Abstract
Neurodegenerative diseases are chronic conditions occurring when neurons die in specific brain regions that lead to loss of movement or cognitive functions. Despite the progress in understanding the mechanisms of this pathology, currently no cure exists to treat these types of diseases: for some of them the only help is alleviating the associated symptoms. Mitochondrial dysfunction has been shown to be involved in the pathogenesis of most the neurodegenerative disorders. The fast and transient permeability of mitochondria (the mitochondrial permeability transition, mPT) has been shown to be an initial step in the mechanism of apoptotic and necrotic cell death, which acts as a regulator of tissue regeneration for postmitotic neurons as it leads to the irreparable loss of cells and cell function. In this study, we review the role of the mitochondrial permeability transition in neuronal death in major neurodegenerative diseases, covering the inductors of mPTP opening in neurons, including the major ones—free radicals and calcium—and we discuss perspectives and difficulties in the development of a neuroprotective strategy based on the inhibition of mPTP in neurodegenerative disorders.
1. Introduction
One of the biggest unsolved problems in modern medicine are neurodegenerative diseases, which, due to the aging of the population in most countries worldwide, have become a serious challenge for society. Two of the most common neurodegenerative disorders—Alzheimer’s disease and Parkinson’s disease—were described for the first time more than 100 and 200 years ago, respectively, and are still incurable [1,2]. However, significant progress in the understanding of the molecular and cellular mechanisms of neurodegeneration in the last few decades has been prompted by the discovery of the toxins which could lead to specific neuronal loss as well as the discovery of the genes causing familial forms of these diseases. This enables the generation of toxic and transgenic cellular and animal models of neurodegenerative diseases which aid not only in the study of the pathogenesis of the diseases but also serve as test platforms for potential therapeutic strategies. Although the clinical manifestation and molecular mechanisms of the pathology in each neurodegenerative disease are different, all of them include mitochondrial dysfunction and the involvement of protein misfolding in their etiopathology [3].
Mitochondrial dysfunction is strongly associated with Parkinson’s disease—mitochondrial toxins have been found to induce Parkinson’s disease in animals; moreover, proteins which are encoded by genes associated with Parkinson’s disease are mitochondrial or mitochondria-associated [3,4]. Various forms of mitochondrial dysfunction have also been found in Alzheimer’s disease, ALS, frontotemporal dementia and other neurological conditions [5,6,7,8]. However, the term “mitochondrial dysfunction” may include changes in a variety of functions in the mitochondria, from the alteration of the mitochondrial electron transport chain (ETC) to the overproduction of reactive oxygen species (ROS) [9] and defects in the mitochondrial Ca2+ transport [10,11]. The combination of cellular and mitochondrial pathological events triggers the mechanism of cell death which initiates with mitochondrial swelling and the release of pro-apoptotic proteins [12]. Considering this, neuronal cell death and neurodegeneration starts from a mitochondrial event known as the mitochondrial permeability transition, a non-selective permeability of the mitochondrial membrane which leads to the swelling of the mitochondria, followed by protein release (including cytochrome c) and subsequent cell death.
Despite much evidence for this pathway, the direct link between mPTP and neurodegeneration is still debatable for several reasons: (a) the nature of the mitochondrial permeability transition and the molecular structure of the pore are still controversial; (b) the detection of mPT in live cells or tissues is methodologically very difficult; (c) the threshold of the mPTP opening that triggers cell death, considering the possible role of mPTP in the physiology, is still under investigation.
The inability of neurons from the central nervous system to regenerate makes the clarification of the mechanism of apoptosis initiation vitally important for the development of neuroprotective strategies. Here, we review the possible structure of mPTP, the mechanism of its activation and discuss how this mitochondrial event leads to neuronal loss in the major neurodegenerative diseases.
2. Permeability Transition
The non-selective permeability of mitochondrial membranes (permeability transition, PT) is characterized by an increase in the permeability of mitochondrial membranes for various ions and substances whose molecular weight does not exceed 1500 Daltons (Da). This phenomenon is accompanied by the loss of membrane potential, the dissipation of the proton gradient across the inner membrane and mitochondrial swelling. Uncontrolled swelling leads to the rupture of the mitochondrial outer membrane. The phenomenon of mitochondrial swelling was discovered and actively studied in the 1950s and 1960s. Using isolated mitochondria, it was found that Ca2+, fatty acids, inorganic phosphate and substances that induce oxidative stress contribute to mitochondrial swelling, while Mg2+ and adenine nucleotides suppress this process [13,14,15,16,17,18]. In 1979, Haworth and Hunter characterized the main indicators of Ca2+-induced membrane transition and named the potential structure that triggers the mitochondrial PT a PT pore—mPTP [19,20,21]. However, the discovery of the mPTP inhibitor cyclosporine A (CsA) [22] helped to link this exclusively mitochondrial phenomenon to the physiology and pathology of the cells. CsA is a cyclic non-ribosomal polypeptide consisting of 11 amino acids produced by the soil fungus Beauveria nivea. CsA is widely used as an immunosuppressant, inhibiting peptidyl-prolyl cis–trans isomerases [23,24] as well as its mitochondrial form, cyclophilin D (CypD) [23]. Over the 70-year history of mPTP research, numerous activators and inhibitors of this pore have been discovered but CsA is still the most commonly used inhibitor of mPTP [25]. The size of the pore was identified using solutions of molecules with different sizes, while the electrophysiological assessment, based only on the inner membrane currents (using mitoplasts), helped to identify the current flowing through the pore [26]. For many researchers, the term mPTP is directly associated with pathology and cell death; however, transient mPTP opening (flickering) is an essential process for maintaining mitochondrial physiology, and is involved in the efflux of matrix Ca2+ and ROS [27].
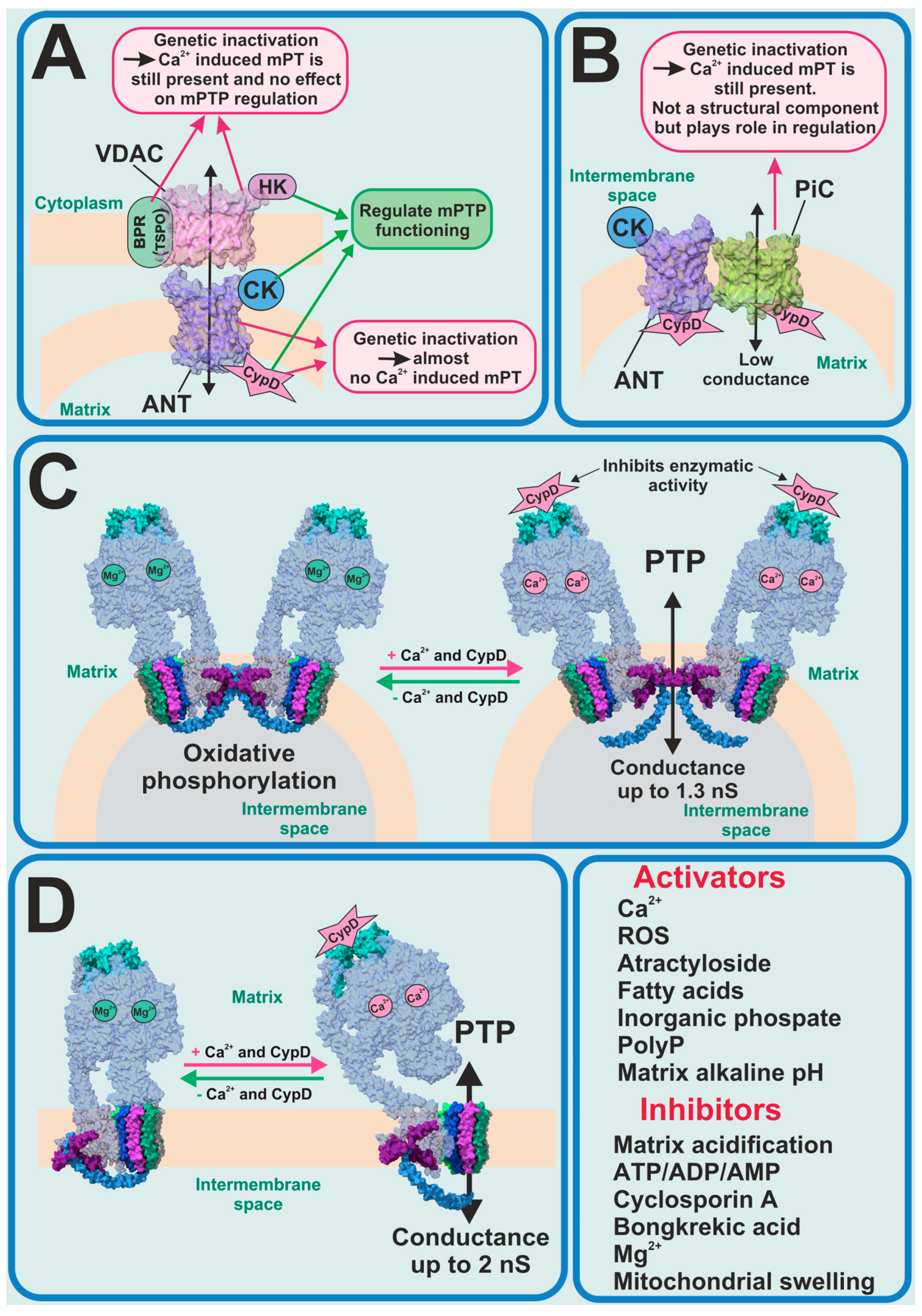
For several decades, the molecular nature of mPTP was unknown and scientists tried to identify the key pore-forming constituents of mPTP (Figure 1). The first candidates for this role were the ATP/ADP transporter (ANT) as a pore-forming protein in the inner membrane and the voltage-dependent anion channel (VDAC) as a pore-forming protein in the outer membrane. In this model, the translocator protein, hexokinase 2 (HK2), the mitochondrial creatine kinase (MtCK) and CypD were assigned a stabilizing and regulatory role (Figure 1A). A hypothesis about ANT involvement in the PTP formation was made due to the discovery that ANT inhibitors such as atractyloside and bongkrekic acid affect the PTP activity. Moreover, the molecular complex of ANT with VDAC, HK2, MtCK and CypD, isolated from the mitochondria and reconstituted into liposomes, have shown biophysical properties resembling those of PTP [28,29,30]. However, in one study, when genetically inactivated, the two isoforms of ANT could not inhibit calcium-dependent mPT [31]. The observed mPT may have been inhibited by CsA and activated by thiol oxidants. However, in 2019 it was shown that the genetic deletion of the three isoforms of ANT (Ant1, Ant2 and Ant4) and CypD abolished Ca2+-induced mPTP formation in mice mitochondria [32], which brought ANT back on the radar of scientists as a potential pore-forming component.
