Aptamer–ODN Chimeras: Enabling Cell-Specific ODN Targeting Therapy
Abstract
1. Introduction
2. Aptamers
2.1. Introduction and Application
2.2. The Internalizing Capacity of Aptamers
2.3. Aptamers as Vehicles for Targeted Delivery of ODN
3. Aptamer–ODN Chimeras
3.1. Aptamer-siRNA Chimeras
3.2. Aptamer-ASO Chimeras
3.3. Aptamer-miRNA Chimeras
3.4. Aptamer-saRNA Chimeras
3.5. Aptamer-Decoy Chimeras
3.6. Aptamer-sgRNA Chimeras
3.7. Aptamer-CpG Chimeras
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ODN | oligonucleotide |
| siRNA | small interfering RNA |
| saRNA | small activating RNA |
| miRNA | microRNA |
| sgRNA | single guide RNA |
| shRNA | short hairpin RNA |
| ASO | antisense oligonucleotide |
| ApDCs | aptamer-drug conjugates |
| AMO | Anti-miRNA ODN |
| RNAi | RNA interference |
| Ago 2 | Argonaute 2 |
| RNase H | Ribonuclease H |
| RIBOTAC | RNA-inducible ribonuclease-targeted chimera |
| PROTAC | protein degradation-targeted chimera |
| 3′-UTR | 3′ Untranslated Region |
| FDA | US Food and Drug Administration |
| EMA | European Medicines Agency |
| SELEX | systematic evolution of ligands by exponential enrichment |
| WC-SELEX | whole cell SELEX |
| FACS-WC-SELEX | fluorescence-activated cell sorting-assisted WC-SELEX |
| MC-WC-SELEX | microfluidic chip system-assisted WC-SELEX |
| 3D-WC-SELEX | three-dimensional culture-assisted WC-SELEX |
| NA-WC-SELEX | nanomaterial-assisted WC-SELEX |
| RME | receptor-mediated endocytosis |
| CME | clathrin-mediated endocytosis |
| CvME | caveolae-mediated endocytosis |
| DSB | double-stranded DNA break |
| NHEJ | non-homologous end-joining |
| HDR | homology-directed repair |
| 3WJ | the three-way junction motif |
| pRNA | packaging RNA |
| VEGF | vascular endothelial growth factor |
| TfR | transferrin receptor |
| PTK7 | protein tyrosine kinase 7 |
| mCRPC | metastatic castration-resistant prostate cancer |
| PSMA | prostate-specific membrane antigen |
| Bcl-2 | B-cell lymphoma 2 |
| Plk1 | polo-like kinase 1 |
| DOX | doxorubicin |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| CEBPA | CCAAT/Enhancer Binding Protein Alpha |
| PDAC | pancreatic ductal adenocarcinoma |
| NF-κB | nuclear factor κB |
| EGFR | epidermal growth factor receptor |
| PD-L1 | programmed death-ligand 1 |
| PD-1 | programmed cell death protein 1 |
| RCA | rolled-loop amplification |
| VSV-G | vesicular stomatitis virus G glycoprotein |
| ABP | aptamer-binding protein |
| AML | acute myeloid leukemia |
| TLR9 | Toll-like receptor 9 |
| DSPE | 1,2-distearoyl-sn-glycero-3-phospho-ethanolamine |
| HDL | high-density lipoprotein |
| Apo-AI | Apolipoprotein AI |
| SRBI | scavenger receptor class B type I |
| NIR | near-infrared |
References
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Mangla, P.; Vicentini, Q.; Biscans, A. Therapeutic Oligonucleotides: An Outlook on Chemical Strategies to Improve Endosomal Trafficking. Cells 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Vinjamuri, B.P.; Pan, J.; Peng, P. A Review on Commercial Oligonucleotide Drug Products. J. Pharm. Sci. 2024, 113, 1749–1768. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Zhang, Y.; Patil, S.; Kaur, K. Metabolic Stability and Targeted Delivery of Oligonucleotides: Advancing RNA Therapeutics Beyond The Liver. J. Med. Chem. 2025, 68, 6870–6896. [Google Scholar] [CrossRef]
- Fabrega, C.; Avino, A.; Navarro, N.; Jorge, A.F.; Grijalvo, S.; Eritja, R. Lipid and Peptide-Oligonucleotide Conjugates for Therapeutic Purposes: From Simple Hybrids to Complex Multifunctional Assemblies. Pharmaceutics 2023, 15, 320. [Google Scholar] [CrossRef]
- Khairnar, P.; Kolipaka, T.; Pandey, G.; Phatale, V.; Shah, S.; Srinivasarao, D.A.; Saraf, S.; Srivastava, S. Nanosponge-Mediated Oligonucleotide Delivery: A Cutting-Edge Technology Towards Cancer Management. J. Drug Deliv. Sci. Technol. 2024, 91, 105226. [Google Scholar] [CrossRef]
- Nuzzo, S.; Roscigno, G.; Affinito, A.; Ingenito, F.; Quintavalle, C.; Condorelli, G. Potential and Challenges of Aptamers as Specific Carriers of Therapeutic Oligonucleotides for Precision Medicine in Cancer. Cancers 2019, 11, 1521. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, X.; Lu, P.Y.; Rosato, R.R.; Tan, W.; Zu, Y. Oligonucleotide aptamers: New tools for targeted cancer therapy. Mol. Ther. Nucleic Acids 2014, 3, e182. [Google Scholar] [CrossRef]
- Fan, R.; Tao, X.; Zhai, X.; Zhu, Y.; Li, Y.; Chen, Y.; Dong, D.; Yang, S.; Lv, L. Application of aptamer-drug delivery system in the therapy of breast cancer. Biomed. Pharmacother. 2023, 161, 114444. [Google Scholar] [CrossRef]
- Soldevilla, M.M.; Meraviglia-Crivelli de Caso, D.; Menon, A.P.; Pastor, F. Aptamer-iRNAs as Therapeutics for Cancer Treatment. Pharmaceuticals 2018, 11, 108. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Moradi, Z.; Abnous, K.; Taghdisi, S.M.; Zamanian, J.; Moshiri, M.; Etemad, D.; Etemad, L.; Kesharwani, P.; Sahebkar, A. Designing multivalent aptamers: Recent advancements in diagnostic and therapeutic approaches for cancer treatment. J. Drug Deliv. Sci. Technol. 2025, 105, 106614. [Google Scholar] [CrossRef]
- Elskens, J.P.; Elskens, J.M.; Madder, A. Chemical Modification of Aptamers for Increased Binding Affinity in Diagnostic Applications: Current Status and Future Prospects. Int. J. Mol. Sci. 2020, 21, 4522. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.M.; Liu, H.; Kuai, H.; Peng, R.; Mo, L.; Zhang, X.B. Aptamer-integrated DNA nanostructures for biosensing, bioimaging and cancer therapy. Chem. Soc. Rev. 2016, 45, 2583–2602. [Google Scholar] [CrossRef]
- U, A.P.; Raj, G.; John, J.; Mohan, K.M.; John, F.; George, J. Aptamers: Features, Synthesis and Applications. Chem. Biodivers. 2023, 20, e202301008. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, Z.; Zhang, Q.; Li, G.; Song, M.; Peng, R. Aptamer-functionalized nucleic acid nanotechnology for biosensing, bioimaging and cancer therapy. Nanoscale 2025, 17, 687–704. [Google Scholar] [CrossRef]
- Bege, M.; Ghanem Kattoub, R.; Borbas, A. The 20th Anniversary of Pegaptanib (MacugenTM), the First Approved Aptamer Medicine: History, Recent Advances and Future Prospects of Aptamers in Therapy. Pharmaceutics 2025, 17, 394. [Google Scholar] [CrossRef]
- He, S.; Du, Y.; Tao, H.; Duan, H. Advances in aptamer-mediated targeted delivery system for cancer treatment. Int. J. Biol. Macromol. 2023, 238, 124173. [Google Scholar] [CrossRef]
- Di, Y.; Wang, P.; Li, C.; Xu, S.; Tian, Q.; Wu, T.; Tian, Y.; Gao, L. Design, Bioanalytical, and Biomedical Applications of Aptamer-Based Hydrogels. Front. Med. 2020, 7, 456. [Google Scholar] [CrossRef]
- Moosavian, S.A.; Sahebkar, A. Aptamer-functionalized liposomes for targeted cancer therapy. Cancer Lett. 2019, 448, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Md, S.; Alhakamy, N.A.; Kesharwani, P. Recent development of aptamer conjugated chitosan nanoparticles as cancer therapeutics. Int. J. Pharm. 2022, 620, 121751. [Google Scholar] [CrossRef]
- Urmi, R.; Banerjee, P.; Singh, M.; Singh, R.; Chhillar, S.; Sharma, N.; Chandra, A.; Singh, N.; Qamar, I. Revolutionizing biomedicine: Aptamer-based nanomaterials and nanodevices for therapeutic applications. Biotechnol. Rep. 2024, 42, e00843. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Gao, T.; Lu, Z.; Yin, J.; Zhang, Y.; Pei, R. Aptamer-Targeted Photodynamic Platforms for Tumor Therapy. ACS Appl. Mater. Interfaces 2021, 13, 27749–27773. [Google Scholar] [CrossRef]
- Alamudi, S.H.; Kimoto, M.; Hirao, I. Uptake mechanisms of cell-internalizing nucleic acid aptamers for applications as pharmacological agents. RSC Med. Chem. 2021, 12, 1640–1649. [Google Scholar] [CrossRef]
- Wan, L.Y.; Yuan, W.F.; Ai, W.B.; Ai, Y.W.; Wang, J.J.; Chu, L.Y.; Zhang, Y.Q.; Wu, J.F. An exploration of aptamer internalization mechanisms and their applications in drug delivery. Expert. Opin. Drug Deliv. 2019, 16, 207–218. [Google Scholar] [CrossRef]
