Clinical Evidence of Mesenchymal Stromal Cells for Cerebral Palsy: Scoping Review with Meta-Analysis of Efficacy in Gross Motor Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Eligibility Criteria
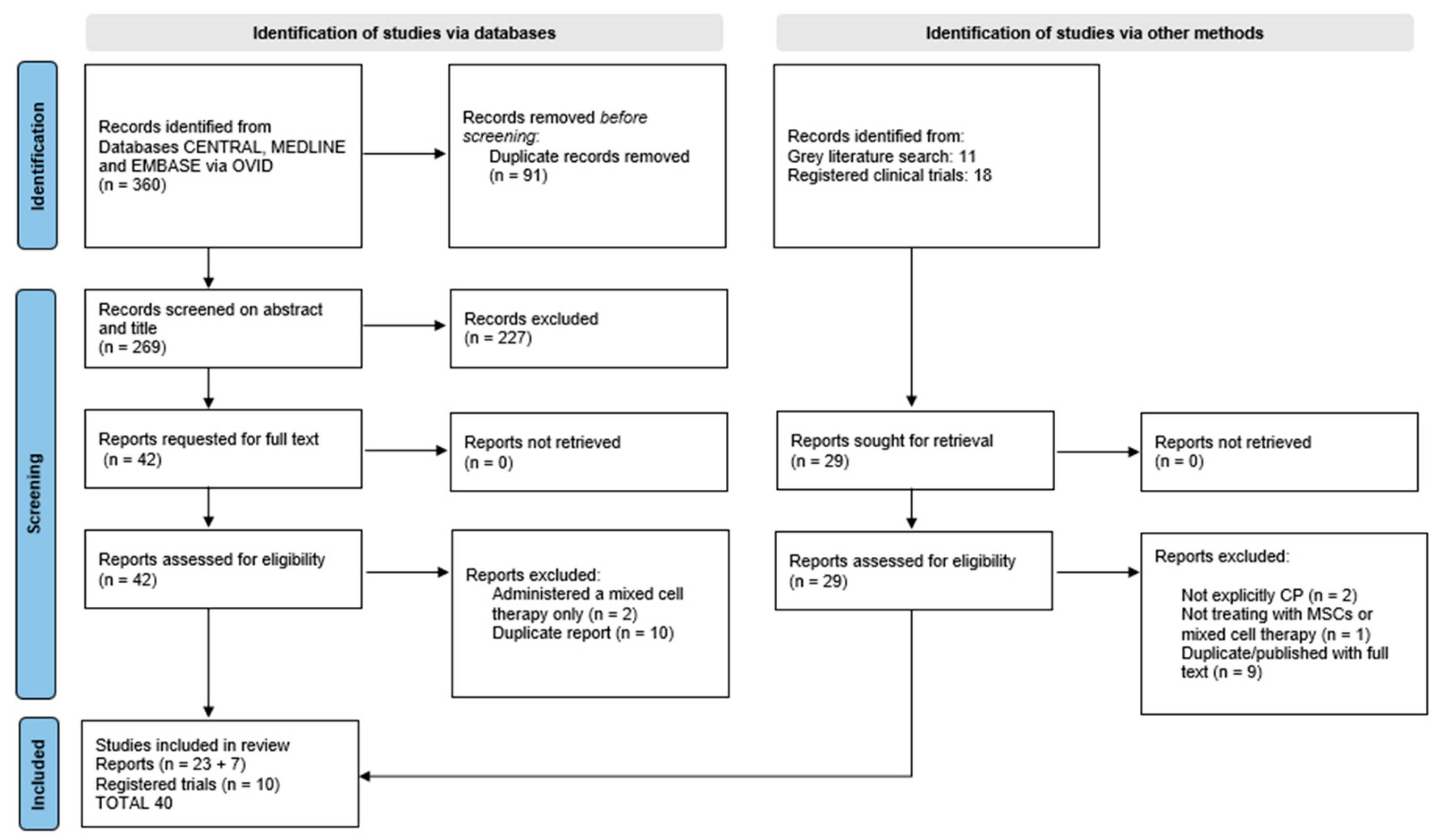
2.3. Study Selection
2.4. Data Extraction, Analysis and Reporting
2.4.1. All Included Reports
2.4.2. For Controlled Studies
2.5. Data Reporting
3. Results
3.1. Study Characteristics
3.2. Participants Characteristics
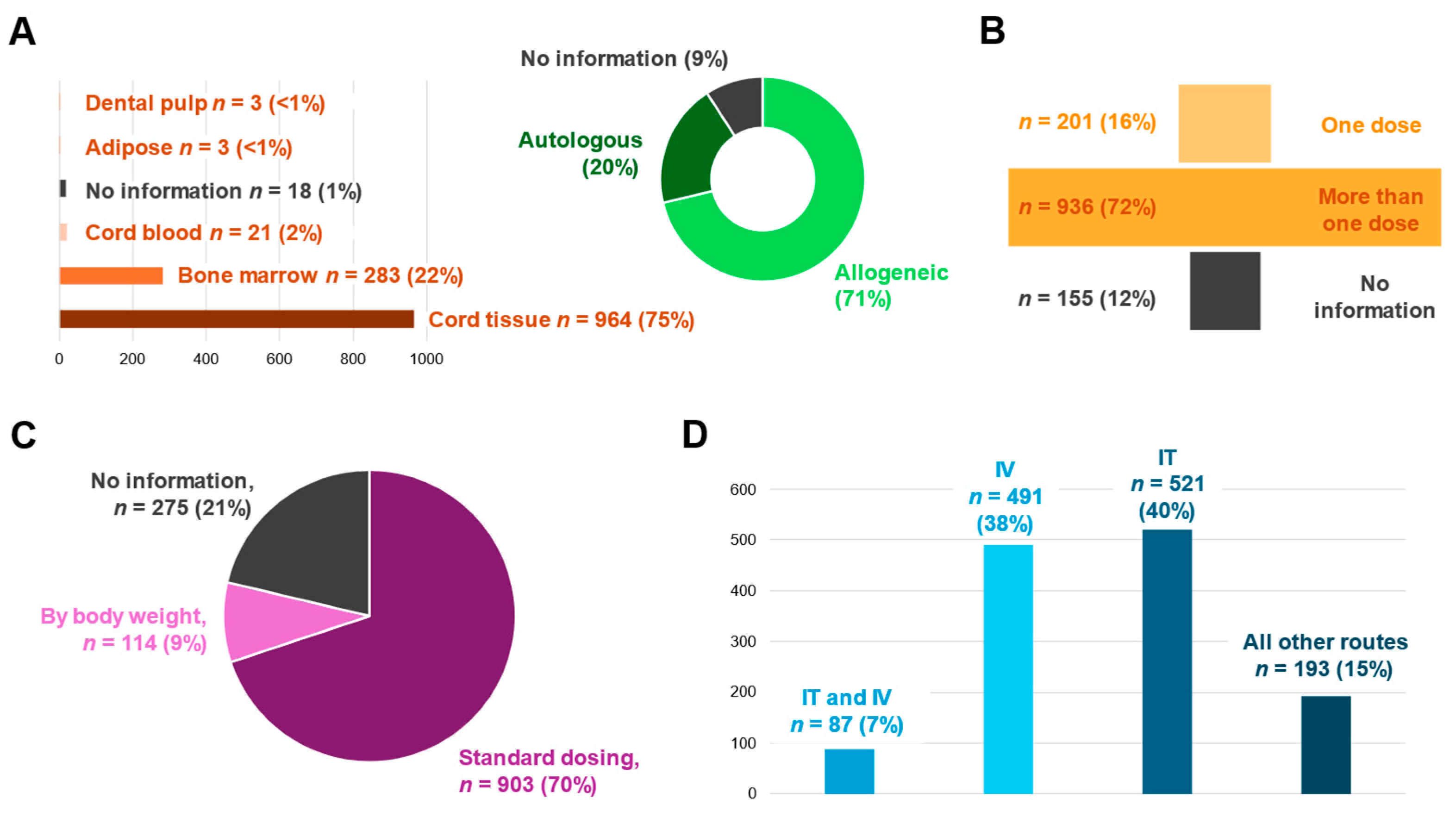
3.3. MSC Characteristics and Treatment Regimen
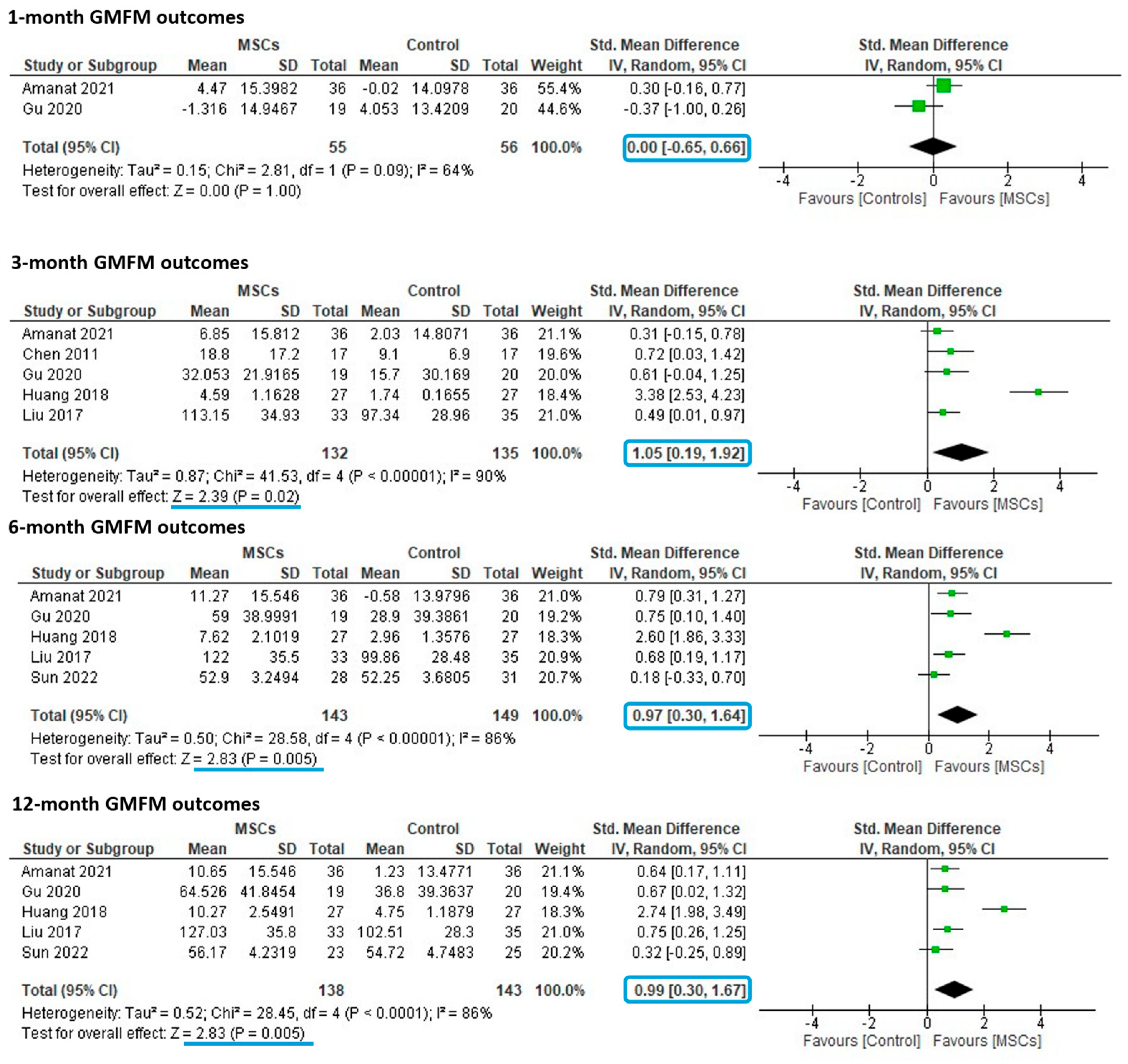
3.4. Meta-Analyses of Controlled Studies
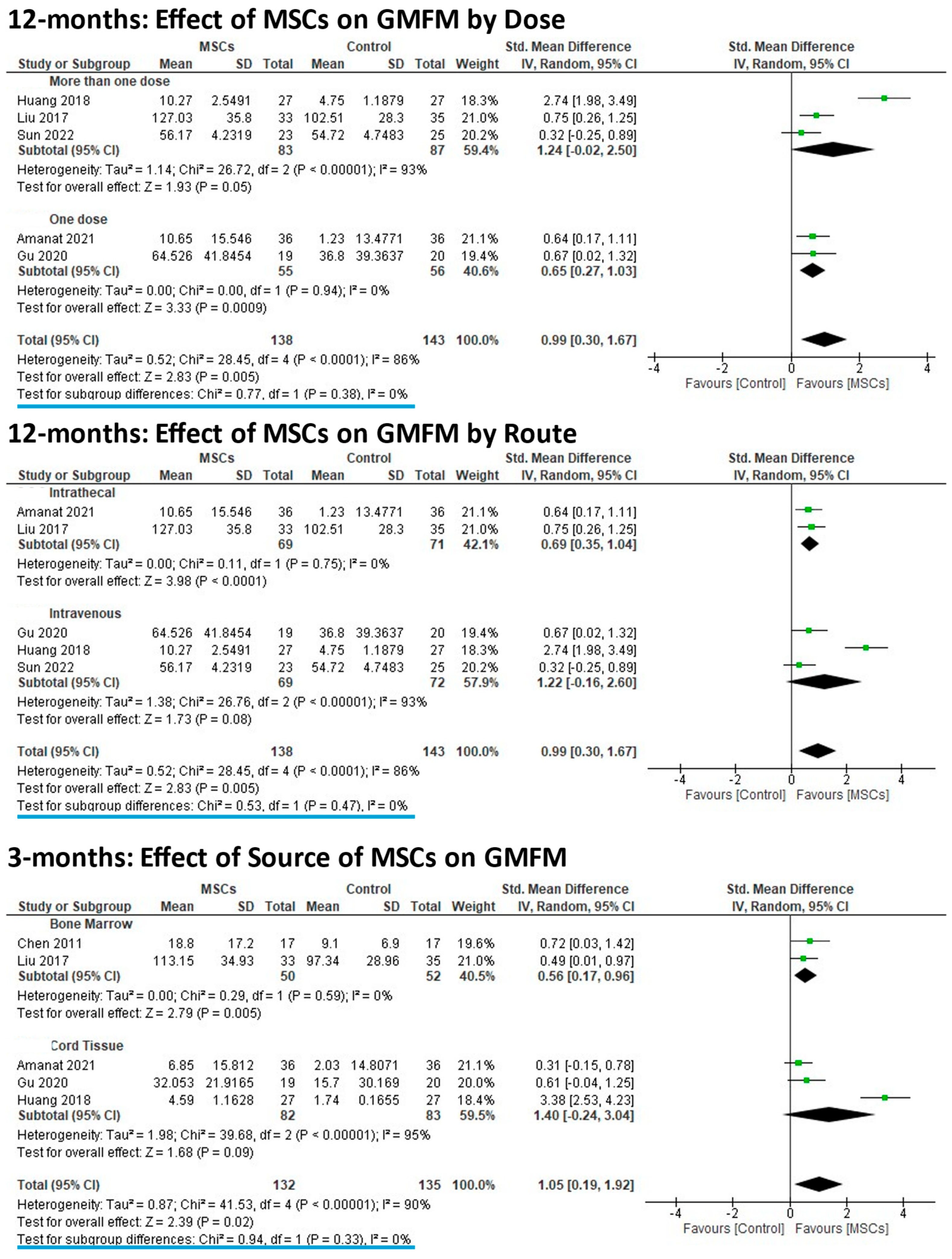
3.5. Subgroup Analyses
3.6. Risk of Bias Assessment
3.7. Safety Assessment
4. Discussion
Limitatons
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal stromal cells |
| CP | Cerebral palsy |
| GMFM | Gross motor function measure |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews Guidelines |
| RevMan | Review Manager |
| CI | Confidence intervals |
| SD | Standard deviation |
| SMD | Standardized mean differences |
| n | Sample/number of participants |
| Auto | Autologous |
| Allo | Allogeneic |
| BM | Bone marrow |
| CT | Cord tissue |
| IT | Intrathecal |
| IV | Intravenous |
| UCB | Umbilical cord blood |
References
- Patel, G.D.; Liu, L.; Li, A.; Yang, Y.-H.; Shen, C.-C.; Brand-Saberi, B.; Yang, X. Mesenchymal stem cell-based therapies for treating well-studied neurological disorders: A systematic review. Front. Med. 2024, 11, 1361723. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. 2007, 109, 8–14, Erratum in: Dev Med Child Neurol. 2007, 49, 480. [Google Scholar] [PubMed]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234. [Google Scholar] [CrossRef]
- Yarygin, K.N.; Namestnikova, D.D.; Sukhinich, K.K.; Gubskiy, I.L.; Majouga, A.G.; Kholodenko, I.V. Cell Therapy of Stroke: Do the Intra-Arterially Transplanted Mesenchymal Stem Cells Cross the Blood-Brain Barrier? Cells 2021, 10, 2997. [Google Scholar] [CrossRef]
- Isaković, J.; Šerer, K.; Barišić, B.; Mitrečić, D. Mesenchymal stem cell therapy for neurological disorders: The light or the dark side of the force? Front. Bioeng. Biotechnol. 2023, 11, 1139359. [Google Scholar] [CrossRef]
