Transient Interphase Microtubules Appear in Differentiating Sponge Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection and Cell Cultivation
2.2. Phylogenetic Analyses
2.3. Transmission Electron Microscopy (TEM)
2.4. Light Microscopy
2.5. Western Blotting Assay
3. Results
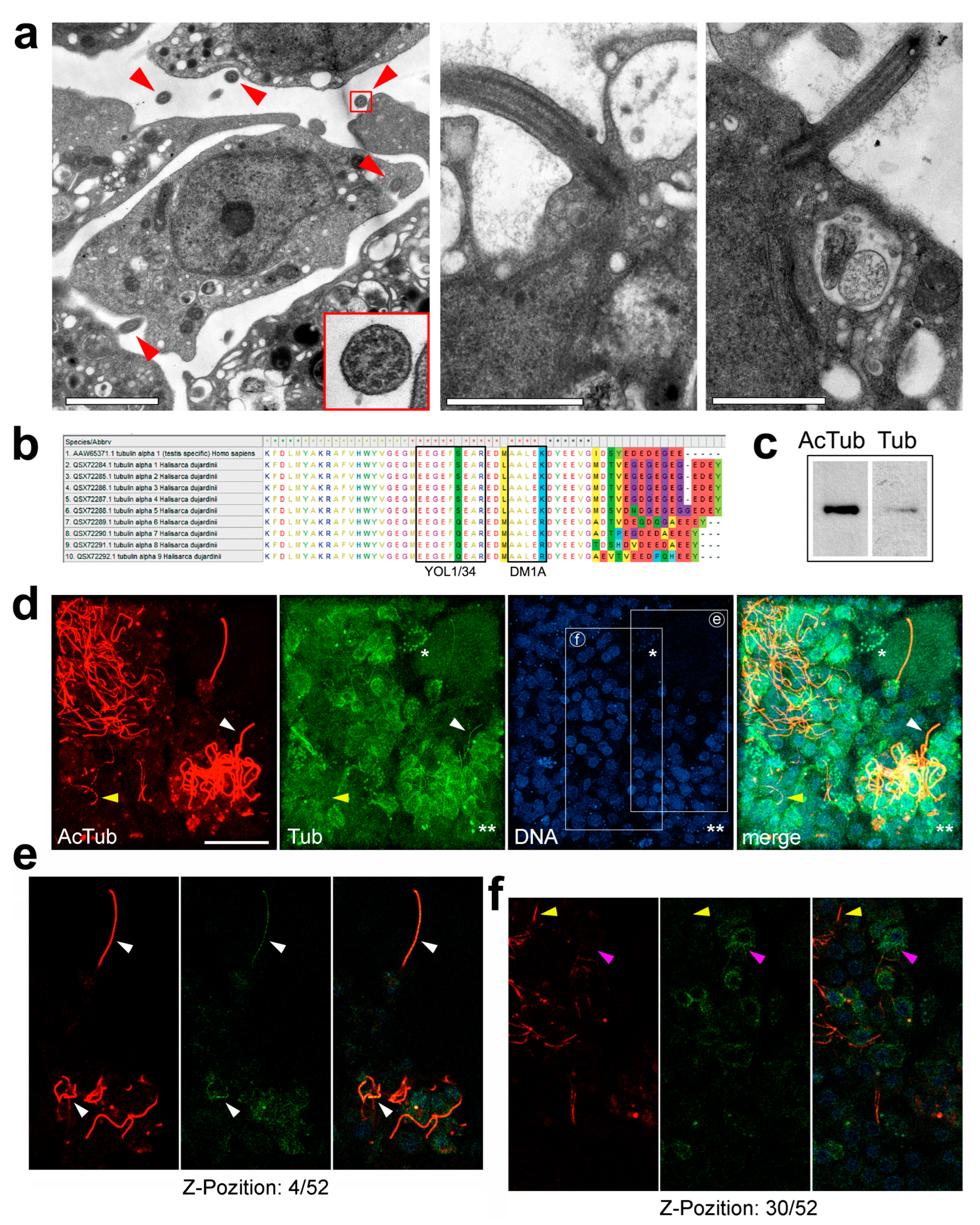
3.1. Tubulins and Dyneins Are Highly Conserved from Sponges to Humans
3.2. Cytoplasmic Microtubules in Sponge Cross-Sections Are Vanishingly Rare
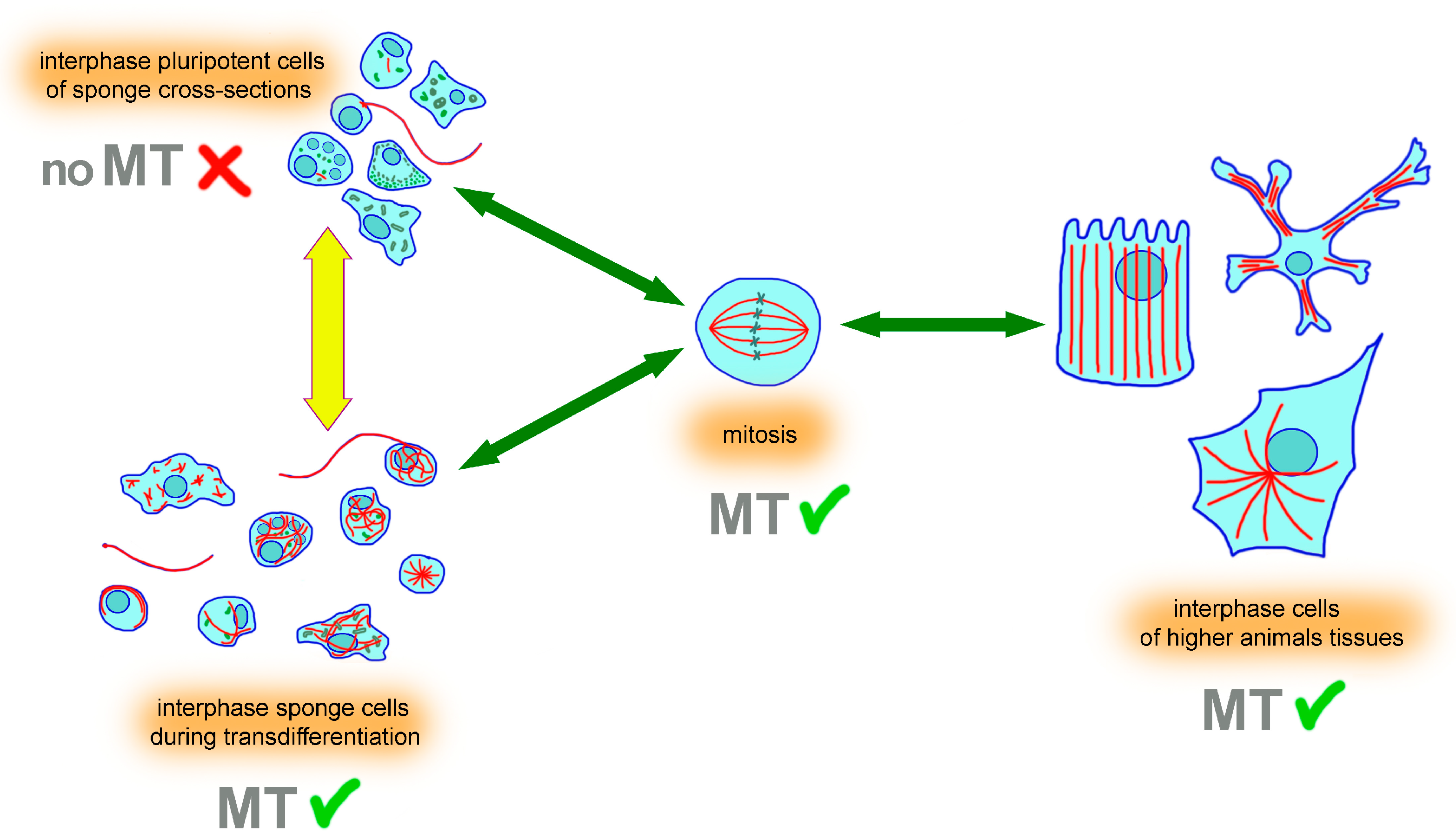
3.3. Microtubules Appear Abundantly in the Cytoplasm of Interphase Sponge Cells during Transdifferentiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dustin, P. Microtubules. Sci. Am. 1980, 243, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.; Allan, V. Microtubule-based membrane movement. Biochim. Biophys. Acta 1998, 1376, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Vale, R.D. The molecular motor toolbox for intracellular transport. Cell 2003, 112, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Welte, M.A. Bidirectional transport along microtubules. Curr. Biol. 2004, 14, R525–R537. [Google Scholar] [CrossRef] [PubMed]
- Barlan, K.; Gelfand, V.I. Microtubule-based transport and the distribution, tethering, and organization of organelles. Cold Spring Harb. Perspect. Biol. 2017, 9, a025817. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, T.; Waterman-Storer, C.M. Cell motility: Can Rho GTPases and microtubules point the way? J. Cell Sci. 2001, 114, 3795–3803. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Manneville, S. Microtubules in cell migration. Annu. Rev. Cell Dev. Biol. 2013, 29, 471–499. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.J.; Dolan, M.F.; Margulis, L. Centrioles and kinetosomes: Form, function, and evolution. Q. Rev. Biol. 2000, 75, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Reiter, J.F. The primary cilium as the cell’s antenna: Signaling at a sensory organelle. Science 2006, 313, 629–633. [Google Scholar] [CrossRef]
- Viswanadha, R.; Sale, W.S.; Porter, M.E. Ciliary motility: Regulation of axonemal dynein motors. Cold Spring Harb. Perspect. Biol. 2017, 9, a018325. [Google Scholar] [CrossRef]
- Sakai, H. Microtubules in mitosis. Cell Struct. Funct. 1994, 19, 57–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walczak, C.E.; Heald, R. Mechanisms of mitotic spindle assembly and function. Int. Rev. Cytol. 2008, 265, 111–158. [Google Scholar] [PubMed]
- McIntosh, J.R. Mitosis. Cold Spring Harb. Perspect. Biol. 2016, 8, a023218. [Google Scholar] [CrossRef]
- Sweeney, H.L.; Holzbaur, E.L.F. Motor proteins. Cold Spring Harb. Perspect. Biol. 2018, 10, a021931. [Google Scholar] [CrossRef] [PubMed]
