Calcium Signalling in Neurological Disorders, with Insights from Miniature Fluorescence Microscopy
Abstract
1. Introduction
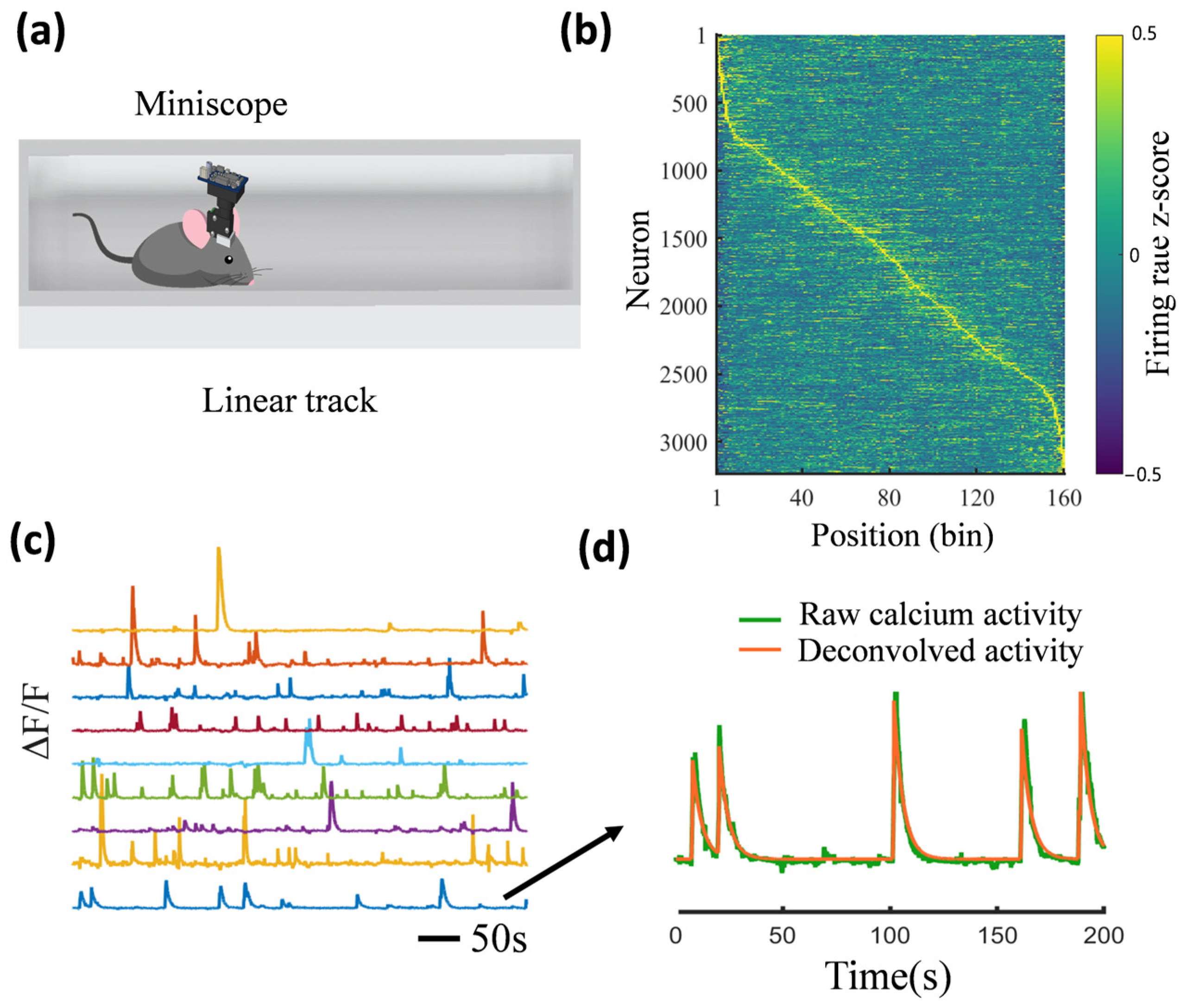
2. Fluorescence Two-Photon Microscopy and Miniature One-Photon Microscopy
3. Calcium Signalling in Neurological Disorders
3.1. Amyotrophic Lateral Sclerosis (ALS)
3.2. Alzheimer’s Disease (AD)
3.3. Parkinson’s Disease (PD)
3.4. Huntington’s Disease (HD)
3.5. Schizophrenia
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mattson, M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000, 1, 120–130. [Google Scholar] [CrossRef]
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell. Mol. Med. 2010, 14, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.M.; Lindemann, L.; Jønch, A.E.; Apostol, G.; Bear, M.F.; Carpenter, R.L.; Crawley, J.N.; Curie, A.; Des Portes, V.; Hossain, F.; et al. Drug development for neurodevelopmental disorders: Lessons learned from fragile X syndrome. Nat. Rev. Drug Discov. 2018, 17, 280–299. [Google Scholar] [CrossRef]
- Youdim, M.B.; Buccafusco, J.J. Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends Pharmacol. Sci. 2005, 26, 27–35. [Google Scholar] [CrossRef]
- Lamptey, R.N.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Parikshak, N.N.; Gandal, M.J.; Geschwind, D.H. Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nat. Rev. Genet. 2015, 16, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Jucker, M.; Walker, L.C. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011, 70, 532–540. [Google Scholar] [CrossRef]
- Cai, H.; Cong, W.N.; Ji, S.; Rothman, S.; Maudsley, S.; Martin, B. Metabolic dysfunction in Alzheimer’s disease and related neurodegenerative disorders. Curr. Alzheimer Res. 2012, 9, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 2018, 70, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zündorf, G.; Reiser, G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid. Redox Signal. 2011, 14, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Calcium signalling remodelling and disease. Biochem. Soc. Trans. 2012, 40, 297–309. [Google Scholar] [CrossRef]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef]
- Lemoine, D.; Jiang, R.; Taly, A.; Chataigneau, T.; Specht, A.; Grutter, T. Ligand-gated ion channels: New insights into neurological disorders and ligand recognition. Chem. Rev. 2012, 112, 6285–6318. [Google Scholar] [CrossRef]
- Capiod, T. Cell proliferation, calcium influx and calcium channels. Biochimie 2011, 93, 2075–2079. [Google Scholar] [CrossRef]
- McAinsh, M.R.; Pittman, J.K. Shaping the calcium signature. New Phytol. 2009, 181, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Stokes, D.L.; Green, N.M. Structure and function of the calcium pump. Annu. Rev. Biophys. Biomol. Struct. 2003, 32, 445–468. [Google Scholar] [CrossRef] [PubMed]
- Philipson, K.D.; Nicoll, D.A. Sodium-calcium exchange: A molecular perspective. Annu. Rev. Physiol. 2000, 62, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, C.; Inesi, G. Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu. Rev. Biochem. 2004, 73, 269–292. [Google Scholar] [CrossRef] [PubMed]
- Kamer, K.J.; Mootha, V.K. The molecular era of the mitochondrial calcium uniporter. Nat. Rev. Mol. Cell Biol. 2015, 16, 545–553. [Google Scholar] [CrossRef]
- Yáñez, M.; Gil-Longo, J.; Campos-Toimil, M. Calcium binding proteins. Calcium Signal. 2012, 740, 461–482. [Google Scholar]
- Xiong, H.; Tang, F.; Guo, Y.; Xu, R.; Lei, P. Neural circuit changes in neurological disorders: Evidence from in vivo two-photon imaging. Ageing Res. Rev. 2023, 87, 101933. [Google Scholar] [CrossRef]
