The Ivermectin Related Compound Moxidectin Can Target Apicomplexan Importin α and Limit Growth of Malarial Parasites
Abstract
1. Introduction
2. Materials and Methods
2.1. Protein Expression and Purification
2.2. Compounds
2.3. AlphaScreen
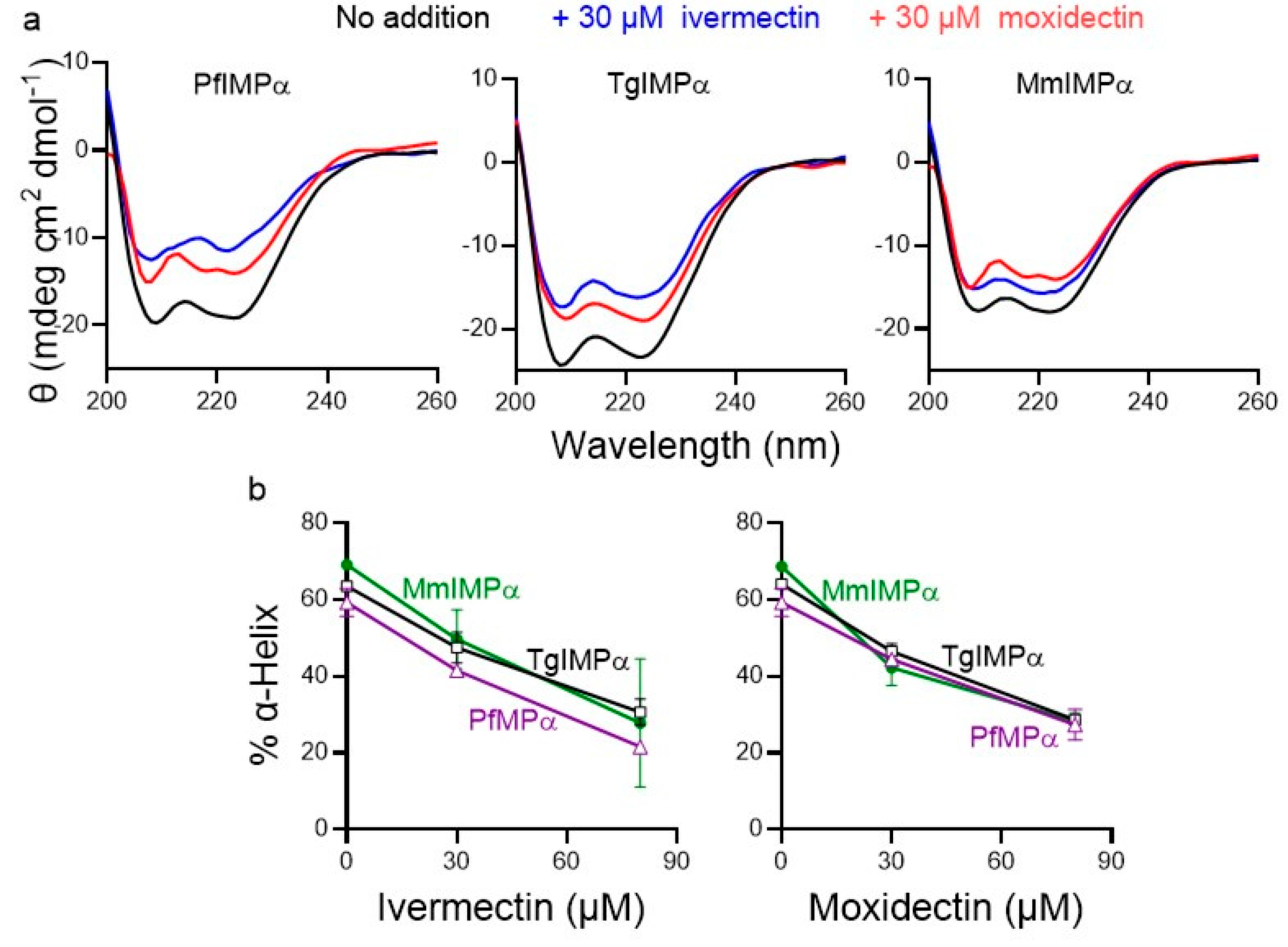
2.4. Circular Dichroism (CD) Spectroscopy
2.5. Intrinsic Tryptophan Fluorescence Assays
2.6. P. falciparum Culture
2.7. Drug Susceptibility Assays for P. falciparum Using HRP2 (Histidine-Rich Protein 2) ELISA
2.8. T. gondii Tachyzoite Culture and Growth Inhibition Assay
2.9. MTT Assay for Host Cell Cytotoxicity
2.10. Statistical Analysis
3. Results
3.1. Moxidectin Can Block NLS Recognition by Apicomplexan IMPαs
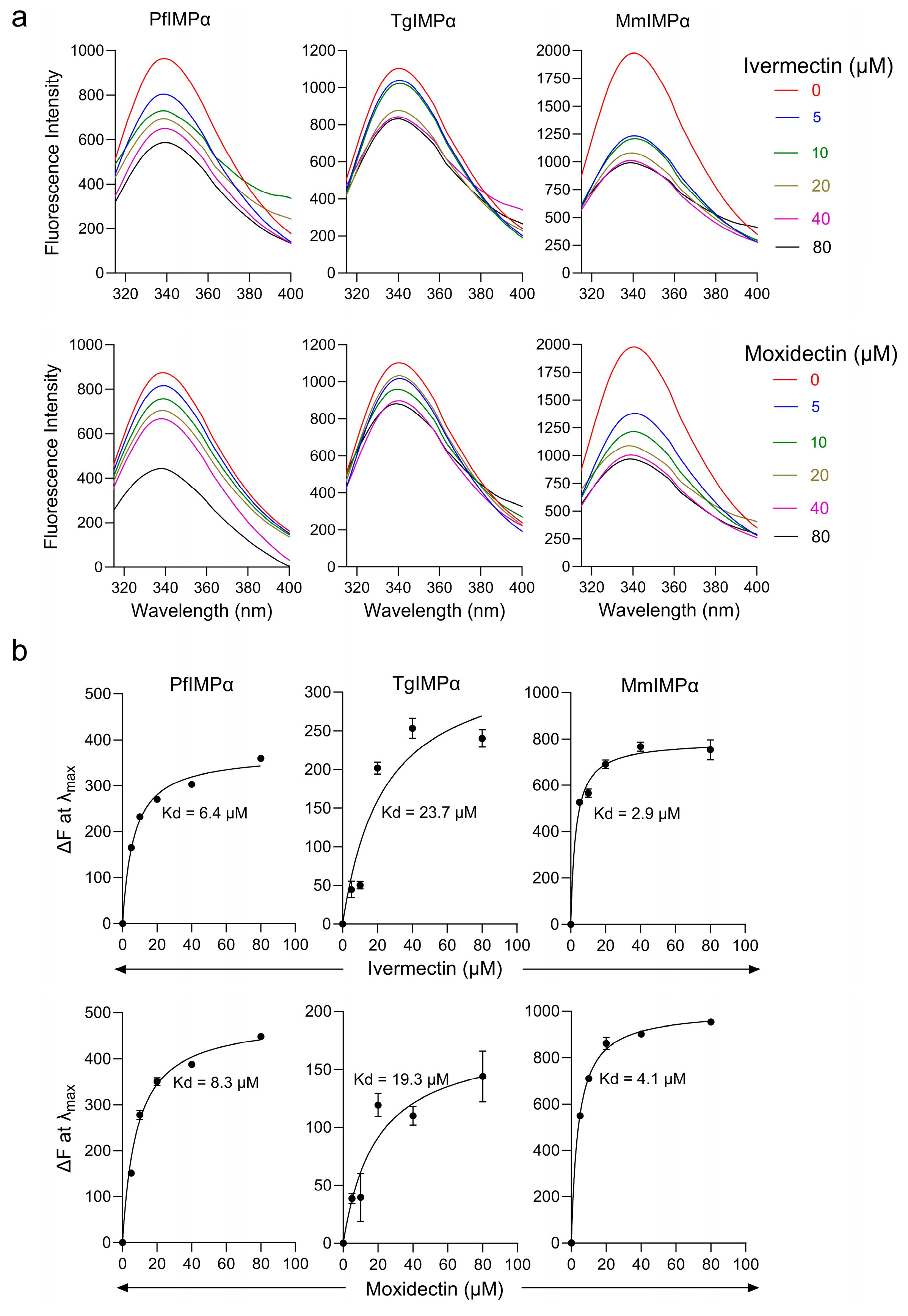
3.2. Moxidectin Can Bind Directly to Apicomplexan IMPα Proteins
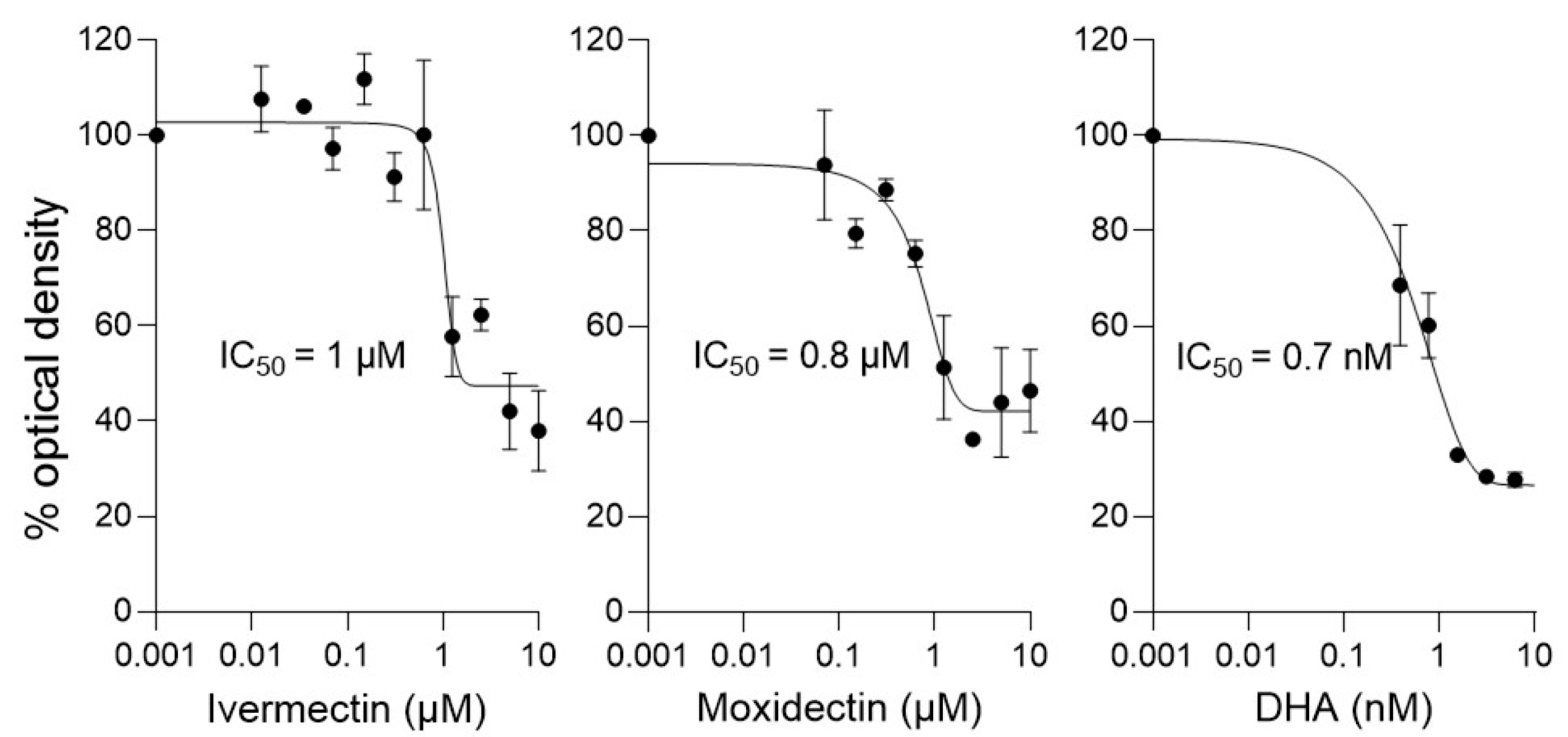
3.3. Like Ivermectin, Moxidectin Can Limit Proliferation of P. falciparum
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kosyna, F.K.; Depping, R. Controlling the Gatekeeper: Therapeutic Targeting of Nuclear Transport. Cells 2018, 7, 221. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, A.J.; Jans, D.A. Regulation of Nucleocytoplasmic Trafficking of Viral Proteins: An Integral Role in Pathogenesis? Biochim. Biophys. Acta-Mol. Cell Res. 2011, 1813, 2176–2190. [Google Scholar] [CrossRef] [PubMed]
- Köhler, M.; Speck, C.; Christiansen, M.; Bischoff, F.R.; Prehn, S.; Haller, H.; Hartmann, E. Evidence for Distinct Substrate Specificities of Importin α Family Members in Nuclear Protein Import. Mol. Cell. Biol. 1999, 16, 7782–7791. [Google Scholar] [CrossRef]
- Jans, D.A.; Martin, A.J.; Wagstaff, K.M. Inhibitors of Nuclear Transport. Curr. Opin. Cell Biol. 2019, 58, 50–60. [Google Scholar] [CrossRef]
- Stewart, M. Molecular Mechanism of the Nuclear Protein Import Cycle. Nat. Rev. Mol. Cell Biol. 2007, 8, 195–208. [Google Scholar] [CrossRef]
