Hernán, M.A.; Schwarzschild, M.A.; Willett, W.C.; Colditz, G.A.; Speizer, F.E.; Ascherio, A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch. Neurol. 2003, 60, 1059–1064. [Google Scholar] [CrossRef]
- Rangasamy, S.B.; Dasarathi, S.; Pahan, P.; Jana, M.; Pahan, K. Low-dose aspirin upregulates tyrosine hydroxylase and increases dopamine production in dopaminergic neurons: Implications for Parkinson’s disease. J. Neuroimmune Pharmacol. 2019, 14, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, I. Aspirin and ibuprofen could lower risk of LRRK2 Parkinson disease. Nat. Rev. Neurol. 2020, 16, 460. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Cooper, G.; Dunne, S.F.; Dusel, B.; Luan, C.H.; Surmeier, D.J.; Silverman, R.B. CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nat. Commun. 2012, 3, 1146. [Google Scholar] [CrossRef] [PubMed]
- Benkert, J.; Hess, S.; Roy, S.; Beccano-Kelly, D.; Wiederspohn, N.; Duda, J.; Simons, C.; Patil, K.; Gaifullina, A.; Mannal, N.; et al. Cav2.3 channels contribute to dopaminergic neuron loss in a model of Parkinson’s disease. Nat. Commun. 2019, 10, 5094. [Google Scholar] [CrossRef]
- Lin, J.; Pang, D.; Li, C.; Ou, R.; Yu, Y.; Cui, Y.; Huang, J.; Shang, H. Calcium channel blockers and Parkinson’s disease: A systematic review and meta-analysis. Ther. Adv. Neurol. Disord. 2024, 17, 17562864241252713. [Google Scholar] [CrossRef]
- Simon, K.C.; Gao, X.; Chen, H.; Schwarzschild, M.A.; Ascherio, A. Calcium channel blocker use and risk of Parkinson’s disease. Mov. Disord. 2010, 25, 1818–1822. [Google Scholar] [CrossRef]
- Carroll, C.B.; Wyse, R.K.H. Simvastatin as a Potential Disease-Modifying Therapy for Patients with Parkinson’s Disease: Rationale for Clinical Trial, and Current Progress. J. Park. Dis. 2017, 7, 545–568. [Google Scholar] [CrossRef]
- Yan, J.; Qiao, L.; Tian, J.; Liu, A.; Wu, J.; Huang, J.; Shen, M.; Lai, X. Effect of statins on Parkinson’s disease: A systematic review and meta-analysis. Medicine 2019, 98, e14852. [Google Scholar] [CrossRef]
- Simmering, J.E.; Welsh, M.J.; Liu, L.; Narayanan, N.S.; Pottegård, A. Association of Glycolysis-Enhancing α-1 Blockers with Risk of Developing Parkinson Disease. JAMA Neurol. 2021, 78, 407–413. [Google Scholar] [CrossRef]