- Lv, C.; Yang, C.; Ding, D.; Sun, Y.; Wang, R.; Han, D.; Tan, W. Endocytic Pathways and Intracellular Transport of Aptamer-Drug Conjugates in Live Cells Monitored by Single-Particle Tracking. Anal. Chem. 2019, 91, 13818–13823. [Google Scholar] [CrossRef]
- Tanaka, K.; Okuda, T.; Kasahara, Y.; Obika, S. Base-modified aptamers obtained by cell-internalization SELEX facilitate cellular uptake of an antisense oligonucleotide. Mol. Ther. Nucleic Acids 2021, 23, 440–449. [Google Scholar] [CrossRef]
- Gopinath, S.C.; Lakshmipriya, T.; Chen, Y.; Arshad, M.K.; Kerishnan, J.P.; Ruslinda, A.R.; Al-Douri, Y.; Voon, C.H.; Hashim, U. Cell-targeting aptamers act as intracellular delivery vehicles. Appl. Microbiol. Biotechnol. 2016, 100, 6955–6969. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, C.; Wang, Y.; Chen, G. Research progress of whole-cell-SELEX selection and the application of cell-targeting aptamer. Mol. Biol. Rep. 2022, 49, 7979–7993. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Gao, H.; Chen, J.; Jiang, H. Progress in Aptamer Research and Future Applications. ChemistryOpen 2025, e202400463. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Zhuo, Z.; Pan, Y.; Yu, Y.; Li, F.; Liu, J.; Wang, L.; Wu, X.; Li, D.; Wan, Y.; et al. Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces 2021, 13, 9500–9519. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, Y.; Xu, X.; Liu, Y.; Lin, B.; Zhang, M.; Zhang, J.; Wan, S.; Yang, C.; Tan, W. Aptamer-Based Detection of Circulating Targets for Precision Medicine. Chem. Rev. 2021, 121, 12035–12105. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.H.; Elsherbiny, M.E.; Emara, M. Updates on Aptamer Research. Int. J. Mol. Sci. 2019, 20, 2511. [Google Scholar] [CrossRef]
- Pandey, S.K.; Parul, M.; Santhanam, M. Aptamer-guided Selective Delivery of Therapeutics to Breast Cancer Cells Expressing Specific Biomarkers. Curr. Cancer Ther. Rev. 2024, 20, 434–460. [Google Scholar] [CrossRef]
- McNamara, J.O., 2nd; Andrechek, E.R.; Wang, Y.; Viles, K.D.; Rempel, R.E.; Gilboa, E.; Sullenger, B.A.; Giangrande, P.H. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 2006, 24, 1005–1015. [Google Scholar] [CrossRef]
- Jiao, Y.; Xu, P.; Luan, S.; Wang, X.; Gao, Y.; Zhao, C.; Fu, P. Molecular imaging and treatment of PSMA-positive prostate cancer with (99m)Tc radiolabeled aptamer-siRNA chimeras. Nucl. Med. Biol. 2022, 104–105, 28–37. [Google Scholar] [CrossRef]
- Rosch, J.C.; Hoogenboezem, E.N.; Sorets, A.G.; Duvall, C.L.; Lippmann, E.S. Albumin-Binding Aptamer Chimeras for Improved siRNA Bioavailability. Cell. Mol. Bioeng. 2022, 15, 161–173. [Google Scholar] [CrossRef]
- Zhang, L.; Mu, C.; Zhang, T.; Yang, D.; Wang, C.; Chen, Q.; Tang, L.; Fan, L.; Liu, C.; Shen, J.; et al. Development of targeted therapy therapeutics to sensitize triple-negative breast cancer chemosensitivity utilizing bacteriophage phi29 derived packaging RNA. J. Nanobiotechnol. 2021, 19, 13. [Google Scholar] [CrossRef]
- Rehmani, H.; Li, Y.; Li, T.; Padia, R.; Calbay, O.; Jin, L.; Chen, H.; Huang, S. Addiction to protein kinase Ci due to PRKCI gene amplification can be exploited for an aptamer-based targeted therapy in ovarian cancer. Signal Transduct. Target. Ther. 2020, 5, 140. [Google Scholar] [CrossRef]
- Zhang, L.; Mu, C.; Zhang, T.; Wang, Y.; Wang, Y.; Fan, L.; Liu, C.; Chen, H.; Shen, J.; Wei, K.; et al. Systemic Delivery of Aptamer-Conjugated XBP1 siRNA Nanoparticles for Efficient Suppression of HER2+ Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 32360–32371. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Song, R.; Sabbagh, A.; Marisetty, A.; Shukla, N.; Fang, D.; Najem, H.; Ott, M.; Long, J.; Zhai, L.; et al. Cell-directed aptamer therapeutic targeting for cancers including those within the central nervous system. Oncoimmunology 2022, 11, 2062827. [Google Scholar] [CrossRef] [PubMed]
- Velema, W.A.; Lu, Z. Chemical RNA Cross-Linking: Mechanisms, Computational Analysis, and Biological Applications. JACS Au 2023, 3, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Paskeh, M.D.A.; Entezari, M.; Bidooki, S.H.; Ghaleh, V.J.; Rezaei, S.; Hejazi, E.S.; Kakavand, A.; Behroozaghdam, M.; Movafagh, A.; et al. siRNA and targeted delivery systems in breast cancer therapy. Clin. Transl. Oncol. 2023, 25, 1167–1188. [Google Scholar] [CrossRef]
- Saw, P.E.; Song, E.W. siRNA therapeutics: A clinical reality. Sci. China Life Sci. 2020, 63, 485–500. [Google Scholar] [CrossRef]
- Alshaer, W.; Zureigat, H.; Al Karaki, A.; Al-Kadash, A.; Gharaibeh, L.; Hatmal, M.M.; Aljabali, A.A.A.; Awidi, A. siRNA: Mechanism of action, challenges, and therapeutic approaches. Eur. J. Pharmacol. 2021, 905, 174178. [Google Scholar] [CrossRef]
- Friedrich, M.; Aigner, A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. BioDrugs 2022, 36, 549–571. [Google Scholar] [CrossRef]
- Zhang, M.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X.B. The growth of siRNA-based therapeutics: Updated clinical studies. Biochem. Pharmacol. 2021, 189, 114432. [Google Scholar] [CrossRef]
- Dong, Y.; Siegwart, D.J.; Anderson, D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019, 144, 133–147. [Google Scholar] [CrossRef]
- Ku, S.H.; Jo, S.D.; Lee, Y.K.; Kim, K.; Kim, S.H. Chemical and structural modifications of RNAi therapeutics. Adv. Drug Deliv. Rev. 2016, 104, 16–28. [Google Scholar] [CrossRef]
- Chu, T.C.; Twu, K.Y.; Ellington, A.D.; Levy, M. Aptamer mediated siRNA delivery. Nucleic Acids Res. 2006, 34, e73. [Google Scholar] [CrossRef] [PubMed]
- Dassie, J.P.; Liu, X.Y.; Thomas, G.S.; Whitaker, R.M.; Thiel, K.W.; Stockdale, K.R.; Meyerholz, D.K.; McCaffrey, A.P.; McNamara, J.O., 2nd; Giangrande, P.H. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat. Biotechnol. 2009, 27, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Pastor, F.; Kolonias, D.; Giangrande, P.H.; Gilboa, E. Induction of tumour immunity by targeted inhibition of nonsense-mediated mRNA decay. Nature 2010, 465, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, E. Expression of new antigens on tumor cells by inhibiting nonsense-mediated mRNA decay. Immunol. Res. 2013, 57, 44–51. [Google Scholar] [CrossRef]
- Ni, X.; Zhang, Y.; Zennami, K.; Castanares, M.; Mukherjee, A.; Raval, R.R.; Zhou, H.; DeWeese, T.L.; Lupold, S.E. Systemic Administration and Targeted Radiosensitization via Chemically Synthetic Aptamer-siRNA Chimeras in Human Tumor Xenografts. Mol. Cancer Ther. 2015, 14, 2797–2804. [Google Scholar] [CrossRef]
- Liu, H.Y.; Yu, X.; Liu, H.; Wu, D.; She, J.X. Co-targeting EGFR and survivin with a bivalent aptamer-dual siRNA chimera effectively suppresses prostate cancer. Sci. Rep. 2016, 6, 30346. [Google Scholar] [CrossRef]
- Guo, L.; Shi, D.; Shang, M.; Sun, X.; Meng, D.; Liu, X.; Zhou, X.; Li, J. Utilizing RNA nanotechnology to construct negatively charged and ultrasound-responsive nanodroplets for targeted delivery of siRNA. Drug Deliv. 2022, 29, 316–327. [Google Scholar] [CrossRef]
- Berezhnoy, A.; Castro, I.; Levay, A.; Malek, T.R.; Gilboa, E. Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. J. Clin. Investig. 2014, 124, 188–197. [Google Scholar] [CrossRef]
- Rajagopalan, A.; Berezhnoy, A.; Schrand, B.; Puplampu-Dove, Y.; Gilboa, E. Aptamer-Targeted Attenuation of IL-2 Signaling in CD8(+) T Cells Enhances Antitumor Immunity. Mol. Ther. 2017, 25, 54–61. [Google Scholar] [CrossRef]
- Zhou, J.; Li, H.; Li, S.; Zaia, J.; Rossi, J.J. Novel dual inhibitory function aptamer-siRNA delivery system for HIV-1 therapy. Mol. Ther. 2008, 16, 1481–1489. [Google Scholar] [CrossRef]
- Zhou, J.; Swiderski, P.; Li, H.; Zhang, J.; Neff, C.P.; Akkina, R.; Rossi, J.J. Selection, characterization and application of new RNA HIV gp 120 aptamers for facile delivery of Dicer substrate siRNAs into HIV infected cells. Nucleic Acids Res. 2009, 37, 3094–3109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, H.; Zhang, J.; Piotr, S.; Rossi, J. Development of cell-type specific anti-HIV gp120 aptamers for siRNA delivery. J. Vis. Exp. JoVE 2011, 23, 2954. [Google Scholar] [CrossRef]