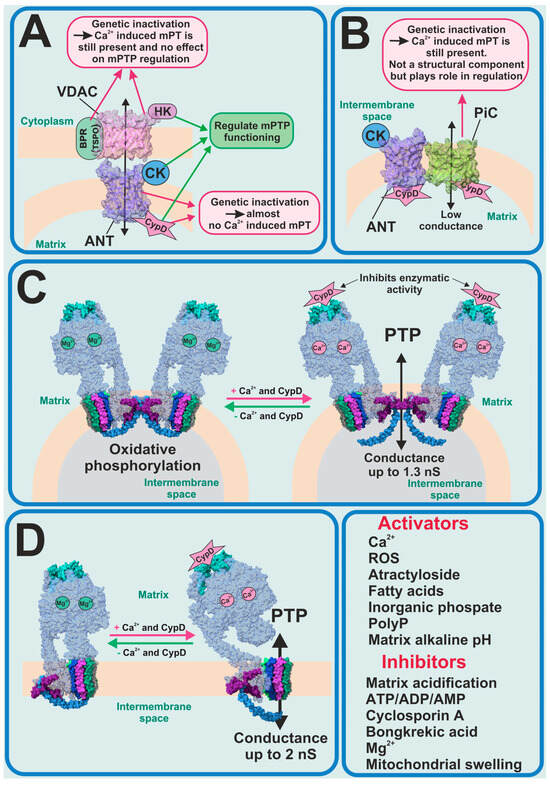
Figure 1.
Previous and current PTP candidates. (A) ANT–VDAC model of PTP—partially refuted; (B) mitochondria phosphate carrier model of PTP—refuted; FOF1-ATP synthase as candidate for PTP-forming protein in its dimeric (C) and monomeric (D) forms.
From the very beginning until now CypD has been the only protein whose forming role in the PTP phenomenon has been 100% proven. In this regard, when new experimental data shows the interaction of CypD with any mitochondrial inner membrane proteins, this protein immediately begins to be considered as a potential mPTP-forming protein. In 2008 it was found that CypD can directly interact with the phosphate carrier (PiC) and ANT; thus, PiC became the new PTP-forming candidate (Figure 1B) [33]. However, an earlier electrophysiological evaluation of PiC purified and reconstituted into giant liposomes showed that it is able to generate Ca2+-induced currents, with a mean conductance ranging from 25 to 40 pS in different experimental conditions, which is much lower compared to PTP currents previously observed [34]. Recent work, using genetic ablation, has shown that PiC is not an obligatory part of PTP, but certainly participates in its regulation [35]. Moreover, phosphate is one of the major regulators of the mPTP opening along with the endogenous inorganic polyphosphate (polyP). Thus, the reduction of the mitochondrial polyP by the overexpression of mitochondrially targeted polyphosphatase has been shown to inhibit Ca2+-induced but not ROS-induced PTP and to protect mammalian neurons and astrocytes from Ca2+-induced and beta-amyloid-induced cell death [36], which was also confirmed in isolated rat liver mitochondria [37]. It should be noted that long-chain polyPs induce mitochondrial depolarization and apoptotic cell death, which can be prevented by CsA [38].
One of the most recent and promising findings is that the mitochondrial F1FO-ATP synthase could potentially form the mPTP [39,40] (Figure 1C,D). However, despite the number of studies directly or indirectly supporting the hypothesis of PTP formation by the ATP synthase, it remains unclear how this complex enzyme switches from ATP synthase/ase into mPTP.
It should be noted that the potential mPT players are not restricted to mitochondrial proteins. Thus, palmitic acid not only induces CsA-dependent mPT but also initiates apoptosis [41,42].
However, despite the multiple candidates for molecular contributors to the mPTP complex or for serving as inductors or inhibitors of the pore opening, the evidence implicating mPTP in cellular and whole animal pathology remains unequivocally contingent upon Ca2+ or redox dynamics, which in turn are dependent on CsA and CypD. However, despite the fact that CsA is a very efficient CypD inhibitor, it is not used in clinics as an mPTP inhibitor. CsA is an FDA-approved immunosuppressive agent, which might be used during solid organ transplantations, for rheumatoid arthritis, psoriasis, ALS, nephrotic syndrome, graft vs. host disease, etc. Moreover, CsA is quite toxic, and its application has a lot of side effects, so research and development of new non-immunosuppressive CypD and mPTP inhibitors remains an important and challenging task. Therefore, in the pursuit of discovering novel inhibitors targeting CypD and mPTP, researchers have used various approaches, ranging from the large-scale screening of substances available in FDA-approved and other libraries [43,44] to the de novo development of new peptides [45]. Some of the new mPTP inhibitors (Ebselen) have already proved their efficiency in mouse models of Alzheimer’s disease [44].
3. Alzheimer’s Disease
The most common neurodegenerative disease, Alzheimer’s disease, is also the most common cause of dementia. Major histopathological features of this disease include extracellular senile plaques (consisting of the aggregated β-amyloid) and intracellular neurofibrillary tangles (formed by aggregated tau protein). Familial forms of the disease are caused by mutations in the amyloid precursor’s proteins or presenilins, leading to the misfolding and aggregation of β-amyloid; importantly, the aggregated β-amyloid is neurotoxic and for decades has been used for the induction of a cellular model of Alzheimer’s disease [46,47].
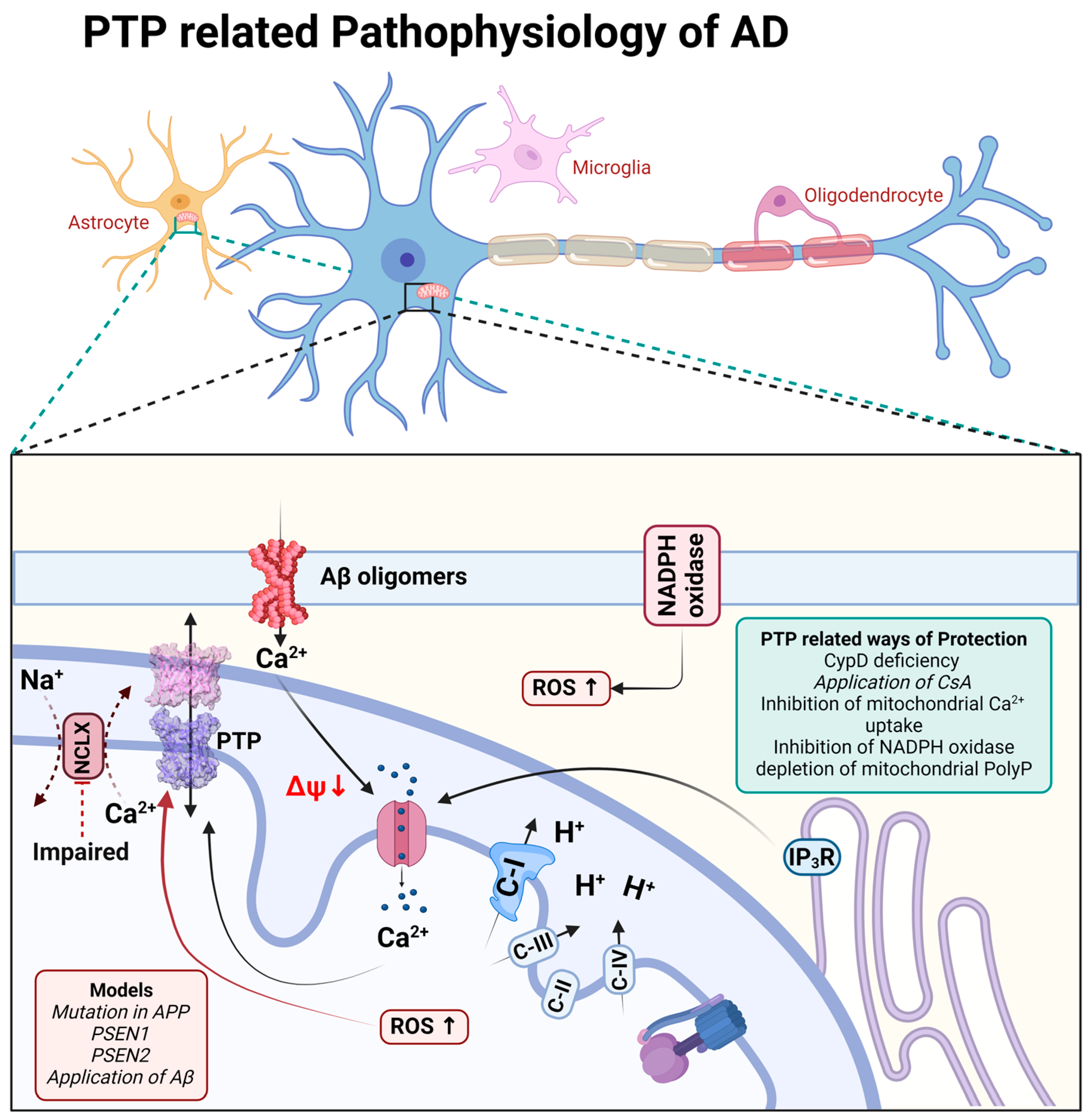
β-amyloid in aggregated form has been shown to be membrane-active and forms calcium channels in artificial membranes and in the membranes of astrocytes [48,49] as well as directly induces the opening of mPTP in experiments with isolated brain mitochondria [50,51]. In astrocytes, the combination of a β-amyloid-induced calcium signal and ROS overproduction has led to mitochondrial depolarization, which was dependent on CsA, inhibitors of mitochondrial calcium uptake, or it was blocked in astrocytes from CypD knockout mice [5,52,53].
