Dux Is Dispensable for Skeletal Muscle Regeneration: A Study Inspired by a “Red Flagged” Publication and Editorial Oversight
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Models
2.2. Cardiotoxin (CTX)-Induced Muscle Injury
2.3. Histology
2.4. RNA Isolation and RT-qPCR
2.5. RNA-Seq Data Processing and Visualization
2.6. Statistics
3. Results
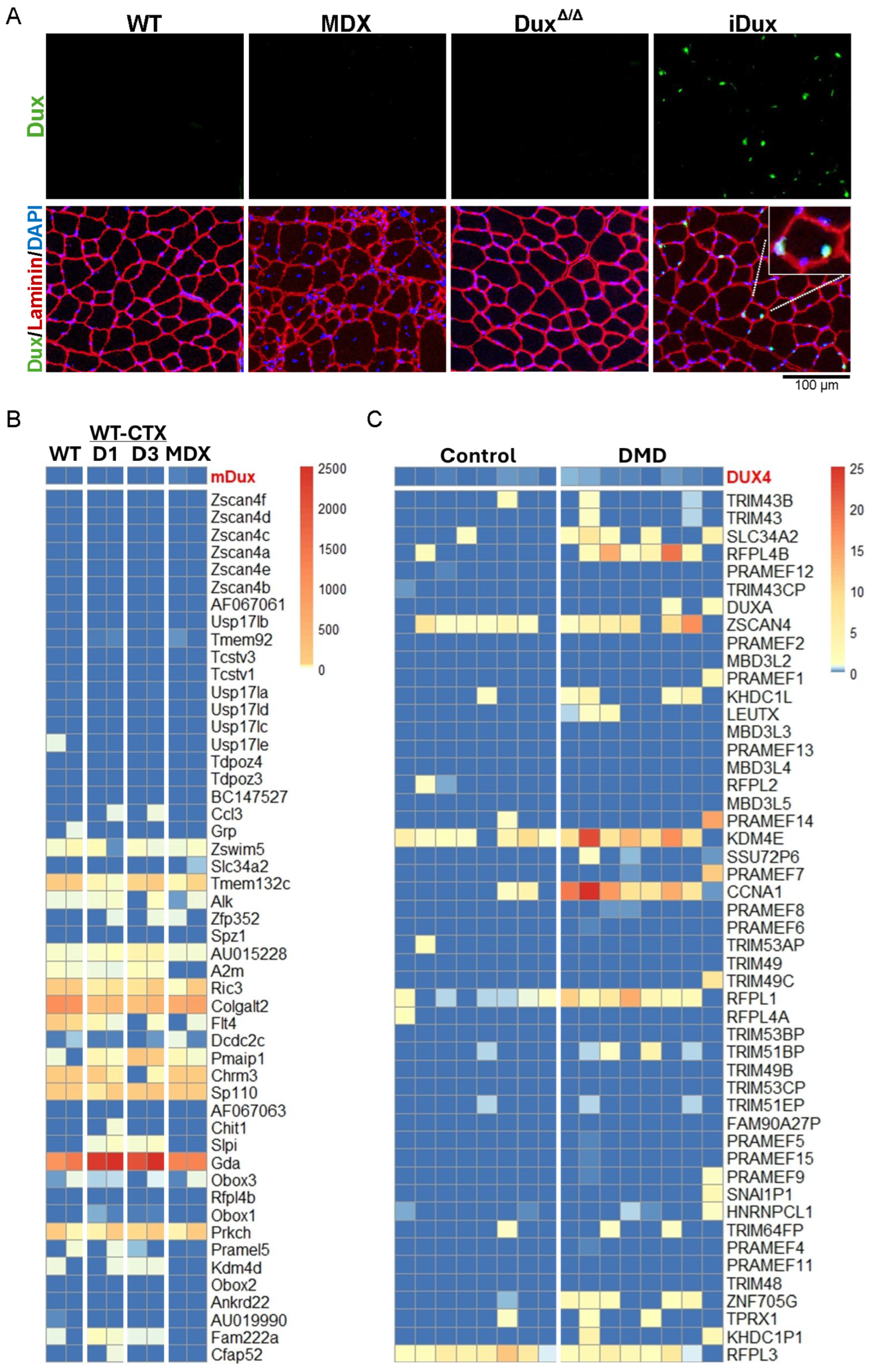
3.1. Lack of Dux Expression in Skeletal Muscle of Mdx Mice and DUX4 in DMD
3.2. Dux Does Not Contribute to Muscle Regeneration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leidenroth, A.; Hewitt, J.E. A family history of DUX4: Phylogenetic analysis of DUXA, B, C and Duxbl reveals the ancestral DUXgene. BMC Evol. Biol. 2010, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Clapp, J.; Mitchell, L.M.; Bolland, D.J.; Fantes, J.; Corcoran, A.E.; Scotting, P.J.; Armour, J.A.L.; Hewitt, J.E. Evolutionary conservation of a coding function for D4Z4, the tandem DNA repeat mutated in facioscapulohumeral muscular dystrophy. Am. J. Hum. Genet. 2007, 81, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Bosnakovski, D.; Toso, E.A.; Ener, E.T.; Gearhart, M.D.; Yin, L.; Lüttmann, F.F.; Magli, A.; Shi, K.; Kim, J.; Aihara, H.; et al. Antagonism among DUX family members evolved from an ancestral toxic single homeodomain protein. iScience 2023, 26, 107823. [Google Scholar] [CrossRef] [PubMed]
- Whiddon, J.L.; Langford, A.T.; Wong, C.-J.; Zhong, J.W.; Tapscott, S.J. Conservation and innovation in the DUX4-family gene network. Nat. Genet. 2017, 49, 935–940. [Google Scholar] [CrossRef]
- Hendrickson, P.G.; Doráis, J.A.; Grow, E.J.; Whiddon, J.L.; Lim, J.-W.; Wike, C.L.; Weaver, B.D.; Pflueger, C.; Emery, B.R.; Wilcox, A.L.; et al. Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat. Genet. 2017, 49, 925–934. [Google Scholar] [CrossRef]
- De Iaco, A.; Planet, E.; Coluccio, A.; Verp, S.; Duc, J.; Trono, D. DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat. Genet. 2017, 49, 941–945. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Gearhart, M.D.; Choi, S.H.; Kyba, M. Dux facilitates post-implantation development, but is not essential for zygotic genome activationdagger. Biol. Reprod. 2021, 104, 83–93. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y. Loss of DUX causes minor defects in zygotic genome activation and is compatible with mouse development. Nat. Genet. 2019, 51, 947–951. [Google Scholar] [CrossRef]
- De Iaco, A.; Verp, S.; Offner, S.; Grun, D.; Trono, D. DUX is a non-essential synchronizer of zygotic genome activation. Development 2020, 147, dev177725. [Google Scholar] [CrossRef]
- Eckersley-Maslin, M.; Alda-Catalinas, C.; Blotenburg, M.; Kreibich, E.; Krueger, C.; Reik, W. Dppa2 and Dppa4 directly regulate the Dux-driven zygotic transcriptional program. Genes Dev. 2019, 33, 194–208. [Google Scholar] [CrossRef]