- Zhou, J.; Shu, Y.; Guo, P.; Smith, D.D.; Rossi, J.J. Dual functional RNA nanoparticles containing phi29 motor pRNA and anti-gp120 aptamer for cell-type specific delivery and HIV-1 inhibition. Methods 2011, 54, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Neff, C.P.; Zhou, J.; Remling, L.; Kuruvilla, J.; Zhang, J.; Li, H.; Smith, D.D.; Swiderski, P.; Rossi, J.J.; Akkina, R. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4(+) T cell decline in humanized mice. Sci. Transl. Med. 2011, 3, 66ra66. [Google Scholar] [CrossRef]
- Zhou, J.; Neff, C.P.; Swiderski, P.; Li, H.; Smith, D.D.; Aboellail, T.; Remling-Mulder, L.; Akkina, R.; Rossi, J.J. Functional in vivo delivery of multiplexed anti-HIV-1 siRNAs via a chemically synthesized aptamer with a sticky bridge. Mol. Ther. 2013, 21, 192–200. [Google Scholar] [CrossRef]
- Zhou, J.; Lazar, D.; Li, H.; Xia, X.; Satheesan, S.; Charlins, P.; O’Mealy, D.; Akkina, R.; Saayman, S.; Weinberg, M.S.; et al. Receptor-targeted aptamer-siRNA conjugate-directed transcriptional regulation of HIV-1. Theranostics 2018, 8, 1575–1590. [Google Scholar] [CrossRef]
- Zhou, J.; Satheesan, S.; Li, H.; Weinberg, M.S.; Morris, K.V.; Burnett, J.C.; Rossi, J.J. Cell-specific RNA aptamer against human CCR5 specifically targets HIV-1 susceptible cells and inhibits HIV-1 infectivity. Chem. Biol. 2015, 22, 379–390. [Google Scholar] [CrossRef]
- Wheeler, L.A.; Trifonova, R.; Vrbanac, V.; Basar, E.; McKernan, S.; Xu, Z.; Seung, E.; Deruaz, M.; Dudek, T.; Einarsson, J.I.; et al. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. J. Clin. Investig. 2011, 121, 2401–2412. [Google Scholar] [CrossRef]
- Qiu, C.; Peng, W.K.; Shi, F.; Zhang, T. Bottom-up assembly of RNA nanoparticles containing phi29 motor pRNA to silence the asthma STAT5b gene. Genet. Mol. Res. 2012, 11, 3236–3245. [Google Scholar] [CrossRef]
- Zhu, Q.; Shibata, T.; Kabashima, T.; Kai, M. Inhibition of HIV-1 protease expression in T cells owing to DNA aptamer-mediated specific delivery of siRNA. Eur. J. Med. Chem. 2012, 56, 396–399. [Google Scholar] [CrossRef]
- Wheeler, L.A.; Vrbanac, V.; Trifonova, R.; Brehm, M.A.; Gilboa-Geffen, A.; Tanno, S.; Greiner, D.L.; Luster, A.D.; Tager, A.M.; Lieberman, J. Durable knockdown and protection from HIV transmission in humanized mice treated with gel-formulated CD4 aptamer-siRNA chimeras. Mol. Ther. 2013, 21, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Chung, W.H.; Cheng, Y.F.; Ying, N.W.; Peck, K.; Chen, Y.T.; Hung, S.I. A new nucleic acid-based agent inhibits cytotoxic T lymphocyte-mediated immune disorders. J. Allergy Clin. Immunol. 2013, 132, 713–722.e11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tiemann, K.; Chomchan, P.; Alluin, J.; Swiderski, P.; Burnett, J.; Zhang, X.; Forman, S.; Chen, R.; Rossi, J. Dual functional BAFF receptor aptamers inhibit ligand-induced proliferation and deliver siRNAs to NHL cells. Nucleic Acids Res. 2013, 41, 4266–4283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, H.; Tan, Y.; Wu, X.; Li, S.; Shi, Y. A U87-EGFRvIII cell-specific aptamer mediates small interfering RNA delivery. Biomed. Rep. 2014, 2, 495–499. [Google Scholar] [CrossRef]
- Lai, W.Y.; Wang, W.Y.; Chang, Y.C.; Chang, C.J.; Yang, P.C.; Peck, K. Synergistic inhibition of lung cancer cell invasion, tumor growth and angiogenesis using aptamer-siRNA chimeras. Biomaterials 2014, 35, 2905–2914. [Google Scholar] [CrossRef]
- Cheng, H.; Hong, S.; Wang, Z.; Sun, N.; Wang, T.; Zhang, Y.; Chen, H.; Pei, R. Self-assembled RNAi nanoflowers via rolling circle transcription for aptamer-targeted siRNA delivery. J. Mater. Chem. B 2018, 6, 4638–4644. [Google Scholar] [CrossRef]
- Meraviglia-Crivelli, D.; Villanueva, H.; Menon, A.P.; Zheleva, A.; Moreno, B.; Villalba-Esparza, M.; Pastor, F. A pan-tumor-siRNA aptamer chimera to block nonsense-mediated mRNA decay inflames and suppresses tumor progression. Mol. Ther. Nucleic Acids 2022, 29, 413–425. [Google Scholar] [CrossRef]
- Subramanian, N.; Kanwar, J.R.; Athalya, P.K.; Janakiraman, N.; Khetan, V.; Kanwar, R.K.; Eluchuri, S.; Krishnakumar, S. EpCAM aptamer mediated cancer cell specific delivery of EpCAM siRNA using polymeric nanocomplex. J. Biomed. Sci. 2015, 22, 4. [Google Scholar] [CrossRef]
- Wang, T.; Gantier, M.P.; Xiang, D.; Bean, A.G.; Bruce, M.; Zhou, S.F.; Khasraw, M.; Ward, A.; Wang, L.; Wei, M.Q.; et al. EpCAM Aptamer-mediated Survivin Silencing Sensitized Cancer Stem Cells to Doxorubicin in a Breast Cancer Model. Theranostics 2015, 5, 1456–1472. [Google Scholar] [CrossRef]
- Subramanian, N.; Kanwar, J.R.; Kanwar, R.K.; Sreemanthula, J.; Biswas, J.; Khetan, V.; Krishnakumar, S. EpCAM Aptamer-siRNA Chimera Targets and Regress Epithelial Cancer. PLoS ONE 2015, 10, e0132407. [Google Scholar] [CrossRef]
- Gilboa-Geffen, A.; Hamar, P.; Le, M.T.; Wheeler, L.A.; Trifonova, R.; Petrocca, F.; Wittrup, A.; Lieberman, J. Gene Knockdown by EpCAM Aptamer-siRNA Chimeras Suppresses Epithelial Breast Cancers and Their Tumor-Initiating Cells. Mol. Cancer Ther. 2015, 14, 2279–2291. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Pang, L.; Wang, H.; Xu, C.; Shah, H.; Guo, P.; Shu, D.; Qian, S.Y. Specific delivery of delta-5-desaturase siRNA via RNA nanoparticles supplemented with dihomo-gamma-linolenic acid for colon cancer suppression. Redox Biol. 2019, 21, 101085. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Shah, H.; Wang, H.; Shu, D.; Qian, S.Y.; Sathish, V. EpCAM-Targeted 3WJ RNA Nanoparticle Harboring Delta-5-Desaturase siRNA Inhibited Lung Tumor Formation via DGLA Peroxidation. Mol. Ther. Nucleic Acids 2020, 22, 222–235. [Google Scholar] [CrossRef]
- Thiel, K.W.; Hernandez, L.I.; Dassie, J.P.; Thiel, W.H.; Liu, X.; Stockdale, K.R.; Rothman, A.M.; Hernandez, F.J.; McNamara, J.O., 2nd; Giangrande, P.H. Delivery of chemo-sensitizing siRNAs to HER2+-breast cancer cells using RNA aptamers. Nucleic Acids Res. 2012, 40, 6319–6337. [Google Scholar] [CrossRef]
- Yu, X.; Ghamande, S.; Liu, H.; Xue, L.; Zhao, S.; Tan, W.; Zhao, L.; Tang, S.C.; Wu, D.; Korkaya, H.; et al. Targeting EGFR/HER2/HER3 with a Three-in-One Aptamer-siRNA Chimera Confers Superior Activity against HER2(+) Breast Cancer. Mol. Ther. Nucleic Acids 2018, 10, 317–330. [Google Scholar] [CrossRef]
- Xue, L.; Maihle, N.J.; Yu, X.; Tang, S.C.; Liu, H.Y. Synergistic Targeting HER2 and EGFR with Bivalent Aptamer-siRNA Chimera Efficiently Inhibits HER2-Positive Tumor Growth. Mol. Pharm. 2018, 15, 4801–4813. [Google Scholar] [CrossRef]
- Kim, M.W.; Jeong, H.Y.; Kang, S.J.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Im, C.S.; Song, I.H.; Lee, T.S.; Lee, J.S.; et al. Anti-EGF Receptor Aptamer-Guided Co-Delivery of Anti-Cancer siRNAs and Quantum Dots for Theranostics of Triple-Negative Breast Cancer. Theranostics 2019, 9, 837–852. [Google Scholar] [CrossRef]
- Yang, L.; Li, Z.; Binzel, D.W.; Guo, P.; Williams, T.M. Targeting oncogenic KRAS in non-small cell lung cancer with EGFR aptamer-conjugated multifunctional RNA nanoparticles. Mol. Ther. Nucleic Acids 2023, 33, 559–571. [Google Scholar] [CrossRef]
- Xu, X.; Li, L.; Li, X.; Tao, D.; Zhang, P.; Gong, J. Aptamer-protamine-siRNA nanoparticles in targeted therapy of ErbB3 positive breast cancer cells. Int. J. Pharm. 2020, 590, 119963. [Google Scholar] [CrossRef]