It should be noted that the triggering of cell death through mPTP has also been demonstrated in the familial forms of Alzheimer’s disease. Thus, presenilin-1 mutation is linked to perturbated calcium homeostasis and oxyradical production, which trigger mPTP and induce caspases activation [54]; in addition, both presenilin-1- and presenilin-2-associated mutations increase the gain of function of IP3 receptors and the open probability of mPTP [55] (Figure 2).
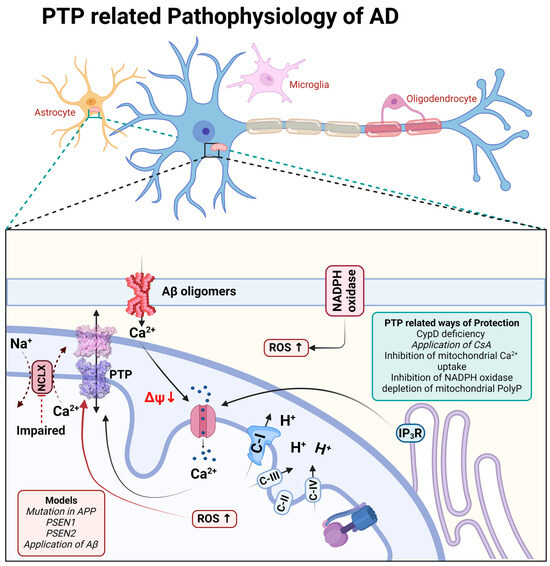
Figure 2.
Schematic representation of PTP-related pathophysiological processes during Alzheimer’s disease.
The role of mPTP in neuronal loss through Alzheimer’s disease is also shown in experiments with animal models of this disorder. Thus, CypD deficiency protects neuronal function and ameliorates learning and memory deficits in β-amyloid and APP-mutated mice [56,57]. Interestingly, CypD deficiency protects the function of FOF1-ATP synthase, one of the candidates for constituents of mPTP [58].
Importantly, the major trigger for mPTP—mitochondrial calcium overload—is connected to the inhibition of the mitochondrial calcium efflux in the pathology [10,59,60]. Importantly, the molecular inhibition of the mitochondrial calcium uptake mitigates the pathology in neurodegeneration [61], and the pharmacological sequestration of the mitochondrial calcium uptake protects against excitotoxicity [62] and dementia in Alzheimer’s disease models (Figure 2).
The induction of mPTP in Alzheimer’s disease requires several factors. Thus, despite the mitochondrial calcium overload, the inhibition of reactive oxygen species in NADPH oxidase, upon the application of β-amyloid, completely blocks mitochondrial depolarization [5]. However, the direct delivery of singlet oxygen to the mitochondria in live cells without mitochondrial calcium overload may not induce the opening of mPTP and cell death [53,63] (Figure 2).
Based on the importance of mPTP in the pathology of Alzheimer’s disease, a number of studies have focused on the study of novel molecules for the inhibition of the permeability transition and neuroprotection [44,64].
4. Parkinson’s Disease
Parkinson’s disease is the most common movement disorder and the second most common neurodegenerative disease. It is characterized by the loss of dopaminergic neurons in substantia nigra, as are all other neurodegenerative disorders associated with the deposition of misfolded proteins (i.e., aggregated α-synuclein) as intracellular inclusions, or Lewy bodies. More than any other neurodegenerative disorder, it is associated with mitochondria, due to the induction in Parkinson’s disease of mitochondrial toxins and mitochondrial localization, or the connection to proteins encoded by the Parkinson’s genes [3]. However, not all mitochondrial toxins or mutations in complex I lead to Parkinson’s disease [65,66].
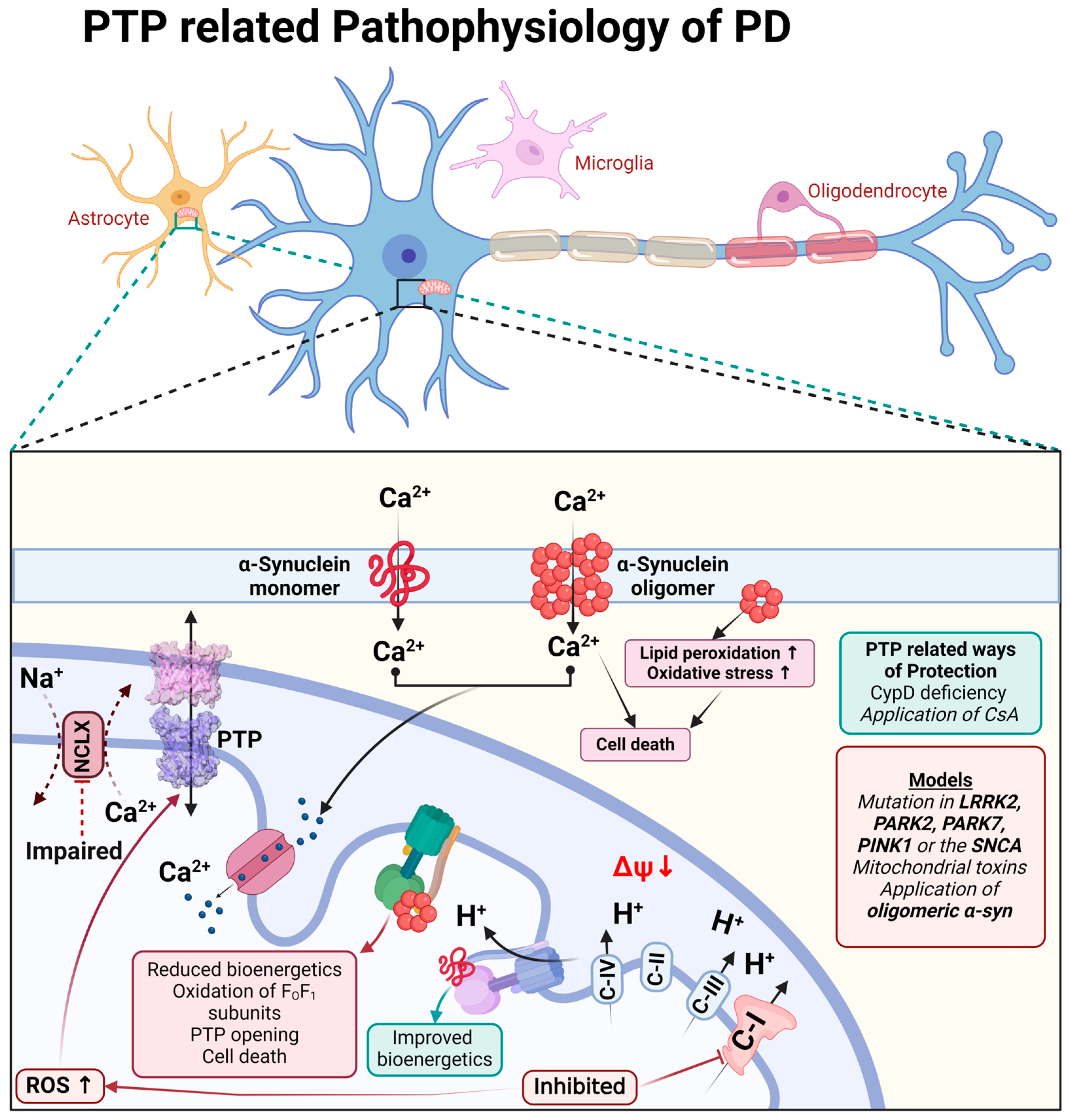
The physiological function of monomeric α-synuclein is connected to signal transduction but also to the function of the mitochondrial FOF1-ATP synthase. Binding to this enzyme, monomeric α-synuclein increases the efficiency of ATP synthesis [67]. Both monomeric and oligomeric (toxic) α-synucleins are able to incorporate themselves into the plasma membrane and induce a calcium signal [68,69]. However, only oligomeric α-synuclein (not fibrils or monomers) produces reactive oxygen species [69,70]. The oligomeric α-synuclein form, which can produce ROS, binds to the FO-F1-ATP synthase in the same way as physiological α-synuclein monomers and oxidases, an enzyme which triggers mPTP opening and cell death [71], and which also confirms the possible involvement of complex V in the mPTP structure (Figure 3).
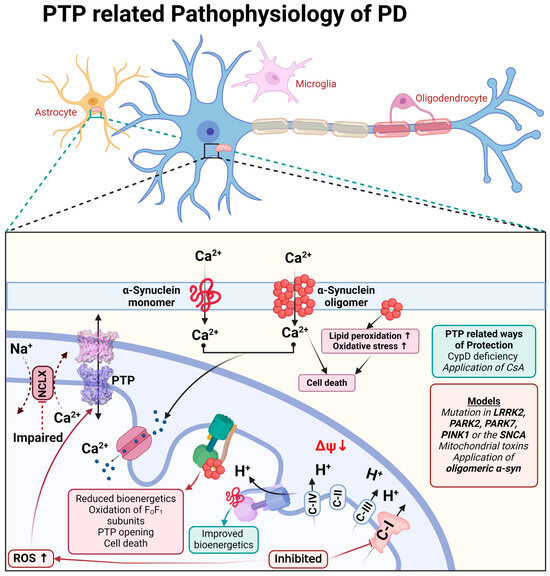
Figure 3.
Schematic representation of PTP-related pathophysiological processes during Parkinson’s disease.