- Yutin, N.; Koonin, E.V. Archaeal origin of tubulin. Biol. Direct. 2012, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Santana-Molina, C.; Del Saz-Navarro, D.; Devos, D.P. Early origin and evolution of the FtsZ/tubulin protein family. Front. Microbiol. 2023, 13, 1100249. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.G.; Borisy, G.G. Evolution of the multi-tubulin hypothesis. BioEssays 1997, 19, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Fulton, C.; Simpson, P.A. Selective synthesis and utilization of flagellar tubulin. The multi-tubulin hypothesis. Cell Motil. 1976, 3, 987–1005. [Google Scholar]
- Matthews, K.A.; Rees, D.; Kaufman, T.C. A functionally specialized alpha-tubulin is required for oocyte meiosis and cleavage mitoses in Drosophila. Development 1993, 117, 977–991. [Google Scholar] [CrossRef]
- Hoyle, H.D.; Raff, E.C. Two Drosophila beta tubulin isoforms are not functionally equivalent. J. Cell Biol. 1990, 111, 1009–1026. [Google Scholar] [CrossRef]
- Savage, C.; Hamelin, M.; Culotti, J.G.; Coulson, A.; Albertson, D.G.; Chalfie, M. mec-7 is a beta-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes Dev. 1989, 3, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A. The axonemal microtubules of the Chlamydomonas flagellum differ in tubulin isoform content. J. Cell Sci. 1998, 111, 313–320. [Google Scholar] [CrossRef]
- Goodson, H.V.; Jonasson, E.M. Microtubules and Microtubule-Associated Proteins. Cold Spring Harb. Perspect. Biol. 2018, 10, a022608. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Akhmanova, A. Microtubule-Organizing Centers. Annu. Rev. Cell Dev. Biol. 2017, 33, 51–75. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.D.; Feldman, J.L. Microtubule-organizing centers: From the centrosome to non-centrosomal sites. Curr. Opin. Cell Biol. 2017, 44, 93–101. [Google Scholar] [CrossRef]
- Nishita, M.; Satake, T.; Minami, Y.; Suzuki, A. Regulatory mechanisms and cellular functions of non-centrosomal microtubules. J. Biochem. 2017, 162, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meza, I.; Talamás-Rohana, P.; Vargas, M.A. The cytoskeleton of Entamoeba histolytica: Structure, function, and regulation by signaling pathways. Arch. Med. Res. 2006, 37, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.J. The role of actin, actomyosin and microtubules in defining cell shape during the differentiation of Naegleria amebae into flagellates. Eur. J. Cell Biol. 2007, 86, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.J. The structure of the mitotic spindle and nucleolus during mitosis in the amebo-flagellate Naegleria. PLoS ONE 2012, 7, e34763. [Google Scholar] [CrossRef][Green Version]