- Dunn, J.; McCuaig, R.D.; Tan, A.H.Y.; Tu, W.; Wu, F.; Wagstaff, K.M.; Zafar, A.; Ali, S.; Diwakar, H.; Dahlstrom, J.E.; et al. Selective Targeting of Protein Kinase C (PKC)-θ Nuclear Translocation Reduces Mesenchymal Gene Signatures and Reinvigorates Dysfunctional CD8+ T Cells in Immunotherapy-Resistant and Metastatic Cancers. Cancers 2022, 14, 1596. [Google Scholar] [CrossRef]
- Gajewska, K.; Lescesen, H.; Ramialison, M.; Wagstaff, K.M.; Jans, D.A. Nuclear transporter Importin-13 plays a key role in the oxidative stress transcriptional response. Nat. Commun. 2021, 12, 5904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Williamson, N.A.; Duvick, L.; Lee, A.; Orr, H.T.; Korlin-Downs, A.; Yang, P.; Mok, Y.-F.; Jans, D.A.; Bogoyevitch, M.A. The ataxin-1 interactome reveals direct connection with multiple disrupted nuclear transport pathways. Nat. Commun. 2020, 11, 3343. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.J.; Jans, D.A. Antivirals that target the host IMPα/β1-virus interface. Biochem. Soc. Trans. 2021, 49, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Crump, A.; Omura, S. Ivermectin, wonder drug from Japan: The human use perspective. Proc. Jpn. Acad. 2011, 87, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin Is a Specific Inhibitor of Importin α/β -Mediated Nuclear Import Able to Inhibit Replication of HIV-1 and Dengue Virus. Biochem. J. 2012, 856, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-Approved Drug Ivermectin Inhibits the Replication of SARS-CoV-2 in Vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Thomas, D.R.; Lundberg, L.; Pinkham, C.; Shechter, S.; Debono, A.; Baell, J.; Wagstaff, K.M.; Hick, C.A.; Kehn-hall, K.; Jans, D.A. Identification of Novel Antivirals Inhibiting Recognition of Venezuelan Equine Encephalitis Virus Capsid Protein by the Importin α/β1 Heterodimer through High-Throughput Screening. Antivir. Res. 2018, 151, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.Y.; Atkinson, S.C.; Fraser, J.E.; Wang, C.; Maher, B.; Roman, N.; Forwood, J.K.; Wagstaff, K.M.; Borg, N.A.; Jans, D.A. Novel