The toxicity of the inhibitors of complex I which induce Parkinson’s disease can be partially blocked by CsA or CypD deficiency, confirming the involvement of permeability transition in neurodegeneration [72,73,74].
Familial forms of Parkinson’s disease—PINK1 and LRRK2 mutations—have slower mitochondrial calcium efflux due to the inhibition of the enzyme NCLX [75,76,77] that renders these cells vulnerable to dopamine due to calcium overload and ROS production, leading to mPTP opening [78] (Figure 3).
The familial forms of Parkinson’s disease show no strong phenotype in rodents but induce Parkinson’s-like movements in Drosophila. CypD mutation ameliorates oxidative stress-induced defects in a Drosophila DJ-1 null mutant that links the phenotype and neuronal loss to the mitochondrial permeability transition [79].
5. Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is associated with damage to upper and lower motor neurons in the cerebral cortex and spinal cord, as well as skeletal muscle dystrophy [80]. The prevalence of ALS, according to different data, ranges from 0.6 to 6 cases per 100,000 people, with a significant difference between ethnic groups [81]. The average age of diagnosis is 55–65 years, but familial forms are characterized by an earlier onset (47–52 years). The average life expectancy of patients is 2–3 years from the time of diagnosis. The rapidly developing symptomatology of the disease includes weakness, twitching, spasticity and respiratory failure. About 5–10% of registered cases of ALS are related to familial forms [82,83].
Mitochondrial dysfunction, including that which is associated with mPTP opening, is considered to be one of the key causes of death in both motor neurons and muscle cells in ALS [84,85]. Such ALS-related pathological processes as excitotoxicity [86] with a reduced calcium buffering capacity of the cytosol and mitochondria [87], overproduction of ROS and ONOO- [88], nitration of proteins including CypD and ANT [89], ATP level decrease, disturbances in mitochondrial dynamics [90], mitochondrial swelling and vacuolization [91], appear as triggers and consequences of mPTP opening [92]. Mouse models of ALS also show increased expression of mPTP-associated proteins (VDAC, ANT, CypD) with increased accumulation in mitochondria [89]. Moreover, these changes are often found in both motor neurons and muscle cells [93]. The neurotoxicity of the mutant protein TDP-43 penetrating into mitochondria may be related to the mPTP opening, mtDNA release and the activation of inflammation via the cGAS/STING pathway caused by increased mtROS production [94].
The role of mPTP is also confirmed by the change in pathology progression when modulating individual proteins influencing the pore. For example, mice with SOD1 mutations and lacking the Ppif gene encoding CypD showed improved mitochondrial function and morphology, decreased accumulation of the mutant protein and motor neuron death [87]. In the SOD1G93A model, the increased expression of Bcl-2 inhibiting mPTP opening resulted in a delayed onset of the pathology, as well as an increased lifespan of transgenic animals [95].
mPTP plays a dual role relative to cell life and death, and to date there is no definite explanation of the mPTP opening effect on the viability of motor neurons and muscle cells; moreover, some data are controversial. For a number of models, the classical pathway of apoptosis has been shown to increase the activity of caspase-1 and caspase-3 with a concomitant increase in CypD accumulation in defective mitochondria [96]. In addition, the ability to bind the anti-apoptotic protein Bcl-2 [97] and to increase the expression of pro-apoptotic proteins Bim and Bax [98] was revealed for mutant SOD1. A decreased rate of symptom development and an increased lifespan of SOD1-transgenic mice was obtained by the genetic knockout of Bim, Bax and Bak, and by the inhibition of caspases [99,100]. However, in cell models with the mutant TDP-43 protein, a relatively small increase in apoptotic cells was observed [101]. It has also been shown that despite the development of chromatin condensation, the morphology of dead cells differs from the classical pattern of apoptosis [91]. The changes in the mitochondrial state occurring in ALS, which may be associated with mPTP opening, in some cases do not lead to the development of apoptosis [88]; in particular, they do not activate caspase-1 and caspase-3 [91].
The efficacy of the ALS treatment strategies used in modern medical practice is extremely low. Riluzole [102], which reduces the effect of excitotoxicity, as well as the radical scavenger Edaravone [103], allow for delaying the progression of disease symptoms for several months. Therefore, the shown association of mPTP with ALS makes the pore an attractive therapeutic target.
Thus, the use of CypD inhibitors, in particular the administration of CsA (intraventricular or intraperitoneal with increasing permeability of the blood–brain barrier) can lead to an enhanced lifespan of SOD1G93A model animals. CsA also reduces mitochondrial depolarization in the motor neurons of SOD1G93A and SOD1G85R models under repetitive stimulation [104]. The stabilization of mitochondrial creatine kinase and the inhibition of mPTP opening may explain the improved motor function and increased survival of SOD1G93A-transgenic mice on a creatine-enriched diet [105]. The application of another mPTP inhibitor—GNX-4728—increased calcium retention capacity, decreased mitochondrial swelling and the motor neuron death rate and resulted in an almost two-fold increase in the lifespan of animals carrying the mutant G37R allele of the SOD1 gene [106]. The application of the mitochondrial-targeted neuroprotector Olesoxime (TRO19622) has also shown promising results with ALS cellular and animal models. The application of Olesoxime increased motoneuron viability, reduced glial cell degeneration, delayed the onset of the pathology phenotype and increased the survival of the animals [107]. Many authors suggest that the positive effect of Olesoxime on mPTP is related to its ability to bind to TSPO and VDAC [107]; however, previous research has shown that these proteins do not play any significant role in PTP [108,109]. Considering the nature of the motoneurons, mPTP opening is the initial trigger for neuronal loss in ALS.
6. Frontotemporal Dementia
Frontotemporal dementia (FTD) has a number of phenotypic manifestations (the most common are behavioral (bvFTD) and language variants (lvFTD)) [110,111] and is characterized by the selective degeneration of the frontal and/or temporal lobe [112]. FTD is the second cause of degenerative dementia after Alzheimer’s disease (AD), distinguished by an earlier onset with diagnosis between the ages of 45–65 years [113]. With a prevalence of 15–22 cases per 100,000 people [114], familial forms account for 30–50% of patients with FTD [112]. Often, fFTDs are associated with ALS and AD or other tauopathies-related genes [115,116]. Mutations in ABCA7, CTSF [117] and PGRN [118] are specific to FTD. The main published FTD research has been performed on cellular (iPSC-derived neurons in particular) and animal models mainly with mutations of the MAPT gene encoding tau protein.
One study on tau−/− mice, which showed improvement in the mitochondrial function and cognitive abilities of animals with a significant decrease in the level of CypD expression, indicates that there is a connection between tau and mPTP regulation and, consequently, cell viability [119]. Meanwhile, the presence of mutant tau in cells leads to pore opening conditions. These include oxidative stress, impaired calcium homeostasis, inflammation and excitotoxicity [120]. In particular, iPSC-derived neurons with a 10 + 16 MAPT mutation leading to FTD-associated protein splicing have been shown to exhibit increased ΔΨm due to complex V reversal mode, increased ROS production and finally cell death [6]. The primary neuroglial cultures treated with tau K18, as well as the iPSC-derived neurons with the 10 + 16 MAPT mutation, have shown impaired calcium homeostasis manifested in the oscillations of Ca2+ concentration in the cytoplasm, as well as in the decreased rate of Ca2+ efflux from mitochondria. As a result, the cells appear to be more sensitive to the mPTP opening-associated apoptosis stimulated by the calcium overload after physiological stimuli [60]. The significant reduction of apoptosis in the presence of MitoTempo suggests a key role for mitochondrial ROS in this process [121]. In addition to stimulating oxidative stress in cells with 10 + 16 MAPT mutation, mtROS lead to the increased expression of AMPA and NMDA receptors, which can also be reduced by mitochondrial antioxidants [122]. In a mouse model, it was shown that full-length tau with the A152T mutation causes NMDA receptor-related excitotoxicity with increased extracellular glutamate, which can be reduced by stimulating the astrocytic glutamate uptake via the EAAT2/GLT1 transporter [123]. An increase in intracellular calcium and subsequent neuronal death have also been shown for tau oligomers with the R406W mutation, which reduces the microtubule binding of tau, as well as for the 10 + 14C→T mutation, which increases the 4R/3R ratio [124].
FTD-related tau mutations, which result in altered 4R/3R ratios and reduce the protein’s ability to bind to the microtubules, increase the sensitivity of neurons to apoptosis associated with an increase in intracellular calcium [125]. Studies using selective inhibitors show a regulatory role of mutant tau with respect to L-type calcium channels [126].
The increase in caspase activity that occurs as a result of mPTP opening may itself lead to the increased neurotoxicity of tau. Neurotoxic fragments, particularly NH2-26-44, which form as a result of tau hydrolysis, bind to cytochrome c oxidase and ANT and lead to the uncoupling of oxidative phosphorylation [127].
At the same time, it should be noted that tau can also trigger alternative mechanisms of apoptosis with similarities to mPTP opening. Camilleri et al. [128] have shown that mitochondrial swelling, cytochrome c release and the loss of ΔΨm under the influence of mutant hTau46 were associated with an increase in mitochondrial membrane permeability due to the interaction of tau with cardiolipin [128]. Thus, mPTP opening marks the initial step in the neurodegeneration of FTD.
7. Huntington’s Disease
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disease, caused by a mutation that expands a specific sequence of nucleotides (CAG repeats) within exon 1 of the HTT gene. This mutation causes the production of an abnormal protein, the mutant huntingtin (mHtt), which can lead to the development of symptoms such as chorea, cognitive decline and psychiatric issues [129]. The mHtt is able to initiate a cascade of molecular damage processes, which eventually leads to mitochondrial dysfunction, ROS formation and increased oxidative stress [130,131,132]. The mitochondria in the striatum may be considered the place where various stimuli are integrated, including calcium homeostasis [3,133].