- Lemmers, R.J.; Tawil, R.; Petek, L.M.; Balog, J.; Block, G.J.; Santen, G.W.E.; Amell, A.M.; van der Vliet, P.J.; Almomani, R.; Straasheijm, K.R.; et al. Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulo-humeral muscular dystrophy type 2. Nat. Genet. 2012, 44, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- van Overveld, P.G.; Lemmers, R.J.F.L.; Sandkuijl, L.A.; Enthoven, L.; Winokur, S.T.; Bakels, F.; Padberg, G.W.; van Ommen, G.-J.B.; Frants, R.R.; van der Maarel, S.M. Hypomethylation of D4Z4 in 4q-linked and non-4q-linked facioscapulohumeral muscular dystrophy. Nat. Genet. 2003, 35, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Wijmenga, C.; Hewitt, J.E.; Sandkuijl, L.A.; Clark, L.N.; Wright, T.J.; Dauwerse, H.G.; Gruter, A.M.; Hofker, M.H.; Moerer, P.; Williamson, R.; et al. Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nat. Genet. 1992, 2, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Lemmers, R.J.; van der Vliet, P.J.; Klooster, R.; Sacconi, S.; Camaño, P.; Dauwerse, J.G.; Snider, L.; Straasheijm, K.R.; Jan van Ommen, G.; Padberg, G.W.; et al. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science 2010, 329, 1650–1653. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Xu, Z.; Gang, E.J.; Galindo, C.L.; Liu, M.; Simsek, T.; Garner, H.R.; Agha-Mohammadi, S.; Tassin, A.; Coppée, F.; et al. An isogenetic myoblast expression screen identifies DUX4-mediated FSHD-associated molecular pathologies. EMBO J. 2008, 27, 2766–2779. [Google Scholar] [CrossRef]
- Kowaljow, V.; Marcowycz, A.; Ansseau, E.; Conde, C.B.; Sauvage, S.; Mattéotti, C.; Arias, C.; Corona, E.D.; Nuñez, N.G.; Leo, O.; et al. The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein. Neuromuscul. Disord. 2007, 17, 611–623. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Gearhart, M.D.; Toso, E.A.; Ener, E.T.; Choi, S.H.; Kyba, M. Low level DUX4 expression disrupts myogenesis through deregulation of myogenic gene expression. Sci. Rep. 2018, 8, 16957. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Daughters, R.S.; Xu, Z.; Slack, J.M.; Kyba, M. Biphasic myopathic phenotype of mouse DUX, an ORF within conserved FSHD-related repeats. PLoS ONE 2009, 4, e7003. [Google Scholar] [CrossRef]
- Eidahl, J.O.; Giesige, C.R.; Domire, J.S.; Wallace, L.M.; Fowler, A.M.; Guckes, S.M.; Garwick-Coppens, S.E.; Labhart, P.; Harper, S.Q. Mouse Dux is myotoxic and shares partial functional homology with its human paralog DUX4. Hum. Mol. Genet. 2016, 25, 4577–4589. [Google Scholar] [CrossRef]
- Vega-Sendino, M.; Lüttmann, F.F.; Olbrich, T.; Chen, Y.; Kuenne, C.; Stein, P.; Tillo, D.; Carey, G.I.; Zhong, J.; Savy, V.; et al. The homeobox transcription factor DUXBL controls exit from totipotency. Nat. Genet. 2024, 56, 697–709. [Google Scholar] [CrossRef]