- Schu, S.; Nosov, M.; O’Flynn, L.; Shaw, G.; Treacy, O.; Barry, F.; Murphy, M.; O’Brien, T.; Ritter, T. Immunogenicity of allogeneic mesenchymal stem cells. Cell. Mol. Med. 2012, 16, 2094–2103. [Google Scholar] [CrossRef]
- Li, J.; Yawno, T.; Sutherland, A.E.; Gurung, S.; Paton, M.; McDonald, C.; Tiwari, A.; Pham, Y.; Castillo-Melendez, M.; Jenkin, G.; et al. Preterm umbilical cord blood derived mesenchymal stem/stromal cells protect preterm white matter brain development against hypoxia-ischemia. Exp. Neurol. 2018, 308, 120–131. [Google Scholar] [CrossRef]
- Nair, S.; Rocha-Ferreira, E.; Fleiss, B.; Nijboer, C.H.; Gressens, P.; Mallard, C.; Hagberg, H. Neuroprotection offered by mesenchymal stem cells in perinatal brain injury: Role of mitochondria, inflammation, and reactive oxygen species. J. Neurochem. 2021, 158, 59–73. [Google Scholar] [CrossRef]
- Paton, M.C.B.; Allison, B.J.; Fahey, M.C.; Li, J.; Sutherland, A.E.; Pham, Y.; Nitsos, I.; Bischof, R.J.; Moss, T.J.; Polglase, G.R.; et al. Umbilical cord blood versus mesenchymal stem cells for inflammation-induced preterm brain injury in fetal sheep. Pediatr. Res. 2019, 86, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Vaes, J.E.G.; Vink, M.A.; de Theije, C.G.M.; Hoebeek, F.E.; Benders, M.; Nijboer, C.H.A. The Potential of Stem Cell Therapy to Repair White Matter Injury in Preterm Infants: Lessons Learned From Experimental Models. Front. Physiol. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, B.; Chhatbar, P.Y.; Dong, Y.; Alawieh, A.; Lowe, F.; Hu, X.; Feng, W. Mesenchymal Stem Cell Therapy in Stroke: A Systematic Review of Literature in Pre-Clinical and Clinical Research. Cell Transplant. 2018, 27, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Chiossone, L.; Conte, R.; Spaggiari, G.M.; Serra, M.; Romei, C.; Bellora, F.; Becchetti, F.; Andaloro, A.; Moretta, L.; Bottino, C. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem. Cells 2016, 34, 1909–1921. [Google Scholar] [CrossRef]
- Pang, S.H.M.; D’Rozario, J.; Mendonca, S.; Bhuvan, T.; Payne, N.L.; Zheng, D.; Hisana, A.; Wallis, G.; Barugahare, A.; Powell, D.; et al. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nat. Commun. 2021, 12, 6495. [Google Scholar] [CrossRef]