- Aharoni, D.; Hoogland, T.M. Circuit investigations with open-source miniaturized microscopes: Past, present and future. Front. Cell. Neurosci. 2019, 13, 141. [Google Scholar] [CrossRef]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef]
- Paredes, R.M.; Etzler, J.C.; Watts, L.T.; Zheng, W.; Lechleiter, J.D. Chemical calcium indicators. Methods 2008, 46, 143–151. [Google Scholar] [CrossRef]
- Mank, M.; Griesbeck, O. Genetically encoded calcium indicators. Chem. Rev. 2008, 108, 1550–1564. [Google Scholar] [CrossRef]
- Orellana, J.A.; von Bernhardi, R.; Giaume, C.; Saez, J.C. Glial hemichannels and their involvement in aging and neurodegenerative diseases. Rev. Neurosci. 2012, 23, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E. Enhancing ion-selective polymeric membrane electrodes by instrumental control. TrAC Trends Anal. Chem. 2014, 53, 98–105. [Google Scholar] [CrossRef]
- Williams, K.R.; Hashemi, N.N.; Riddley, M.; Clarke, G.; Igwe, N.; Elnagib, D.; Montazami, R. Progress of graphene devices for electrochemical biosensing in electrically excitable cells. Prog. Biomed. Eng. 2021, 3, 022003. [Google Scholar] [CrossRef]
- Sun, D.; French, C.; Unnithan, R.R. Optical Brain–Computer Interface: Using a Miniscope to Detect Multi-Neuronal Dynamics During Cognition-Related Events; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Ghosh, K.K.; Burns, L.D.; Cocker, E.D.; Nimmerjahn, A.; Ziv, Y.; Gamal, A.E.; Schnitzer, M.J. Miniaturized integration of a fluorescence microscope. Nat. Methods 2011, 8, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Amiri, M.; Unnithan, R.R.; French, C. Protocol for calcium imaging and analysis of hippocampal CA1 activity evoked by non-spatial stimuli. STAR Protoc. 2024, 5, 103110. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Unnithan, R.R.; French, C. Scopolamine impairs spatial information recorded with “miniscope” calcium imaging in hippocampal place cells. Front. Neurosci. 2021, 15, 640350. [Google Scholar] [CrossRef]
- Zhou, P.; Resendez, S.L.; Rodriguez-Romaguera, J.; Jimenez, J.C.; Neufeld, S.Q.; Giovannucci, A.; Friedrich, J.; Pnevmatikakis, E.A.; Stuber, G.D.; Hen, R.; et al. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. eLife 2018, 7, e28728. [Google Scholar] [CrossRef] [PubMed]
- de Groot, A.; van den Boom, B.J.; van Genderen, R.M.; Coppens, J.; van Veldhuijzen, J.; Bos, J.; Hoedemaker, H.; Negrello, M.; Willuhn, I.; De Zeeuw, C.I.; et al. NINscope, a versatile miniscope for multi-region circuit investigations. eLife 2020, 9, e49987. [Google Scholar] [CrossRef]
- Yanny, K.; Antipa, N.; Liberti, W.; Dehaeck, S.; Monakhova, K.; Liu, F.L.; Shen, K.; Ng, R.; Waller, L. Miniscope3D: Optimized single-shot miniature 3D fluorescence microscopy. Light Sci. Appl. 2020, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.M.; Resendez, S.L.; Chen, K.S.; Favero, M.; Liang-Guallpa, J.; Nassi, J.J.; Neufeld, S.Q.; Visscher, K.; Ghosh, K.K. Miniature microscopes for manipulating and recording in vivo brain activity. Microscopy 2021, 70, 399–414. [Google Scholar] [CrossRef]
- Guo, C.; Blair, G.J.; Sehgal, M.; Sangiuliano Jimka, F.N.; Bellafard, A.; Silva, A.J.; Golshani, P.; Basso, M.A.; Blair, H.T.; Aharoni, D. Miniscope-LFOV: A large-field-of-view, single-cell-resolution, miniature microscope for wired and wire-free imaging of neural dynamics in freely behaving animals. Sci. Adv. 2023, 9, eadg3918. [Google Scholar] [CrossRef]