Increased mitochondrial calcium is one of the major factors for mPTP activation. Mitochondria isolated from cells obtained from both HD patients and HD mouse models are more sensitive to Ca2+ overloads, i.e., they have a significantly lower threshold required to trigger mPTP [134,135,136,137,138,139]. Choo et al. have demonstrated that mHtt directly induces the mPTP in isolated mouse liver mitochondria. This effect is completely prevented by CsA and exogenous ATP. It has been suggested that mHtt may promote mPTP induction by increasing the binding affinity of the ANT to mPTP activators, such as calcium and CsA, the main regulator of mPTP induction. It also prevents the binding of mPTP inhibitors, such as adenine nucleotides [135].
An artificial chromosome (YAC) transgenic mouse model of HD has also demonstrated the relationship between calcium signaling impairment and apoptosis in medium spiny neurons (MSN) in HD. The stimulation of glutamate receptors causes abnormal calcium reactions in human dopaminergic neurons (HD MSN) and cytosolic Ca2+ overload. Over time, the capacity of mitochondria to store Ca2+ is exceeded, leading to the opening of the mPTP, the release of cytochrome c into the cell and apoptosis [136].
Studies on mitochondria from mutant (STHdhQ111/Q111) huntingtin-expressing cells of striatal origin have shown that these cells have mitochondrial Ca2+ handling defects and that the increased sensitivity to Ca2+ induced mitochondrial permeabilization. At the same time, the mitochondrial dysfunction caused by Ca2+ overload can be reversed using CsA [138]. The study of the muscles and muscle mitochondria of 14-to-16-week-old R6/2 mice, as a model for HD, allowed for the confirmation of the decreased stability of HD mitochondria against the Ca2+-induced permeability transition [139].
Similar observations were obtained in neuronal cultures, in which it was shown that mHtt reduced the content of mitochondrial Ca2+ calcium by increasing the susceptibility to mPTP induction [140].
Gellerich et al. conducted research on the mitochondria in the brains of transgenic HD rats with the 51-glutamine repeat (htt 51Q) and found that the mitochondrial toxicity of htt 51Q appears to be affecting the regulatory binding sites of Ca2+-induced mitochondrial carrier proteins, such as Aralar and mPTP. This ultimately leads to energy depletion, cell death and tissue atrophy [137].
R. Quintanilla has made a significant contribution to our understanding of the role of mPTP in mitochondrial damage caused by mHtt. When observing isolated mitochondria from cell lines obtained from mutant huntingtin knock-in (HdhQ111/Q111) mice, it was found that mitochondrial dysfunction in these cells could be triggered by cumulative cytosolic Ca2+ increases. These effects could contribute to the progression of the striatal neuron death that is observed in HD. At the same time, a Ca2+ overload was discovered in these mutant cells, which led to the discovery of the mPTP. However, this event could have been prevented by pretreating the cells with CsA [141].
The neuroprotective effects of mPTP inhibitors have also proven their role in HD. The mitochondria of HD patients have elevated levels of CypD activity; moreover, CypD activity increases throughout the progression of the disease [142]. The application of CsA—a selective inhibitor of CypD and mPTP—has protective effects in a mouse model of the disease [143]. Additionally, the application of another mPTP inhibitor—GNX-4728—significantly reduced the level of apoptosis in mouse striatum cells (STHdh) expressing endogenous levels of the wild-type huntingtin (STHdh7/7) or mutant (STHdh111/111) proteins [144].
However, it should be mentioned that other researchers have not been able to detect a significant contribution of mPTP to mitochondrial damage in HD [145,146,147,148]. Discrepancies in the observed data may be attributed to the particular model being investigated, experimental research protocols or the necessity to explore additional mechanisms in Huntington’s disease pathogenesis, such as alterations in mitochondrial dynamics [137,149]. However, recent studies also show that presymptomatic YAC128 mouse striatal mitochondria have an altered morphology and Ca2+ handling [150].
In conclusion, it is important to note that the mechanisms leading to neuronal death in HD have not been fully elucidated, and the question of whether mHtt increases the sensitivity of brain mitochondria and, in particular, the mitochondria of neurons to Ca2+ -induced damage, remains open.
8. Epilepsy, Multiple Sclerosis and mtDNA Mutations
Epilepsy is one of the common neurological diseases. Epilepsy is a chronic neurological disorder characterized by unprovoked and repeated seizures that occurs in millions of people globally [151]. Oxidative stress and mitochondrial dysfunction are frequently observed in epilepsy [152]. Several forms of epilepsy are associated with impaired mitochondrial function and increased ROS generation [153]. Recently, it was shown that seizure-induced calcium oscillations in primary neuronal co-cultures negatively affect mitochondrial bioenergetics, and the application of CsA prevented seizure-induced mitochondrial depolarization, thus showing the involvement of mPTP in current mitochondrial dysfunction [154]. Another group showed that ketone bodies, which are the main effectors of anti-seizure effects in the ketogenic diet modulate mPTP by increasing the calcium retention capacity of brain mitochondria isolated from the Kcna1-null mice [155].
In addition, a possible connection has been shown between the increase in the resistance of mitochondria to the induction of the opening of the mPTP and the decrease in the severity of the convulsive state in pilocarpine-induced status epilepticus and the stimulation of neurogenesis in the adult brain of mice, which makes it possible to use mitoprotective strategies in the search for anticonvulsants and C [156,157,158].
Thus, it is now generally accepted that mitochondria and the process of mPTP opening are a key step in the initiation of cell death and, accordingly, a promising target in the search for neuroprotective drugs. In this regard, the use of mitochondria as a target for the creation of a new generation of drugs that can have a neuroprotective effect, as well as act as cognitive and metabolic stimulants, is of particular scientific interest and can be of great practical importance.
Multiple sclerosis (MS) is a demyelinating, inflammatory-mediated and neurodegenerative disease affecting the central nervous system. It causes loss of neurons and damage to axons, leading to the atrophy of the central nervous system and permanent neurological and clinical disability. It has been proven that the key factors of axonal degeneration are mitochondrial dysfunction and the irreversible opening of the mPPT [159]. As previously mentioned, CypD is a key regulator of the mPPT. Forte et al. have shown that the genetic inactivation of CypD reduces axonal damage and improves disease severity in an experimental autoimmune encephalomyelitis (EAE) model of MS. The model mice lacking CyPD developed EAE, but unlike the WT mice, they partially recovered [160]. Su et al. suggested that the physiological trigger for the opening of mPTP in MS may be exposure to ROS. In the presence of pathological levels of ROS, the expression of the adaptor protein p66shc in the mitochondrial intermembrane space was increased [161]. There, p66ShcA oxidizes cytochrome c and reduces oxygen to form mitochondrial ROS, which induces the opening of mPTP. At the same time, the genetic inactivation of p66ShcA has a neuroprotective effect in a murine model of MS [162]. The synthesis of a new mitochondrial Cyp inhibitor, JW47, based on the quinolinium cation associated with cyclosporine, has also confirmed the possibility of protection against neurodegeneration in experimental MS by inhibiting mPTP. When tested on an experimental EAE model, it was shown that JW47 is a highly effective inhibitor of Ca2+-mediated mPTP formation and has neuroprotective properties and slows down the accumulation of disability [163].
The close relationship between mitochondria and neurodegenerative diseases as well as the neurological symptoms of some other pathologies requires attention to an intrinsic cause of mitochondrial dysfunction. Mitochondrial DNA (mtDNA) mutations seem to be one the most important among them. mtDNA encodes 13 ETC polypeptides (seven of complex I, one of complex III, three of complex IV, two of F1FO-ATP synthase) as well as 22 tRNAs and two rRNAs required for protein synthesis, which makes mtDNA quality extremely significant for mitochondrial functions. Located close to the respiratory chain, mtDNA is permanently damaged by ROS mostly produced by complexes I and III that, due to the inefficient repair system, leads to the time-dependent accumulation of mtDNA mutation [164]. The latter correlates with the time of onset of age-related pathologies and may be considered one of the causes of sporadic forms of neurodegenerative diseases [165]. Mutations in mtDNA are known in the case of AD [166], PD [167], HD and ALS [168], multiple system atrophy and dementia with Lewy bodies [164], MELAS, LHON, MERRF, Leigh syndrome and encephalomyopathy [169]. It should be noted that the accumulation of mtDNA mutations can be brain region-specific [170].
Many of the consequences of mtDNA mutations (decreased ETC complexes content, OXPHOS efficiency, ATP formation, disturbance in mitochondrial calcium buffering, increased ROS production and oxidative stress development, apoptosis) [171,172] can lead to the mPTP opening. Thus, in analyzing AD brains, the authors of one study speculate about a higher sensibility to mPTP opening in cells harboring mutations of the mtDNA control region [166]. The mutations m.9821Adel (in MT-TR gene), m.T6589C (MT-COX1), m.13887Cins (MT-ND6) and m.G12273A (MT-ND5) in neurons were associated with ETC complexes deficiency and a change in Ca2+ handling [172]. The increased frequency of mtDNA damage and subsequent increase of apoptosis was shown in an HD in vitro model with mutant huntingtin expression [173]. In the mouse model mutant huntingtin was associated with a two-fold higher level of mtDNA mutations, mitochondrial calcium overload and elevated superoxide production that can lead to mPTP opening [174]. In SOD1-transgenic mice, damaged mtDNA was involved in subsequent motor neuron death [175]. According to the research [176], the influence of mtDNA mutations can be linked to the interactions of aberrant mitochondrially encoded proteins with pro- and anti-apoptotic Bak, Bax and Bcl-2, regulating outer mitochondrial membrane permeability and cytochrome c release.
Unfortunately, most of the models of neurodegenerative diseases used do not allow us to determine whether DNA mutations were the primary cause of the consequences, or if they are the result of another trigger influence.