- Claus, C.; Slavin, M.; Ansseau, E.; Lancelot, C.; Bah, K.; Lassche, S.; Fiévet, M.; Greco, A.; Tomaiuolo, S.; Tassin, A.; et al. The double homeodomain protein DUX4c is associated with regenerating muscle fibers and RNA-binding proteins. Skelet. Muscle 2023, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Duranti, E.; Villa, C. Influence of Expression in Facioscapulohumeral Muscular Dystrophy and Possible Treatments. Int. J. Mol. Sci. 2023, 24, 9503. [Google Scholar] [CrossRef] [PubMed]
- Ganassi, M.; Figeac, N.; Reynaud, M.; Ortuste Quiroga, H.P.; Zammit, P.S. Antagonism Between DUX4 and DUX4c Highlights a Pathomechanism Operating Through β-Catenin in Facioscapulohumeral Muscular Dystrophy. Front. Cell Dev. Biol. 2022, 10, 802573. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhai, W.; Zhang, R.; Cai, N. Deletion of Dux ameliorates muscular dystrophy in mdx mice by attenuating oxidative stress via Nrf2. FASEB J. 2024, 38, e23771. [Google Scholar] [CrossRef]
- Banerji, C.R.S.; Greco, A.; Joosten, L.A.B.; van Engelen, B.G.M.; Zammit, P.S. The FSHD muscle-blood biomarker: A circulating transcriptomic biomarker for clinical severity in facioscapulohumeral muscular dystrophy. Brain Commun. 2023, 5, fcad221. [Google Scholar] [CrossRef]
- Banerji, C.R.S.; Henderson, D.; Tawil, R.N.; Zammit, P.S. Skeletal muscle regeneration in facioscapulohumeral muscular dys-trophy is correlated with pathological severity. Hum. Mol. Genet. 2020, 29, 2746–2760. [Google Scholar] [CrossRef]
- Wang, L.H.; Friedman, S.D.; Shaw, D.; Snider, L.; Wong, C.-J.; Budech, C.B.; Poliachik, S.L.; Gove, N.E.; Lewis, L.M.; Campbell, A.E.; et al. MRI-informed muscle biopsies correlate MRI with pathology and DUX4 target gene expression in FSHD. Hum. Mol. Genet. 2019, 28, 476–486. [Google Scholar] [CrossRef]
- Bernhard, E.K.; Sabel, A.; Gigerenzer, G.; Bilc, M. Fake Publications in Biomedical Science: Red-flagging Method Indicates Mass Production. medRxiv 2023. medRxiv:2023.05.06.23289563. [Google Scholar]
- Singh Chawla, D. Fake research papers flagged by analysing authorship trends. Nature, 2024; online ahead of print. [Google Scholar] [CrossRef]
- Porter, S.J.; Mcintosh, L.D. Identifying fabricated networks within authorship-for-sale enterprises. Sci. Rep. 2024, 14, 29569. [Google Scholar] [CrossRef]
- Byrne, J.A.; Christopher, J. Digital magic, or the dark arts of the 21 century-how can journals and peer reviewers detect man-uscripts and publications from paper mills? Febs Lett. 2020, 594, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Bosnakovski, D.; Chan, S.S.K.; Recht, O.O.; Hartweck, L.M.; Gustafson, C.J.; Athman, L.L.; Lowe, D.A.; Kyba, M. Muscle pathology from stochastic low level DUX4 expression in an FSHD mouse model. Nat. Commun. 2017, 8, 550. [Google Scholar] [CrossRef] [PubMed]