- Guo, C.; Wang, A.; Cheng, H.; Chen, L. New imaging instrument in animal models: Two-photon miniature microscope and large field of view miniature microscope for freely behaving animals. J. Neurochem. 2023, 164, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; Van Den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 1–19. [Google Scholar] [CrossRef]
- Rowland, L.P.; Shneider, N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001, 344, 1688–1700. [Google Scholar] [CrossRef]
- Brown, C.A.; Lally, C.; Kupelian, V.; Flanders, W.D. Estimated prevalence and incidence of amyotrophic lateral sclerosis and SOD1 and C9orf72 genetic variants. Neuroepidemiology 2021, 55, 342–353. [Google Scholar] [CrossRef]
- Sreedharan, J.; Brown, R.H., Jr. Amyotrophic lateral sclerosis: Problems and prospects. Ann. Neurol. 2013, 74, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, V.; Petrozziello, T.; Secondo, A. Calcium dyshomeostasis and lysosomal Ca2+ dysfunction in amyotrophic lateral sclerosis. Cells 2019, 8, 1216. [Google Scholar] [CrossRef]
- Filadi, R.; Pizzo, P. Mitochondrial calcium handling and neurodegeneration: When a good signal goes wrong. Curr. Opin. Physiol. 2020, 17, 224–233. [Google Scholar] [CrossRef]
- Grosskreutz, J.; Van Den Bosch, L.; Keller, B.U. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium 2010, 47, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Stoica, R.; Paillusson, S.; Gomez-Suaga, P.; Mitchell, J.C.; Lau, D.H.; Gray, E.H.; Sancho, R.M.; Vizcay-Barrena, G.; De Vos, K.J.; Shaw, C.E.; et al. ALS/FTD-associated FUS activates GSK-3β to disrupt the VAPB–PTPIP 51 interaction and ER–mitochondria associations. EMBO Rep. 2016, 17, 1326–1342. [Google Scholar] [CrossRef]
- Stoica, R.; De Vos, K.J.; Paillusson, S.; Mueller, S.; Sancho, R.M.; Lau, K.F.; Vizcay-Barrena, G.; Lin, W.L.; Xu, Y.F.; Lewis, J.; et al. ER–mitochondria associations are regulated by the VAPB–PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat. Commun. 2014, 5, 3996. [Google Scholar] [CrossRef] [PubMed]
- Appel, S.H.; Beers, D.; Siklos, L.; Engelhardt, J.I.; Mosier, D.R. Calcium: The darth vader of ALS. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2001, 2, 47–54. [Google Scholar] [CrossRef]
- Damiano, M.; Starkov, A.A.; Petri, S.; Kipiani, K.; Kiaei, M.; Mattiazzi, M.; Flint Beal, M.; Manfredi, G. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J. Neurochem. 2006, 96, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bosch, L.; Van Damme, P.; Bogaert, E.; Robberecht, W. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2006, 1762, 1068–1082. [Google Scholar] [CrossRef]
- Beers, D.R.; Ho, B.; Siklós, L.; Alexianu, M.E.; Mosier, D.R.; Mohamed, A.H.; Otsuka, Y.; Kozovska, M.E.; McAlhany, R.E.; Smith, R.G.; et al. Parvalbumin overexpression alters immune-mediated increases in intracellular calcium, and delays disease onset in a transgenic model of familial amyotrophic lateral sclerosis. J. Neurochem. 2001, 79, 499–509. [Google Scholar] [CrossRef]
- Reiner, A.; Medina, L.; Figueredo-Cardenas, G.; Anfinson, S. Brainstem motoneuron pools that are selectively resistant in amyotrophic lateral sclerosis are preferentially enriched in parvalbumin: Evidence from monkey brainstem for a calcium-mediated mechanism in sporadic ALS. Exp. Neurol. 1995, 131, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Iacopino, A.M.; Christakos, S. Corticosterone regulates calbindin-D28k mRNA and protein levels in rat hippocampus. J. Biol. Chem. 1990, 265, 10177–10180. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Hideyama, T.; Yamashita, T.; Aizawa, H. AMPA receptor-mediated neuronal death in sporadic ALS. Neuropathology 2010, 30, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Kalia, S.K.; Salter, M.W. NMDA receptors in clinical neurology: Excitatory times ahead. Lancet Neurol. 2008, 7, 742–755. [Google Scholar] [CrossRef]
