Harmonizing the Gut Microbiome and Cellular Immunotherapies: The Next Leap in Cancer Treatment
Abstract
:1. Current Landscape of the Gut Microbiome and Immunotherapies
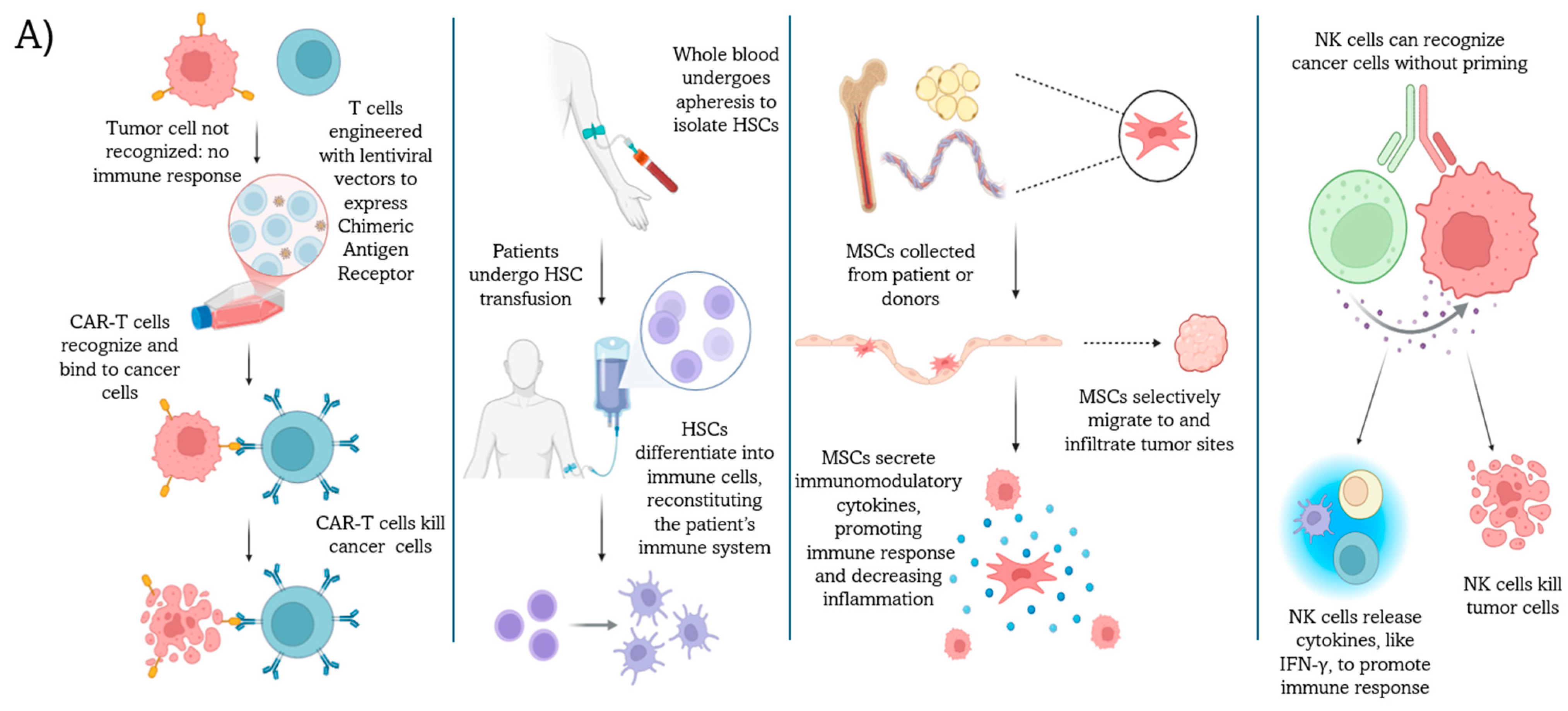
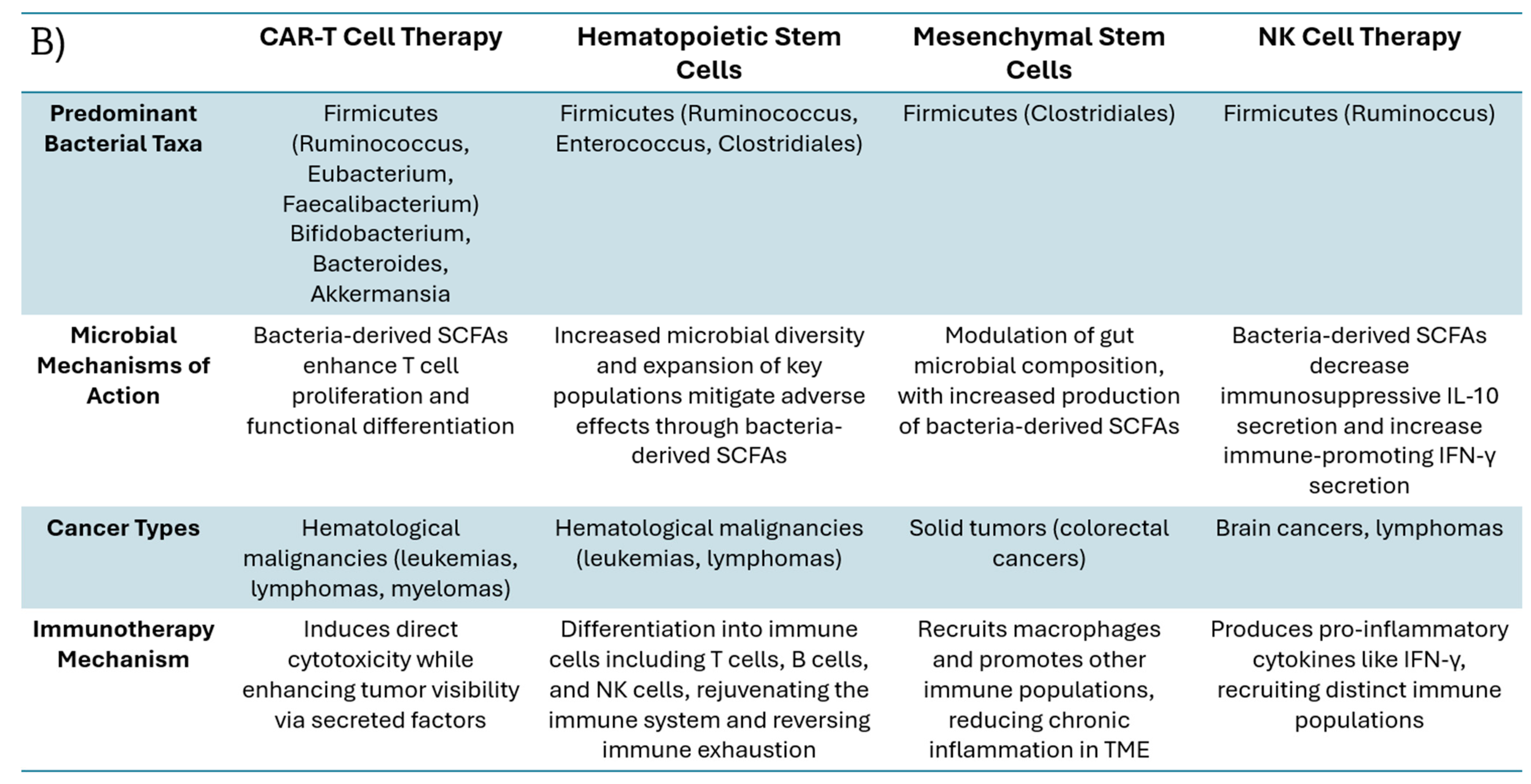
2. Cell-Based Immunotherapies and Influential Gut Bacteria Populations
3. Short-Chain Fatty Acids (SCFAs) and Other Metabolites: How Bacteria Affect Cellular Immunotherapies
4. The Role of Antibiotics in Cell-Based Immunotherapies
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E. Diversity of bacteria within the human gut and its contribution to the functional unity of holobionts. NPJ Biofilms Microbiomes 2024, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Larkinson, M.L.Y.; Clarke, T.B. Immunological design of commensal communities to treat intestinal infection and inflammation. PLoS Pathog. 2021, 17, e1009191. [Google Scholar] [CrossRef]
- Caballero, S.; Pamer, E.G. Microbiota-mediated inflammation and antimicrobial defense in the intestine. Annu. Rev. Immunol. 2015, 33, 227–256. [Google Scholar] [CrossRef]
- Sarkar, D.; Fisher, P.B. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006, 236, 13–23. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef]
- Sun, J.Y.; Yin, T.L.; Zhou, J.; Xu, J.; Lu, X.J. Gut microbiome and cancer immunotherapy. J. Cell Physiol. 2020, 235, 4082–4088. [Google Scholar] [CrossRef]
- Li, W.; Deng, Y.; Chu, Q.; Zhang, P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019, 447, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kiousi, D.E.; Kouroutzidou, A.Z.; Neanidis, K.; Karavanis, E.; Matthaios, D.; Pappa, A.; Galanis, A. The Role of the Gut Microbiome in Cancer Immunotherapy: Current Knowledge and Future Directions. Cancers 2023, 15, 2101. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shah, K. The Potential of the Gut Microbiome to Reshape the Cancer Therapy Paradigm: A Review. JAMA Oncol. 2022, 8, 1059–1067. [Google Scholar] [CrossRef]
- Huang, C.; Li, M.; Liu, B.; Zhu, H.; Dai, Q.; Fan, X.; Mehta, K.; Huang, C.; Neupane, P.; Wang, F.; et al. Relating Gut Microbiome and Its Modulating Factors to Immunotherapy in Solid Tumors: A Systematic Review. Front. Oncol. 2021, 11, 642110. [Google Scholar] [CrossRef]
- Stein-Thoeringer, C.K.; Saini, N.Y.; Zamir, E.; Blumenberg, V.; Schubert, M.L.; Mor, U.; Fante, M.A.; Schmidt, S.; Hayase, E.; Hayase, T.; et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat. Med. 2023, 29, 906–916. [Google Scholar] [CrossRef]