- Ren, K.; Liu, Y.; Wu, J.; Zhang, Y.; Zhu, J.; Yang, M.; Ju, H. A DNA dual lock-and-key strategy for cell-subtype-specific siRNA delivery. Nat. Commun. 2016, 7, 13580. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, S.H.; Hwang, Y.; Yoo, H.; Jung, H.; Kim, S.H.; Mok, H. Multivalent Aptamer-RNA Conjugates for Simple and Efficient Delivery of Doxorubicin/siRNA into Multidrug-Resistant Cells. Macromol. Biosci. 2017, 17, 1600343. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, M.; Guo, Y.; Tu, K.; Wu, W.; Wang, J.; Tong, X.; Wu, W.; Qi, L.; Shi, D. Targeted chimera delivery to ovarian cancer cells by heterogeneous gold magnetic nanoparticle. Nanotechnology 2017, 28, 025101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, S.; Yu, Q.; Wei, S.; Liu, M.; Wei, J.; Huang, Y.; Huang, X.; Li, P.; Qin, Q. Characterization of Novel Aptamers Specifically Directed to Red-Spotted Grouper Nervous Necrosis Virus (RGNNV)-Infected Cells for Mediating Targeted siRNA Delivery. Front. Microbiol. 2020, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, W.; Liu, M.; Li, M.; Zhuo, X.; Feng, L.; Wang, G.; Li, P. Aptamer-mediated targeted siRNA delivery against grouper iridovirus infection. Aquaculture 2021, 544, 737148. [Google Scholar] [CrossRef]
- Herrmann, A.; Priceman, S.J.; Swiderski, P.; Kujawski, M.; Xin, H.; Cherryholmes, G.A.; Zhang, W.; Zhang, C.; Lahtz, C.; Kowolik, C.; et al. CTLA4 aptamer delivers STAT3 siRNA to tumor-associated and malignant T cells. J. Clin. Investig. 2014, 124, 2977–2987. [Google Scholar] [CrossRef]
- Hussain, A.F.; Tur, M.K.; Barth, S. An aptamer-siRNA chimera silences the eukaryotic elongation factor 2 gene and induces apoptosis in cancers expressing alphavbeta3 integrin. Nucleic Acid Ther. 2013, 23, 203–212. [Google Scholar] [CrossRef]
- Esposito, C.L.; Nuzzo, S.; Catuogno, S.; Romano, S.; de Nigris, F.; de Franciscis, V. STAT3 Gene Silencing by Aptamer-siRNA Chimera as Selective Therapeutic for Glioblastoma. Mol. Ther. Nucleic Acids 2018, 10, 398–411. [Google Scholar] [CrossRef]
- Esposito, C.L.; Nuzzo, S.; Ibba, M.L.; Ricci-Vitiani, L.; Pallini, R.; Condorelli, G.; Catuogno, S.; de Franciscis, V. Combined Targeting of Glioblastoma Stem-Like Cells by Neutralizing RNA-Bio-Drugs for STAT3. Cancers 2020, 12, 1434. [Google Scholar] [CrossRef]
- Ibba, M.L.; Ciccone, G.; Rotoli, D.; Coppola, G.; Fiorelli, A.; Catuogno, S.; Esposito, C.L. STAT3 silencing by an aptamer-based strategy hampers the crosstalk between NSCLC cells and cancer-associated fibroblasts. Mol. Ther. Nucleic Acids 2023, 32, 111–126. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, F.; Hao, X.; Bai, S.; Hao, J. Inhibition of monocyte adhesion to brain-derived endothelial cells by dual functional RNA chimeras. Mol. Ther. Nucleic Acids 2014, 3, e209. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, H.; Jain, A.; Zhang, L.; Liu, C.; Cheng, K. Discovery of Aptamer Ligands for Hepatic Stellate Cells Using SELEX. Theranostics 2017, 7, 2982–2995. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, J.; Cai, G.; Chen, W.; Zhou, Z. Aptamer/Adenosine Kinase Chimera Promotes Angiogenesis Through Regulating M2-Type Monocytes/Macrophages Polarization. J. Biomater. Tissue Eng. 2018, 8, 1439–1448. [Google Scholar] [CrossRef]
- Soldevilla, M.M.; Villanueva, H.; Bendandi, M.; Inoges, S.; Lopez-Diaz de Cerio, A.; Pastor, F. 2-fluoro-RNA oligonucleotide CD40 targeted aptamers for the control of B lymphoma and bone-marrow aplasia. Biomaterials 2015, 67, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Chou, Y.K.; Zhang, X.; Meza-Romero, R.; Yomogida, K.; Benedek, G.; Chu, C.Q. CD4 aptamer-RORgammat shRNA chimera inhibits IL-17 synthesis by human CD4(+) T cells. Biochem. Biophys. Res. Commun. 2014, 452, 1040–1045. [Google Scholar] [CrossRef]
- Shi, X.; Song, P.; Tao, S.; Zhang, X.; Chu, C.Q. Silencing RORgammat in Human CD4(+) T cells with CD30 aptamer-RORgammat shRNA Chimera. Sci. Rep. 2019, 9, 10375. [Google Scholar] [CrossRef]
- Pang, K.M.; Castanotto, D.; Li, H.; Scherer, L.; Rossi, J.J. Incorporation of aptamers in the terminal loop of shRNAs yields an effective and novel combinatorial targeting strategy. Nucleic Acids Res. 2018, 46, e6. [Google Scholar] [CrossRef]
- Ni, X.; Zhang, Y.; Ribas, J.; Chowdhury, W.H.; Castanares, M.; Zhang, Z.; Laiho, M.; DeWeese, T.L.; Lupold, S.E. Prostate-targeted radiosensitization via aptamer-shRNA chimeras in human tumor xenografts. J. Clin. Investig. 2011, 121, 2383–2390. [Google Scholar] [CrossRef]
- Zeng, T.; Xie, Y.; Chai, K.; Sang, H. The Application of Prostate Specific Membrane Antigen in the Diagnosis and Treatment of Prostate Cancer: Status and Challenge. OncoTargets Ther. 2024, 17, 991–1015. [Google Scholar] [CrossRef]
- Cavalu, S.; Abdelhamid, A.M.; Saber, S.; Elmorsy, E.A.; Hamad, R.S.; Abdel-Reheim, M.A.; Yahya, G.; Salama, M.M. Cell cycle machinery in oncology: A comprehensive review of therapeutic targets. FASEB J. 2024, 38, e23734. [Google Scholar] [CrossRef]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 protein family: Attractive targets for cancer therapy. Apoptosis 2023, 28, 20–38. [Google Scholar] [CrossRef]
- Wullner, U.; Neef, I.; Eller, A.; Kleines, M.; Tur, M.K.; Barth, S. Cell-specific induction of apoptosis by rationally designed bivalent aptamer-siRNA transcripts silencing eukaryotic elongation factor 2. Curr. Cancer Drug Targets 2008, 8, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Yu, X.; Zhao, L.; Garrett, A.; Wu, D.; Liu, H.Y. Targeted Delivery of AR-V7 siRNA with Bivalent PSMA Aptamers Effectively Suppresses the Growth of Enzalutamide-Resistant Prostate Cancer. Mol. Pharm. 2024, 21, 5749–5760. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Wang, Y.; Li, R.; Chen, W.; Wan, L.; Cai, P. The HER family as therapeutic targets in colorectal cancer. Crit. Rev. Oncol. Hematol. 2022, 174, 103681. [Google Scholar] [CrossRef]
- Jacobsen, H.J.; Poulsen, T.T.; Dahlman, A.; Kjaer, I.; Koefoed, K.; Sen, J.W.; Weilguny, D.; Bjerregaard, B.; Andersen, C.R.; Horak, I.D.; et al. Pan-HER, an Antibody Mixture Simultaneously Targeting EGFR, HER2, and HER3, Effectively Overcomes Tumor Heterogeneity and Plasticity. Clin. Cancer Res. 2015, 21, 4110–4122. [Google Scholar] [CrossRef]
- Liao, Y.C.; Cheng, T.C.; Tu, S.H.; Chang, J.; Guo, P.; Chen, L.C.; Ho, Y.S. Tumor targeting and therapeutic assessments of RNA nanoparticles carrying alpha9-nAChR aptamer and anti-miR-21 in triple-negative breast cancers. Mol. Ther. Nucleic Acids 2023, 33, 351–366. [Google Scholar] [CrossRef]
- Shu, D.; Li, H.; Shu, Y.; Xiong, G.; Carson, W.E., 3rd; Haque, F.; Xu, R.; Guo, P. Systemic Delivery of Anti-miRNA for Suppression of Triple Negative Breast Cancer Utilizing RNA Nanotechnology. ACS Nano 2015, 9, 9731–9740. [Google Scholar] [CrossRef]
- Yin, H.; Xiong, G.; Guo, S.; Xu, C.; Xu, R.; Guo, P.; Shu, D. Delivery of Anti-miRNA for Triple-Negative Breast Cancer Therapy Using RNA Nanoparticles Targeting Stem Cell Marker CD133. Mol. Ther. 2019, 27, 1252–1261. [Google Scholar] [CrossRef]
- Zhao, J.; Niu, N.; Yang, F.; Liu, H.; Qi, W. Preparation, characterisation, and in vitro cancer-suppression function of RNA nanoparticles carrying miR-301b-3p Inhibitor. IET Nanobiotechnol. 2023, 17, 224–233. [Google Scholar] [CrossRef]
- Xu, C.; Haque, F.; Jasinski, D.L.; Binzel, D.W.; Shu, D.; Guo, P. Favorable biodistribution, specific targeting and conditional endosomal escape of RNA nanoparticles in cancer therapy. Cancer Lett. 2018, 414, 57–70. [Google Scholar] [CrossRef]
- Liu, X.; Duan, D.; Wang, Y.; Liu, J.; Duan, D. Advancements in 3WJ-based RNA nanotechnology and its application for cancer diagnosis and therapy. Front. Biosci. 2022, 27, 61. [Google Scholar] [CrossRef]
- Isenmann, M.; Stoddart, M.J.; Schmelzeisen, R.; Gross, C.; Della Bella, E.; Rothweiler, R.M. Basic Principles of RNA Interference: Nucleic Acid Types and In Vitro Intracellular Delivery Methods. Micromachines 2023, 14, 1321. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.C.; Pietrobon, V.; Peng, M.; Wang, S.; Zhao, L.; Marincola, F.M.; Cai, Q. Current strategies employed in the manipulation of gene expression for clinical purposes. J. Transl. Med. 2022, 20, 535. [Google Scholar] [CrossRef] [PubMed]
- Shalbi, F.; Ali, A.R. A mini-review on integrase inhibitors: The cornerstone of next-generation HIV treatment. Eur. J. Med. Chem. 2024, 279, 116900. [Google Scholar] [CrossRef] [PubMed]
- Scoles, D.R.; Minikel, E.V.; Pulst, S.M. Antisense oligonucleotides: A primer. Neurol. Genet. 2019, 5, e323. [Google Scholar] [CrossRef]