- Jayakar, S.S.; Dikshit, M. AMPA receptor regulation mechanisms: Future target for safer neuroprotective drugs. Int. J. Neurosci. 2004, 114, 695–734. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xie, W.; Le, W.; Beers, D.R.; He, Y.; Henkel, J.S.; Simpson, E.P.; Yen, A.A.; Xiao, Q.; Appel, S.H. Activated microglia initiate motor neuron injury by a nitric oxide and glutamate-mediated mechanism. J. Neuropathol. Exp. Neurol. 2004, 63, 964–977. [Google Scholar] [CrossRef]
- Liang, B.; Thapa, R.; Zhang, G.; Moffitt, C.; Zhang, Y.; Zhang, L.; Johnston, A.; Ruby, H.P.; Barbera, G.; Wong, P.C.; et al. Aberrant neural activity in prefrontal pyramidal neurons lacking TDP-43 precedes neuron loss. Prog. Neurobiol. 2022, 215, 102297. [Google Scholar] [CrossRef]
- Brailoiu, E.; Barr, J.L.; Wittorf, H.N.; Inan, S.; Unterwald, E.M.; Brailoiu, G.C. Modulation of the Blood–Brain Barrier by Sigma-1R Activation. Int. J. Mol. Sci. 2024, 25, 5147. [Google Scholar] [CrossRef] [PubMed]
- Femminella, G.D.; Thayanandan, T.; Calsolaro, V.; Komici, K.; Rengo, G.; Corbi, G.; Ferrara, N. Imaging and molecular mechanisms of Alzheimer’s disease: A review. Int. J. Mol. Sci. 2018, 19, 3702. [Google Scholar] [CrossRef] [PubMed]
- Schrank, S.; Barrington, N.; Stutzmann, G.E. Calcium-handling defects and neurodegenerative disease. Cold Spring Harb. Perspect. Biol. 2020, 12, a035212. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Basak, J.M.; Holtzman, D.M. The role of apolipoprotein E in Alzheimer’s disease. Neuron 2009, 63, 287–303. [Google Scholar] [CrossRef]
- Mahley, R.W.; Huang, Y.; Weisgraber, K.H. Detrimental effects of apolipoprotein E4: Potential therapeutic targets in Alzheimer’s disease. Curr. Alzheimer Res. 2007, 4, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Sampaio, V.F.A.; Lameu, C.; Ulrich, H. Calcium signalling: A common target in neurological disorders and neurogenesis. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2019; Volume 95, pp. 25–33. [Google Scholar]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimer’s Res. Ther. 2014, 6, 37. [Google Scholar] [CrossRef]
- Karran, E.; Hardy, J. A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann. Neurol. 2014, 76, 185. [Google Scholar] [CrossRef] [PubMed]
- Bezprozvanny, I.; Mattson, M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008, 31, 454–463. [Google Scholar] [CrossRef]
- Demuro, A.; Parker, I. Cytotoxicity of intracellular aβ42 amyloid oligomers involves Ca2+ release from the endoplasmic reticulum by stimulated production of inositol trisphosphate. J. Neurosci. 2013, 33, 3824–3833. [Google Scholar] [CrossRef] [PubMed]
- Kuchibhotla, K.V.; Goldman, S.T.; Lattarulo, C.R.; Wu, H.Y.; Hyman, B.T.; Bacskai, B.J. Aβ plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 2008, 59, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Leissring, M.A.; Paul, B.A.; Parker, I.; Cotman, C.W.; LaFerla, F.M. Alzheimer’s Presenilin-1 Mutation Potentiates Inositol 1, 4, 5-Trisphosphate-Mediated Calcium Signaling in Xenopus. J. Neurochem. 1999, 72, 1061–1068. [Google Scholar]
