Unlocking the Role of Metabolic Pathways in Brain Metastatic Disease
Abstract
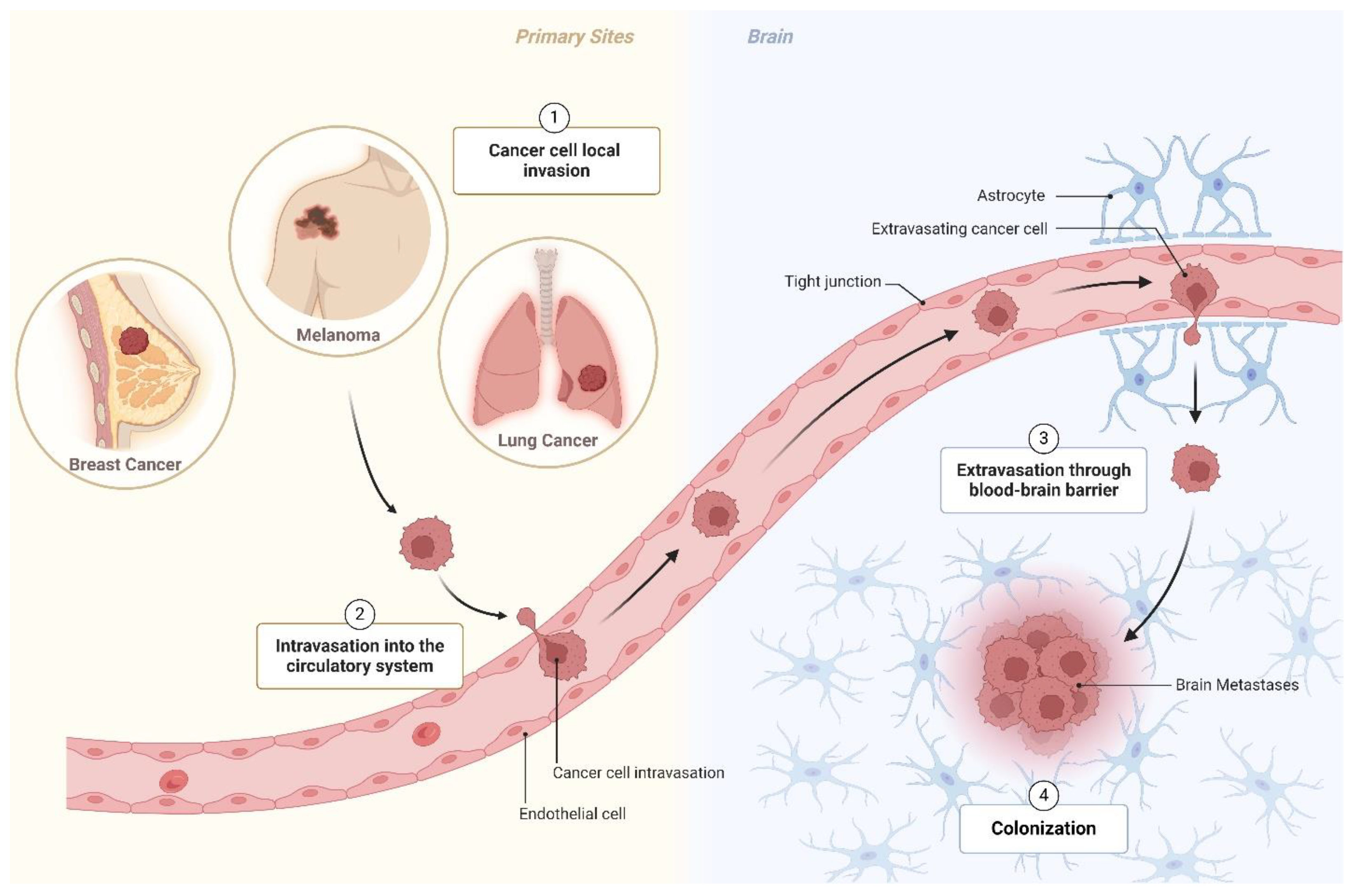
1. Introduction
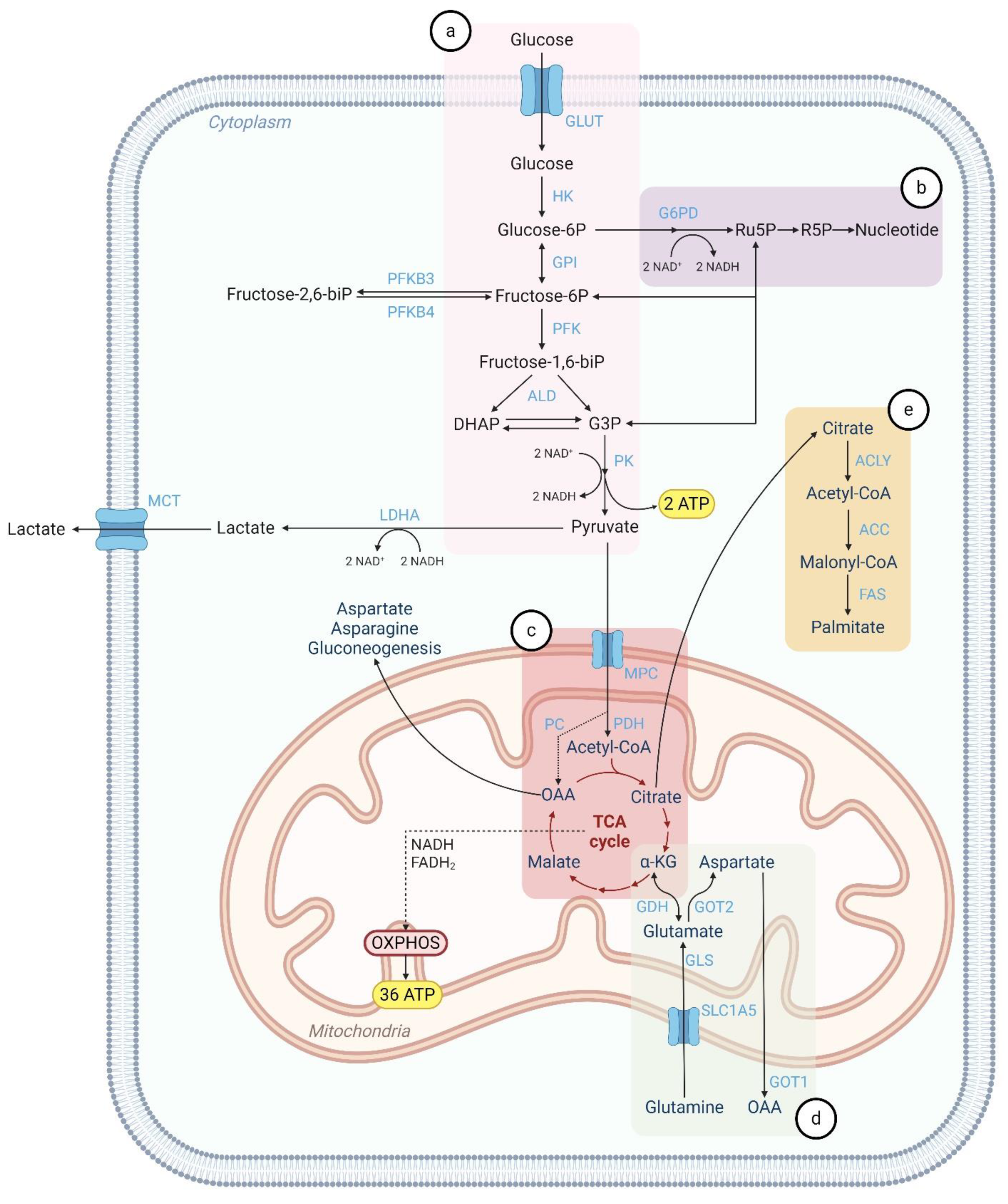
2. Metabolic Pathways in Normal Cells
2.1. Glycolysis
2.2. Pentose Phosphate Pathway (PPP)
2.3. Mitochondrial Metabolism
2.4. Glutaminolysis
2.5. Lipid Metabolism
3. Metabolic Reprogramming in Cancer Cells
4. Metabolic Adaptation in Brain Metastatic Disease
4.1. Glycolysis and TCA Cycle
4.2. Glutaminolysis
4.3. Lipid Metabolism
4.4. Metabolic Interactions Between the Brain Microenvironment and BM
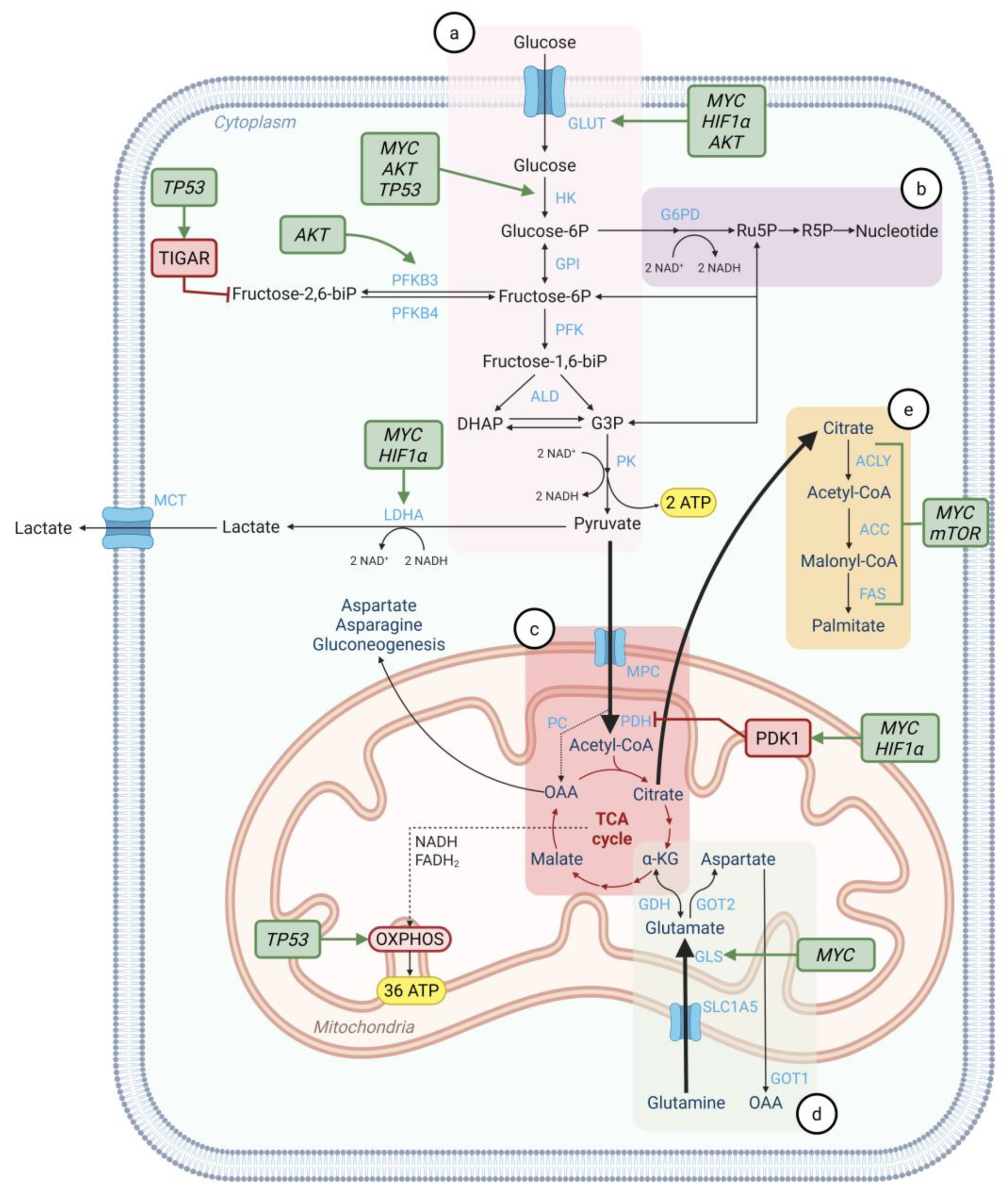
5. Regulation of Cancer Metabolism by Oncogenes and Tumor Suppressors
6. Current Preclinical Models of Brain Metastasis to Test New Therapies
6.1. In Vitro Models
6.2. In Vivo Models
7. Targeting Metabolic Mediators for Future BM Therapeutics
8. Discussion and Future Directions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Mani, K.; Deng, D.; Lin, C.; Wang, M.; Hsu, M.L.; Zaorsky, N.G. Causes of Death among People Living with Metastatic Cancer. Nat. Commun. 2024, 15, 1519. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The Distribution of Secondary Growths in Cancer of the Breast. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Izadi, N.; Solár, P.; Hašanová, K.; Zamani, A.; Akbar, M.S.; Mrázová, K.; Bartošík, M.; Kazda, T.; Hrstka, R.; Joukal, M. Breaking Boundaries: Role of the Brain Barriers in Metastatic Process. Fluids Barriers CNS 2025, 22, 3. [Google Scholar] [CrossRef]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S. Brain Metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef]
- Bergers, G.; Fendt, S.M. The Metabolism of Cancer Cells during Metastasis. Nat. Rev. Cancer 2021, 21, 162–180. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Otto Warburg, B.; Wind, F.; Negelein, N. The Metabolism of Tumors in The Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Barba, I.; Carrillo-Bosch, L.; Seoane, J. Targeting the Warburg Effect in Cancer: Where Do We Stand? Int. J. Mol. Sci. 2024, 25, 3142. [Google Scholar] [CrossRef]
- Jaworska, M.; Szczudło, J.; Pietrzyk, A.; Shah, J.; Trojan, S.E.; Ostrowska, B.; Kocemba-Pilarczyk, K.A. The Warburg Effect: A Score for Many Instruments in the Concert of Cancer and Cancer Niche Cells. Pharmacol. Rep. 2023, 75, 876–890. [Google Scholar] [CrossRef]