- Crooke, S.T.; Baker, B.F.; Crooke, R.M.; Liang, X.H. Antisense technology: An overview and prospectus. Nat. Rev. Drug Discov. 2021, 20, 427–453. [Google Scholar] [CrossRef]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef]
- Quemener, A.M.; Bachelot, L.; Forestier, A.; Donnou-Fournet, E.; Gilot, D.; Galibert, M.D. The powerful world of antisense oligonucleotides: From bench to bedside. Wiley Interdiscip. Rev. RNA 2020, 11, e1594. [Google Scholar] [CrossRef]
- Kim, Y. Drug Discovery Perspectives of Antisense Oligonucleotides. Biomol. Ther. 2023, 31, 241–252. [Google Scholar] [CrossRef]
- Huang, S.; Hao, X.Y.; Li, Y.J.; Wu, J.Y.; Xiang, D.X.; Luo, S. Nonviral delivery systems for antisense oligonucleotide therapeutics. Biomater. Res. 2022, 26, 49. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Munusamy, S.; Chakraborty, N.; Kim, J.O. Nano drug delivery systems for antisense oligonucleotides (ASO) therapeutics. J. Control. Release 2022, 352, 861–878. [Google Scholar] [CrossRef]
- Ruchi, R.; Raman, G.M.; Kumar, V.; Bahal, R. Evolution of antisense oligonucleotides: Navigating nucleic acid chemistry and delivery challenges. Expert. Opin. Drug Discov. 2025, 20, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Kotula, J.W.; Pratico, E.D.; Ming, X.; Nakagawa, O.; Juliano, R.L.; Sullenger, B.A. Aptamer-mediated delivery of splice-switching oligonucleotides to the nuclei of cancer cells. Nucleic Acid Ther. 2012, 22, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Han, D.; Chen, T.; Peng, L.; Zhu, G.; You, M.; Qiu, L.; Sefah, K.; Zhang, X.; Tan, W. Building a multifunctional aptamer-based DNA nanoassembly for targeted cancer therapy. J. Am. Chem. Soc. 2013, 135, 18644–18650. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, C.; Cansiz, S.; Wu, C.; Xu, J.; Cui, C.; Liu, Y.; Hou, W.; Wang, Y.; Zhang, L.; et al. Self-assembly of DNA nanohydrogels with controllable size and stimuli-responsive property for targeted gene regulation therapy. J. Am. Chem. Soc. 2015, 137, 1412–1415. [Google Scholar] [CrossRef]
- Hong, S.; Sun, N.; Liu, M.; Wang, J.; Pei, R. Building a chimera of aptamer–antisense oligonucleotide for silencing galectin-1 gene. RSC Adv. 2016, 6, 112445–112450. [Google Scholar] [CrossRef]
- Dai, Z.; Li, J.; Lin, Y.; Wang, Z.; Huang, Y. Facile Construction of a Solely-DNA-Based System for Targeted Delivery of Nucleic Acids. Nanomaterials 2021, 11, 1967. [Google Scholar] [CrossRef]
- Luo, F.; Yang, G.; Bai, X.; Yuan, D.; Li, L.; Wang, D.; Lu, X.; Cheng, Y.; Wang, Y.; Song, X.; et al. Anti-tumor effect of PD-L1-targeting antagonistic aptamer-ASO delivery system with dual inhibitory function in immunotherapy. Cell Chem. Biol. 2023, 30, 1390–1401.e6. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, S.; Song, W.; Bai, X.; Li, L.; Luo, F.; Cheng, Y.; Wang, D.; Wang, Y.; Chen, J.; et al. Efficient Targeted Delivery of Bifunctional Circular Aptamer-ASO Chimera to Suppress the SARS-CoV-2 Proliferation and Inflammation. Small 2023, 19, e2207066. [Google Scholar] [CrossRef]
- Thongchot, S.; Aksonnam, K.; Thuwajit, P.; Yenchitsomanus, P.T.; Thuwajit, C. Nucleolin-based targeting strategies in cancer treatment: Focus on cancer immunotherapy (Review). Int. J. Mol. Med. 2023, 52, 81. [Google Scholar] [CrossRef]
- Van den Avont, A.; Sharma-Walia, N. Anti-nucleolin aptamer AS1411: An advancing therapeutic. Front. Mol. Biosci. 2023, 10, 1217769. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Li, G.; Wang, J.; Liu, M.; Wang, Z.; Song, Y.; Zhang, X.; Wang, X. PD-L1: Expression regulation. Blood Sci. 2023, 5, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, W.; Xu, Z.P.; Gu, W. PD-L1 Distribution and Perspective for Cancer Immunotherapy-Blockade, Knockdown, or Inhibition. Front. Immunol. 2019, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Kitai, H.; Suzuki, H.I. Network Regulation of microRNA Biogenesis and Target Interaction. Cells 2023, 12, 306. [Google Scholar] [CrossRef]
- Pagoni, M.; Cava, C.; Sideris, D.C.; Avgeris, M.; Zoumpourlis, V.; Michalopoulos, I.; Drakoulis, N. miRNA-Based Technologies in Cancer Therapy. J. Pers. Med. 2023, 13, 1586. [Google Scholar] [CrossRef]
- Samad, A.F.A.; Kamaroddin, M.F. Innovative approaches in transforming microRNAs into therapeutic tools. Wiley Interdiscip. Rev. RNA 2023, 14, e1768. [Google Scholar] [CrossRef]
- Catuogno, S.; Di Martino, M.T.; Nuzzo, S.; Esposito, C.L.; Tassone, P.; de Franciscis, V. An Anti-BCMA RNA Aptamer for miRNA Intracellular Delivery. Mol. Ther. Nucleic Acids 2019, 18, 981–990. [Google Scholar] [CrossRef]
- Catuogno, S.; Rienzo, A.; Di Vito, A.; Esposito, C.L.; de Franciscis, V. Selective delivery of therapeutic single strand antimiRs by aptamer-based conjugates. J. Control. Release 2015, 210, 147–159. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Bahreyni, A.; Lavaee, P.; Moosavian, S.A.; Taghdisi, S.M. A smart ATP-responsive chemotherapy drug-free delivery system using a DNA nanostructure for synergistic treatment of breast cancer in vitro and in vivo. J. Drug Target. 2020, 28, 852–859. [Google Scholar] [CrossRef]
- Ramezanpour, M.; Daei, P.; Tabarzad, M.; Khanaki, K.; Elmi, A.; Barati, M. Preliminary study on the effect of nucleolin specific aptamer-miRNA let-7d chimera on Janus kinase-2 expression level and activity in gastric cancer (MKN-45) cells. Mol. Biol. Rep. 2019, 46, 207–215. [Google Scholar] [CrossRef]
- Russo, V.; Paciocco, A.; Affinito, A.; Roscigno, G.; Fiore, D.; Palma, F.; Galasso, M.; Volinia, S.; Fiorelli, A.; Esposito, C.L.; et al. Aptamer-miR-34c Conjugate Affects Cell Proliferation of Non-Small-Cell Lung Cancer Cells. Mol. Ther. Nucleic Acids 2018, 13, 334–346. [Google Scholar] [CrossRef]
- Esposito, C.L.; Nuzzo, S.; Kumar, S.A.; Rienzo, A.; Lawrence, C.L.; Pallini, R.; Shaw, L.; Alder, J.E.; Ricci-Vitiani, L.; Catuogno, S.; et al. A combined microRNA-based targeted therapeutic approach to eradicate glioblastoma stem-like cells. J. Control. Release 2016, 238, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Iaboni, M.; Russo, V.; Fontanella, R.; Roscigno, G.; Fiore, D.; Donnarumma, E.; Esposito, C.L.; Quintavalle, C.; Giangrande, P.H.; de Franciscis, V.; et al. Aptamer-miRNA-212 Conjugate Sensitizes NSCLC Cells to TRAIL. Mol. Ther. Nucleic Acids 2016, 5, e289. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.L.; Cerchia, L.; Catuogno, S.; De Vita, G.; Dassie, J.P.; Santamaria, G.; Swiderski, P.; Condorelli, G.; Giangrande, P.H.; de Franciscis, V. Multifunctional aptamer-miRNA conjugates for targeted cancer therapy. Mol. Ther. 2014, 22, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, Y.; Yang, D.; Wu, C.; Li, H. Preparation, characterization, and in vitro tumor-suppressive effect of anti-miR-21-equipped RNA nanoparticles. Biochem. Biophys. Res. Commun. 2021, 558, 107–113. [Google Scholar] [CrossRef]
- Dai, F.; Zhang, Y.; Zhu, X.; Shan, N.; Chen, Y. The anti-chemoresistant effect and mechanism of MUC1 aptamer-miR-29b chimera in ovarian cancer. Gynecol. Oncol. 2013, 131, 451–459. [Google Scholar] [CrossRef]
- Nuzzo, S.; Catuogno, S.; Capuozzo, M.; Fiorelli, A.; Swiderski, P.; Boccella, S.; de Nigris, F.; Esposito, C.L. Axl-Targeted Delivery of the Oncosuppressor miR-137 in Non-small-Cell Lung Cancer. Mol. Ther. Nucleic Acids 2019, 17, 256–263. [Google Scholar] [CrossRef]
- Li, J.; Qiu, L.; Xie, S.; Zhang, J.; Zhang, L.; Liu, H.; Li, J.; Zhang, X.; Tan, W. Engineering a customized nanodrug delivery system at the cellular level for targeted cancer therapy. Sci. China Chem. 2018, 61, 497–504. [Google Scholar] [CrossRef]
- Wu, X.; Ding, B.; Gao, J.; Wang, H.; Fan, W.; Wang, X.; Zhang, W.; Wang, X.; Ye, L.; Zhang, M.; et al. Second-generation aptamer-conjugated PSMA-targeted delivery system for prostate cancer therapy. Int. J. Nanomed. 2011, 6, 1747–1756. [Google Scholar] [CrossRef]