- Tu, H.; Nelson, O.; Bezprozvanny, A.; Wang, Z.; Lee, S.F.; Hao, Y.H.; Serneels, L.; De Strooper, B.; Yu, G.; Bezprozvanny, I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell 2006, 126, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Castro, J.; Saneyoshi, T.; Matsuno, H.; Sur, M.; Hayashi, Y. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron 2014, 82, 444–459. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Hyman, B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Meyer-Luehmann, M.; Osetek, J.D.; Jones, P.B.; Stern, E.A.; Bacskai, B.J.; Hyman, B.T. Impaired spine stability underlies plaque-related spine loss in an Alzheimer’s disease mouse model. Am. J. Pathol. 2007, 171, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Chakroborty, S.; Hill, E.S.; Christian, D.T.; Helfrich, R.; Riley, S.; Schneider, C.; Kapecki, N.; Mustaly-Kalimi, S.; Seiler, F.A.; Peterson, D.A.; et al. Reduced presynaptic vesicle stores mediate cellular and network plasticity defects in an early-stage mouse model of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 7. [Google Scholar] [CrossRef]
- Kishi, T.; Matsunaga, S.; Oya, K.; Nomura, I.; Ikuta, T.; Iwata, N. Memantine for Alzheimer’s disease: An updated systematic review and meta-analysis. J. Alzheimer’s Dis. 2017, 60, 401–425. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Johnston, K.G.; Crapser, J.; Green, K.N.; Ha, N.M.L.; Tenner, A.J.; Holmes, T.C.; Nitz, D.A.; Xu, X. Degenerate mapping of environmental location presages deficits in object-location encoding and memory in the 5xFAD mouse model for Alzheimer’s disease. Neurobiol. Dis. 2023, 176, 105939. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, L.; Baglietto-Vargas, D.; Kamalipour, P.; Ye, Q.; LaFerla, F.M.; Nitz, D.A.; Holmes, T.C.; Xu, X. Spatial coding defects of hippocampal neural ensemble calcium activities in the triple-transgenic Alzheimer’s disease mouse model. Neurobiol. Dis. 2022, 162, 105562. [Google Scholar] [CrossRef]
- Murano, T.; Nakajima, R.; Nakao, A.; Hirata, N.; Amemori, S.; Murakami, A.; Kamitani, Y.; Yamamoto, J.; Miyakawa, T. Multiple types of navigational information are independently encoded in the population activities of the dentate gyrus neurons. Proc. Natl. Acad. Sci. USA 2022, 119, e2106830119. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, J.; Jerbi, K. How critical is brain criticality? Trends Neurosci. 2022, 45, 820–837. [Google Scholar] [CrossRef]
- Habibollahi, F.; Sun, D.; Burkitt, A.N.; French, C. Neural Networks Are Tuned Near Criticality During a Cognitive Task and Distanced from Criticality in a Psychopharmacological Model of Alzheimer’s Disease. Neurobiol. Dis. 2023, 176, 105939. [Google Scholar]
- Zhou, H.; Li, H.; Gowravaram, N.; Quan, M.; Kausar, N.; Gomperts, S.N. Disruption of hippocampal neuronal circuit function depends upon behavioral state in the APP/PS1 mouse model of Alzheimer’s disease. Sci. Rep. 2022, 12, 21022. [Google Scholar] [CrossRef] [PubMed]
- Bublak, P.; Redel, P.; Sorg, C.; Kurz, A.; Förstl, H.; Müller, H.J.; Schneider, W.X.; Finke, K. Staged decline of visual processing capacity in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2011, 32, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Parka, A.; Degel, C.; Dreyer, J.; Richter, U.; Hall, B.; Bastlund, J.F.; Laursen, B.; Nedergaard, M.; Sotty, F.; Botta, P. Early impairments of visually-driven neuronal ensemble dynamics in the rTg4510 tauopathy mouse model. Neurobiol. Dis. 2023, 178, 106012. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yu, M.; Lin, R.; Wang, Y.; Zhuo, Z.; Cheng, N.; Wang, M.; Tang, Y.; Wang, L.; Hou, S.T. Rhythmic light flicker rescues hippocampal low gamma and protects ischemic neurons by enhancing presynaptic plasticity. Nat. Commun. 2020, 11, 3012. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Shaik, N.E.K.; Unnithan, R.R.; French, C. Hippocampal cognitive and relational map paradigms explored by multisensory encoding recording with wide-field calcium imaging. iScience 2024, 27, 108603. [Google Scholar] [CrossRef]