- Fulton, C.; Dingle, A.D. Basal bodies, but not centrioles, in Naegleria. J. Cell Biol. 1971, 51, 826–836. [Google Scholar] [CrossRef]
- Ereskovsky, A.V.; Lavrov, A.I. Porifera. In Invertebrate Histology; LaDouceur, E.E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 19–54. [Google Scholar]
- Musser, J.M.; Schippers, K.J.; Nickel, M.; Mizzon, G.; Kohn, A.B.; Pape, C.; Ronchi, P.; Papadopoulos, N.; Tarashansky, A.J.; Hammel, J.U.; et al. Profiling cellular diversity in sponges informs animal cell type and nervous system evolution. Science 2021, 374, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Funayama, N. The cellular and molecular bases of the sponge stem cell systems underlying reproduction, homeostasis and regeneration. Int. J. Dev. Biol. 2018, 62, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Sogabe, S.; Hatleberg, W.L.; Kocot, K.M.; Say, T.E.; Stoupin, D.; Roper, K.E.; Fernandez-Valverde, S.L.; Degnan, S.M.; Degnan, B.M. Pluripotency and the origin of animal multicellularity. Nature 2019, 570, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Borisenko, I.E.; Adamska, M.; Tokina, D.B.; Ereskovsky, A.V. Transdifferentiation is a driving force of regeneration in Halisarca dujardini (Demospongiae, Porifera). PeerJ 2015, 3, e1211. [Google Scholar] [CrossRef] [PubMed]
- Ereskovsky, A.V.; Tokina, D.B.; Saidov, D.M.; Baghdiguian, S.; Le Goff, E.; Lavrov, A.I. Transdifferentiation and Mesenchymal-to-epithelial Transition during Regeneration in Demospongiae (Porifera). J. Exp. Zool. B Mol. Dev. Evol. 2020, 334, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Ereskovsky, A.; Borisenko, I.E.; Bolshakov, F.V.; Lavrov, A.I. Whole-Body Regeneration in Sponges: Diversity, Fine Mechanisms, and Future Prospects. Genes 2021, 12, 506. [Google Scholar] [CrossRef]
- Martinez, P.; Ballarin, L.; Ereskovsky, A.V.; Gazave, E.; Hobmayer, B.; Manni, L.; Rottinger, E.; Sprecher, S.G.; Tiozzo, S.; Varela-Coelho, A.; et al. Articulating the “stem cell niche” paradigm through the lens of non-model aquatic invertebrates. BMC Biol. 2022, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Gaino, E.; Burlando, B.; Zunino, L.; Pansini, M.; Buffa, P. Origin of male gametes from choanocytes in Spongia officinalis (Porifera, Demospongiae). Int. J. Invertebr. Reprod. Dev. 1984, 7, 83–93. [Google Scholar] [CrossRef]
- Gonobobleva, E.L.; Maldonado, M. Choanocyte ultrastructure in Halisarca dujardini (Demospongiae, Halisarcida). J. Morphol. 2009, 270, 615–627. [Google Scholar] [CrossRef]