- Xiang, S.; Gu, H.; Jin, L.; Thorne, R.F.; Zhang, X.D.; Wu, M. LncRNA IDH1-AS1 Links the Functions of c-Myc and HIF1α via IDH1 to Regulate the Warburg Effect. Proc. Natl. Acad. Sci. USA 2018, 115, E1465–E1474. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Byun, J.K.; Choi, Y.K.; Park, K.G. Targeting Glutamine Metabolism as a Therapeutic Strategy for Cancer. Exp. Mol. Med. 2023, 55, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Lamba, N.; Catalano, P.J.; Bi, W.L.; Wen, P.Y.; Haas-Kogan, D.A.; Cagney, D.N.; Aizer, A.A. Predictors of Long-Term Survival among Patients with Brain Metastases. Neuro Oncol. 2022, 24, 494–496. [Google Scholar] [CrossRef]
- Lamba, N.; Wen, P.Y.; Aizer, A.A. Epidemiology of Brain Metastases and Leptomeningeal Disease. Neuro Oncol. 2021, 23, 1447–1456. [Google Scholar] [CrossRef]
- Hügel, M.; Stöhr, J.; Kuhnt, T.; Nägler, F.; Papsdorf, K.; Klagges, S.; Hambsch, P.; Güresir, E.; Nicolay, N.H.; Seidel, C. Long-Term Survival in Patients with Brain Metastases—Clinical Characterization of a Rare Scenario. Strahlenther. Onkol. 2023, 200, 335–345. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Schur, S.; Füreder, L.M.; Gatterbauer, B.; Dieckmann, K.; Widhalm, G.; Hainfellner, J.; Zielinski, C.C.; Birner, P.; Bartsch, R.; et al. Descriptive Statistical Analysis of a Real Life Cohort of 2419 Patients with Brain Metastases of Solid Cancers. ESMO Open 2016, 1, 24. [Google Scholar] [CrossRef]
- Chen, E.I.; Hewel, J.; Krueger, J.S.; Tiraby, C.; Weber, M.R.; Kralli, A.; Becker, K.; Yates, J.R.; Felding-Habermann, B. Adaptation of Energy Metabolism in Breast Cancer Brain Metastases. Cancer Res. 2007, 67, 1472–1486. [Google Scholar] [CrossRef]
- Palmieri, D.; Fitzgerald, D.; Shreeve, S.M.; Hua, E.; Bronder, J.L.; Weil, R.J.; Davis, S.; Stark, A.M.; Merino, M.J.; Kurek, R.; et al. Analyses of Resected Human Brain Metastases of Breast Cancer Reveal the Association between Up-Regulation of Hexokinase 2 and Poor Prognosis. Mol. Cancer Res. 2009, 7, 1438–1445. [Google Scholar] [CrossRef]
- McGeehan, R.E.; Cockram, L.A.; Littlewood, D.T.J.; Keatley, K.; Eccles, D.M.; An, Q. Deep Sequencing Reveals the Mitochondrial DNA Variation Landscapes of Breast-to-Brain Metastasis Blood Samples. Mitochondrial DNA A DNA Mapp. Seq. Anal. 2018, 29, 703–713. [Google Scholar] [CrossRef]
- Fischer, G.M.; Jalali, A.; Kircher, D.A.; Lee, W.C.; McQuade, J.L.; Haydu, L.E.; Joon, A.Y.; Reuben, A.; de Macedo, M.P.; Carapeto, F.C.L.; et al. Molecular Profiling Reveals Unique Immune and Metabolic Features of Melanoma Brain Metastases. Cancer Discov. 2019, 9, 628–645. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, K.; Malgulwar, P.B.; Fischer, G.M.; Hu, X.; Mao, X.; Song, X.; Hernandez, S.D.; Zhang, X.H.F.; Zhang, J.; Parra, E.R.; et al. Multi-Omic Molecular Profiling Reveals Potentially Targetable Abnormalities Shared across Multiple Histologies of Brain Metastasis. Acta Neuropathol. 2021, 141, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Kamer, I.; Steuerman, Y.; Daniel-Meshulam, I.; Perry, G.; Izraeli, S.; Perelman, M.; Golan, N.; Simansky, D.; Barshack, I.; Nun, A.B.; et al. Predicting Brain Metastasis in Early Stage Non-Small Cell Lung Cancer Patients by Gene Expression Profiling. Transl. Lung Cancer Res. 2020, 9, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lee, H.J.; Wu, X.; Huo, L.; Kim, S.J.; Xu, L.; Wang, Y.; He, J.; Bollu, L.R.; Gao, G.; et al. Gain of Glucose-Independent Growth upon Metastasis of Breast Cancer Cells to the Brain. Cancer Res. 2015, 75, 554–565. [Google Scholar] [CrossRef]
- Nygaard, V.; Prasmickaite, L.; Vasiliauskaite, K.; Clancy, T.; Hovig, E. Melanoma Brain Colonization Involves the Emergence of a Brain-Adaptive Phenotype. Oncoscience 2014, 1, 82–94. [Google Scholar] [CrossRef]
- Hyuk Yoon, J.; Seo, Y.; Suk Jo, Y.; Lee, S.; Cho, E.; Cazenave-Gassiot, A.; Shin, Y.-S.; Hee Moon, M.; Joo An, H.; Wenk, M.R.; et al. Brain Lipidomics: From Functional Landscape to Clinical Significance. Sci. Adv. 2022, 8, eadc9317. [Google Scholar]
- Jin, X.; Demere, Z.; Nair, K.; Ali, A.; Ferraro, G.B.; Natoli, T.; Deik, A.; Petronio, L.; Tang, A.A.; Zhu, C.; et al. A Metastasis Map of Human Cancer Cell Lines. Nature 2020, 588, 331–336. [Google Scholar] [CrossRef]
- Zhao, Q.; Lin, X.; Wang, G. Targeting SREBP-1-Mediated Lipogenesis as Potential Strategies for Cancer. Front. Oncol. 2022, 12, 952371. [Google Scholar] [CrossRef]
- Li, Y.Q.; Sun, F.Z.; Li, C.X.; Mo, H.N.; Zhou, Y.T.; Lv, D.; Zhai, J.T.; Qian, H.L.; Ma, F. RARRES2 Regulates Lipid Metabolic Reprogramming to Mediate the Development of Brain Metastasis in Triple Negative Breast Cancer. Mil. Med. Res. 2023, 10, 34. [Google Scholar] [CrossRef]
- Ferraro, G.B.; Ali, A.; Luengo, A.; Kodack, D.P.; Deik, A.; Abbott, K.L.; Bezwada, D.; Blanc, L.; Prideaux, B.; Jin, X.; et al. Fatty Acid Synthesis Is Required for Breast Cancer Brain Metastasis. Nat. Cancer 2021, 2, 414–428. [Google Scholar] [CrossRef]
- Cordero, A.; Kanojia, D.; Miska, J.; Panek, W.K.; Xiao, A.; Han, Y.; Bonamici, N.; Zhou, W.; Xiao, T.; Wu, M.; et al. FABP7 Is a Key Metabolic Regulator in HER2+ Breast Cancer Brain Metastasis. Oncogene 2019, 38, 6445–6460. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Watters, A.; Cheng, N.; Perry, C.E.; Xu, K.; Alicea, G.M.; Parris, J.L.D.; Baraban, E.; Ray, P.; Nayak, A.; et al. Polyunsaturated Fatty Acids from Astrocytes Activate PPARγ Signaling in Cancer Cells to Promote Brain Metastasis. Cancer Discov. 2019, 9, 1720–1735. [Google Scholar] [CrossRef]