- Firestone, J.A.; Lundin, J.I.; Powers, K.M.; Smith-Weller, T.; Franklin, G.M.; Swanson, P.D.; Longstreth, W.T., Jr.; Checkoway, H. Occupational factors and risk of Parkinson’s disease: A population-based case-control study. Am. J. Ind. Med. 2010, 53, 217–223. [Google Scholar] [CrossRef]
- Haaxma, C.A.; Borm, G.F.; van der Linden, D.; Kappelle, A.C.; Bloem, B.R. Artistic occupations are associated with a reduced risk of Parkinson’s disease. J. Neurol. 2015, 262, 2171–2176. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Miyake, Y.; Fukushima, W.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Occupational risk factors for Parkinson’s disease: A case-control study in Japan. BMC Neurol. 2011, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Petit, P.; Berger, F.; Bonneterre, V.; Vuillerme, N. Investigating Parkinson’s disease risk across farming activities using data mining and large-scale administrative health data. npj Park. Dis. 2025, 11, 13. [Google Scholar] [CrossRef]
- Pitz, V.; Makarious, M.B.; Bandres-Ciga, S.; Iwaki, H.; Singleton, A.B.; Nalls, M.; Heilbron, K.; Blauwendraat, C. Analysis of rare Parkinson’s disease variants in millions of people. npj Park. Dis. 2024, 10, 11. [Google Scholar] [CrossRef]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef]
- Belvisi, D.; Pellicciari, R.; Fabbrini, A.; Costanzo, M.; Pietracupa, S.; De Lucia, M.; Modugno, N.; Magrinelli, F.; Dallocchio, C.; Ercoli, T.; et al. Risk factors of Parkinson disease: Simultaneous assessment, interactions, and etiologic subtypes. Neurology 2020, 95, e2500–e2508. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu-Jones, B.K.; Frau, F.; Bozzi, S.; Chandross, K.J.; Peterschmitt, M.J.; Cohen, C.; Coulovrat, C.; Kumar, D.; Kruger, M.J.; Lipnick, S.L.; et al. Disease progression strikingly differs in research and real-world Parkinson’s populations. npj Park. Dis. 2024, 10, 58. [Google Scholar] [CrossRef]
- Zarkali, A.; Thomas, G.E.C.; Zetterberg, H.; Weil, R.S. Neuroimaging and fluid biomarkers in Parkinson’s disease in an era of targeted interventions. Nat. Commun. 2024, 15, 5661. [Google Scholar] [CrossRef]
- Hällqvist, J.; Bartl, M.; Dakna, M.; Schade, S.; Garagnani, P.; Bacalini, M.G.; Pirazzini, C.; Bhatia, K.; Schreglmann, S.; Xylaki, M.; et al. Plasma proteomics identify biomarkers predicting Parkinson’s disease up to 7 years before symptom onset. Nat. Commun. 2024, 15, 4759. [Google Scholar] [CrossRef]