- Pozdnyakov, I.R.; Karpov, S.A. Kinetid Structure in Choanocytes of Sponges (Heteroscleromorpha): Toward the Ancestral Kinetid of Demospongiae. J. Morphol. 2016, 277, 925–934. [Google Scholar] [CrossRef]
- Sokolova, A.M.; Pozdnyakov, I.R.; Ereskovsky, A.V.; Karpov, S.A. Kinetid structure in larval and adult stages of the demosponges Haliclona aquaeductus (Haplosclerida) and Halichondria panicea (Suberitida). Zoomorphology 2019, 138, 171–184. [Google Scholar] [CrossRef]
- Melnikov, N.P.; Bolshakov, F.V.; Frolova, V.S.; Skorentseva, K.V.; Ereskovsky, A.V.; Saidova, A.A.; Lavrov, A.I. Tissue homeostasis in sponges: Quantitative analysis of cell proliferation and apoptosis. J. Exp. Zool. B Mol. Dev. Evol. 2022, 338, 360–381. [Google Scholar] [CrossRef] [PubMed]
- Wachtmann, D.; Stockem, W.; Weissenfels, N. Cytoskeletal organization and cell organelle transport in basal epithelial cells of the freshwater sponge Ephydatia fluviatilis. Cell Tissue Res. 1990, 261, 145–154. [Google Scholar] [CrossRef]
- Weissenfels, N.; Wachtmann, D.; Stockem, W. The role of microtubules for the movement of mitochondria in pinacocytes of fresh-water sponges (Spongillidae, Porifera). Eur. J. Cell Biol. 1990, 52, 310–314. [Google Scholar] [PubMed]
- Wachtmann, D.; Stockem, W. Significance of the cytoskeleton for cytoplasmic organization and cell organelle dynamics in epithelial cells of fresh-water sponges. Protoplasma 1992, 169, 107–119. [Google Scholar] [CrossRef]
- Riesgo, A.; Santodomingo, N.; Koutsouveli, V.; Kumala, L.; Leger, M.M.; Leys, S.P.; Funch, P. Molecular machineries of ciliogenesis, cell survival, and vasculogenesis are differentially expressed during regeneration in explants of the demosponge Halichondria panacea. BMC Genom. 2022, 23, 858. [Google Scholar] [CrossRef] [PubMed]
- Adameyko, K.; Burakov, A.V.; Finoshin, A.D.; Mikhailov, K.V.; Kravchuk, O.I.; Kozlova, O.S.; Gornostaev, N.G.; Cherkasov, A.V.; Erokhov, P.A.; Indeykina, M.I.; et al. Conservative and Atypical Ferritins of Sponges. Int. J. Mol. Sci. 2021, 22, 8635. [Google Scholar] [CrossRef]
- Fokin, A.I.; Zhapparova, O.N.; Burakov, A.V.; Nadezhdina, E.S. Centrosome-derived microtubule radial array, PCM-1 protein, and primary cilia formation. Protoplasma 2019, 256, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutierrez, S.; Silla-Martinez, J.M.; Gabaldon, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Velle, K.B.; Kennard, A.S.; Trupinić, M.; Ivec, A.; Swafford, A.J.M.; Nolton, E.; Rice, L.M.; Tolić, I.M.; Fritz-Laylin, L.K.; Wadsworth, P. Naegleria’s mitotic spindles are built from unique tubulins and highlight core spindle features. Curr. Biol. 2022, 32, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Vallee, R.B.; Williams, J.C.; Varma, D.; Barnhart, L.E. Dynein: An ancient motor protein involved in multiple modes of transport. J. Neurobiol. 