9. Conclusions and Perspectives
There are no doubts about the involvement of mPTP in the mechanism of neurodegeneration. It acts as an initial trigger for apoptotic neuronal death, and some data suggest that the opening of mPTP may be induced in the processes of necrosis or ferroptosis after the initial damage of the plasma membrane. Considering this, the inhibition of mPTP is a promising therapeutic target for the prevention of neuronal loss in neurodegenerative disorders. Multiple in vitro and in vivo studies demonstrate neuroprotection by the molecular or pharmacological inhibition of the mPTP [177]. However, cyclosporine A was used in medicine for decades in patients after transplantation surgery and this treatment did not show any significant changes in the cognitive decline of the patients or even show an overall impaired cognitive function compared with controls [178]. The deficiency of CypD also induces an alteration in mitochondrial energy metabolism. On the other hand, some compounds which induce mPTP opening and the activation of apoptosis, such as the electrogenic calcium ionophore ferutinin [179,180], are used as selective estrogen receptor modulators, without any neurotoxic effects [181].
However, the development of a neuroprotective strategy using inhibitors of mPTP has a number of challenges that are connected to the uncertainty of the structure of the pore and thus to a delay in the development of more specific pharmacological agents. The opening of mPTP is the last step before the initiation of the process of cell death, and the prevention of the conditions that lead to permeability transition (mitochondrial calcium overload, ROS overproduction) may be a more efficient strategy for neuroprotection.
Author Contributions
Conceptualization, A.Y.A., A.Y.B., A.Y.V., P.R.A., E.V.P. and A.V.D.; writing—original draft preparation, A.Y.A., A.Y.B., A.Y.V., P.R.A., A.V.D. and E.V.P.; writing—review and editing, A.Y.A., A.V.D. and A.Y.B.; visualization, A.Y.A. and A.Y.B.; supervision, A.Y.A.; project administration, E.V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (Grant # 22-15-00317 (A.Y.V.)) and А-ИРВ-2021-465 (A.Y.B.) research grant of the Agency for Innovative Development under the Ministry of Higher Education, Science and Innovation of the Republic of Uzbekistan.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
ALS—amyotrophic lateral sclerosis; ANT—ATP/ADP transporter; CyPD—cyclophilin D; CsA—cyclosporine A; ETC—electron transport chain; FTD—frontotemporal dementia; HD—Huntington’s disease; mPT—mitochondrial permeability transition; mPTP—mitochondrial permeability transition pore; VDAC—voltage-dependent anion channel; ROS—reactive oxygen species; PiC—phosphate carrier; MtCK—mitochondrial creatine kinase.
References
- Spillantini, M.G.; Goedert, M. Neurodegeneration and the ordered assembly of alpha-synuclein. Cell Tissue Res. 2018, 373, 137–148. [Google Scholar] [CrossRef]
- Rollo, J.; Crawford, J.; Hardy, J. A dynamical systems approach for multiscale synthesis of Alzheimer’s pathogenesis. Neuron 2023, 111, 2126–2139. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Berezhnov, A.V.; Fedotova, E.I.; Zinchenko, V.P.; Dolgacheva, L.P. Interaction of misfolded proteins and mitochondria in neurodegenerative disorders. Biochem. Soc. Trans. 2017, 45, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H. Mitochondrial diseases. Lancet 2012, 379, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Canevari, L.; Duchen, M.R. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Esteras, N.; Rohrer, J.D.; Hardy, J.; Wray, S.; Abramov, A.Y. Mitochondrial hyperpolarization in iPSC-derived neurons from patients of FTDP-17 with 10+16 MAPT mutation leads to oxidative stress and neurodegeneration. Redox Biol. 2017, 12, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Ludtmann, M.H.R.; Arber, C.; Bartolome, F.; de Vicente, M.; Preza, E.; Carro, E.; Houlden, H.; Gandhi, S.; Wray, S.; Abramov, A.Y. Mutations in valosin-containing protein (VCP) decrease ADP/ATP translocation across the mitochondrial membrane and impair energy metabolism in human neurons. J. Biol. Chem. 2017, 292, 8907–8917. [Google Scholar] [CrossRef]
- Ghosh, R.; Wood-Kaczmar, A.; Dobson, L.; Smith, E.J.; Sirinathsinghji, E.C.; Kriston-Vizi, J.; Hargreaves, I.P.; Heaton, R.; Herrmann, F.; Abramov, A.Y.; et al. Expression of mutant exon 1 huntingtin fragments in human neural stem cells and neurons causes inclusion formation and mitochondrial dysfunction. FASEB J. 2020, 34, 8139–8154. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef]
- Abeti, R.; Abramov, A.Y. Mitochondrial Ca2+ in neurodegenerative disorders. Pharmacol. Res. 2015, 99, 377–381. [Google Scholar] [CrossRef]
- Baev, A.Y.; Vinokurov, A.Y.; Novikova, I.N.; Dremin, V.V.; Potapova, E.V.; Abramov, A.Y. Interaction of Mitochondrial Calcium and ROS in Neurodegeneration. Cells 2022, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.V.; Shilov, E.S.; Mufazalov, I.A.; Gogvadze, V.; Nedospasov, S.A.; Zhivotovsky, B. Cytochrome c: The Achilles’ heel in apoptosis. Cell Mol. Life Sci. 2012, 69, 1787–1797. [Google Scholar] [CrossRef]
- Azzi, A.; Azzone, G.F. Swelling and shrinkage phenomena in liver mitochondria. II. Low amplitude swelling-shrinkage cycles. Biochim. Biophys. Acta 1965, 105, 265–278. [Google Scholar] [CrossRef]
- Azzi, A.; Azzone, G.F. Swelling and shrinkage phenomena in liver mitochondria. I. Large amplitude swelling induced by inorganic phosphate and by ATP. Biochim. Biophys. Acta 1965, 105, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Azzone, G.F.; Azzi, A. Volume changes in liver mitochondria. Proc. Natl. Acad. Sci. USA 1965, 53, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Brenner-Holzach, O.; Raaflaub, J. Correlation between the swelling of isolated mitochondria and decomposition of intramitochondrial adenosine nucleotides (ATP, ADP, AMP, CoA). Helv. Physiol. Pharmacol. Acta 1954, 12, 242–252. [Google Scholar]
- Chappell, J.B.; Crofts, A.R. Calcium Ion Accumulation and Volume Changes of Isolated Liver Mitochondria. Calcium Ion-Induced Swelling. Biochem. J. 1965, 95, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Fluharty, A.L.; Sanadi, D.R. On the Mechanism of Oxidative Phosphorylation. IV. Mitochondrial Swelling Caused by Arsenite in Combination with 2,3-Dimercaptopropanol and by Cadmium Ion*. Biochemistry 1962, 1, 276–281. [Google Scholar] [CrossRef]
- Hunter, D.R.; Haworth, R.A. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch. Biochem. Biophys. 1979, 195, 453–459. [Google Scholar] [CrossRef]
- Haworth, R.A.; Hunter, D.R. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch. Biochem. Biophys. 1979, 195, 460–467. [Google Scholar] [CrossRef]
- Hunter, D.R.; Haworth, R.A. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch. Biochem. Biophys. 1979, 195, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Broekemeier, K.M.; Dempsey, M.E.; Pfeiffer, D.R. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J. Biol. Chem. 1989, 264, 7826–7830. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Hayano, T.; Suzuki, M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature 1989, 337, 473–475. [Google Scholar] [CrossRef]
- Handschumacher, R.E.; Harding, M.W.; Rice, J.; Drugge, R.J.; Speicher, D.W. Cyclophilin: A specific cytosolic binding protein for cyclosporin A. Science 1984, 226, 544–547. [Google Scholar] [CrossRef]
- Bonora, M.; Giorgi, C.; Pinton, P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell Biol. 2022, 23, 266–285. [Google Scholar] [CrossRef]
- Petronilli, V.; Szabo, I.; Zoratti, M. The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett. 1989, 259, 137–143. [Google Scholar] [CrossRef]
- Carraro, M.; Bernardi, P. The mitochondrial permeability transition pore in Ca2+ homeostasis. Cell Calcium 2023, 111, 102719. [Google Scholar] [CrossRef]
- McEnery, M.W.; Snowman, A.M.; Trifiletti, R.R.; Snyder, S.H. Isolation of the mitochondrial benzodiazepine receptor: Association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. USA 1992, 89, 3170–3174. [Google Scholar] [CrossRef] [PubMed]
- Beutner, G.; Ruck, A.; Riede, B.; Brdiczka, D. Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim. Biophys. Acta 1998, 1368, 7–18. [Google Scholar] [CrossRef]
- Ruck, A.; Dolder, M.; Wallimann, T.; Brdiczka, D. Reconstituted adenine nucleotide translocase forms a channel for small molecules comparable to the mitochondrial permeability transition pore. FEBS Lett. 1998, 426, 97–101. [Google Scholar] [CrossRef]