- Paton, M.C.B.; Finch-Edmondson, M.; Dale, R.C.; Fahey, M.C.; Nold-Petry, C.A.; Nold, M.F.; Griffin, A.R.; Novak, I. Persistent Inflammation in Cerebral Palsy: Pathogenic Mediator or Comorbidity? A Scoping Review. J. Clin. Med. 2022, 11, 7368. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef]
- Eggenberger, S.; Boucard, C.; Schoeberlein, A.; Guzman, R.; Limacher, A.; Surbek, D.; Mueller, M. Stem cell treatment and cerebral palsy: Systemic review and meta-analysis. World J. Stem Cells 2019, 11, 891–903. [Google Scholar] [CrossRef]
- Xie, B.; Chen, M.; Hu, R.; Han, W.; Ding, S. Therapeutic Evidence of Human Mesenchymal Stem Cell Transplantation for Cerebral Palsy: A Meta-Analysis of Randomized Controlled Trials. Stem Cells Int. 2020, 2020, 5701920. [Google Scholar] [CrossRef]
- Sun, J.M.; Case, L.E.; McLaughlin, C.; Burgess, A.; Skergan, N.; Crane, S.; Jasien, J.M.; Mikati, M.A.; Troy, J.; Kurtzberg, J. Motor function and safety after allogeneic cord blood and cord tissue-derived mesenchymal stromal cells in cerebral palsy: An open-label, randomized trial. Dev. Med. Child Neurol. 2022, 64, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Couto, P.S.; Bersenev, A.; Verter, F. The first decade of advanced cell therapy clinical trials using perinatal cells (2005–2015). Regen. Med. 2017, 12, 953–968. [Google Scholar] [CrossRef]
- Paton, M.C.B.; Finch-Edmondson, M.; Fahey, M.C.; London, J.; Badawi, N.; Novak, I. Fifteen years of human research using stem cells for cerebral palsy: A review of the research landscape. J. Paediatr. Child Health 2021, 57, 295–296. [Google Scholar] [CrossRef]
- Abo Elkheir, W.; Gabr, H. Neuro-regenerative effect of autologous mesenchymal stem cell therapy in cerebral palsy patients: Pilot clinical trial. Cytotherapy 2014, 16, S15. [Google Scholar] [CrossRef]
- Amanat, M.; Majmaa, A.; Zarrabi, M.; Nouri, M.; Akbari, M.G.; Moaiedi, A.R.; Ghaemi, O.; Zamani, F.; Najafi, S.; Badv, R.S.; et al. Clinical and imaging outcomes after intrathecal injection of umbilical cord tissue mesenchymal stem cells in cerebral palsy: A randomized double-blind sham-controlled clinical trial. Stem Cell Res. Ther. 2021, 12, 439. [Google Scholar] [CrossRef]