Flavivirus Antiviral That Targets The Host Nuclear Transport Importin α/β1 Heterodimer. Cells 2019, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Shechter, S.; Thomas, D.R.; Lundberg, L.; Pinkham, C.; Lin, S.; Wagstaff, K.M.; Debono, A.; Kehn-hall, K.; Jans, D.A. Novel Inhibitors Targeting Venezuelan Equine Encephalitis Virus Capsid Protein Identified Using In Silico Structure-Based-Drug-Design. Sci. Rep. 2017, 7, 17705. [Google Scholar] [CrossRef]
- Yang, S.N.Y.; Atkinson, S.C.; Wang, C.; Lee, A.; Bogoyevitch, M.A.; Borg, N.A.; Jans, D.A. The Broad Spectrum Antiviral Ivermectin Targets the Host Nuclear Transport Importin α/b1 Heterodimer. Antivir. Res. 2020, 177, 104760. [Google Scholar] [CrossRef]
- Walunj, S.B.; Dias, M.M.; Kaur, C.; Wagstaff, K.M.; Dey, V.; Hick, C.; Patankar, S.; Jans, D.A. High-Throughput Screening to Identify Inhibitors of Plasmodium falciparum Importin α. Cells 2022, 11, 1201. [Google Scholar] [CrossRef]
- Walunj, S.B.; Wang, C.; Wagstaff, K.M.; Patankar, S.; Jans, D.A. Conservation of Importin a Function in Apicomplexans: Ivermectin and GW5074 Target Plasmodium falciparum Importin a and Inhibit Parasite Growth in Culture. Int. J. Mol. Sci. 2022, 23, 13899. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.M.; Fidock, D.A.; Kyle, D.E.; Kappe, S.H.I.; Alonso, P.L.; Collins, F.H.; Duffy, P.E. Malaria: Progress, Perils, and Prospects for Eradication. J. Clin. Investig. 2008, 118, 1266–1276. [Google Scholar] [CrossRef]
- Tse, E.G.; Korsik, M.; Todd, M.H. The Past, Present and Future of Antimalarial Medicines. Malar. J. 2019, 18, 93. [Google Scholar] [CrossRef]
- Blasco, B.; Leroy, D.; Fidock, D.A. Antimalarial Drug Resistance: Linking Plasmodium falciparum Parasite Biology to the Clinic. Nat. Med. 2017, 23, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, M.; Mikaeili Galeh, T.; Moosazadeh, M.; Sarvi, S.; Dodangeh, S.; Javidnia, J.; Sharif, M.; Daryani, A. The Global Serological Prevalence of Toxoplasma gondii in Felids during the Last Five Decades (1967–2017): A Systematic Review and Meta-Analysis. Parasites Vectors 2020, 13, 82. [Google Scholar] [CrossRef]