- Ma, Z.L.; Wang, Z.L.; Zhang, F.Y.; Liu, H.X.; Mao, L.H.; Yuan, L. Biomarkers of Parkinson’s Disease: From Basic Research to Clinical Practice. Aging Dis. 2024, 15, 1813–1830. [Google Scholar] [CrossRef]
- Yamashita, K.Y.; Bhoopatiraju, S.; Silverglate, B.D.; Grossberg, G.T. Biomarkers in Parkinson’s disease: A state of the art review. Biomark. Neuropsychiatry 2023, 9, 100074. [Google Scholar] [CrossRef]
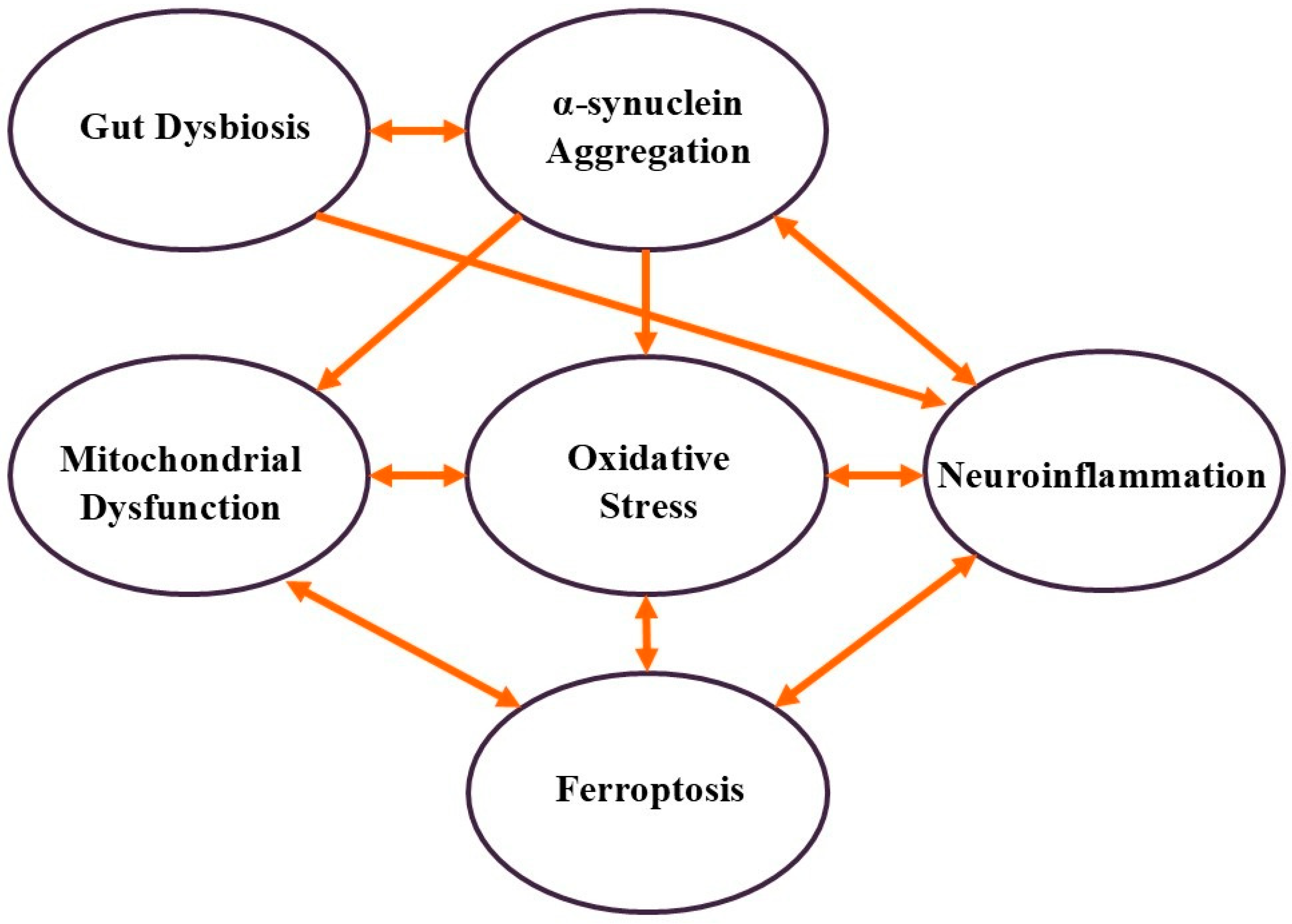
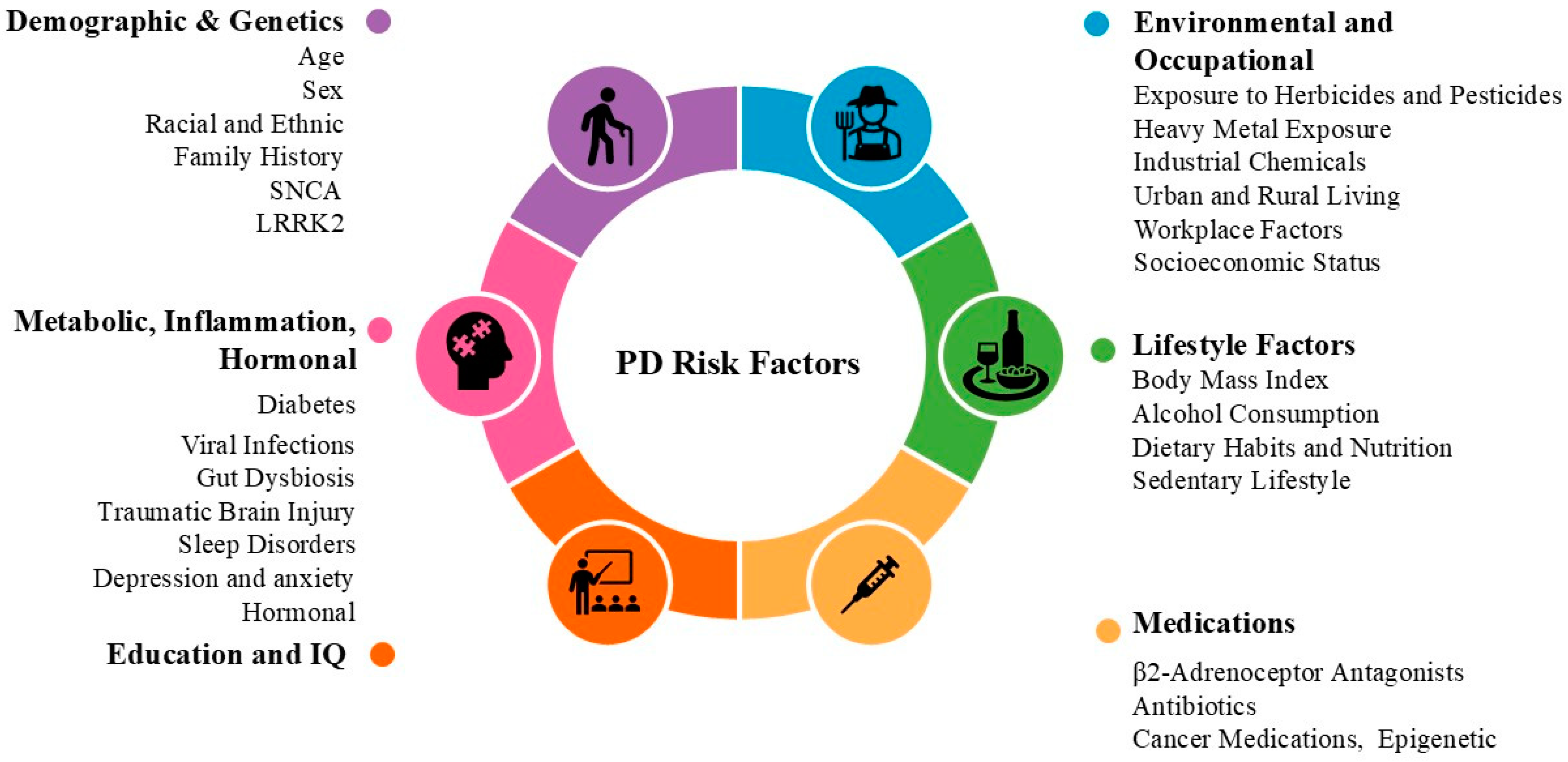
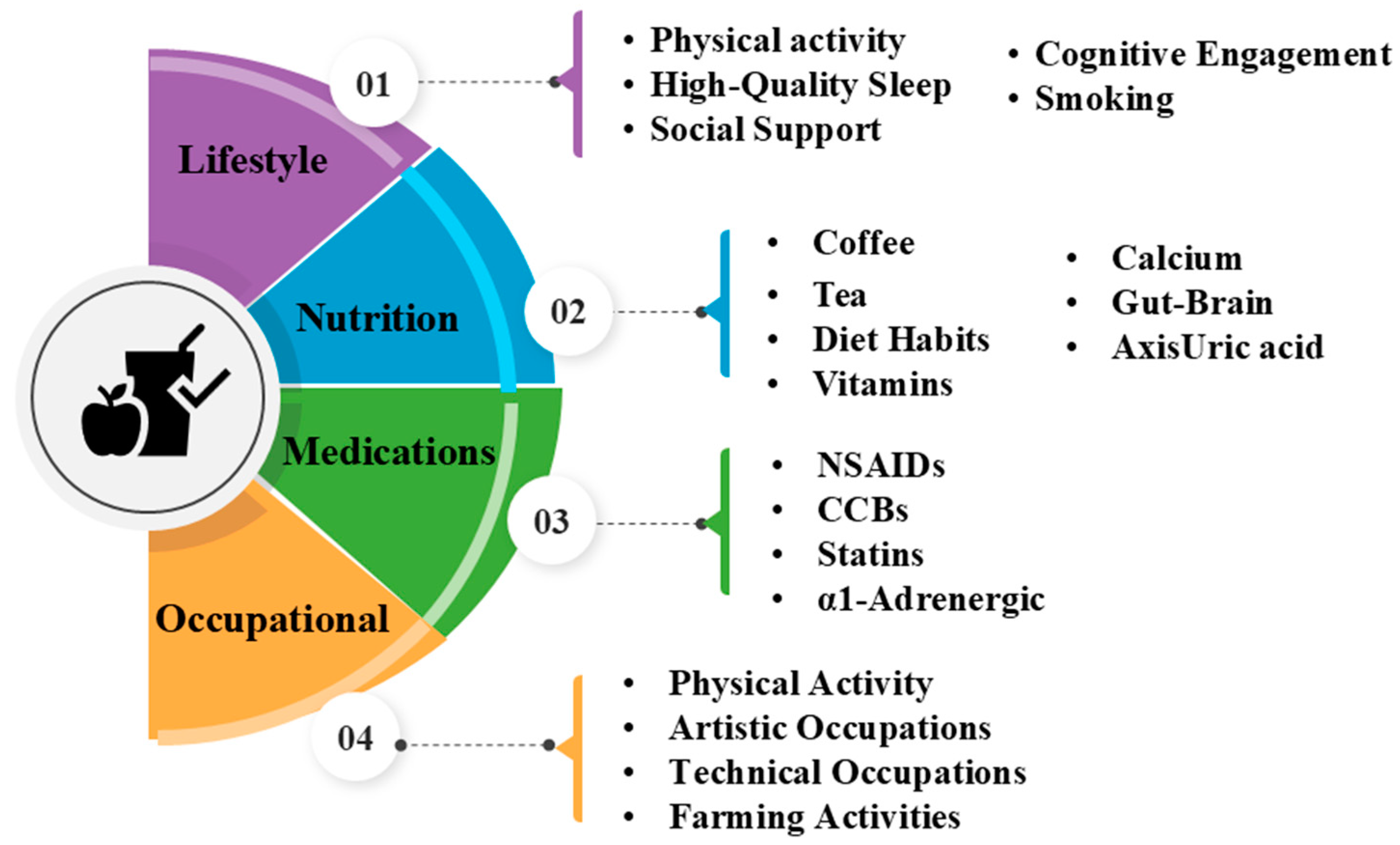
| Mechanism | Factors |
|---|---|
| α-Synuclein Aggregation | - Genetics: SNCA gene mutation (early-onset PD, Lewy body formation) |
| - Environmental: Heavy metals (mercury, manganese, lead) | |
| - Infections: Viral infections (trigger immune responses) | |
| - Gut Dysbiosis: Disrupts gut–brain axis, promotes misfolding | |
| - Non-Motor Symptoms: Anosmia, REM sleep behavior disorder | |
| Oxidative Stress | - Aging: Natural increase in oxidative damage |
| - Genetics: PARK7 (DJ-1) mutation (oxidative stress defense failure) | |
| - Environmental: Pesticides, herbicides, heavy metals, air pollution | |
| - Metabolic Factors: Low LDL-C (loss of antioxidant protection) | |
| - Mixed Effects: Hyperuricemia (antioxidant but linked to decline) | |
| Ferroptosis | - Aging: Iron accumulation in substantia nigra |
| - Heavy Metals: Iron, manganese, lead (redox imbalance) | |
| - Dietary Factors: Dairy consumption (potential increased iron load) | |
| - Industrial Chemicals: Solvents (trichloroethylene) | |
| Mitochondrial Dysfunction | - Genetics: PINK1, PRKN (Parkin) mutations (defective mitophagy) |
| - Environmental: Herbicides, pesticides, heavy metals, solvents | |
| - Metabolic Factors: Insulin dysregulation, diabetes medications | |
| Neuroinflammation | - Aging: Increased pro-inflammatory cytokines |
| - Sex Differences: Men at higher risk (estrogen is neuroprotective) | |
| - Genetics: PARK7 (DJ-1) gene (linked to inflammation) | |
| - Infections: Viral infections, chronic inflammatory diseases | |
| - Environmental: Heavy metals, air pollution | |
| - Psychological: Chronic stress, depression, anxiety (HPA axis) | |
| Gut Dysbiosis | - Gut–Brain Axis: Links gastrointestinal and neurodegenerative issues |