- Boyali, O.; Kabatas, S.; Civelek, E.; Ozdemir, O.; Bahar-Ozdemir, Y.; Kaplan, N.; Savrunlu, E.C.; Karaoz, E. Allogeneic mesenchymal stem cells may be a viable treatment modality in cerebral palsy. World J. Clin. Cases 2024, 12, 1585–1596. [Google Scholar] [CrossRef]
- Chen, W.m.; Hu, Y.x.; Yin, J.y.; Lu, J.j.; Wang, J.; Zou, Q.y. Effect of autologous bone marrow mesenchymal stem cell transplantation on motor development of cerebral palsy children. J. Clin. Rehabil. Tissue Eng. Res. 2011, 15, 7589–7592. [Google Scholar] [CrossRef]
- Chroscinska-Kawczyk, M.; Zdolinska-Malinowska, I.; Boruczkowski, D. The Impact of Umbilical Cord Mesenchymal Stem Cells on Motor Function in Children with Cerebral Palsy: Results of a Real-world, Compassionate use Study. Stem Cell Rev. Rep. 2024, 20, 1636–1649. [Google Scholar] [CrossRef]
- Dong, H.; Li, G.; Shang, C.; Yin, H.; Luo, Y.; Meng, H.; Li, X.; Wang, Y.; Lin, L.; Zhao, M. Umbilical cord mesenchymal stem cell (UC-MSC) transplantations for cerebral palsy. Am. J. Transl. Res. 2018, 10, 901–906. [Google Scholar] [PubMed]
- Du, K.; Luan, Z.; Qu, S.-q.; Liu, W.-p.; Wang, Z.-y.; Yang, Y.-x.; Yang, H. Clinical outcome of bone marrow mesenchymal stem cells transplantation in children with severe cerebral palsy. J. Clin. Pediatr. 2011, 29, 55–58. [Google Scholar] [CrossRef]
- Fu, X.; Hua, R.; Wang, X.; Wang, P.; Yi, L.; Yu, A.; Yang, J.; Li, Y.; An, Y. Synergistic Improvement in Children with Cerebral Palsy Who Underwent Double-Course Human Wharton’s Jelly Stem Cell Transplantation. Stem Cells Int. 2019, 2019, 7481069. [Google Scholar] [CrossRef] [PubMed]
- Gabr, H. Intrathecal Autologous Bone Marrow Derived MSC Therapy in Cerebral Palsy: Safety and Short Term Efficacy. Am. J. Biosci. Bioeng. 2015, 3, 24. [Google Scholar] [CrossRef]
- Gu, J.; Huang, L.; Zhang, C.; Wang, Y.; Zhang, R.; Tu, Z.; Wang, H.; Zhou, X.; Xiao, Z.; Liu, Z.; et al. Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: A randomized, controlled trial. Stem Cell Res. Ther. 2020, 11, 43. [Google Scholar] [CrossRef]
- Hastuti, S.; Sartika, C.R.; Haifa, R.; Naura, N.; Hasibuan, A.; Devi, D.; Pertiwi, V. Mesenchymal Stem/Stromal Cells: Therapeutic Potential of Umbilical Cord Mesenchymal Stem Cell in Patient With Cerebral Palsy: A Case Report. Cytotherapy 2023, 25, S58. [Google Scholar] [CrossRef]