- Sabin, A.B. Toxoplasmic encephalitis in children. J. Am. Med. Assoc. 1941, 116, 801–807. [Google Scholar] [CrossRef]
- Alday, P.H.; Doggett, J.S. Drugs in Development for Toxoplasmosis: Advances, Challenges, and Current Status. Drug Des. Dev. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef]
- Frankel, M.B.; Knoll, L.J. Functional Analysis of Key Nuclear Trafficking Components Reveals an Atypical Ran Network Required for Parasite Pathogenesis. Mol. Microbiol. 2008, 70, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Frankel, M.B.; Knoll, L.J. The Ins and Outs of Nuclear Trafficking: Unusual Aspects in Apicomplexan Parasites. DNA Cell Biol. 2009, 28, 277–284. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Otto, T.D.; Oberstaller, J.; Liao, X.; Adapa, S.R.; Udenze, K.; Bronner, I.F.; Casandra, D.; Mayho, M.; et al. Uncovering the Essential Genes of the Human Malaria Parasite Plasmodium falciparum by Saturation Mutagenesis. Science 2018, 360, eaap7847. [Google Scholar] [CrossRef] [PubMed]
- Sidik, S.M.; Huet, D.; Ganesan, S.M.; Huynh, M.H.; Wang, T.; Nasamu, A.S.; Thiru, P.; Saeij, J.P.J.; Carruthers, V.B.; Niles, J.C.; et al. A Genome-Wide CRISPR Screen in Toxoplasma Identifies Essential Apicomplexan Genes. Cell 2016, 166, 1423–1435.e12. [Google Scholar] [CrossRef]
- González Canga, A.; Sahagún Prieto, A.M.; Diez Liébana, M.J.; Fernández Martínez, N.; Sierra Vega, M.; García Vieitez, J.J. The pharmacokinetics and interactions of ivermectin in humans—A mini-review. AAPS J. 2008, 10, 42–46. [Google Scholar] [CrossRef]
- Jans, D.A.; Wagstaff, K.M. Ivermectin as a Broad-Spectrum Host-Directed Antiviral: The Real Deal? Cells 2020, 9, 2100. [Google Scholar] [CrossRef]