- Deshpande, K.; Martirosian, V.; Nakamura, B.N.; Iyer, M.; Julian, A.; Eisenbarth, R.; Shao, L.; Attenello, F.; Neman, J. Neuronal Exposure Induces Neurotransmitter Signaling and Synaptic Mediators in Tumors Early in Brain Metastasis. Neuro Oncol. 2022, 24, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Michael, I.P.; Zhang, P.; Saghafinia, S.; Knott, G.; Jiao, W.; McCabe, B.D.; Galván, J.A.; Robinson, H.P.C.; Zlobec, I.; et al. Synaptic Proximity Enables NMDAR Signalling to Promote Brain Metastasis. Nature 2019, 573, 526–531. [Google Scholar] [CrossRef]
- Gan, S.; Macalinao, D.G.; Shahoei, S.H.; Tian, L.; Jin, X.; Basnet, H.; Bibby, C.; Muller, J.T.; Atri, P.; Seffar, E.; et al. Distinct Tumor Architectures and Microenvironments for the Initiation of Breast Cancer Metastasis in the Brain. Cancer Cell 2024, 42, 1693–1712.e24. [Google Scholar] [CrossRef]
- Okawa, T.; Hara, K.; Goto, M.; Kikuchi, M.; Kogane, M.; Hatakeyama, H.; Tanaka, H.; Shirane, D.; Akita, H.; Hisaka, A.; et al. Effects on Metabolism in Astrocytes Caused by Cgamp, Which Imitates the Initial Stage of Brain Metastasis. Int. J. Mol. Sci. 2021, 22, 9028. [Google Scholar] [CrossRef]
- Dong, Y.; Tu, R.; Liu, H.; Qing, G. Regulation of Cancer Cell Metabolism: Oncogenic MYC in the Driver’s Seat. Signal Transduct. Target. Ther. 2020, 5, 124. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.; Li, Y.; Wei, S.; He, G.; Liu, J.; Li, X.; Yang, S.; Li, D.; Lin, W.; et al. Glutamine Addiction in Tumor Cell: Oncogene Regulation and Clinical Treatment. Cell Commun. Signal. 2024, 22, 12. [Google Scholar] [CrossRef]
- Gao, P.; Tchernyshyov, I.; Chang, T.C.; Lee, Y.S.; Kita, K.; Ochi, T.; Zeller, K.I.; De Marzo, A.M.; Van Eyk, J.E.; Mendell, J.T.; et al. C-Myc Suppression of MiR-23a/b Enhances Mitochondrial Glutaminase Expression and Glutamine Metabolism. Nature 2009, 458, 762–765. [Google Scholar] [CrossRef]
- Gouw, A.M.; Margulis, K.; Liu, N.S.; Raman, S.J.; Mancuso, A.; Toal, G.G.; Tong, L.; Mosley, A.; Hsieh, A.L.; Sullivan, D.K.; et al. The MYC Oncogene Cooperates with Sterol-Regulated Element-Binding Protein to Regulate Lipogenesis Essential for Neoplastic Growth. Cell Metab. 2019, 30, 556–572.e5. [Google Scholar] [CrossRef]