| - Chronic Constipation: Early symptom, linked to PD progression | |
| - Antibiotic Use: Macrolides, lincosamides (gut microbiome disruption) | |
| - Probiotics/Diet: Possible protective role in PD |
| Factor | Recommendation for Healthy People | Recommendation for PD Patients | Health System Consideration |
|---|---|---|---|
| Age | Regular screenings for neurodegenerative markers, cognitive assessments | Regular check-ups to monitor disease progression and adjust treatment | Prioritize neurodegenerative screenings for older adults |
| Sex | Engage in regular screening, focus on motor and non-motor symptoms | Tailored care for gender-specific risks | Targeted health campaigns focusing on men for early diagnosis |
| Race and Ethnicity | Participate in community-based health programs and screenings | Targeted interventions for early diagnosis and treatment access | Implement targeted screenings for high-risk racial and ethnic groups |
| Family History and Genetics | Consider genetic counseling and predictive testing (e.g., LRRK2) | Explore targeted therapies for genetically predisposed individuals | Offer genetic counseling and predictive testing for families with PD history |
| Cognitive and Educational Factors | Engage in mentally stimulating activities (e.g., reading, memory exercises) | Incorporate cognitive therapies and brain-training exercises | Encourage educational programs and cognitive activities for brain health |
| Exposure to Toxins | Use protective gear in high-risk occupations and reduce toxin exposure | Regular consultations to monitor toxin exposure and neurological health | Advocate for safer pesticide use, enforce safety regulations in industries |
| Air Pollution | Use air purifiers and masks in high-pollution areas | Focus on reducing exposure to air pollutants | Promote clean air policies and raise awareness about air pollution risks |
| Socioeconomic Disparities | Seek affordable preventive screenings | Expand access to healthcare for underserved communities | Offer subsidized screenings and care in underserved areas |
| Nutrition and Diet | Adopt a diet rich in antioxidants, healthy fats, and anti-inflammatory foods | Follow a specialized diet with omega-3s, antioxidants, and vitamin E | Encourage nutritional counseling for those at risk and PD patients |