- Ooi, E.E. Repurposing Ivermectin as an Anti-Dengue Drug. Clin. Infect. Dis. 2021, 72, e594–e595. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.; Lawrie, T.A.; Dowswell, T.; Fordham, E.J.; Mitchell, S.; Hill, S.R.; Tham, T.C. Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis to Inform Clinical Guidelines. Am. J. Ther. 2021, 28, e434–e460. [Google Scholar] [CrossRef]
- Foy, B.D.; Alout, H.; Seaman, J.A.; Rao, S.; Magalhaes, T.; Wade, M.; Parikh, S.; Soma, D.; Sagna, B.; Fournet, F.; et al. Efficacy and Risk of Harms of Repeat Ivermectin Mass Drug Administrations for Control of Malaria (RIMDAMAL): A Cluster-Randomised Trial. Lancet 2019, 393, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, A.L.; Bastiaens, G.J.H.; Tiono, A.B.; Guelbéogo, W.M.; Kobylinski, K.C.; Ouédraogo, A.; Barry, A.; Bougouma, E.C.; Nebie, I.; Ouattara, M.S.; et al. Efficacy and Safety of the Mosquitocidal Drug Ivermectin to Prevent Malaria Transmission after Treatment: A Double-Blind, Randomized, Clinical Trial. Clin. Infect. Dis. 2015, 60, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, V.P.; Hofmann, D.; Savathdy, V.; Xayavong, P.; Norkhankhame, C.; Huy, R.; Khieu, V.; Sayasone, S.; Hattendorf, J.; Keiser, J. Efficacy and Safety of Moxidectin Compared with Ivermectin against Strongyloides Stercoralis Infection in Adults in Laos and Cambodia: A Randomised, Double-Blind, Non-Inferiority, Phase 2b/3 Trial. Lancet Infect. Dis. 2023, 24, 196–205. [Google Scholar] [CrossRef]
- Cobb, R.; Boeckh, A. Moxidectin: A Review of Chemistry, Pharmacokinetics and Use in Horses. Parasit. Vectors 2009, 2, S5. [Google Scholar] [CrossRef] [PubMed]
- Prichard, R.K.; Geary, T.G. Perspectives on the Utility of Moxidectin for the Control of Parasitic Nematodes in the Face of Developing Anthelmintic Resistance. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 69–83. [Google Scholar] [CrossRef]
- Tang, M.; Hu, X.; Wang, Y.; Yao, X.; Zhang, W.; Yu, C.; Cheng, F.; Li, J.; Fang, Q. Ivermectin, a Potential Anticancer Drug Derived from an Anti-parasitic Drug. Pharmacol. Res. 2021, 163, 105207. [Google Scholar] [CrossRef]