2004, 58, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Schroer, T.A.; Szilak, I.; Steuer, E.R.; Sheetz, M.P.; Cleveland, D.W. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J. Cell Biol. 1991, 115, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- King, S.J.; Schroer, T.A. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat. Cell Biol. 2000, 2, 20–24. [Google Scholar] [CrossRef]
- Janke, C.; Magiera, M.M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 307–326. [Google Scholar] [CrossRef]
- Fulton, C. Chapter 13 Amebo-flagellates as research partners: The laboratory biology of Naegleria and Tetramitus. In Methods in Cell Biology; Prescott, D.M., Ed.; Academic Press: Cambridge, MA, USA, 1970; pp. 341–476. [Google Scholar]
- Fritz-Laylin, L.K.; Assaf, Z.J.; Chen, S.; Cande, W.Z. Naegleria gruberi de novo basal body assembly occurs via stepwise incorporation of conserved proteins. Eukaryot. Cell 2010, 9, 860–865. [Google Scholar] [CrossRef][Green Version]
- Fulton, C. Naegleria: A research partner for cell and developmental biology. J. Eukaryot. Microbiol. 1993, 40, 520–532. [Google Scholar] [CrossRef]
- Lee, J.H.; Walsh, C.J. Transcriptional regulation of coordinate changes in flagellar mRNAs during differentiation of Naegleria gruberi amebae into flagellates. Mol. Cell. Biol. 1988, 8, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Cho, J.; Cheon, H.; Paik, S.; Lee, J. Cloning and characterization of a divergent alpha-tubulin that is expressed specifically in dividing amebae of Naegleria gruberi. Gene 2002, 293, 77–86. [Google Scholar] [CrossRef]
- Schuster, F.L. Ultrastructure of mitosis in the amoeboflagellate Naegleria gruberi. Tissue Cell 1975, 7, 1–11. [Google Scholar] [CrossRef]
- Burakov, A.; Vorobjev, I.; Semenova, I.; Cowan, A.; Carson, J.; Wu, Y.; Rodionov, V. Persistent growth of microtubules at low density. Mol. Biol. Cell. 2021, 32, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Kravchuk, O.I.; Burakov, A.V.; Gornostaev, N.G.; Mikhailov, K.V.; Adameyko, K.I.; Finoshin, A.D.; Georgiev, A.A.; Mikhailov, V.S.; Yeryukova, Y.E.; Rubinovsky, G.A.; et al. Histone Deacetylases in the Process of Halisarca dujardini Cell Reaggregation. Rus. J. Dev. Biol. 2021, 52, 319–333. [Google Scholar] [CrossRef]
- Hubbert, C.; Guardiola, A.; Shao, R.; Kawaguchi, Y.; Ito, A.; Nixon, A.; Yoshida, M.; Wang, X.-F.; Yao, T.-P. HDAC6 is a microtubule-associated deacetylase. Nature 2002, 417, 455–458. [Google Scholar] [CrossRef]