- Hirano, A.; Sano, M.; Urushihata, N.; Tanemura, H.; Oki, K.; Suzaki, E. Assessment of safety and feasibility of human allogeneic adipose-derived mesenchymal stem cells in a pediatric patient. Pediatr. Res. 2018, 84, 575–577. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, C.; Gu, J.; Wu, W.; Shen, Z.; Zhou, X.; Lu, H. A Randomized, Placebo-Controlled Trial of Human Umbilical Cord Blood Mesenchymal Stem Cell Infusion for Children With Cerebral Palsy. Cell Transpl. 2018, 27, 325–334. [Google Scholar] [CrossRef]
- Li, M.; Yu, A.; Zhang, F.; Dai, G.; Cheng, H.; Wang, X.; An, Y. Treatment of one case of cerebral palsy combined with posterior visual pathway injury using autologous bone marrow mesenchymal stem cells. J. Transl. Med. 2012, 10, 100. [Google Scholar] [CrossRef]
- Liu, X.; Fu, X.; Dai, G.; Wang, X.; Zhang, Z.; Cheng, H.; Zheng, P.; An, Y. Comparative analysis of curative effect of bone marrow mesenchymal stem cell and bone marrow mononuclear cell transplantation for spastic cerebral palsy. J. Transl. Med. 2017, 15, 48. [Google Scholar] [CrossRef]
- Liu, Y.s.; Wang, X.d.; Yang, J.; Hua, R.r.; Cheng, H.b.; Liu, Y.f.; An, Y.h. Effect evaluation of autologous bone marrow mesenchymal stem cell transplantation on motor function in children with cerebral palsy. Chin. J. Tissue Eng. Res. 2012, 16, 4290–4295. [Google Scholar] [CrossRef]
- Miao, X.; Wu, X.; Shi, W. Umbilical cord mesenchymal stem cells in neurological disorders: A clinical study. Indian J. Biochem. Biophys. 2015, 52, 140–146. [Google Scholar] [PubMed]
- Okur, S.C.; Erdogan, S.; Demir, C.S.; Gunel, G.; Karaoz, E. The Effect of Umbilical Cord-derived Mesenchymal Stem Cell Transplantation in a Patient with Cerebral Palsy: A Case Report. Int. J. Stem Cells 2018, 11, 141–147. [Google Scholar] [CrossRef]
- Rojek, M.; Piatek, M.; Zdolinska-Malinowska, I.; Boruczkowski, D. (Eds.) The Polish Experience of Wharton’s Jelly-derived Mesenchymal Stem Cell Treatment of Paediatric Patients. In Bone Marrow Transplantation; Springernature Campus: London, UK, 2020. [Google Scholar]
- Venkataramana, N.; Pal, R.; Rao, S.; Naik, A. A Pilot Study on Safety and Efficacy of Mesenchymal Stem Cells for Children with Cerebral Palsy. In Proceedings of the 39th Annual Meeting of the International Society for Pediatric Neurosurgery, Goa, India, 16–20 October 2011. Childs Nerv Syst 2011, 27, 1751–1850. [Google Scholar] [CrossRef]