- Bosnakovski, D.; Shams, A.S.; Yuan, C.; da Silva, M.T.; Ener, E.T.; Baumann, C.W.; Lindsay, A.J.; Verma, M.; Asakura, A.; Lowe, D.A.; et al. Transcriptional and cytopathological hallmarks of FSHD in chronic DUX4-expressing mice. J. Clin. Inves. 2020, 130, 2465–2477. [Google Scholar] [CrossRef] [PubMed]
- Grow, E.J.; Weaver, B.D.; Smith, C.M.; Guo, J.; Stein, P.; Shadle, S.C.; Hendrickson, P.G.; Johnson, N.E.; Butterfield, R.J.; Menafra, R.; et al. p53 convergently activates Dux/DUX4 in embryonic stem cells and in facioscapulohumeral muscular dystrophy cell models. Nat. Genet. 2021, 53, 1207–1220. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Snider, L.; Geng, L.N.; Lemmers, R.J.L.F.; Kyba, M.; Ware, C.B.; Nelson, A.M.; Tawil, R.; Filippova, G.N.; van der Maarel, S.M.; Tapscott, S.J.; et al. Facioscapulohumeral dystrophy: Incomplete suppression of a retrotransposed gene. PLoS Genet. 2010, 6, e1001181. [Google Scholar] [CrossRef]
- Jones, T.I.; Chen, J.C.J.; Rahimov, F.; Homma, S.; Arashiro, P.; Beermann, M.L.; King, O.D.; Miller, J.B.; Kunkel, L.M.; Emerson, C.P., Jr.; et al. Facioscapulohumeral muscular dystrophy family studies of DUX4 expression: Evidence for disease modifiers and a quantitative model of pathogenesis. Hum. Mol. Genet. 2012, 21, 4419–4430. [Google Scholar] [CrossRef]
- Nieves-Rodriguez, S.; Barthélémy, F.; Woods, J.D.; Douine, E.D.; Wang, R.T.; Scripture-Adams, D.D.; Chesmore, K.N.; Galasso, F.; Miceli, M.C.; Nelson, S.F. Transcriptomic analysis of paired healthy human skeletal muscles to identify modulators of disease severity in DMD. Front. Genet. 2023, 14, 1216066. [Google Scholar] [CrossRef]
- Malecova, B.; Gatto, S.; Etxaniz, U.; Passafaro, M.; Cortez, A.; Nicoletti, C.; Giordani, L.; Torcinaro, A.; De Bardi, M.; Bicciato, S.; et al. Dynamics of cellular states of fibro-adipogenic progenitors during myogenesis and muscular dystrophy. Nat. Commun. 2018, 9, 3670. [Google Scholar] [CrossRef] [PubMed]
- Banerji, C.R.S.; Panamarova, M.; Hebaishi, H.; White, R.B.; Relaix, F.; Severini, S.; Zammit, P.S. PAX7 target genes are globally repressed in facioscapulohumeral muscular dystrophy skeletal muscle. Nat. Commun. 2017, 8, 2152. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.Z.; Snider, L.; Balog, J.; Lemmers, R.J.L.F.; Van Der Maarel, S.M.; Tawil, R.; Tapscott, S.J. DUX4-induced gene expression is the major molecular signature in FSHD skeletal muscle. Hum. Mol. Genet. 2014, 23, 5342–5352. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xiahou, Z.; Li, Z.; Zhang, Z.; Song, Y.; Wang, Y. Identification of hub genes and therapeutic siRNAs to develop novel adjunctive therapy for Duchenne muscular dystrophy. BMC Musculoskelet. Disord. 2024, 25, 386. [Google Scholar] [CrossRef]
- Choi, S.H.; Gearhart, M.D.; Cui, Z.; Bosnakovski, D.; Kim, M.; Schennum, N.; Kyba, M. DUX4 recruits p300/CBP through its C-terminus and induces global H3K27 acetylation changes. Nucleic Acids Res. 2016, 44, 5161–5173. [Google Scholar] [CrossRef]