- Tanno, T.; Zhang, P.; Bailey, C.; Wang, Y.; Ittiprasert, W.; Devenport, M.; Zheng, P.; Liu, Y. A novel aptamer-based small RNA delivery platform and its application to cancer therapy. Genes Dis. 2023, 10, 1075–1089. [Google Scholar] [CrossRef]
- Haj-Yahia, S.; Nandi, A.; Benhamou, R.I. Targeted Degradation of Structured RNAs via Ribonuclease-Targeting Chimeras (RiboTacs). Expert. Opin. Drug Discov. 2023, 18, 929–942. [Google Scholar] [CrossRef]
- Dey, S.K.; Jaffrey, S.R. RIBOTACs: Small Molecules Target RNA for Degradation. Cell Chem. Biol. 2019, 26, 1047–1049. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wu, Q.; Wang, F.; Liu, Y.; Zhang, H.; Yang, C.; Zhu, Z. Aptamer-RIBOTAC Strategy Enabling Tumor-Specific Targeted Degradation of MicroRNA for Precise Cancer Therapy. Small Methods 2024, 8, e2400349. [Google Scholar] [CrossRef]
- Kwok, A.; Raulf, N.; Habib, N. Developing small activating RNA as a therapeutic: Current challenges and promises. Ther. Deliv. 2019, 10, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Bednarz, P.T.; Oberli, M.A.; Veiseh, O. Small activating RNA delivery in vivo: Challenges, prospects, and lessons learned from siRNA delivery. Nano Res. 2024, 17, 8990–9002. [Google Scholar] [CrossRef]
- Tan, C.P.; Sinigaglia, L.; Gomez, V.; Nicholls, J.; Habib, N.A. RNA Activation—A Novel Approach to Therapeutically Upregulate Gene Transcription. Molecules 2021, 26, 6530. [Google Scholar] [CrossRef]
- Voutila, J.; Reebye, V.; Roberts, T.C.; Protopapa, P.; Andrikakou, P.; Blakey, D.C.; Habib, R.; Huber, H.; Saetrom, P.; Rossi, J.J.; et al. Development and Mechanism of Small Activating RNA Targeting CEBPA, a Novel Therapeutic in Clinical Trials for Liver Cancer. Mol. Ther. 2017, 25, 2705–2714. [Google Scholar] [CrossRef]
- Yoon, S.; Huang, K.W.; Reebye, V.; Mintz, P.; Tien, Y.W.; Lai, H.S.; Saetrom, P.; Reccia, I.; Swiderski, P.; Armstrong, B.; et al. Targeted Delivery of C/EBPalpha -saRNA by Pancreatic Ductal Adenocarcinoma-specific RNA Aptamers Inhibits Tumor Growth In Vivo. Mol. Ther. 2016, 24, 1106–1116. [Google Scholar] [CrossRef]
- Yoon, S.; Huang, K.W.; Andrikakou, P.; Vasconcelos, D.; Swiderski, P.; Reebye, V.; Sodergren, M.; Habib, N.; Rossi, J.J. Targeted Delivery of C/EBPalpha-saRNA by RNA Aptamers Shows Anti-tumor Effects in a Mouse Model of Advanced PDAC. Mol. Ther. Nucleic Acids 2019, 18, 142–154. [Google Scholar] [CrossRef]
- Li, C.; Jiang, W.; Hu, Q.; Li, L.C.; Dong, L.; Chen, R.; Zhang, Y.; Tang, Y.; Thrasher, J.B.; Liu, C.B.; et al. Enhancing DPYSL3 gene expression via a promoter-targeted small activating RNA approach suppresses cancer cell motility and metastasis. Oncotarget 2016, 7, 22893–22910. [Google Scholar] [CrossRef]
- Van Simaeys, D.; De La Fuente, A.; Zilio, S.; Zoso, A.; Kuznetsova, V.; Alcazar, O.; Buchwald, P.; Grilli, A.; Caroli, J.; Bicciato, S.; et al. RNA aptamers specific for transmembrane p24 trafficking protein 6 and Clusterin for the targeted delivery of imaging reagents and RNA therapeutics to human beta cells. Nat. Commun. 2022, 13, 1815. [Google Scholar] [CrossRef]
- Porciani, D.; Tedeschi, L.; Marchetti, L.; Citti, L.; Piazza, V.; Beltram, F.; Signore, G. Aptamer-Mediated Codelivery of Doxorubicin and NF-kappaB Decoy Enhances Chemosensitivity of Pancreatic Tumor Cells. Mol. Ther. Nucleic Acids 2015, 4, e235. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Al-Waili, D.; Hassan, A.; Fan, G.C.; Xin, M.; Hao, J. Inhibition of cerebral vascular inflammation by brain endothelium-targeted oligodeoxynucleotide complex. Neuroscience 2016, 329, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Wang, L. Iron metabolism and the tumor microenvironment: A new perspective on cancer intervention and therapy (Review). Int. J. Mol. Med. 2025, 55, 39. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Rossi, J.J. Treatment of Pancreatic Cancer by Aptamer Conjugated C/EBPalpha-saRNA. Adv. Exp. Med. Biol. 2017, 983, 173–188. [Google Scholar] [CrossRef]
- Mahjoubin-Tehran, M.; Atkin, S.L.; Bezsonov, E.E.; Jamialahmadi, T.; Sahebkar, A. Harnessing the Therapeutic Potential of Decoys in Non-Atherosclerotic Cardiovascular Diseases: State of the Art. J. Cardiovasc. Dev. Dis. 2021, 8, 103. [Google Scholar] [CrossRef]
- Yamakawa, K.; Nakano-Narusawa, Y.; Hashimoto, N.; Yokohira, M.; Matsuda, Y. Development and Clinical Trials of Nucleic Acid Medicines for Pancreatic Cancer Treatment. Int. J. Mol. Sci. 2019, 20, 4224. [Google Scholar] [CrossRef]
- Datsyuk, J.K.; Paudel, K.R.; Rajput, R.; Kokkinis, S.; El Sherkawi, T.; Singh, S.K.; Gupta, G.; Chellappan, D.K.; Yeung, S.; Hansbro, P.M.; et al. Emerging applications and prospects of NFkappaB decoy oligodeoxynucleotides in managing respiratory diseases. Chem.-Biol. Interact. 2023, 385, 110737. [Google Scholar] [CrossRef]
- Mehta, M.; Paudel, K.R.; Shukla, S.D.; Allam, V.; Kannaujiya, V.K.; Panth, N.; Das, A.; Parihar, V.K.; Chakraborty, A.; Ali, M.K.; et al. Recent trends of NFkappaB decoy oligodeoxynucleotide-based nanotherapeutics in lung diseases. J. Control. Release 2021, 337, 629–644. [Google Scholar] [CrossRef]
- Yao, X.; Lyu, P.; Yoo, K.; Yadav, M.K.; Singh, R.; Atala, A.; Lu, B. Engineered extracellular vesicles as versatile ribonucleoprotein delivery vehicles for efficient and safe CRISPR genome editing. J. Extracell. Vesicles 2021, 10, e12076. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Zhu, Z.; Ding, C.; Chen, S.; Liu, R.; Fan, H.; Chen, Y.; Li, H. Novel CD123 polyaptamer hydrogel edited by Cas9/sgRNA for AML-targeted therapy. Drug Deliv. 2021, 28, 1166–1178. [Google Scholar] [CrossRef]
- Han, Y.; Ding, B.; Zhao, Z.; Zhang, H.; Sun, B.; Zhao, Y.; Jiang, L.; Zhou, J.; Ding, Y. Immune lipoprotein nanostructures inspired relay drug delivery for amplifying antitumor efficiency. Biomaterials 2018, 185, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, J.; Duan, J.; Yi, H.; Liu, J.; Song, H.; Zhang, Z.; Shi, J.; Zhang, K. Enrichment and sensing tumor cells by embedded immunomodulatory DNA hydrogel to inhibit postoperative tumor recurrence. Nat. Commun. 2023, 14, 4511. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Deng, L.; Li, S. [Application of nanoparticles in CRISPR/Cas9-based gene therapy]. Sheng Wu Gong Cheng Xue Bao 2022, 38, 2087–2104. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; El-Tanani, M.; Tambuwala, M.M. Principles of CRISPR-Cas9 technology: Advancements in genome editing and emerging trends in drug delivery. J. Drug Deliv. Sci. Technol. 2024, 92, 105338. [Google Scholar] [CrossRef]
- Razavi, Z.; Soltani, M.; Souri, M.; van Wijnen, A.J. CRISPR innovations in tissue engineering and gene editing. Life Sci. 2024, 358, 123120. [Google Scholar] [CrossRef]
- Yang, W.; Yan, J.; Zhuang, P.; Ding, T.; Chen, Y.; Zhang, Y.; Zhang, H.; Cui, W. Progress of delivery methods for CRISPR-Cas9. Expert. Opin. Drug Deliv. 2022, 19, 913–926. [Google Scholar] [CrossRef]
- Whitley, J.A.; Cai, H. Engineering extracellular vesicles to deliver CRISPR ribonucleoprotein for gene editing. J. Extracell. Vesicles 2023, 12, e12343. [Google Scholar] [CrossRef]
- Lu, Z.; Yao, X.; Lyu, P.; Yadav, M.; Yoo, K.; Atala, A.; Lu, B. Lentiviral Capsid-Mediated Streptococcus pyogenes Cas9 Ribonucleoprotein Delivery for Efficient and Safe Multiplex Genome Editing. CRISPR J. 2021, 4, 914–928. [Google Scholar] [CrossRef]
- Dey, S.; Basu, S.; Ranjan, A. Revisiting the Role of CD63 as Pro-Tumorigenic or Anti-Tumorigenic Tetraspanin in Cancers and its Theragnostic Implications. Adv. Biol. 2023, 7, e2300078. [Google Scholar] [CrossRef]
- Lee, J.; Le, Q.V.; Yang, G.; Oh, Y.K. Cas9-edited immune checkpoint blockade PD-1 DNA polyaptamer hydrogel for cancer immunotherapy. Biomaterials 2019, 218, 119359. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, M.; Yang, Y.; Wang, G.; Che, F.; Li, Q.; Zhang, L. CD123 thioaptamer protects against sepsis via the blockade between IL-3/CD123 in a cecal ligation and puncture rat model. Nucleosides Nucleotides Nucleic Acids 2021, 40, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, H.; Duan, M.; Yang, Y.; Wang, G.; Che, F.; Liu, B.; He, W.; Li, Q.; Zhang, L. SS30, a novel thioaptamer targeting CD123, inhibits the growth of acute myeloid leukemia cells. Life Sci. 2019, 232, 116663. [Google Scholar] [CrossRef] [PubMed]