- Shoob, S.; Buchbinder, N.; Shinikamin, O.; Gold, O.; Baeloha, H.; Langberg, T.; Zarhin, D.; Shapira, I.; Braun, G.; Habib, N.; et al. Deep brain stimulation of thalamic nucleus reuniens promotes neuronal and cognitive resilience in an Alzheimer’s disease mouse model. Nat. Commun. 2023, 14, 7002. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and treatment of Parkinson disease: A review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Kim, S.; Pajarillo, E.; Nyarko-Danquah, I.; Aschner, M.; Lee, E. Role of astrocytes in Parkinson’s disease associated with genetic mutations and neurotoxicants. Cells 2023, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Suaga, P.; Bravo-San Pedro, J.M.; González-Polo, R.A.; Fuentes, J.M.; Niso-Santano, M. ER–mitochondria signaling in Parkinson’s disease. Cell Death Dis. 2018, 9, 337. [Google Scholar] [CrossRef]
- Calì, T.; Ottolini, D.; Negro, A.; Brini, M. α-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J. Biol. Chem. 2012, 287, 17914–17929. [Google Scholar] [CrossRef]
- Paillusson, S.; Gomez-Suaga, P.; Stoica, R.; Little, D.; Gissen, P.; Devine, M.J.; Noble, W.; Hanger, D.P.; Miller, C.C. α-Synuclein binds to the ER–mitochondria tethering protein VAPB to disrupt Ca2+ homeostasis and mitochondrial ATP production. Acta Neuropathol. 2017, 134, 129–149. [Google Scholar] [CrossRef]
- Hurley, M.J.; Brandon, B.; Gentleman, S.M.; Dexter, D.T. Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins. Brain 2013, 136, 2077–2097. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.S.; Guzman, J.N.; Ilijic, E.; Mercer, J.N.; Rick, C.; Tkatch, T.; Meredith, G.E.; Surmeier, D.J. ‘Rejuvenation’protects neurons in mouse models of Parkinson’s disease. Nature 2007, 447, 1081–1086. [Google Scholar] [CrossRef]
- Ilijic, E.; Guzman, J.N.; Surmeier, D.J. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol. Dis. 2011, 43, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.N.; Ilijic, E.; Yang, B.; Sanchez-Padilla, J.; Wokosin, D.; Galtieri, D.; Kondapalli, J.; Schumacker, P.T.; Surmeier, D.J. Systemic isradipine treatment diminishes calcium-dependent mitochondrial oxidant stress. J. Clin. Investig. 2018, 128, 2266–2280. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Ríos, P.; Gómez-Suaga, P.; Fdez, E.; Hilfiker, S. Upstream deregulation of calcium signaling in Parkinson’s disease. Front. Mol. Neurosci. 2014, 7, 53. [Google Scholar]
- Yuan, H.H.; Chen, R.J.; Zhu, Y.H.; Peng, C.L.; Zhu, X.R. The neuroprotective effect of overexpression of calbindin-D 28k in an animal model of Parkinson’s disease. Mol. Neurobiol. 2013, 47, 117–122. [Google Scholar] [CrossRef]
- Parker, J.G.; Marshall, J.D.; Ahanonu, B.; Wu, Y.W.; Kim, T.H.; Grewe, B.F.; Zhang, Y.; Li, J.Z.; Ding, J.B.; Ehlers, M.D.; et al. Diametric neural ensemble dynamics in parkinsonian and dyskinetic states. Nature 2018, 557, 177–182. [Google Scholar] [CrossRef]
- Trevathan, J.K.; Asp, A.J.; Nicolai, E.N.; Trevathan, J.M.; Kremer, N.A.; Kozai, T.D.; Cheng, D.; Schachter, M.J.; Nassi, J.J.; Otte, S.L.; et al. Calcium imaging in freely moving mice during electrical stimulation of deep brain structures. J. Neural Eng. 2021, 18, 026008. [Google Scholar] [CrossRef]
- Sun, Y.; Tong, H.; Yang, T.; Liu, L.; Li, X.J.; Li, S. Insights into white matter defect in Huntington’s disease. Cells 2022, 11, 3381. [Google Scholar] [CrossRef] [PubMed]