- Kokoszka, J.E.; Waymire, K.G.; Levy, S.E.; Sligh, J.E.; Cai, J.; Jones, D.P.; MacGregor, G.R.; Wallace, D.C. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 2004, 427, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Karch, J.; Bround, M.J.; Khalil, H.; Sargent, M.A.; Latchman, N.; Terada, N.; Peixoto, P.M.; Molkentin, J.D. Inhibition of mitochondrial permeability transition by deletion of the ANT family and CypD. Sci. Adv. 2019, 5, eaaw4597. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.W.; Varanyuwatana, P.; Halestrap, A.P. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J. Biol. Chem. 2008, 283, 26312–26323. [Google Scholar] [CrossRef] [PubMed]
- Herick, K.; Kramer, R.; Luhring, H. Patch clamp investigation into the phosphate carrier from Saccharomyces cerevisiae mitochondria. Biochim. Biophys. Acta 1997, 1321, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Aguilar, M.; Douglas, D.L.; Gibson, A.K.; Domeier, T.L.; Molkentin, J.D.; Baines, C.P. Genetic manipulation of the cardiac mitochondrial phosphate carrier does not affect permeability transition. J. Mol. Cell. Cardiol. 2014, 72, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Fraley, C.; Diao, C.T.; Winkfein, R.; Colicos, M.A.; Duchen, M.R.; French, R.J.; Pavlov, E. Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death. Proc. Natl. Acad. Sci. USA 2007, 104, 18091–18096. [Google Scholar] [CrossRef]
- Baev, A.Y.; Negoda, A.; Abramov, A.Y. Modulation of mitochondrial ion transport by inorganic polyphosphate—essential role in mitochondrial permeability transition pore. J. Bioenerg. Biomembr. 2017, 49, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Baev, A.Y.; Berezhnov, A.V.; Abramov, A.Y. Role of inorganic polyphosphate in mammalian cells: From signal transduction and mitochondrial metabolism to cell death. Biochem. Soc. Trans. 2016, 44, 40–45. [Google Scholar] [CrossRef]
- Giorgio, V.; von Stockum, S.; Antoniel, M.; Fabbro, A.; Fogolari, F.; Forte, M.; Glick, G.D.; Petronilli, V.; Zoratti, M.; Szabo, I.; et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. USA 2013, 110, 5887–5892. [Google Scholar] [CrossRef]
- Bonora, M.; Bononi, A.; De Marchi, E.; Giorgi, C.; Lebiedzinska, M.; Marchi, S.; Patergnani, S.; Rimessi, A.; Suski, J.M.; Wojtala, A.; et al. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle 2013, 12, 674–683. [Google Scholar] [CrossRef]
- Mironova, G.D.; Gritsenko, E.; Gateau-Roesch, O.; Levrat, C.; Agafonov, A.; Belosludtsev, K.; Prigent, A.F.; Muntean, D.; Dubois, M.; Ovize, M. Formation of palmitic acid/Ca2+ complexes in the mitochondrial membrane: A possible role in the cyclosporin-insensitive permeability transition. J. Bioenerg. Biomembr. 2004, 36, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Belosludtsev, K.; Saris, N.E.; Andersson, L.C.; Belosludtseva, N.; Agafonov, A.; Sharma, A.; Moshkov, D.A.; Mironova, G.D. On the mechanism of palmitic acid-induced apoptosis: The role of a pore induced by palmitic acid and Ca2+ in mitochondria. J. Bioenerg. Biomembr. 2006, 38, 113–120. [Google Scholar] [CrossRef]
- Peterson, A.A.; Rangwala, A.M.; Thakur, M.K.; Ward, P.S.; Hung, C.; Outhwaite, I.R.; Chan, A.; Usanov, D.L.; Mootha, V.K.; Seeliger, M.A.; et al. Discovery and molecular basis of subtype-selective cyclophilin inhibitors. Nat. Chem. Biol. 2022, 18, 1184. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Akhter, F.; Roy, A.; Chen, D.; Turner, B.; Wang, Y.; Clemente, N.; Wang, C.; Swerdlow, R.H.; Battaile, K.P.; et al. New cyclophilin D inhibitor rescues mitochondrial and cognitive function in Alzheimer’s disease. Brain 2023, 26, awad432. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, T.; Lai, X.; Xie, H.; Tang, H.; Wu, S.; Li, Y. Rational design peptide inhibitors of Cyclophilin D as a potential treatment for acute pancreatitis. Medicine 2023, 102, e36188. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Ionov, M.; Pavlov, E.; Duchen, M.R. Membrane cholesterol content plays a key role in the neurotoxicity of β-amyloid: Implications for Alzheimer’s disease. Aging Cell 2011, 10, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Arispe, N.; Rojas, E.; Pollard, H.B. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: Blockade by tromethamine and aluminum. Proc. Natl. Acad. Sci. USA 1993, 90, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Canevari, L.; Duchen, M.R. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 5088–5095. [Google Scholar] [CrossRef]
- Moreira, P.I.; Santos, M.S.; Moreno, A.; Rego, A.C.; Oliveira, C. Effect of amyloid beta-peptide on permeability transition pore: A comparative study. J. Neurosci. Res. 2002, 69, 257–267. [Google Scholar] [CrossRef]
- Bachurin, S.O.; Shevtsova, E.P.; Kireeva, E.G.; Oxenkrug, G.F.; Sablin, S.O. Mitochondria as a Target for Neurotoxins and Neuroprotective Agents. Ann. N. Y. Acad. Sci. 2003, 993, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Abeti, R.; Abramov, A.Y.; Duchen, M.R. β-amyloid activates PARP causing astrocytic metabolic failure and neuronal death. Brain 2011, 134, 1658–1672. [Google Scholar] [CrossRef]
- Shevtsova, E.F.; Angelova, P.R.; Stelmashchuk, O.A.; Esteras, N.; Vasil’eva, N.A.; Maltsev, A.V.; Shevtsov, P.N.; Shaposhnikov, A.V.; Fisenko, V.P.; Bachurin, S.O.; et al. Pharmacological sequestration of mitochondrial calcium uptake protects against dementia and β-amyloid neurotoxicity. Sci. Rep. 2022, 12, 12766. [Google Scholar] [CrossRef]
- Keller, J.N.; Guo, Q.; Holtsberg, F.W.; Bruce-Keller, A.J.; Mattson, M.P. Increased Sensitivity to Mitochondrial Toxin-Induced Apoptosis in Neural Cells Expressing Mutant Presenilin-1 Is Linked to Perturbed Calcium Homeostasis and Enhanced Oxyradical Production. J. Neurosci. 1998, 18, 4439–4450. [Google Scholar] [CrossRef]
- Toglia, P.; Ullah, G. The gain-of-function enhancement of IP3-receptor channel gating by familial Alzheimer’s disease-linked presenilin mutants increases the open probability of mitochondrial permeability transition pore. Cell Calcium 2016, 60, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Guo, L.; Fang, F.; Chen, D.; A Sosunov, A.; McKhann, M.G.; Yan, Y.; Wang, C.; Zhang, H.; Molkentin, J.D.; et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 2008, 14, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Guo, L.; Zhang, W.; Rydzewska, M.; Yan, S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol. Aging 2011, 32, 398–406. [Google Scholar] [CrossRef]
- Gauba, E.; Chen, H.; Guo, L.; Du, H. Cyclophilin D deficiency attenuates mitochondrial F1Fo ATP synthase dysfunction via OSCP in Alzheimer’s disease. Neurobiol. Dis. 2019, 121, 138–147. [Google Scholar] [CrossRef]
- Jadiya, P.; Kolmetzky, D.W.; Tomar, D.; Di Meco, A.; Lombardi, A.A.; Lambert, J.P.; Luongo, T.S.; Ludtmann, M.H.; Praticò, D.; Elrod, J.W. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat. Commun. 2019, 10, 3885. [Google Scholar] [CrossRef]
- Britti, E.; Ros, J.; Esteras, N.; Abramov, A.Y. Tau inhibits mitochondrial calcium efflux and makes neurons vulnerable to calcium-induced cell death. Cell Calcium 2020, 86, 102150. [Google Scholar] [CrossRef]
- Twyning, M.J.; Tufi, R.; Gleeson, T.P.; Kolodziej, K.M.; Campesan, S.; Terriente-Felix, A.; Collins, L.; De Lazzari, F.; Giorgini, F.; Whitworth, A.J. Partial loss of MCU mitigates pathology in vivo across a diverse range of neurodegenerative disease models. Cell Rep. 2024, 43, 113681. [Google Scholar] [CrossRef] [PubMed]
- Belosludtsev, K.N.; Sharipov, R.R.; Boyarkin, D.P.; Belosludtseva, N.V.; Dubinin, M.V.; Krasilnikova, I.A.; Bakaeva, Z.V.; Zgodova, A.E.; Pinelis, V.G.; Surin, A.M. The effect of DS16570511, a new inhibitor of mitochondrial calcium uniporter, on calcium homeostasis, metabolism, and functional state of cultured cortical neurons and isolated brain mitochondria. BBA-Gen Subj. 2021, 1865, 129847. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Vinogradova, D.; Neganova, M.E.; Serkova, T.P.; Sokolov, V.V.; Bachurin, S.O.; Shevtsova, E.F.; Abramov, A.Y. Pharmacological Sequestration of Mitochondrial Calcium Uptake Protects Neurons against Glutamate Excitotoxicity. Mol. Neurobiol. 2019, 56, 2244–2255. [Google Scholar] [CrossRef]
- Griffiths, K.K.; Wang, A.; Jonas, E.A.; Levy, R.J. Sulfide quinone oxidoreductase contributes to voltage sensing of the mitochondrial permeability transition pore. FASEB J. 2024, 38, e23494. [Google Scholar] [CrossRef] [PubMed]
- Greenamyre, J.T.; Sherer, T.B.; Betarbet, R.; Panov, A.V. Complex I and Parkinson’s Disease. IUBMB Life 2001, 52, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Angelova, P.R. Cellular mechanisms of complex I-associated pathology. Biochem. Soc. Trans. 2019, 47, 1963–1969. [Google Scholar] [CrossRef]