- Su-Tobon, Q.; Fan, J.; Goldstein, M.; Feeney, K.; Ren, H.; Autissier, P.; Wang, P.; Huang, Y.; Mohanty, U.; Niu, J. CRISPR-Hybrid: A CRISPR-Mediated Intracellular Directed Evolution Platform for RNA Aptamers. Nat. Commun. 2025, 16, 595. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, X.; Shi, Y.; Wang, W.; Huang, Z.; Tong, Y.; Zou, X.; Xu, Y.; Dai, Z. CRISPR/Pepper-tDeg: A Live Imaging System Enables Non-Repetitive Genomic Locus Analysis with One Single-Guide RNA. Adv. Sci. 2024, 11, e2402534. [Google Scholar] [CrossRef]
- Wang, S.; Su, J.H.; Zhang, F.; Zhuang, X. An RNA-aptamer-based two-color CRISPR labeling system. Sci. Rep. 2016, 6, 26857. [Google Scholar] [CrossRef]
- Liu, Y.J.; Dou, X.Q.; Wang, F.; Zhang, J.; Wang, X.L.; Xu, G.L.; Xiang, S.S.; Gao, X.; Fu, J.; Song, H.F. IL-4Ralpha aptamer-liposome-CpG oligodeoxynucleotides suppress tumour growth by targeting the tumour microenvironment. J. Drug Target. 2017, 25, 275–283. [Google Scholar] [CrossRef]
- Lu, D.; Di, Z.; Li, L.; Zhao, J.; Zheng, L. An aptamer-driven DNA nanodevice for improved delivery of synthetic immunostimulants. Nano Res. 2024, 17, 9078–9083. [Google Scholar] [CrossRef]
- Sun, L.; Shen, F.; Tian, L.; Tao, H.; Xiong, Z.; Xu, J.; Liu, Z. ATP-Responsive Smart Hydrogel Releasing Immune Adjuvant Synchronized with Repeated Chemotherapy or Radiotherapy to Boost Antitumor Immunity. Adv. Mater. 2021, 33, e2007910. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, Z.; Su, Q.; Fang, L.; Xiang, Q.; Tian, C.; Gao, Q.; Mao, C.; Huang, C.Z.; Zuo, H. Bivalent OX40 Aptamer and CpG as Dual Agonists for Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2025, 17, 7353–7362. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, Z.; Wang, Y.; Zou, J.; Lin, Q.; Duan, Y. One-Step Self-Assembly of Multifunctional DNA Nanohydrogels: An Enhanced and Harmless Strategy for Guiding Combined Antitumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 46479–46489. [Google Scholar] [CrossRef]
- Fan, Q.; Li, Z.; Yin, J.; Xie, M.; Cui, M.; Fan, C.; Wang, L.; Chao, J. Inhalable pH-responsive DNA tetrahedron nanoplatform for boosting anti-tumor immune responses against metastatic lung cancer. Biomaterials 2023, 301, 122283. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, J.; Duan, J.; Ma, Y.; Gao, H.; Zhang, Z.; Liu, J.; Shi, J.; Zhang, K. Photocontrolled Spatiotemporal Delivery of DNA Immunomodulators for Enhancing Membrane-Targeted Tumor Photodynamic Immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 44183–44198. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, N.; Kanwar, J.R.; Akilandeswari, B.; Kanwar, R.K.; Khetan, V.; Krishnakumar, S. Chimeric nucleolin aptamer with survivin DNAzyme for cancer cell targeted delivery. Chem. Commun. 2015, 51, 6940–6943. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, F.; Zhou, W.; Yuan, R.; Xiang, Y. Targeted and direct intracellular delivery of native DNAzymes enables highly specific gene silencing. Chem. Sci. 2020, 11, 8966–8972. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Liu, J.; Yang, X.; Xu, Y.; Shi, J.; Liu, W.; Li, J. Y-Shaped Circular Aptamer–DNAzyme Conjugates for Highly Efficient in Vivo Gene Silencing. CCS Chem. 2020, 2, 631–641. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, S.; Yu, X.; Huang, S.; Liu, H.Y. Simultaneous targeting of CD44 and EpCAM with a bispecific aptamer effectively inhibits intraperitoneal ovarian cancer growth. Theranostics 2017, 7, 1373–1388. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhao, H.; Zhu, T.; Yang, Z.; Xu, H.; Fu, Y.; Lin, F.; Pan, X.; Li, L.; et al. Enhanced in Vivo Blood-Brain Barrier Penetration by Circular Tau-Transferrin Receptor Bifunctional Aptamer for Tauopathy Therapy. J. Am. Chem. Soc. 2020, 142, 3862–3872. [Google Scholar] [CrossRef]
- Zhang, Z.; Kuo, J.C.; Yao, S.; Zhang, C.; Khan, H.; Lee, R.J. CpG Oligodeoxynucleotides for Anticancer Monotherapy from Preclinical Stages to Clinical Trials. Pharmaceutics 2021, 14, 73. [Google Scholar] [CrossRef]
- Kayraklioglu, N.; Horuluoglu, B.; Klinman, D.M. CpG Oligonucleotides as Vaccine Adjuvants. Methods Mol. Biol. 2021, 2197, 51–85. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, Y.; Liu, W.; Huang, Y. Nanomaterial-assisted delivery of CpG oligodeoxynucleotides for boosting cancer immunotherapy. J. Control. Release 2024, 376, 184–199. [Google Scholar] [CrossRef]
- Li, M.; Yao, H.; Yi, K.; Lao, Y.H.; Shao, D.; Tao, Y. Emerging nanoparticle platforms for CpG oligonucleotide delivery. Biomater. Sci. 2024, 12, 2203–2228. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, J.; Gui, T.; Yang, Y.; Feng, T.; Tzvetkov, N.T.; Xu, T.; Gai, Z.; Zhou, Y.; Zhang, J.; et al. SR-BI as a target of natural products and its significance in cancer. Semin. Cancer Biol. 2022, 80, 18–38. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Zhang, X. Aptamers as Versatile Ligands for Biomedical and Pharmaceutical Applications. Int. J. Nanomed. 2020, 15, 1059–1071. [Google Scholar] [CrossRef]
- Xie, S.; Sun, W.; Fu, T.; Liu, X.; Chen, P.; Qiu, L.; Qu, F.; Tan, W. Aptamer-Based Targeted Delivery of Functional Nucleic Acids. J. Am. Chem. Soc. 2023, 145, 7677–7691. [Google Scholar] [CrossRef]
- Dhanya, C.R.; Mary, A.S.; Madhavan, M. Aptamer-siRNA chimeras: Promising tools for targeting HER2 signaling in cancer. Chem. Biol. Drug Des. 2023, 101, 1162–1180. [Google Scholar] [CrossRef]
- Thomas, B.J.; Porciani, D.; Burke, D.H. Cancer immunomodulation using bispecific aptamers. Mol. Ther. Nucleic Acids 2022, 27, 894–915. [Google Scholar] [CrossRef]
- Peng, X.; Liu, Y.; Peng, F.; Wang, T.; Cheng, Z.; Chen, Q.; Li, M.; Xu, L.; Man, Y.; Zhang, Z.; et al. Aptamer-controlled stimuli-responsive drug release. Int. J. Biol. Macromol. 2024, 279, 135353. [Google Scholar] [CrossRef]
- Chung, Y.D.; Tsai, Y.C.; Wang, C.H.; Lee, G.B. Aptamer selection via versatile microfluidic platforms and their diverse applications. Lab Chip 2025, 25, 1047–1080. [Google Scholar] [CrossRef]
- Komarova, N.; Barkova, D.; Kuznetsov, A. Implementation of High-Throughput Sequencing (HTS) in Aptamer Selection Technology. Int. J. Mol. Sci. 2020, 21, 8774. [Google Scholar] [CrossRef]
- Zhu, C.; Feng, Z.; Qin, H.; Chen, L.; Yan, M.; Li, L.; Qu, F. Recent progress of SELEX methods for screening nucleic acid aptamers. Talanta 2024, 266, 124998. [Google Scholar] [CrossRef]
- Kruspe, S.; Giangrande, P.H. Aptamer-siRNA Chimeras: Discovery, Progress, and Future Prospects. Biomedicines 2017, 5, 45. [Google Scholar] [CrossRef] [PubMed]
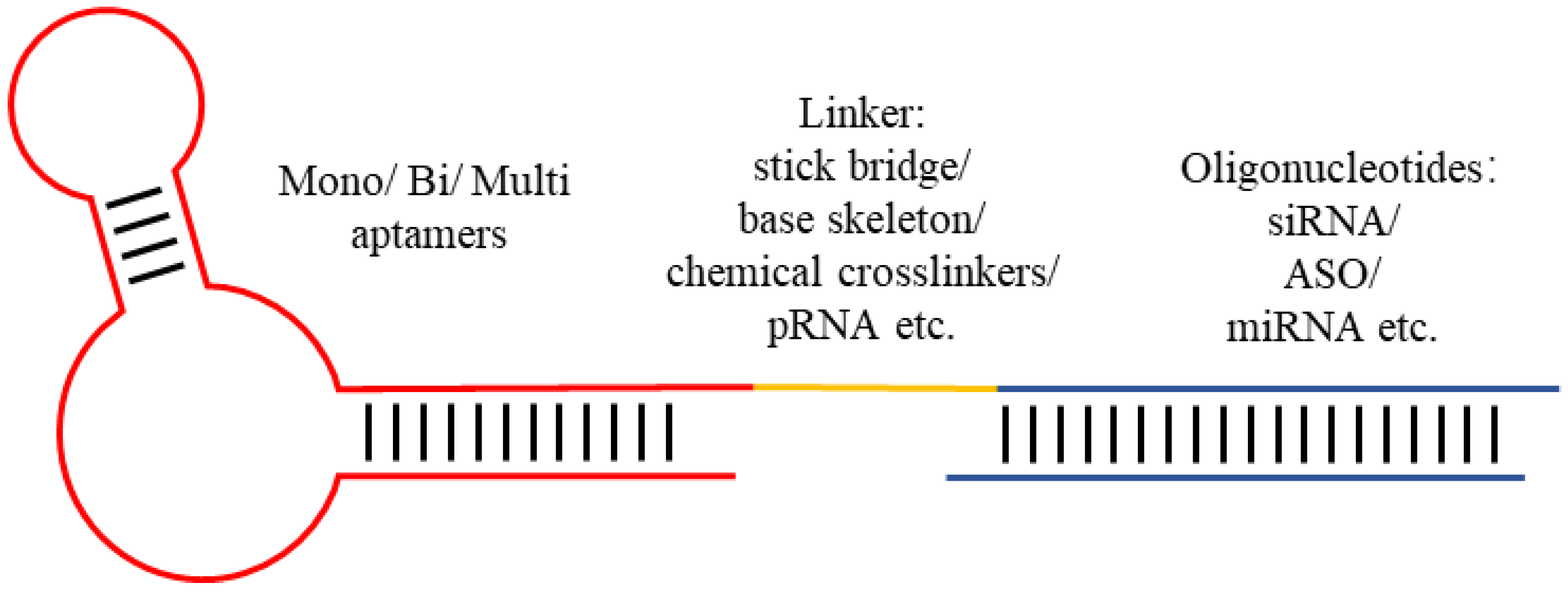
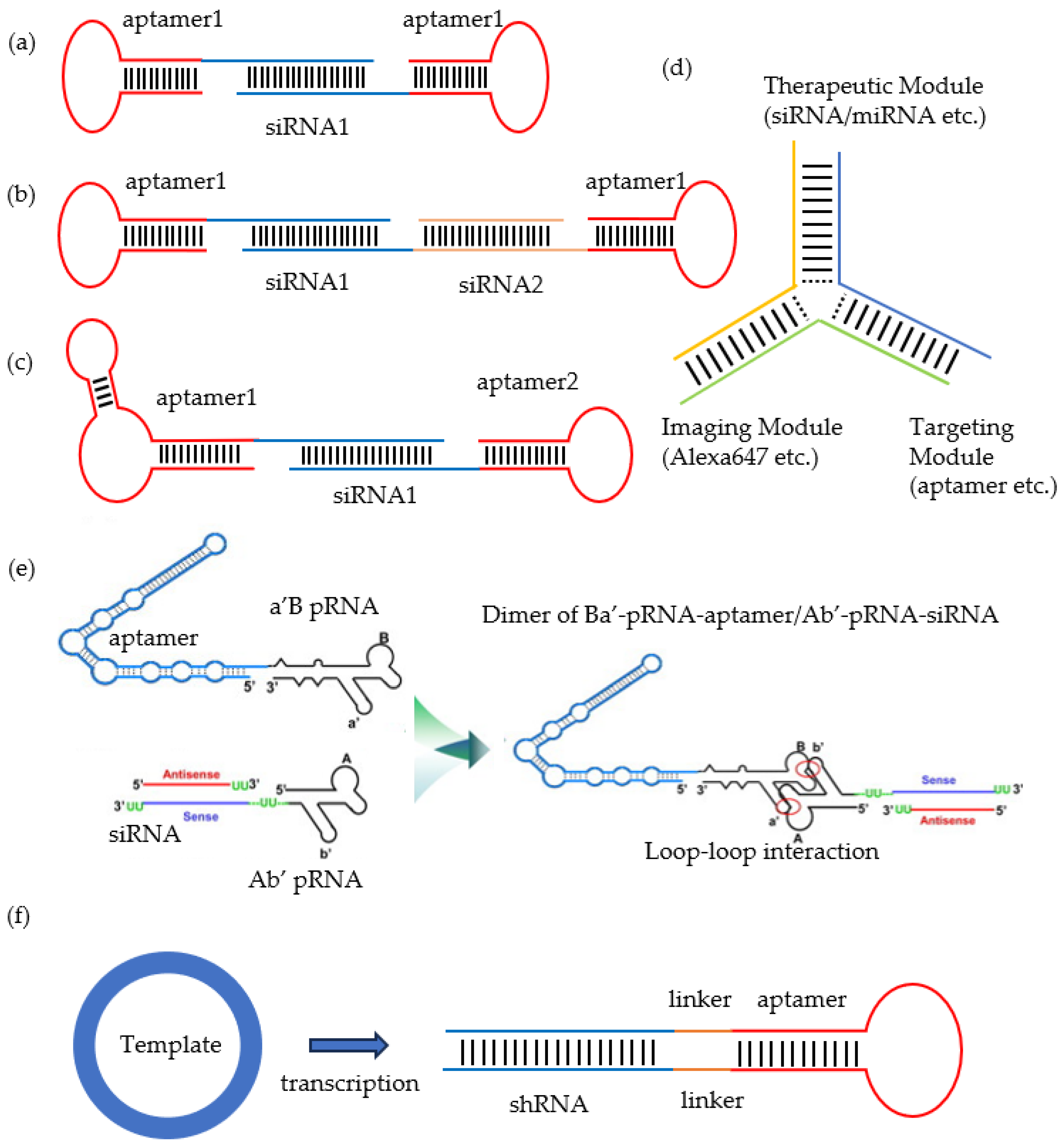

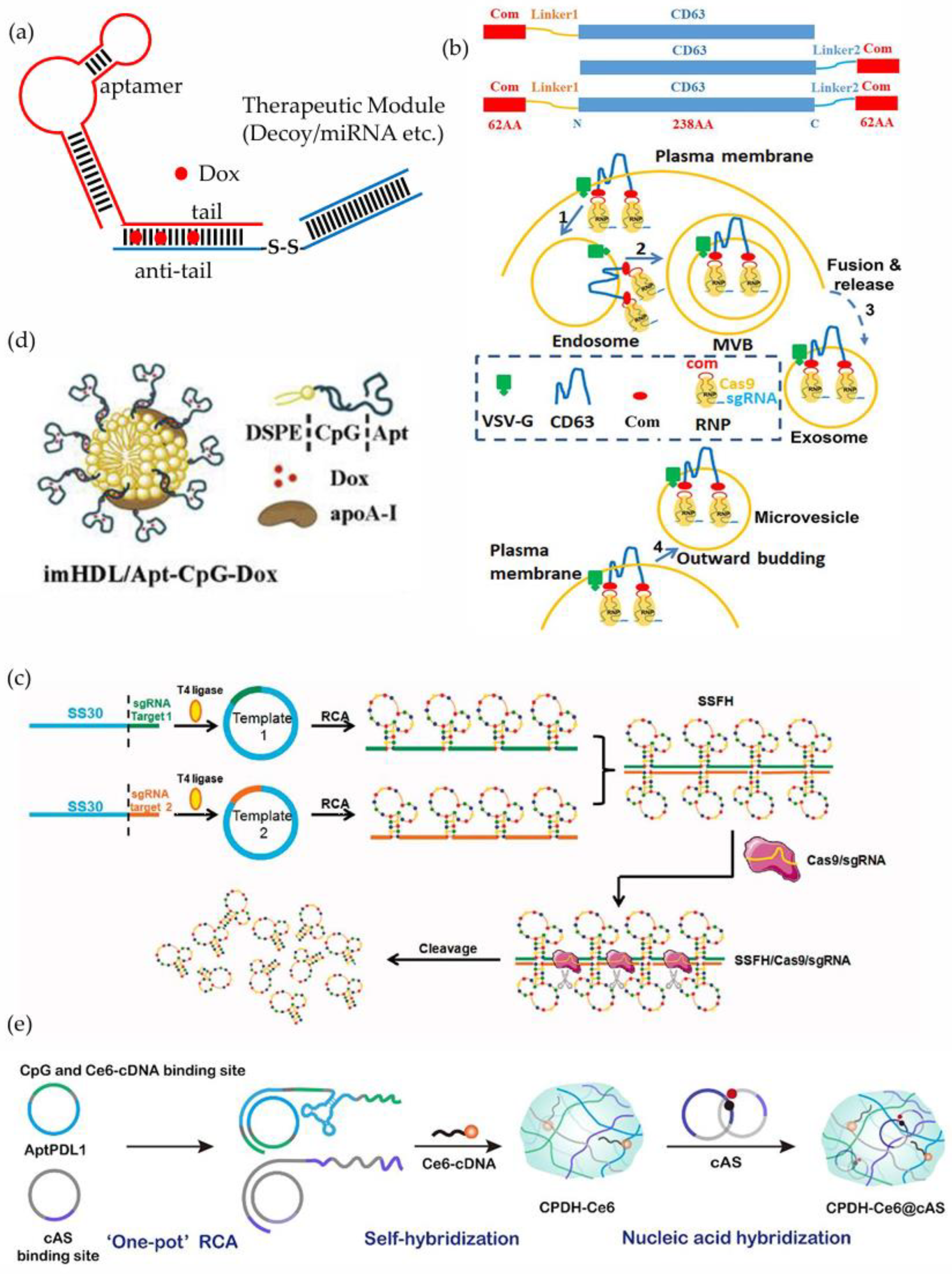
| ODNs | Aptamer’s Name/Target | Target | Disease | Year | Ref. |
|---|---|---|---|---|---|
| siRNA | A9/PMSA | laminA/C | prostate cancer | 2006 | [51] |
| A10/PMSA | PLK1 and BCL2 | prostate cancer | 2006 | [36] | |
| A10-3.2/PSMA | PLK1 | prostate cancer | 2009 | [52] | |
| PSMA aptamers/PSMA | Smg-1/Upf-2 | colon cancer | 2010 | [53] | |
| PSMA aptamers/PSMA | Smg-1/Upf-2 | prostate cancer | 2013 | [54] | |
| A10-3/PSMA | DNA-PK | prostate cancer | 2015 | [55] | |
| PSMA aptamers/PSMA | EGFR and survivin | prostate cancer | 2016 | [56] | |
| A10-3.2/PSMA | MDM2 | prostate cancer | 2022 | [37] | |
| A10-3.2/PSMA | CAT-1 | prostate cancer | 2022 | [57] | |
| 4-1BB aptamer/4-1BB | mTORC1 | antitumor immunity | 2014 | [58] | |
| 4-1BB aptamer/4-1BB | CD25 | antitumor immunity | 2017 | [59] | |
| gp120 aptamer/gp120 glycoprotein | HIV-1 gag p17 | HIV | 2008 | [60] | |
| gp120 aptamer/gp120 glycoprotein | HIV-1 tat/rev | HIV | 2009 | [61] | |
| gp120 aptamer/gp120 glycoprotein | HIV-1 tat/rev | HIV | 2011 | [62] | |