- Cotreau, M.M.; Warren, S.; Ryan, J.L.; Fleckenstein, L.; Vanapalli, S.R.; Brown, K.R.; Rock, D.; Chen, C.Y.; Schwertschlag, U.S. The Anti-parasitic Moxidectin: Safety, Tolerability, and Pharmacokinetics in Humans. J. Clin. Pharmacol. 2003, 43, 1108–1115. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Jans, D.A. Intramolecular Masking of Nuclear Localization Signals: Analysis of Importin Binding Using a Novel AlphaScreen-Based Method. Anal. Biochem. 2006, 348, 49–56. [Google Scholar] [CrossRef]
- Oshokoya, O.O.; Roach, C.A.; Jiji, R.D. Quantification of Protein Secondary Structure Content by Multivariate Analysis of Deep-Ultraviolet Resonance Raman and Circular Dichroism Spectroscopies. Anal. Methods 2014, 6, 1691–1699. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Kurth, F.; Pongratz, P.; Bélard, S.; Mordmüller, B.; Kremsner, P.G.; Ramharter, M. In Vitro Activity of Pyronaridine against Plasmodium falciparum and Comparative Evaluation of Antimalarial Drug Susceptibility Assays. Malar. J. 2009, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Noedl, H.; Bronnert, J.; Yingyuen, K.; Attlmayr, B.; Kollaritsch, H.; Fukuda, M. Simple Histidine-Rich Protein 2 Double-Site Sandwich Enzyme-Linked Immunosorbent Assay for Use in Malaria Drug Sensitivity Testing. Antimicrob. Agents Chemother. 2005, 49, 3575–3577. [Google Scholar] [CrossRef]
- Jacot, D.; Meissner, M.; Sheiner, L.; Soldati-Favre, D.; Striepen, B. Genetic Manipulation of Toxoplasma gondii. In Toxoplasma Gondii: The Model Apicomplexan—Perspectives and Methods, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 577–611. [Google Scholar] [CrossRef]
- Striepen, B.; Soldati, D. Genetic Manipulation of Toxoplasma gondii. In Toxoplasma Gondii; Academic Press: Cambridge, MA, USA, 2007; pp. 391–418. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual [Internet]; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2016. [Google Scholar]
- Hürlimann, E.; Hofmann, D.; Keiser, J. Ivermectin and Moxidectin against Soil-Transmitted Helminth Infections. Trends Parasitol. 2023, 39, 272–284. [Google Scholar] [CrossRef]