- Craufurd, D.; Thompson, J.C.; Snowden, J.S. Behavioral changes in Huntington disease. Cogn. Behav. Neurol. 2001, 14, 219–226. [Google Scholar]
- Raymond, L.A. Striatal synaptic dysfunction and altered calcium regulation in Huntington disease. Biochem. Biophys. Res. Commun. 2017, 483, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Bezprozvanny, I. Calcium signaling and neurodegenerative diseases. Trends Mol. Med. 2009, 15, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, P.; Nascimento Da Conceicao, V.; Sun, Y.; Ahamad, N.; Saraiva, L.R.; Selvaraj, S.; Singh, B.B. Calcium signaling regulates autophagy and apoptosis. Cells 2021, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.S.; Guo, C.; Wang, H.; Chen, X.; Bezprozvanny, I. Neuroprotective effects of inositol 1, 4, 5-trisphosphate receptor C-terminal fragment in a Huntington’s disease mouse model. J. Neurosci. 2009, 29, 1257–1266. [Google Scholar] [CrossRef]
- Fan, M.M.; Raymond, L.A. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington’s disease. Prog. Neurobiol. 2007, 81, 272–293. [Google Scholar] [CrossRef]
- Smith-Dijak, A.I.; Sepers, M.D.; Raymond, L.A. Alterations in synaptic function and plasticity in Huntington disease. J. Neurochem. 2019, 150, 346–365. [Google Scholar] [CrossRef] [PubMed]
- Dau, A.; Gladding, C.M.; Sepers, M.D.; Raymond, L.A. Chronic blockade of extrasynaptic NMDA receptors ameliorates synaptic dysfunction and pro-death signaling in Huntington disease transgenic mice. Neurobiol. Dis. 2014, 62, 533–542. [Google Scholar] [CrossRef]
- Okamoto, S.I.; Pouladi, M.A.; Talantova, M.; Yao, D.; Xia, P.; Ehrnhoefer, D.E.; Zaidi, R.; Clemente, A.; Kaul, M.; Graham, R.K.; et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat. Med. 2009, 15, 1407–1413. [Google Scholar] [CrossRef]
- Seto-Ohshima, A.; Lawson, E.; Emson, P.C.; Mountjoy, C.Q.; Carrasco, L.H. Loss of matrix calcium-binding protein-containing neurons in Huntington’s disease. Lancet 1988, 331, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Schwaller, B. Cytosolic Ca2+ buffers. Cold Spring Harb. Perspect. Biol. 2010, 2, a004051. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.; Peng, A.; Levine, M.S.; Cepeda, C. Calcium imaging: A versatile tool to examine Huntington’s disease mechanisms and progression. Front. Neurosci. 2022, 16, 1040113. [Google Scholar] [CrossRef]
- Donzis, E.J.; Estrada-Sánchez, A.M.; Indersmitten, T.; Oikonomou, K.; Tran, C.H.; Wang, C.; Latifi, S.; Golshani, P.; Cepeda, C.; Levine, M.S. Cortical network dynamics is altered in mouse models of Huntington’s disease. Cereb. Cortex 2020, 30, 2372–2388. [Google Scholar] [CrossRef]
- Saha, S.; Chant, D.; McGrath, J. A systematic review of mortality in schizophrenia: Is the differential mortality gap worsening over time? Arch. Gen. Psychiatry 2007, 64, 1123–1131. [Google Scholar] [CrossRef]
- Liu, C.; Kanazawa, T.; Tian, Y.; Mohamed Saini, S.; Mancuso, S.; Mostaid, M.S.; Takahashi, A.; Zhang, D.; Zhang, F.; Yu, H.; et al. The schizophrenia genetics knowledgebase: A comprehensive update of findings from candidate gene studies. Transl. Psychiatry 2019, 9, 205. [Google Scholar] [CrossRef]
- Meehl, P.E. Schizotaxia, schizotypy, schizophrenia. In Schizophrenia; Routledge: London, UK, 2017; pp. 21–46. [Google Scholar]
- Escudero, I.; Johnstone, M. Genetics of schizophrenia. Curr. Psychiatry Rep. 2014, 16, 502. [Google Scholar] [CrossRef]