| gp120 aptamer/gp120 glycoprotein | HIV-1 tat/rev | HIV | 2011 | [63] | |
| gp120 aptamer/gp120 glycoprotein | HIV-1 tat/rev | HIV | 2011 | [64] | |
| gp120 aptamer/gp120 glycoprotein | HIV-1 tat/rev | HIV | 2013 | [65] | |
| gp120 aptamer/gp120 glycoprotein | HIV-1 LTR | HIV | 2018 | [66] | |
| CCR5 aptamer/CCR5 | TNPO3 | HIV | 2015 | [67] | |
| CD4 aptamer/CD4 | HIV genes and/or CCR5 | HIV | 2011 | [68] | |
| CD4 aptamer/CD4 | STAT5b | asthma | 2012 | [69] | |
| CD4 aptamer/CD4 | HIV-PR | HIV | 2012 | [70] | |
| CD4 aptamer/CD4 | CCR5 | HIV | 2013 | [71] | |
| CD8AP17s/CD8 | GNLY | immune diseases | 2013 | [72] | |
| BAFF-R aptamer/BAFF-R | STAT3 | non-Hodgkin’s lymphoma | 2013 | [73] | |
| aptamer 32/U87-EGFRvIII | c-Met | gioma | 2014 | [74] | |
| aptNCL/nucleolin | SLUG and NRP1 | lung cancer | 2014 | [75] | |
| AS1411/nucleolin | Bcl-2 | breast cancer | 2018 | [76] | |
| AS1411/nucleolin | OPN | gioma | 2022 | [42] | |
| AS1411/nucleolin | SMG1 | antitumor immunity | 2022 | [77] | |
| EpCAM aptamer/EpCAM | EpCAM | breast cancer/retinoblastoma | 2015 | [78] | |
| EpCAM aptamer/EpCAM | survivin | breast cancer | 2015 | [79] | |
| EpCAM aptamer/EpCAM | EpCAM | epithelial cancer | 2015 | [80] | |
| EpCAM aptamer/EpCAM | PLK1 | breast cancer | 2015 | [81] | |
| EpCAM aptamer/EpCAM | D5D | colon cancer | 2019 | [82] | |
| EpCAM aptamer/EpCAM | PKCι | breast cancer | 2020 | [40] | |
| EpCAM aptamer/EpCAM | D5D | lung cancer | 2020 | [83] | |
| HER2 aptamer/HER2 | Bcl-2 | breast cancer | 2012 | [84] | |
| HER2, HER3 aptamer/HER2, HER3 | EGFR | breast cancer | 2018 | [85] | |
| HER2 aptamer/HER2 | EGFR | breast cancer | 2018 | [86] | |
| HER2 aptamer/HER2 | XBP1 | breast cancer | 2020 | [41] | |
| EGFR aptamer/EGFR | Bcl-2 and PKC-ι | breast cancer | 2019 | [87] | |
| EGFR aptamer/EGFR | XBP1 | breast cancer | 2021 | [39] | |
| EGFR aptamer/EGFR | KRAS | non-small cell lung cancer | 2023 | [88] | |
| ErbB3 aptamer/ErbB3 | survivin | breast cancer | 2020 | [89] | |
| sgc8c and sgc4f/PTK7 | VEGF | leukemia | 2016 | [90] | |
| mucin-1 aptamers/mucin-1 (MUC1) | BCL2 | multidrug-resistant breast cancer | 2017 | [91] | |
| VEGF aptamer/VEGF | notch 3 | ovarian cancer | 2017 | [92] | |
| GBN/RGNNV coat protein (CP) protein | viral CP | viral nervous necrosis | 2020 | [93] | |
| LYGV1c/coat protein (CP) protein | SGIV MCP and VP19 | grouper iridovirus infection | 2021 | [94] | |
| CTLA4 aptamer/CTLA4 | STAT3 | colorectal cancer | 2014 | [95] | |
| αvβ3 aptamer/αvβ3 | EEF2 | prostate cancer/cervical cancer/glioblastoma | 2013 | [96] | |
| Gint4.T/PDGFRβ | STAT3 | glioblastoma | 2018 | [97] | |
| Gint4.T/PDGFRβ | STAT3 | glioblastoma | 2020 | [98] | |
| Gint4.T/PDGFRβ | STAT3 | non-small cell lung cancer | 2023 | [99] | |
| FB4/TfR | ICAM-1 | neuroinflammatory disease | 2014 | [100] | |
| IGFIIR aptamer/IGFIIR | PCBP2 | antifibrotic | 2017 | [101] | |
| monocytes/macrophages aptamer/monocytes/macrophages | ADK | immune diseases | 2018 | [102] | |
| shRNA | CD40 aptamer/CD40 | SMG1 | B lymphoma and bone-marrow aplasia | 2015 | [103] |
| CD40 aptamer/CD40 | RORγt | immune diseases | 2014 | [104] | |
| CD30 aptamer/CD30 | RORγt | autoimmune inflammatory diseases | 2019 | [105] | |
| S3R3/integrase | HIV-1 tat/rev | HIV | 2018 | [106] | |
| A10/PSMA | DNAPK | prostate cancer | 2011 | [107] |
| ODNs | Aptamer’s Name/Target | Target | Disease | Year | Ref. |
|---|---|---|---|---|---|
| ASO | AS1411/nucleolin | luciferase | pancreatic cancer | 2012 | [132] |
| TfR aptamer/TfR | caspase-3 | ischemic stroke | 2013 | [133] | |
| S6 aptamer/A549 | MMP-9 | lung cancer | 2015 | [134] | |
| AS1411/nucleolin | galectin-1 | breast cancer | 2016 | [135] | |
| Apt-2/LAMP1 | MALAT1 | lung cancer | 2021 | [28] | |
| AS1411/nucleolin | c-myc | lung cancer | 2021 | [136] | |
| PA9-1/PD-L1 | PD-L1 | antitumor immunity | 2023 | [137] | |
| SApt/SARS-CoV-2 spike protein | SARS-CoV-2 nucleocapsid | SARS-CoV-2 | 2023 | [138] |
| ODNs | Aptamer’s Name/Target | Target | Disease | Year | Ref. |
|---|---|---|---|---|---|
| miRNA | CD133 aptamer/CD133 | Anti-miR21 | triple-negative breast cancer | 2019 | [117] |
| EGFR aptamer/EGFR | Anti-miR21 | breast cancer | 2015 | [116] | |
| EGFR aptamer/EGFR | Anti-miR21 | breast cancer | 2021 | [154] | |
| A549 aptamer/A549 | miR-301b-3p | lung adenocarcinoma | 2023 | [118] | |
| α9-nAChR aptamer/α9-nAChR | Anti-miR21 | breast cancer | 2023 | [115] | |
| MUC1 aptamer/MUC1 | PTEN | ovarian cancer | 2013 | [155] | |
| GL21.T/Axl | HMGA2 | non-small cell lung cancer | 2014 | [153] | |
| GL21.T/Axl | miR-222 | glioblastoma | 2015 | [147] | |
| GL21.T and Gint4.T/PDGFRβ and Axl | miR-137 and anti-miR-10b | glioblastoma | 2016 | [151] | |
| GL21.T/Axl | PED/PEA-15 | non-small cell lung cancer | 2016 | [152] | |
| GL21.T/Axl | AXL | non-small cell lung cancer | 2018 | [150] | |
| GL21.T/Axl | Axl and the miR-137 | non-small cell lung cancer | 2019 | [156] | |
| AS1411/nucleolin | c-raf-1 | non-small cell lung cancer | 2018 | [157] | |
| AS1411/nucleolin | miRNA let-7d | gastric cancer | 2019 | [149] | |
| A10–3.2/PSMA | Bcl-2, cyclin D1, Wnt3a | prostate cancer | 2011 | [158] | |
| cKIT aptamer/cKIT | Ezh2 and Bak1 | breast cancer | 2023 | [159] | |
| apt69.T/B cell maturation antigen | miR-137 and miR-122 | multiple myeloma | 2019 | [146] |
| ODNs | Aptamer’s Name/Target | Target | Disease | Year | Ref. |
|---|---|---|---|---|---|
| saRNA | P19/P1 aptamer/PDAC | C/EBPα | pancreatic cancer | 2016 | [167] |
| TR14/hTfR | C/EBPα | prostate cancer | 2019 | [168] | |
| A10-3.2/PSMA | DPYSL3 | prostate cancer | 2016 | [169] | |
| m12-3773/clusterin and 1-717/TMED6 | XIAP | prostate cancer | 2022 | [170] | |
| decoy | c2.min/hTfR | NF-κB | pancreatic cancer | 2015 | [171] |
| GS-24/TfR | NF-κB | cerebrovascular inflammation | 2015 | [172] |
| ODNs | Aptamer’s Name/Target | Target | Disease | Year | Ref. |
|---|---|---|---|---|---|
| cpg | IL-4Rα/IL-4Rα receptor | CpG-induced pathways | antitumor immunity | 2017 | [196] |
| AS1411/nucleolin | CpG-induced pathways | antitumor immunity | 2018 | [181] | |
| AS1411/nucleolin | CpG-induced pathways | antitumor immunity | 2024 | [197] | |
| Aapt/ATP | CpG-induced pathways | antitumor immunity | 2021 | [198] | |
| ROX40 aptamer/ROX40 | CpG-induced pathways | antitumor immunity | 2025 | [199] | |
| MUC1 aptamer/MUC1 | CpG-induced pathways | antitumor immunity | 2019 | [200] | |
| PD-L1 aptamer/PD-L1 | CpG-induced pathways | metastatic lung cancer | 2023 | [201] | |
| PD-L1 aptamer/PD-L1 | CpG-induced pathways | antitumor immunity | 2022 | [202] | |
| PD-L1 aptamer/PD-L1 | CpG-induced pathways | post-operative tumor recurrence | 2023 | [182] | |
| others | com/Com | sgRNA | duchenne muscular dystrophy | 2021 | [179] |
| PD-1 aptamer/PD-1 | sgRNA | antitumor immunity | 2019 | [190] | |
| SS30/CD123 | sgRNA | acute myeloid leukemia | 2019 | [180] | |
| NCL-APT/nucleolin | survivin | retinoblastoma | 2021 | [203] | |
| MUC1 aptamer/MUC1 | Egr-1 | breast cancer | 2020 | [204] | |
| Sgc8/PTK7 | MET | lung cancer | 2020 | [205] | |
| CD44-EpCAM aptamer/CD44 and EpCAM | CD44 and EpCAM | ovarian cancer | 2017 | [206] | |
| TfR-aptamer/TfR | Tau | tauopathy | 2020 | [207] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, B.; Zhu, Q. Aptamer–ODN Chimeras: Enabling Cell-Specific ODN Targeting Therapy. Cells 2025, 14, 697. https://doi.org/10.3390/cells14100697
Xia B, Zhu Q. Aptamer–ODN Chimeras: Enabling Cell-Specific ODN Targeting Therapy. Cells. 2025; 14(10):697. https://doi.org/10.3390/cells14100697
Chicago/Turabian StyleXia, Bei, and Qubo Zhu. 2025. "Aptamer–ODN Chimeras: Enabling Cell-Specific ODN Targeting Therapy" Cells 14, no. 10: 697. https://doi.org/10.3390/cells14100697
APA StyleXia, B., & Zhu, Q. (2025). Aptamer–ODN Chimeras: Enabling Cell-Specific ODN Targeting Therapy. Cells, 14(10), 697. https://doi.org/10.3390/cells14100697