- Vij, R.; Kim, H.; Park, H.; Cheng, T.; Lotfi, D.; Chang, D. Multi-Dose Hope Biosciences-Adipose-Derived Mesenchymal Stem Cell (HB-adMSC) Therapy for a Patient with Cerebral Palsy: A Case Report. Int. J. Stem Cell Res. Ther. 2023, 10, 78. [Google Scholar] [CrossRef]
- Wang, J.-G.; Yin, Z.-M.; Wen, H.; Wang, L.-Z.; Chen, Z. Application of Umbilical Cord Mesenchumal Stem Cells in Cerebral Palsy Treatment: Report of 51 Cases. Chin. Gen. Pract. 2011, 14, 2446–2447. [Google Scholar]
- Wang, L.; Ji, H.; Zhou, J.; Xie, J.; Zhong, Z.; Li, M.; Bai, W.; Li, N.; Zhang, Z.; Wang, X.; et al. Therapeutic potential of umbilical cord mesenchymal stromal cells transplantation for cerebral palsy: A case report. Case Rep. Transpl. 2013, 2013, 146347. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, H.; Hua, R.; Yang, J.; Dai, G.; Zhang, Z.; Wang, R.; Qin, C.; An, Y. Effects of bone marrow mesenchymal stromal cells on gross motor function measure scores of children with cerebral palsy: A preliminary clinical study. Cytotherapy 2013, 15, 1549–1562. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Hua, R.; Yang, J.; Zheng, P.; Niu, X.; Cheng, H.; Dai, G.; Liu, X.; Zhang, Z.; et al. Effect of umbilical cord mesenchymal stromal cells on motor functions of identical twins with cerebral palsy: Pilot study on the correlation of efficacy and hereditary factors. Cytotherapy 2015, 17, 224–231. [Google Scholar] [CrossRef]
- Wang, X.-d.; Yang, J.; Li, M. Effects of bone marrow mesenchymal stem cell graft on gross motor function in children withcerebral palsy. J China-Jpn. Friendsh. Hosp. 2010, 24, 337–342. [Google Scholar]
- Wu, F.; Yang, J.-y.; Zhang, M.; Chen, C.-h.; Yang, Z.-g.; Sheng, Y.-x.; Liang, H.-w.; Chi, Q.; Yu-ting, W.; Yang, W.-z. Effect of umbilical cord blood mesenchymal stem cell transplantation on nervous system function in 20 cerebral palsy children. J. Clin. Rehabil. Tissue Eng. Res. 2008, 12, 3198–3200. [Google Scholar]
- Yang, H.; Zhang, R.; Du, L.; Li, D. Effect of Umbilical Cord Mesenchymal Stem Cell Transplantation Therapy for Cerebral Palsy on Motor Function. Prog. Mod. Biomed. 2012, 12, 91–98. [Google Scholar]