- Miller, J.H.; Singh, A.J.; Northcote, P.T. Microtubule-Stabilizing Drugs from Marine Sponges: Focus on Peloruside A and Zampanolide. Mar. Drugs 2010, 8, 1059–1079. [Google Scholar] [CrossRef]
- Kanakkanthara, A.; Northcote, P.T.; Miller, J.H. Peloruside A: A lead non-taxoid-site microtubule stabilizing agent with potential activity against cancer, neurodegeneration, and autoimmune disease. Nat. Prod. Rep. 2016, 33, 549. [Google Scholar] [CrossRef]
- Souza, W. Prokaryotic cells: Structural organization of the cytoskeleton and organelles. Mem. Inst. Oswaldo Cruz. 2012, 107, 283–293. [Google Scholar] [CrossRef][Green Version]
- Kim, K.W. Prokaryotic cytoskeletons: In situ and ex situ structures and cellular locations. Antonie Van Leeuwenhoek 2019, 112, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Pogliano, J. The bacterial cytoskeleton. Curr. Opin. Cell Biol. 2008, 20, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Pilhofer, M.; Ladinsky, M.S.; McDowall, A.W.; Petroni, G.; Jensen, G.J. Microtubules in Bacteria: Ancient tubulins build a five-protofilament homolog of the eukaryotic cytoskeleton. PLoS Biol. 2011, 9, e1001213. [Google Scholar] [CrossRef] [PubMed]
- Montababa, E.; Agard, D.A. Bacterial tubulin TubZ-Bt transitions between a two-stranded intermediate and a fourstranded filament upon GTP hydrolysis. Proc. Natl. Acad. Sci. USA 2014, 111, 3407–3412. [Google Scholar] [CrossRef]
- Aylett, C.H.S.; Wang, Q.; Michie, K.A.; Amos, L.A.; Lowe, J. Filament structure of bacterial tubulin homologue TubZ. Proc. Natl. Acad. Sci. USA 2010, 107, 19766–19771. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golyshev, S.A.; Lyupina, Y.V.; Kravchuk, O.I.; Mikhailov, K.V.; Gornostaev, N.G.; Burakov, A.V. Transient Interphase Microtubules Appear in Differentiating Sponge Cells. Cells 2024, 13, 736. https://doi.org/10.3390/cells13090736
Golyshev SA, Lyupina YV, Kravchuk OI, Mikhailov KV, Gornostaev NG, Burakov AV. Transient Interphase Microtubules Appear in Differentiating Sponge Cells. Cells. 2024; 13(9):736. https://doi.org/10.3390/cells13090736
Chicago/Turabian StyleGolyshev, Sergei A., Yulia V. Lyupina, Oksana I. Kravchuk, Kirill V. Mikhailov, Nicolay G. Gornostaev, and Anton V. Burakov. 2024. "Transient Interphase Microtubules Appear in Differentiating Sponge Cells" Cells 13, no. 9: 736. https://doi.org/10.3390/cells13090736
APA StyleGolyshev, S. A., Lyupina, Y. V., Kravchuk, O. I., Mikhailov, K. V., Gornostaev, N. G., & Burakov, A. V. (2024). Transient Interphase Microtubules Appear in Differentiating Sponge Cells. Cells, 13(9), 736. https://doi.org/10.3390/cells13090736