| Physical Activity | Engage in aerobic and balance-enhancing exercises | Regular physical therapy and exercise regimens to manage motor symptoms | Promote physical activity and integrate therapy into PD management plans |
| Alcohol Consumption | Limit excessive alcohol intake | Consult healthcare providers to monitor alcohol use and interactions | Educate on the risks of excessive alcohol intake and offer reduction resources |
| Body Weight and Metabolic Health | Maintain a healthy weight through diet and exercise | Monitor BMI and metabolic health to manage obesity-related risks | Regular metabolic health and BMI screenings as part of preventive care |
| Diabetes and Hypertension | Maintain blood sugar levels and blood pressure | Manage blood pressure and glucose levels to support neurological function | Emphasize managing diabetes and hypertension in PD prevention and care |
| Cholesterol and Triglycerides | Manage lipid levels with diet and medications | Ensure cholesterol and triglyceride levels are in check to support brain health | Regular lipid screenings as part of PD management |
| Metabolic Syndrome | Address with weight management and healthier lifestyle choices | Manage weight and diet to reduce inflammation and support brain health | Provide metabolic screenings and counseling for those at risk |
| Neuroinflammation and Chronic Diseases | Anti-inflammatory treatments: Consider incorporating anti-inflammatory foods (e.g., curcumin, flavonoids) into the diet to reduce inflammation. | Anti-inflammatory treatments: PD patients could benefit from anti-inflammatory medications (e.g., NSAIDs) or dietary supplements to help manage PD-related inflammation. | Promote research into anti-inflammatory treatments and provide guidance on inflammation management for PD patients. |
| Chronic Inflammatory Diseases & Autoimmune Conditions | Manage chronic inflammatory diseases like arthritis early to prevent complications that may contribute to neurodegeneration. | Manage autoimmune conditions with appropriate treatments to reduce inflammation and minimize their impact on neurological health. | Provide integrated care between neurologists and specialists in autoimmune diseases to ensure better management of PD-related inflammation. |