- NCT01929434, Efficacy of Stem Cell Transplantation Compared to Rehabilitation Treatment of Children With Cerebral Paralysis. National Library of Medicine (US). Available online: https://clinicaltrials.gov/study/NCT01929434 (accessed on 11 February 2025).
- IRCT20110628006907N18, A Triple Blinded, Parallel Randomized Clinical Trial Phase II&III of 3 Times Intrathecal Injections of an Umbilical Cord-Derived Mesenchymal Stromal Cell Product “WhartoCell” in Children with Cerebral Palsy. Iranian Registry of Clinical Trials (IR). Available online: https://irct.behdasht.gov.ir/trial/68950 (accessed on 10 February 2025).
- Tanaka, M.; Miyazawa, H.; Terashima, R.; Ikuma, M. Conditional early approval for new drug applications in Japan: Current and emerging issues. Clin. Transl. Sci. 2023, 16, 1289–1293. [Google Scholar] [CrossRef]
- Cyranoski, D.; Sipp, D.; Mallik, S.; Rasko, J.E.J. Too little, too soon: Japan’s experiment in regenerative medicine deregulation. Cell Stem Cell 2023, 30, 913–916. [Google Scholar] [CrossRef]
- Oeffinger, D.; Bagley, A.; Rogers, S.; Gorton, G.; Kryscio, R.; Abel, M.; Damiano, D.; Barnes, D.; Tylkowski, C. Outcome tools used for ambulatory children with cerebral palsy: Responsiveness and minimum clinically important differences. Dev. Med. Child Neurol. 2008, 50, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Finch-Edmondson, M.; Paton, M.C.B.; Honan, I.; Karlsson, P.; Stephenson, C.; Chiu, D.; Reedman, S.; Griffin, A.R.; Morgan, C.; Novak, I. Are We Getting It Right? A Scoping Review of Outcomes Reported in Cell Therapy Clinical Studies for Cerebral Palsy. J. Clin. Med. 2022, 11, 7319. [Google Scholar] [CrossRef]
- Nouri, M.; Zarrabi, M.; Masoumi, S.; Khodadoust, E.; Majmae, A.; Amanat, M.; Ashrafi, M.R.; Vosough, M. Cell-Based Therapy for Cerebral Palsy: A Puzzle in Progress. Cell J. 2025, 26, 569–574. [Google Scholar] [CrossRef]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transpl. 2019, 28, 801–812. [Google Scholar] [CrossRef]
- NCT03414697, Transplantation of Umbilical Cord-Derived Mesenchymal Stem Cells Via Different Routes. National Library of Medicine (US). Available online: https://clinicaltrials.gov/study/NCT03414697?id=NCT03414697&rank=1 (accessed on 11 February 2025).