- Maj, C.; Minelli, A.; Giacopuzzi, E.; Sacchetti, E.; Gennarelli, M. The role of metabotropic glutamate receptor genes in schizophrenia. Curr. Neuropharmacol. 2016, 14, 540–550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levitt, P.; Ebert, P.; Mirnics, K.; Nimgaonkar, V.L.; Lewis, D.A. Making the case for a candidate vulnerability gene in schizophrenia: Convergent evidence for regulator of G-protein signaling 4 (RGS4). Biol. Psychiatry 2006, 60, 534–537. [Google Scholar] [CrossRef]
- Eastwood, S.L.; Harrison, P.J. Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res. Bull. 2001, 55, 569–578. [Google Scholar] [CrossRef]
- Lintunen, J.; Lähteenvuo, M.; Tiihonen, J.; Tanskanen, A.; Taipale, H. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophr. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; McGrath, J.J.; Reynolds, G.P. Neuronal calcium-binding proteins and schizophrenia. Schizophr. Res. 2002, 57, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhang, L.; Barbera, G.; Fang, W.; Zhang, J.; Chen, X.; Chen, R.; Li, Y.; Lin, D.T. Distinct and dynamic on and off neural ensembles in the prefrontal cortex code social exploration. Neuron 2018, 100, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Grieco, S.F.; Qiao, X.; Johnston, K.G.; Chen, L.; Nelson, R.R.; Lai, C.; Holmes, T.C.; Xu, X. Neuregulin signaling mediates the acute and sustained antidepressant effects of subanesthetic ketamine. Transl. Psychiatry 2021, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Masuda, F.K.; Aery Jones, E.A.; Sun, Y.; Giocomo, L.M. Ketamine evoked disruption of entorhinal and hippocampal spatial maps. Nat. Commun. 2023, 14, 6285. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Yu, Y.; Habibollahi, F.; Unnithan, R.R.; French, C. Real-time multimodal sensory detection using widefield hippocampal calcium imaging. Commun. Eng. 2023, 2, 91. [Google Scholar] [CrossRef]


| Neurological Disorders | Miniscope Device | Year | Authors |
|---|---|---|---|
| ALS | Customised | 2022 | Liang et al. [65] |
| ALS | Doric Lenses | 2024 | Brailoiu et al. [66] |
| AD | UCLA | 2023 | Zhang et al. [86] |
| AD | UCLA | 2022 | Lin et al. [87] |
| AD | Inscopix | 2022 | Murano et al. [88] |
| AD | UCLA | 2023 | Habibollahi et al. [90] |
| AD | UCLA | 2023 | Sun et al. [38] |
| AD | Inscopix | 2022 | Zhou et al. [91] |
| AD | Inscopix | 2023 | Parka et al. [93] |
| AD | Inscopix | 2023 | Shoob et al. [96] |
| PD | Inscopix | 2018 | Parker et al. [110] |
| PD | Inscopix | 2021 | Trevathan et al. [111] |
| HD | UCLA | 2022 | Barry et al. [124] |
| Schizophrenia | Customised | 2018 | Liang et al. [135] |
| Schizophrenia | UCLA | 2021 | Grieco et al. [136] |
| Schizophrenia | UCLA | 2023 | Masuda et al. [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Amiri, M.; Meng, Q.; Unnithan, R.R.; French, C. Calcium Signalling in Neurological Disorders, with Insights from Miniature Fluorescence Microscopy. Cells 2025, 14, 4. https://doi.org/10.3390/cells14010004
Sun D, Amiri M, Meng Q, Unnithan RR, French C. Calcium Signalling in Neurological Disorders, with Insights from Miniature Fluorescence Microscopy. Cells. 2025; 14(1):4. https://doi.org/10.3390/cells14010004
Chicago/Turabian StyleSun, Dechuan, Mona Amiri, Qi Meng, Ranjith R. Unnithan, and Chris French. 2025. "Calcium Signalling in Neurological Disorders, with Insights from Miniature Fluorescence Microscopy" Cells 14, no. 1: 4. https://doi.org/10.3390/cells14010004
APA StyleSun, D., Amiri, M., Meng, Q., Unnithan, R. R., & French, C. (2025). Calcium Signalling in Neurological Disorders, with Insights from Miniature Fluorescence Microscopy. Cells, 14(1), 4. https://doi.org/10.3390/cells14010004