| Preventive Vaccinations | Get regular vaccinations (e.g., flu shots) to prevent infections that may increase the risk of neurodegeneration. | Preventive vaccinations and antiviral therapies may reduce the risk of infections that can exacerbate PD symptoms. | Ensure access to vaccines and antiviral treatments for PD patients to reduce the likelihood of infection-induced complications. |
| Traumatic Brain Injury (TBI) | Follow safety measures in high-risk occupations (e.g., wearing helmets in contact sports or construction). | Monitor for any history of traumatic brain injury, as it may accelerate neurodegeneration in PD patients. | Implement policies for mandatory concussion screening and neurocognitive assessments for individuals in high-risk occupations. |
| Sleep Disorders | Prioritize good sleep hygiene and seek medical advice for sleep disturbances. | Screen for sleep disorders like REM sleep behavior disorder and use treatments like melatonin or CPAP therapy to improve sleep quality. | Incorporate sleep disorder screenings into routine neurological assessments for both healthy individuals at risk and PD patients. |
| Loss of Smell (Anosmia) | Stay aware of any early signs of anosmia and seek medical consultation if symptoms develop. | Use routine smell tests during neurological assessments to detect high-risk individuals and diagnose early PD. | Offer routine smell tests as part of early PD detection, especially for individuals at risk of developing PD. |
| Stress, Depression, and Anxiety | Manage chronic stress through lifestyle changes, including exercise and mindfulness practices. | Engage in psychological support like cognitive behavioral therapy (CBT) and consider medications (e.g., SSRIs) to manage depression and anxiety. | Integrate mental health support services into PD care and emphasize early interventions for stress and depression in both healthy individuals and PD patients. |
| Gut–Brain Axis and Microbiome Health | Maintain a healthy gut by consuming a diet rich in prebiotics, probiotics, and fiber to support brain health. | Work with both neurologists and gastroenterologists to identify gut dysbiosis early and incorporate gut health therapies such as probiotics and dietary changes. | Promote interdisciplinary collaboration between neurologists and gastroenterologists to monitor and manage the gut–brain connection in PD patients. |