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. ‘Off-the-shelf’ allogeneic CAR T cells: Development and challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef]
- Li, C.; Zhao, H.; Cheng, L.; Wang, B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021, 11, 187. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Du, M.; Jiang, H.; Luo, W.; Tang, L.; Kang, Y.; Xu, J.; Wu, Z.; Wang, X.; et al. Allogenic and autologous anti-CD7 CAR-T cell therapies in relapsed or refractory T-cell malignancies. Blood Cancer J. 2023, 13, 61. [Google Scholar] [CrossRef]
- Claude Gorin, N. Autologous stem cell transplantation versus alternative allogeneic donor transplants in adult acute leukemias. Semin. Hematol 2016, 53, 103–110. [Google Scholar] [CrossRef]
- Yano, M.; Sharpe, C.; Lance, J.R.; Ravikrishnan, J.; Zapolnik, K.; Mo, X.; Woyach, J.A.; Sampath, D.; Kittai, A.S.; Vasu, S.; et al. Evaluation of allogeneic and autologous membrane-bound IL-21-expanded NK cells for chronic lymphocytic leukemia therapy. Blood Adv. 2022, 6, 5641–5654. [Google Scholar] [CrossRef]
- Smith, M.; Dai, A.; Ghilardi, G.; Amelsberg, K.V.; Devlin, S.M.; Pajarillo, R.; Slingerland, J.B.; Beghi, S.; Herrera, P.S.; Giardina, P.; et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 2022, 28, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, J.; Ni, F.; Yang, Z.; Gui, X.; Bao, Z.; Zhao, H.; Wei, G.; Wang, Y.; Zhang, M.; et al. CAR-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic malignancies. Nat. Commun. 2022, 13, 5313. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Rehman, L.; Rehman, A.; Darbaniyan, F.; Weber, D.M.; Becnel, M.; Gaballa, M.R.; Thomas, S.K.; Lee, H.C.; Chang, C.C.; et al. Longitudinal analysis of gut microbiome and metabolome correlates of response and toxicity with idecabtagene vicleucel. Blood Adv. 2025. ahead of print. [Google Scholar] [CrossRef]
- Peled, J.U.; Gomes, A.L.C.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef]
- Ingham, A.C.; Kielsen, K.; Cilieborg, M.S.; Lund, O.; Holmes, S.; Aarestrup, F.M.; Muller, K.G.; Pamp, S.J. Specific gut microbiome members are associated with distinct immune markers in pediatric allogeneic hematopoietic stem cell transplantation. Microbiome 2019, 7, 131. [Google Scholar] [CrossRef]
- He, R.; Han, C.; Li, Y.; Qian, W.; Hou, X. Cancer-Preventive Role of Bone Marrow-Derived Mesenchymal Stem Cells on Colitis-Associated Colorectal Cancer: Roles of Gut Microbiota Involved. Front. Cell Dev. Biol. 2021, 9, 642948. [Google Scholar] [CrossRef]