- Tan, B.; Opoku, N.; Attah, S.K.; Awadzi, K.; Kuesel, A.C.; Lazdins-Helds, J.; Rayner, C.; Ryg-Cornejo, V.; Sullivan, M.; Fleckenstein, L. Pharmacokinetics of Oral Moxidectin in Individuals with Onchocerca Volvulus Infection. PLoS Negl. Trop. Dis. 2022, 16, e0010005. [Google Scholar] [CrossRef] [PubMed]
- Kobe, B. Autoinhibition by an Internal Nuclear Localization Signal Revealed by the Crystal Structure of Mammalian Importin α. Nat. Struct. Biol. 1999, 6, 388–397. [Google Scholar] [CrossRef]
- Fanara, P.; Hodel, M.R.; Corbett, A.H.; Hodel, A.E. Quantitative Analysis of Nuclear Localization Signal (NLS)-Importin a Interaction through Fluorescence Depolarization. Evidence for auto-inhibitory regulation of NLS binding. J. Biol. Chem. 2000, 275, 21218–21223. [Google Scholar] [CrossRef]
- Sindrewicz, P.; Li, X.; Yates, E.A.; Turnbull, J.E.; Lian, L.Y.; Yu, L.G. Intrinsic Tryptophan Fluorescence Spectroscopy Reliably Determines Galectin-Ligand Interactions. Sci. Rep. 2019, 9, 11851. [Google Scholar] [CrossRef]
- Prichard, R.; Ménez, C.; Lespine, A. Moxidectin and the Avermectins: Consanguinity but Not Identity. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Atif, M.; Estrada-Mondragon, A.; Nguyen, B.; Lynch, J.W.; Keramidas, A. Effects of glutamate and ivermectin on single glutamate-gated chloride channels of the parasitic nematode H. contortus. PLoS Pathog. 2017, 13, e1006663. [Google Scholar] [CrossRef] [PubMed]
- Hibbs, R.; Gouaux, E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 2011, 474, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lynagh, T.; Lynch, J.W. A glycine residue essential for high ivermectin sensitivity in Cys-loop ion channel receptors. Int. J. Parasitol. 2010, 40, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Lynagh, T.; Webb, T.I.; Dixon, C.L.; Cromer, B.A.; Lynch, J.W. Molecular Determinants of Ivermectin Sensitivity at the Glycine Receptor Chloride Channel. J. Biol. Chem. 2011, 286, 43913–43924. [Google Scholar] [CrossRef] [PubMed]