- Lee, H.Y.; Cha, J.; Kim, S.K.; Park, J.H.; Song, K.H.; Kim, P.; Kim, M.Y. C-MYC Drives Breast Cancer Metastasis to the Brain, but Promotes Synthetic Lethality with TRAIL. Mol. Cancer Res. 2019, 17, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, M.; Xia, S.; Zeng, F.; Liu, Q. Systematic and Comprehensive Insights into HIF-1 Stabilization under Normoxic Conditions: Implications for Cellular Adaptation and Therapeutic Strategies in Cancer. Cell Mol. Biol. Lett. 2025, 30, 2. [Google Scholar] [CrossRef] [PubMed]
- Boulahbel, H.; Durán, R.V.; Gottlieb, E. Prolyl Hydroxylases as Regulators of Cell Metabolism. Biochem. Soc. Trans. 2009, 37, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Wicks, E.E.; Semenza, G.L. Hypoxia-Inducible Factors: Cancer Progression and Clinical Translation. J. Clin. Investig. 2022, 132, e159839. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, L.; Huang, B.; Liu, R.; Ren, B. Central Role of Hypoxia-Inducible Factor-1α in Metabolic Reprogramming of Cancer Cells: A Review. Medicine 2024, 103, e40273. [Google Scholar] [CrossRef]
- Ebright, R.Y.; Zachariah, M.A.; Micalizzi, D.S.; Wittner, B.S.; Niederhoffer, K.L.; Nieman, L.T.; Chirn, B.; Wiley, D.F.; Wesley, B.; Shaw, B.; et al. HIF1A Signaling Selectively Supports Proliferation of Breast Cancer in the Brain. Nat. Commun. 2020, 11, 6311. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT Network at the Interface of Oncogenic Signalling and Cancer Metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. MTOR Signaling Pathway and MTOR Inhibitors in Cancer: Progress and Challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Yu, L.; Wu, M.; Zhu, G.; Xu, Y. Emerging Roles of the Tumor Suppressor P53 in Metabolism. Front. Cell Dev. Biol. 2022, 9, 762742. [Google Scholar] [CrossRef]
- AlMaazmi, F.I.; Bou Malhab, L.J.; ElDohaji, L.; Saber-Ayad, M. Deciphering the Controversial Role of TP53 Inducible Glycolysis and Apoptosis Regulator (TIGAR) in Cancer Metabolism as a Potential Therapeutic Strategy. Cells 2025, 14, 598. [Google Scholar] [CrossRef]
- Stambolic, V.; Macpherson, D.; Sas, D.; Lin, Y.; Snow, B.; Jang, Y.; Benchimol, S.; Mak, T.W. Regulation of PTEN Transcription by P53 Onstrate an Active Role for PTEN in Regulation of the Life. Mol. Cell 2001, 8, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Lo Nigro, C.; Vivenza, D.; Monteverde, M.; Lattanzio, L.; Gojis, O.; Garrone, O.; Comino, A.; Merlano, M.; Quinlan, P.R.; Syed, N.; et al. High Frequency of Complex TP53 Mutations in CNS Metastases from Breast Cancer. Br. J. Cancer 2012, 106, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, E.S.; Deshpande, K.; Neman, J.; Winkler, F.; Khasraw, M. The Microenvironment of Brain Metastases from Solid Tumors. Neuro-Oncol. Adv. 2021, 3, v121–v132. [Google Scholar] [CrossRef]