- Fletcher, J.E.; Jiang, M.S.; Gong, Q.H.; Yudkowsky, M.L.; Wieland, S.J. Effects of a cardiotoxin from Naja naja kaouthia venom on skeletal muscle: Involvement of calcium-induced calcium release, sodium ion currents and phospholipases A2 and C. Toxicon 1991, 29, 1489–1500. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Liu, Y. Skeletal Muscle Regeneration in Cardiotoxin-Induced Muscle Injury Models. Int. J. Mol. Sci. 2022, 23, 13380. [Google Scholar] [CrossRef]
- Giovarelli, M.; Arnaboldi, F.; Zecchini, S.; Cornaghi, L.B.; Nava, A.; Sommariva, M.; Clementi, E.G.I.; Gagliano, N. Characterisation of Progressive Skeletal Muscle Fibrosis in the Mdx Mouse Model of Duchenne Muscular Dystrophy: An In Vivo and In Vitro Study. Int. J. Mol. Sci. 2022, 23, 8735. [Google Scholar] [CrossRef]
- Van Noorden, R. How big is science’s fake-paper problem? Nature 2023, 623, 466–467. [Google Scholar] [CrossRef]
- Perez-Neri, I.; Pineda, C.; Sandoval, H. Threats to scholarly research integrity arising from paper mills: A rapid scoping review. Clin. Rheumatol. 2022, 41, 2241–2248. [Google Scholar] [CrossRef]
- Else, H.; Van Noorden, R. The fight against fake-paper factories that churn out sham science. Nature 2021, 591, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Van Noorden, R. More Than 10,000 Research Papers Were Retracted in 2023—A New Record. Nature 2023, 624, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Steen, R.G. Retractions in the medical literature: How many patients are put at risk by flawed research? J. Med. Ethics 2011, 37, 688–692. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Wei, E.; Mitanoska, A.; Gearhart, M.D.; Kyba, M.; Bosnakovski, D. Dux Is Dispensable for Skeletal Muscle Regeneration: A Study Inspired by a “Red Flagged” Publication and Editorial Oversight. Cells 2025, 14, 695. https://doi.org/10.3390/cells14100695
Chen K, Wei E, Mitanoska A, Gearhart MD, Kyba M, Bosnakovski D. Dux Is Dispensable for Skeletal Muscle Regeneration: A Study Inspired by a “Red Flagged” Publication and Editorial Oversight. Cells. 2025; 14(10):695. https://doi.org/10.3390/cells14100695
Chicago/Turabian StyleChen, Kenric, Erdong Wei, Ana Mitanoska, Micah D. Gearhart, Michael Kyba, and Darko Bosnakovski. 2025. "Dux Is Dispensable for Skeletal Muscle Regeneration: A Study Inspired by a “Red Flagged” Publication and Editorial Oversight" Cells 14, no. 10: 695. https://doi.org/10.3390/cells14100695
APA StyleChen, K., Wei, E., Mitanoska, A., Gearhart, M. D., Kyba, M., & Bosnakovski, D. (2025). Dux Is Dispensable for Skeletal Muscle Regeneration: A Study Inspired by a “Red Flagged” Publication and Editorial Oversight. Cells, 14(10), 695. https://doi.org/10.3390/cells14100695