- Liu, A.; Liang, X.; Wang, W.; Wang, C.; Song, J.; Guo, J.; Sun, D.; Wang, D.; Song, M.; Qian, J.; et al. Human umbilical cord mesenchymal stem cells ameliorate colon inflammation via modulation of gut microbiota-SCFAs-immune axis. Stem. Cell Res. Ther. 2023, 14, 271. [Google Scholar] [CrossRef]
- Takamiya, S.; Kawabori, M.; Yamazaki, K.; Yamaguchi, S.; Tanimori, A.; Yamamoto, K.; Ohnishi, S.; Seki, T.; Konno, K.; Tha, K.K.; et al. Intravenous transplantation of amnion-derived mesenchymal stem cells promotes functional recovery and alleviates intestinal dysfunction after spinal cord injury. PLoS ONE 2022, 17, e0270606. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, Q.; Lu, D.; Yue, H.; Zhang, J.; Li, Y.; Zhang, B.; Li, X.; Cao, M. Human mesenchymal stem cells treatment improved hepatic lesions and reversed gut microbiome disorder in non-alcoholic steatohepatitis. Aging 2020, 12, 21660–21673. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Zhao, L.; Zhang, J.; Sun, W.; Chu, J.; Zhao, H.; Yang, C.; Yan, S.; Chen, X.; et al. Unraveling the interplay between mesenchymal stem cells, gut microbiota, and systemic sclerosis: Therapeutic implications. Microbiol. Spectr. 2025. ahead of print. [Google Scholar] [CrossRef]
- Ocansey, D.K.W.; Wang, L.; Wang, J.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Mesenchymal stem cell-gut microbiota interaction in the repair of inflammatory bowel disease: An enhanced therapeutic effect. Clin. Transl. Med. 2019, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Suo, C.; Gu, X.; Shen, S.; Lin, K.; Zhu, C.; Yan, K.; Bian, Z.; Chen, L.; Zhang, T.; et al. AKR1D1 suppresses liver cancer progression by promoting bile acid metabolism-mediated NK cell cytotoxicity. Cell Metab. 2025, 37, 1103–1118. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Du, M.; Li, X.; Gao, J.; Li, Q.; Li, H.; Li, J.; Gao, X.; Cong, H.; Huang, Y.; et al. Upregulation of Lactobacillus spp. in gut microbiota as a novel mechanism for environmental eustress-induced anti-pancreatic cancer effects. Gut. Microbes 2025, 17, 2470372. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Xiang, M.; Tan, J.; Macia, L. Chapter 11—Fatty Acids, Gut Bacteria, and Immune Cell Function. In The Molecular Nutrition of Fats; Patel, V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 151–164. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e4114. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Perez, M.; Buey, B.; Corral, P.; Giraldos, D.; Latorre, E. Microbiota-Derived Short-Chain Fatty Acids Boost Antitumoral Natural Killer Cell Activity. J. Clin. Med. 2024, 13, 3885. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Arroyo, A.; Garcia-Vicente, R.; Morales, M.L.; Gomez-Gordo, R.; Justo, P.; Cuellar, C.; Sanchez-Pina, J.; Lopez, N.; Alonso, R.; et al. Short-Chain Fatty Acid Production by Gut Microbiota Predicts Treatment Response in Multiple Myeloma. Clin. Cancer Res. 2024, 30, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Rehman, A.; Rehman, L.; Darbaniyan, F.; Blumenberg, V.; Schubert, M.L.; Mor, U.; Zamir, E.; Schmidt, S.; Hayase, T.; et al. Antibiotic-induced loss of gut microbiome metabolic output correlates with clinical responses to CAR T-cell therapy. Blood 2025, 145, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Sanmarco, L.M.; Wheeler, M.A.; Gutierrez-Vazquez, C.; Polonio, C.M.; Linnerbauer, M.; Pinho-Ribeiro, F.A.; Li, Z.; Giovannoni, F.; Batterman, K.V.; Scalisi, G.; et al. Gut-licensed IFNgamma(+) NK cells drive LAMP1(+)TRAIL(+) anti-inflammatory astrocytes. Nature 2021, 590, 473–479. [Google Scholar] [CrossRef]
- Mowat, C.; Dhatt, J.; Bhatti, I.; Hamie, A.; Baker, K. Short chain fatty acids prime colorectal cancer cells to activate antitumor immunity. Front. Immunol. 2023, 14, 1190810. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.; Freifeld, A. When to Use Prophylactic Antibiotics in Neutropenic Patients. Oncology 2016, 30, 838. [Google Scholar]
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.G.; Chen, T. Antibiotics for cancer treatment: A double-edged sword. J. Cancer 2020, 11, 5135–5149. [Google Scholar] [CrossRef]
- Fishbein, S.R.S.; Mahmud, B.; Dantas, G. Antibiotic perturbations to the gut microbiome. Nat. Rev. Microbiol. 2023, 21, 772–788. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrington, K.; Shah, K. Harmonizing the Gut Microbiome and Cellular Immunotherapies: The Next Leap in Cancer Treatment. Cells 2025, 14, 708. https://doi.org/10.3390/cells14100708
Harrington K, Shah K. Harmonizing the Gut Microbiome and Cellular Immunotherapies: The Next Leap in Cancer Treatment. Cells. 2025; 14(10):708. https://doi.org/10.3390/cells14100708
Chicago/Turabian StyleHarrington, Kendall, and Khalid Shah. 2025. "Harmonizing the Gut Microbiome and Cellular Immunotherapies: The Next Leap in Cancer Treatment" Cells 14, no. 10: 708. https://doi.org/10.3390/cells14100708
APA StyleHarrington, K., & Shah, K. (2025). Harmonizing the Gut Microbiome and Cellular Immunotherapies: The Next Leap in Cancer Treatment. Cells, 14(10), 708. https://doi.org/10.3390/cells14100708