- Petrovskaya, A.V.; Barykin, E.P.; Tverskoi, A.M.; Varshavskaya, K.B.; Mitkevich, V.A.; Petrushanko, I.Y.; Makarov, A.A. Blood–Brain Barrier Transwell Modeling. Mol. Biol. 2022, 56, 1020–1027. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, S.; Liu, M.; Liu, Y.; Ye, Z.; Zeng, J.; Yao, M. Experimental Models for Cancer Brain Metastasis. Cancer Pathog. Ther. 2024, 2, 15–23. [Google Scholar] [CrossRef]
- Liu, Q.; Bao, H.; Zhang, S.; Li, C.; Sun, G.; Sun, X.; Fu, T.; Wang, Y.; Liang, P. MicroRNA-522-3p Promotes Brain Metastasis in Non-Small Cell Lung Cancer by Targeting Tensin 1 and Modulating Blood-Brain Barrier Permeability. Exp. Cell Res. 2024, 442, 114199. [Google Scholar] [CrossRef]
- Yin, W.; Zhao, Y.; Kang, X.; Zhao, P.; Fu, X.; Mo, X.; Wan, Y.; Huang, Y. BBB-Penetrating Codelivery Liposomes Treat Brain Metastasis of Non-Small Cell Lung Cancer with EGFRT790M Mutation. Theranostics 2020, 10, 6122–6135. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, F.; Wang, G.; Li, Q. CXC Motif Chemokine Receptor Type 4 Disrupts Blood-Brain Barrier and Promotes Brain Metastasis Through Activation of the PI3K/AKT Pathway in Lung Cancer. World Neurosurg. 2022, 166, e369–e381. [Google Scholar] [CrossRef]
- Curtaz, C.J.; Schmitt, C.; Herbert, S.L.; Feldheim, J.; Schlegel, N.; Gosselet, F.; Hagemann, C.; Roewer, N.; Meybohm, P.; Wöckel, A.; et al. Serum-Derived Factors of Breast Cancer Patients with Brain Metastases Alter Permeability of a Human Blood-Brain Barrier Model. Fluids Barriers CNS 2020, 17, 31. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- Qu, F.; Brough, S.C.; Michno, W.; Madubata, C.J.; Hartmann, G.G.; Puno, A.; Drainas, A.P.; Bhattacharya, D.; Tomasich, E.; Lee, M.C.; et al. Crosstalk between Small-Cell Lung Cancer Cells and Astrocytes Mimics Brain Development to Promote Brain Metastasis. Nat. Cell Biol. 2023, 25, 1506–1519. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Nagayach, A.; Patel, H.; Dao, L.; Zhu, H.; Wasylishen, A.R.; Fan, Y.; Kendler, A.; Guo, Z. Utilizing Human Cerebral Organoids to Model Breast Cancer Brain Metastasis in Culture. Breast Cancer Res. 2024, 26, 108. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.S.; Kim, J.S.; Yeo, H.C.; Bae, C.M.; Han, H.J.; Baek, K.; Chang, W.; Lim, K.S.; Yun, S.P.; Shin, I.S.; et al. A Simple Metastatic Brain Cancer Model Using Human Embryonic Stem Cell-Derived Cerebral Organoids. FASEB J. 2020, 34, 16464–16475. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, V.; Linkous, A. Organoids as a Systems Platform for SCLC Brain Metastasis. Front. Oncol. 2022, 12, 881989. [Google Scholar] [CrossRef]