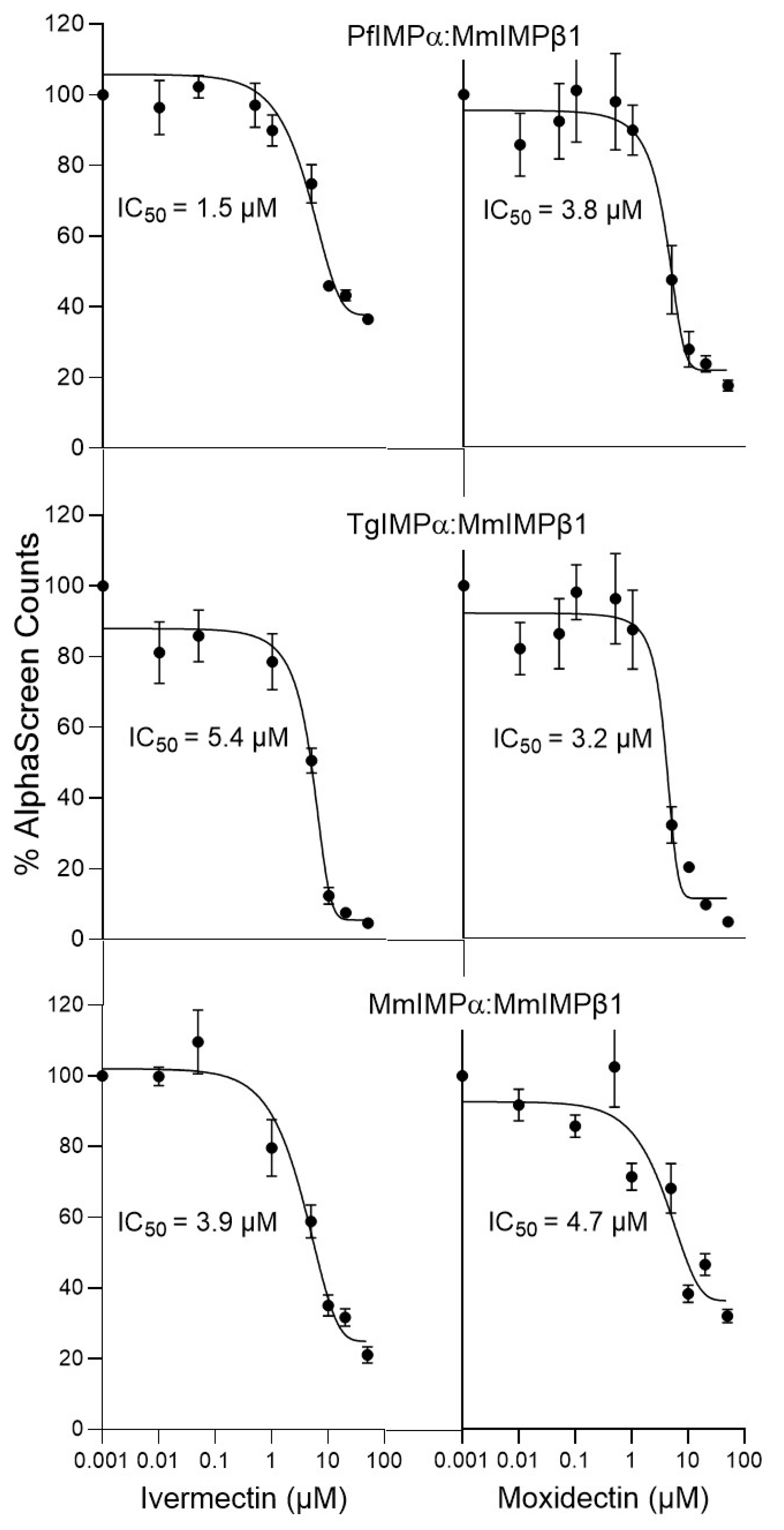
| IC50 (μM) * | ||
|---|---|---|
| Binding Interaction | Ivermectin | Moxidectin |
| PfIMPα:TGS1-NLS-GFP | 5.0 ± 1.3 | 4.8 ± 0.4 |
| TgIMPα:Tag-NLS-GFP | 4.9 ± 0.2 | 3.8 ± 0.8 |
| MmΔIBBIMPα:Tag-NLS-GFP | 6.2 ± 0.2 | 3.6 ± 0.5 |
| PfIMPα:MmIMPβ1 | 1.9 ± 0.2 | 3.4 ± 0.4 |
| TgIMPα:MmIMPβ1 | 6.9 ± 1.2 | 2.7 ± 0.2 |
| MmIMPα:MmIMPβ1 | 3.1 ± 0.5 | 4.0 ± 0.4 |
| Protein | Kd (μM) * | |
|---|---|---|
| Ivermectin | Moxidectin | |
| PfIMPα | 6.4 ± 0.3 | 7.8 ± 1.2 |
| TgIMPα | 24 ± 1.4 | 19 ± 2.2 |
| MmIMPα | 2.9 ± 0.4 | 4.1 ± 0.2 |
| Compound | IC50 (µM) * |
|---|---|
| Ivermectin | 0.7 ± 0.1 |
| Moxidectin | 0.9 ± 0.1 |
| DHA | 0.001 ± 0.0005 |
| Compound | IC50 (µM) * | CC50 (µM) + |
|---|---|---|
| Ivermectin | 29 ± 0.7 | 8 ± 1.8 |
| Moxidectin | 35 ± 3.1 | 10 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walunj, S.B.; Mishra, G.; Wagstaff, K.M.; Patankar, S.; Jans, D.A. The Ivermectin Related Compound Moxidectin Can Target Apicomplexan Importin α and Limit Growth of Malarial Parasites. Cells 2025, 14, 39. https://doi.org/10.3390/cells14010039
Walunj SB, Mishra G, Wagstaff KM, Patankar S, Jans DA. The Ivermectin Related Compound Moxidectin Can Target Apicomplexan Importin α and Limit Growth of Malarial Parasites. Cells. 2025; 14(1):39. https://doi.org/10.3390/cells14010039
Chicago/Turabian StyleWalunj, Sujata B., Geetanjali Mishra, Kylie M. Wagstaff, Swati Patankar, and David A. Jans. 2025. "The Ivermectin Related Compound Moxidectin Can Target Apicomplexan Importin α and Limit Growth of Malarial Parasites" Cells 14, no. 1: 39. https://doi.org/10.3390/cells14010039
APA StyleWalunj, S. B., Mishra, G., Wagstaff, K. M., Patankar, S., & Jans, D. A. (2025). The Ivermectin Related Compound Moxidectin Can Target Apicomplexan Importin α and Limit Growth of Malarial Parasites. Cells, 14(1), 39. https://doi.org/10.3390/cells14010039