- Conrado, D.J.; Karlsson, M.O.; Romero, K.; Sarr, C.; Wilkins, J.J. Open innovation: Towards sharing of data, models and workflows. Eur. J. Pharm. Sci. 2017, 109, S65–S71. [Google Scholar] [CrossRef]
- van Wijk, R.C.; Imperial, M.Z.; Savic, R.M.; Solans, B.P. Pharmacokinetic analysis across studies to drive knowledge-integration: A tutorial on individual patient data meta-analysis (IPDMA). CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 1187–1200. [Google Scholar] [CrossRef]
- Finch-Edmondson, M.; Paton, M.C.B.; Webb, A.; Ashrafi, M.R.; Blatch-Williams, R.K.; Cox, C.S., Jr.; Crompton, K.; Griffin, A.; Kim, M.; Kosmach, S.; et al. Cord blood treatment for children with cerebral palsy: Individual participant data meta-analysis. Pediatrics 2025, e2024068999. [Google Scholar] [CrossRef] [PubMed]
- Jantzie, L.L.; Scafidi, J.; Robinson, S. Stem cells and cell-based therapies for cerebral palsy: A call for rigor. Pediatr. Res. 2018, 83, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Blanc, K.L.; Ciccocioppo, R.; Dagher, G.; Filiano, A.J.; Galipeau, J.; Krampera, M.; Krieger, L.; Lalu, M.M.; Nolta, J.; et al. An International Society for Cell and Gene Therapy Mesenchymal Stromal Cells (MSC) Committee perspectives on International Standards Organization/Technical Committee 276 Biobanking Standards for bone marrow-MSCs and umbilical cord tissue-derived MSCs for research purposes. Cytotherapy 2023, 25, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Ankrum, J.A.; Olson, S.D.; Nolta, J.A. Improved MSC Minimal Criteria to Maximize Patient Safety: A Call to Embrace Tissue Factor and Hemocompatibility Assessment of MSC Products. Stem Cells Transl. Med. 2022, 11, 2–13. [Google Scholar] [CrossRef]
- Webbe, J.W.H.; Duffy, J.M.N.; Afonso, E.; Al-Muzaffar, I.; Brunton, G.; Greenough, A.; Hall, N.J.; Knight, M.; Latour, J.M.; Lee-Davey, C.; et al. Core outcomes in neonatology: Development of a core outcome set for neonatal research. Arch. Dis. Child. -Fetal Neonatal Ed. 2020, 105, 425–431. [Google Scholar] [CrossRef]
- Quirke, F.A.; Ariff, S.; Battin, M.R.; Bernard, C.; Biesty, L.; Bloomfield, F.H.; Daly, M.; Finucane, E.; Healy, P.; Haas, D.M.; et al. COHESION: A core outcome set for the treatment of neonatal encephalopathy. Pediatr. Res. 2024, 95, 922–930. [Google Scholar] [CrossRef]
- Walker, M.F.; Hoffmann, T.C.; Brady, M.C.; Dean, C.M.; Eng, J.J.; Farrin, A.J.; Felix, C.; Forster, A.; Langhorne, P.; Lynch, E.A.; et al. Improving the development, monitoring and reporting of stroke rehabilitation research: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2017, 12, 472–479. [Google Scholar] [CrossRef]
- Paton, M.C.B.; Benders, M.; Blatch-Williams, R.; Dallimore, E.; Edwards, A.; Elwood, N.; Facer, K.; Finch-Edmondson, M.; Garrity, N.; Gordon, A.; et al. Updates on neonatal cell and novel therapeutics: Proceedings of the Second Neonatal Cell Therapies Symposium (2024). Pediatr. Res. 2025. [Google Scholar] [CrossRef]
- Robb, K.P.; Galipeau, J.; Shi, Y.; Schuster, M.; Martin, I.; Viswanathan, S. Failure to launch commercially-approved mesenchymal stromal cell therapies: What’s the path forward? Proceedings of the International Society for Cell & Gene Therapy (ISCT) Annual Meeting Roundtable held in May 2023, Palais des Congrès de Paris, Organized by the ISCT MSC Scientific Committee. Cytotherapy 2024, 26, 413–417. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paton, M.C.B.; Griffin, A.R.; Blatch-Williams, R.; Webb, A.; Verter, F.; Couto, P.S.; Bersenev, A.; Dale, R.C.; Popat, H.; Novak, I.; et al. Clinical Evidence of Mesenchymal Stromal Cells for Cerebral Palsy: Scoping Review with Meta-Analysis of Efficacy in Gross Motor Outcomes. Cells 2025, 14, 700. https://doi.org/10.3390/cells14100700
Paton MCB, Griffin AR, Blatch-Williams R, Webb A, Verter F, Couto PS, Bersenev A, Dale RC, Popat H, Novak I, et al. Clinical Evidence of Mesenchymal Stromal Cells for Cerebral Palsy: Scoping Review with Meta-Analysis of Efficacy in Gross Motor Outcomes. Cells. 2025; 14(10):700. https://doi.org/10.3390/cells14100700
Chicago/Turabian StylePaton, Madison C. B., Alexandra R. Griffin, Remy Blatch-Williams, Annabel Webb, Frances Verter, Pedro S. Couto, Alexey Bersenev, Russell C. Dale, Himanshu Popat, Iona Novak, and et al. 2025. "Clinical Evidence of Mesenchymal Stromal Cells for Cerebral Palsy: Scoping Review with Meta-Analysis of Efficacy in Gross Motor Outcomes" Cells 14, no. 10: 700. https://doi.org/10.3390/cells14100700
APA StylePaton, M. C. B., Griffin, A. R., Blatch-Williams, R., Webb, A., Verter, F., Couto, P. S., Bersenev, A., Dale, R. C., Popat, H., Novak, I., & Finch-Edmondson, M. (2025). Clinical Evidence of Mesenchymal Stromal Cells for Cerebral Palsy: Scoping Review with Meta-Analysis of Efficacy in Gross Motor Outcomes. Cells, 14(10), 700. https://doi.org/10.3390/cells14100700