- Drouin, Z.; Lévesque, F.; Mouzakitis, K.; Labrie, M. Current Preclinical Models of Brain Metastasis. Clin. Exp. Metastasis 2025, 42, 5. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Duan, W.; Xia, S.; Wei, S.; Liu, W.; Wang, Q. Proteomic Reveals Reasons for Acquired Drug Resistance in Lung Cancer Derived Brain Metastasis Based on a Newly Established Multi-Organ Microfluidic Chip Model. Front. Bioeng. Biotechnol. 2020, 8, 612091. [Google Scholar] [CrossRef]
- Liu, W.; Song, J.; Du, X.; Zhou, Y.; Li, Y.; Li, R.; Lyu, L.; He, Y.; Hao, J.; Ben, J.; et al. AKR1B10 (Aldo-Keto Reductase Family 1 B10) Promotes Brain Metastasis of Lung Cancer Cells in a Multi-Organ Microfluidic Chip Model. Acta Biomater. 2019, 91, 195–208. [Google Scholar] [CrossRef]
- Faria, C.C.; Cascão, R.; Custódia, C.; Paisana, E.; Carvalho, T.; Pereira, P.; Roque, R.; Pimentel, J.; Miguéns, J.; Cortes-Ciriano, I.; et al. Patient-Derived Models of Brain Metastases Recapitulate Human Disseminated Disease. Cell Rep. Med. 2022, 3, 100623. [Google Scholar] [CrossRef]
- Lin, X.; DeAngelis, L.M. Treatment of Brain Metastases. J. Clin. Oncol. 2015, 33, 3475–3484. [Google Scholar] [CrossRef]
- Ene, C.I.; Ferguson, S.D. Surgical Management of Brain Metastasis: Challenges and Nuances. Front. Oncol. 2022, 12, 847110. [Google Scholar] [CrossRef]
- Sung, K.S. Clinical Practice Guidelines for Brain Metastasis from Solid Tumors. Brain Tumor Res. Treat. 2024, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, E.M.; Coats, B.S.; Shroads, A.L.; Langaee, T.; Lew, A.; Forder, J.R.; Shuster, J.J.; Wagner, D.A.; Stacpoole, P.W. Phase 1 Trial of Dichloroacetate (DCA) in Adults with Recurrent Malignant Brain Tumors. Investig. New Drugs 2014, 32, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R.; Lee, C.T.; Lopaschuk, G.D.; Puttagunta, L.; Bonnet, S.; et al. A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth. Cancer Cell 2007, 11, 37–51. [Google Scholar] [CrossRef]
- Koltai, T.; Fliegel, L. Dichloroacetate for Cancer Treatment: Some Facts and Many Doubts. Pharmaceuticals 2024, 17, 744. [Google Scholar] [CrossRef] [PubMed]