| Estrogen and Neuroprotection | Postmenopausal women should consult with their healthcare providers about the potential benefits of hormone replacement therapy (HRT). | Postmenopausal women with PD may benefit from HRT for neuroprotection and should discuss this option with their healthcare providers. | Offer regular hormonal evaluations and consider HRT for postmenopausal women at risk of or diagnosed with PD to support brain health. |
| Uric Acid Levels | Maintain healthy uric acid levels through dietary modifications (e.g., reducing purine-rich foods). | Clinicians should monitor uric acid levels in PD patients and consider medication if levels are elevated to reduce neurodegenerative risks. | Regularly monitor uric acid levels in at-risk individuals and PD patients as part of preventive care. |
| Neuroprotective Drug Strategies | Consider discussing emerging treatments, such as GLP-1 receptor agonists, with a healthcare provider if at high risk for PD. | For PD patients, emerging drugs like GLP-1 receptor agonists or β2-adrenoceptor antagonists could be considered as part of the treatment plan. | Encourage research into new neuroprotective drug therapies, and integrate emerging treatments into clinical practice for PD prevention and management. |
| Long-Term Drug Use | Be aware of the potential neurological effects of long-term drug use and discuss any concerns with healthcare providers. | Monitor the neurological effects of long-term medications and consider safer alternatives if necessary. | provide regular assessments of the long-term effects of medications and offer alternative therapies when available. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beheshti, I. Exploring Risk and Protective Factors in Parkinson’s Disease. Cells 2025, 14, 710. https://doi.org/10.3390/cells14100710
Beheshti I. Exploring Risk and Protective Factors in Parkinson’s Disease. Cells. 2025; 14(10):710. https://doi.org/10.3390/cells14100710
Chicago/Turabian StyleBeheshti, Iman. 2025. "Exploring Risk and Protective Factors in Parkinson’s Disease" Cells 14, no. 10: 710. https://doi.org/10.3390/cells14100710
APA StyleBeheshti, I. (2025). Exploring Risk and Protective Factors in Parkinson’s Disease. Cells, 14(10), 710. https://doi.org/10.3390/cells14100710