- Serhan, H.A.; Bao, L.; Cheng, X.; Qin, Z.; Liu, C.J.; Heth, J.A.; Udager, A.M.; Soellner, M.B.; Merajver, S.D.; Morikawa, A.; et al. Targeting Fatty Acid Synthase in Preclinical Models of TNBC Brain Metastases Synergizes with SN-38 and Impairs Invasion. NPJ Breast Cancer 2024, 10, 43. [Google Scholar] [CrossRef]
- Sultanpure, K.A.; Bagade, J.; Bangare, S.L.; Bangare, M.L.; Bamane, K.D.; Patankar, A.J. Internet of Things and Deep Learning Based Digital Twins for Diagnosis of Brain Tumor by Analyzing MRI Images. Meas. Sens. 2024, 33, 101220. [Google Scholar] [CrossRef]
- Sarris, A.L.; Sidiropoulos, E.; Paraskevopoulos, E.; Bamidis, P. Towards a Digital Twin in Human Brain: Brain Tumor Detection Using K-Means. Stud. Health Technol. Inf. Inform. 2023, 302, 1052–1056. [Google Scholar]
- Fekonja, L.S.; Schenk, R.; Schröder, E.; Tomasello, R.; Tomšič, S.; Picht, T. The Digital Twin in Neuroscience: From Theory to Tailored Therapy. Front. Neurosci. 2024, 18, 1454856. [Google Scholar] [CrossRef]
| Study | Drug Name | Mechanism of Action | Metabolic Pathway Affected | Phase of Clinical Trial (Trial ID/Status) | Key Findings |
|---|---|---|---|---|---|
| Dunbar, E.M. et al. (2014) [72] | Dichloroacetate (DCA) | PDK inhibitor | Glycolysis/TCA cycle | Phase 1 (NCT01111097/Completed) | Safe, tolerable, and feasible for chronic administration in adults. |
| Ferraro, G. et al. (2021) [30] | TVB-3166 | Fatty acid synthase (FAS)inhibitor | Lipid metabolism | Preclinical study (N/A) | Increased cancer cell death ex vivo. |
| Serhan, H. et al. (2024) [75] | TVB-2640 | Fatty acid synthase (FAS) inhibitor | Lipid metabolism | Phase 2 (NCT03179904/Ongoing) | Data are still not available. |
| Ferraro, G. et al. (2021) [30] | BI99179 | Fatty acid synthase (FAS) inhibitor | Lipid metabolism | Preclinical study (N/A) | Decreased brain tumor growth in vivo. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, M.; Violante, S.; Cascão, R.; Faria, C.C. Unlocking the Role of Metabolic Pathways in Brain Metastatic Disease. Cells 2025, 14, 707. https://doi.org/10.3390/cells14100707
Pinto M, Violante S, Cascão R, Faria CC. Unlocking the Role of Metabolic Pathways in Brain Metastatic Disease. Cells. 2025; 14(10):707. https://doi.org/10.3390/cells14100707
Chicago/Turabian StylePinto, Madalena, Sara Violante, Rita Cascão, and Claudia C. Faria. 2025. "Unlocking the Role of Metabolic Pathways in Brain Metastatic Disease" Cells 14, no. 10: 707. https://doi.org/10.3390/cells14100707
APA StylePinto, M., Violante, S., Cascão, R., & Faria, C. C. (2025). Unlocking the Role of Metabolic Pathways in Brain Metastatic Disease. Cells, 14(10), 707. https://doi.org/10.3390/cells14100707