- Ludtmann, M.H.; Angelova, P.R.; Ninkina, N.N.; Gandhi, S.; Buchman, V.L.; Abramov, A.Y. Monomeric Alpha-Synuclein Exerts a Physiological Role on Brain ATP Synthase. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 10510–10521. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Ludtmann, M.H.R.; Horrocks, M.H.; Negoda, A.; Cremades, N.; Klenerman, D.; Dobson, C.M.; Wood, N.W.; Pavlov, E.V.; Gandhi, S.; et al. Ca2+ is a key factor in α-synuclein-induced neurotoxicity. J. Cell Sci. 2016, 129, 1792–1801. [Google Scholar] [CrossRef][Green Version]
- Angelova, P.R.; Abramov, A.Y. Alpha-synuclein and beta-amyloid—different targets, same players: Calcium, free radicals and mitochondria in the mechanism of neurodegeneration. Biochem. Biophys. Res. Commun. 2017, 483, 1110–1115. [Google Scholar] [CrossRef]
- Deas, E.; Cremades, N.; Angelova, P.R.; Ludtmann, M.H.; Yao, Z.; Chen, S.; Horrocks, M.H.; Banushi, B.; Little, D.; Devine, M.J.; et al. Alpha-Synuclein Oligomers Interact with Metal Ions to Induce Oxidative Stress and Neuronal Death in Parkinson’s Disease. Antioxid. Redox Signal. 2016, 24, 376–391. [Google Scholar] [CrossRef]
- Ludtmann, M.H.R.; Angelova, P.R.; Horrocks, M.H.; Choi, M.L.; Rodrigues, M.; Baev, A.Y.; Berezhnov, A.V.; Yao, Z.; Little, D.; Banushi, B.; et al. α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat. Commun. 2018, 9, 2293. [Google Scholar] [CrossRef]
- Seaton, T.A.; Cooper, J.M.; Schapira, A.H.V. Cyclosporin inhibition of apoptosis induced by mitochondrial complex I toxins. Brain Res. 1998, 809, 12–17. [Google Scholar] [CrossRef]
- Cassarino, D.S.; Parks, J.K.; Parker, W.D.; Bennett, J.P. The parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 1999, 1453, 49–62. [Google Scholar] [CrossRef]
- Thomas, B.; Banerjee, R.; Starkova, N.N.; Zhang, S.F.; Calingasan, N.Y.; Yang, L.; Wille, E.; Lorenzo, B.J.; Ho, D.J.; Beal, M.F.; et al. Mitochondrial Permeability Transition Pore Component Cyclophilin D Distinguishes Nigrostriatal Dopaminergic Death Paradigms in the MPTP Mouse Model of Parkinson’s Disease. Antioxid. Redox Signal. 2012, 16, 855–868. [Google Scholar] [CrossRef]
- Gandhi, S.; Wood-Kaczmar, A.; Yao, Z.; Plun-Favreau, H.; Deas, E.; Klupsch, K.; Downward, J.; Latchman, D.S.; Tabrizi, S.J.; Wood, N.W.; et al. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol. Cell 2009, 33, 627–638. [Google Scholar] [CrossRef]
- Ludtmann, M.H.R.; Kostic, M.; Horne, A.; Gandhi, S.; Sekler, I.; Abramov, A.Y. LRRK2 deficiency induced mitochondrial Ca2+ efflux inhibition can be rescued by Na+/Ca2+/Li+ exchanger upregulation. Cell Death Dis. 2019, 10, 265. [Google Scholar] [CrossRef]
- Kostic, M.; Ludtmann, M.H.R.; Bading, H.; Hershfinkel, M.; Steer, E.; Chu, C.T.; Abramov, A.Y.; Sekler, I. PKA Phosphorylation of NCLX Reverses Mitochondrial Calcium Overload and Depolarization, Promoting Survival of PINK1-Deficient Dopaminergic Neurons. Cell Rep. 2015, 13, 376–386. [Google Scholar] [CrossRef]
- Gandhi, S.; Vaarmann, A.; Yao, Z.; Duchen, M.R.; Wood, N.W.; Abramov, A.Y. Dopamine induced neurodegeneration in a PINK1 model of Parkinson’s disease. PLoS ONE 2012, 7, e375642012. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kang, K.-h.; Koh, H. Cyclophilin 1 (Cyp1) mutation ameliorates oxidative stress-induced defects in a Drosophila DJ-1 null mutant. Biochem. Biophys. Res. Commun. 2018, 505, 823–829. [Google Scholar] [CrossRef]
- Smith, E.F.; Shaw, P.J.; De Vos, K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2019, 710, 132933. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Cook, C.; Petrucelli, L. Genetic Convergence Brings Clarity to the Enigmatic Red Line in ALS. Neuron 2019, 101, 1057–1069. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Potapova, E.V.; Dremin, V.V.; Dunaev, A.V. Interaction of Oxidative Stress and Misfolded Proteins in the Mechanism of Neurodegeneration. Life 2020, 10, 101. [Google Scholar] [CrossRef]
- Belosludtseva, N.V.; Matveeva, L.A.; Belosludtsev, K.N. Mitochondrial Dyshomeostasis as an Early Hallmark and a Therapeutic Target in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2023, 24, 6833. [Google Scholar] [CrossRef]
- Van Den Bosch, L.; Van Damme, P.; Bogaert, E.; Robberecht, W. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2006, 1762, 1068–1082. [Google Scholar] [CrossRef]
- Parone, P.A.; Cruz, S.D.; Han, J.S.; McAlonis-Downes, M.; Vetto, A.P.; Lee, S.K.; Tseng, E.; Cleveland, D.W. Enhancing Mitochondrial Calcium Buffering Capacity Reduces Aggregation of Misfolded SOD1 and Motor Neuron Cell Death without Extending Survival in Mouse Models of Inherited Amyotrophic Lateral Sclerosis. J. Neurosci. 2013, 33, 4657–4671. [Google Scholar] [CrossRef]
- Martin, L.J.; Liu, Z.; Chen, K.; Price, A.C.; Pan, Y.; Swaby, J.A.; Golden, W.C. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: Mechanisms of mitochondriopathy and cell death. J. Comp. Neurol. 2007, 500, 20–46. [Google Scholar] [CrossRef]
- Martin, L.J.; Gertz, B.; Pan, Y.; Price, A.C.; Molkentin, J.D.; Chang, Q. The mitochondrial permeability transition pore in motor neurons: Involvement in the pathobiology of ALS mice. Exp. Neurol. 2009, 218, 333–346. [Google Scholar] [CrossRef]
- Sasaki, S.; Iwata, M. Mitochondrial Alterations in the Spinal Cord of Patients with Sporadic Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2007, 66, 10–16. [Google Scholar] [CrossRef]
- Martin, L.J. The mitochondrial permeability transition pore: A molecular target for amyotrophic lateral sclerosis therapy. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 186–197. [Google Scholar] [CrossRef]
- Ferraiuolo, L.; Kirby, J.; Grierson, A.J.; Sendtner, M.; Shaw, P.J. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 616–630. [Google Scholar] [CrossRef]
- Luo, G.; Yi, J.; Ma, C.; Xiao, Y.; Yi, F.; Yu, T.; Zhou, J. Defective Mitochondrial Dynamics Is an Early Event in Skeletal Muscle of an Amyotrophic Lateral Sclerosis Mouse Model. PLoS ONE 2013, 8, e82112. [Google Scholar] [CrossRef]
- Yu, C.-H.; Davidson, S.; Harapas, C.R.; Hilton, J.B.; Mlodzianoski, M.J.; Laohamonthonkul, P.; Louis, C.; Low, R.R.J.; Moecking, J.; De Nardo, D.; et al. TDP-43 Triggers Mitochondrial DNA Release via mPTP to Activate cGAS/STING in ALS. Cell 2020, 183, 636–649.e618. [Google Scholar] [CrossRef]
- Kostic, V.; Jackson-Lewis, V.; de Bilbao, F.; Dubois-Dauphin, M.; Przedborski, S. Bcl-2: Prolonging Life in a Transgenic Mouse Model of Familial Amyotrophic Lateral Sclerosis. Science 1997, 277, 559–563. [Google Scholar] [CrossRef]
- Pasinelli, P.; Borchelt, D.R.; Houseweart, M.K.; Cleveland, D.W.; Brown, R.H. Caspase-1 is activated in neural cells and tissue with amyotrophic lateral sclerosis-associated mutations in copper-zinc superoxide dismutase. Proc. Natl. Acad. Sci. USA 1998, 95, 15763–15768. [Google Scholar] [CrossRef]
- Pasinelli, P.; Belford, M.E.; Lennon, N.; Bacskai, B.J.; Hyman, B.T.; Trotti, D.; Brown, R.H. Amyotrophic Lateral Sclerosis-Associated SOD1 Mutant Proteins Bind and Aggregate with Bcl-2 in Spinal Cord Mitochondria. Neuron 2004, 43, 19–30. [Google Scholar] [CrossRef]
- Vukosavic, S.; Dubois-Dauphin, M.; Romero, N.; Przedborski, S. Bax and Bcl-2 Interaction in a Transgenic Mouse Model of Familial Amyotrophic Lateral Sclerosis. J. Neurochem. 1999, 73, 2460–2468. [Google Scholar] [CrossRef]
- Hetz, C.; Thielen, P.; Fisher, J.; Pasinelli, P.; Brown, R.H.; Korsmeyer, S.; Glimcher, L. The proapoptotic BCL-2 family member BIM mediates motoneuron loss in a model of amyotrophic lateral sclerosis. Cell Death Differ. 2007, 14, 1386–1389. [Google Scholar] [CrossRef]
- Soo, K.Y.; Atkin, J.D.; Farg, M.; Walker, A.K.; Horne, M.K.; Nagley, P. Bim Links ER Stress and Apoptosis in Cells Expressing Mutant SOD1 Associated with Amyotrophic Lateral Sclerosis. PLoS ONE 2012, 7, e35413. [Google Scholar] [CrossRef]
- Izumikawa, K.; Nobe, Y.; Yoshikawa, H.; Ishikawa, H.; Miura, Y.; Nakayama, H.; Nonaka, T.; Hasegawa, M.; Egawa, N.; Inoue, H.; et al. TDP-43 stabilises the processing intermediates of mitochondrial transcripts. Sci. Rep. 2017, 7, 7709. [Google Scholar] [CrossRef]
- Bensimon, G.; Lacomblez, L.; Meininger, V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N. Engl. J. Med. 1994, 330, 585–591. [Google Scholar] [CrossRef]
- Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet Neurol 2017, 16, 505–512. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Barrett, J.N.; Garcia-Chacon, L.; David, G.; Barrett, E.F. Repetitive nerve stimulation transiently opens the mitochondrial permeability transition pore in motor nerve terminals of symptomatic mutant SOD1 mice. Neurobiol. Dis. 2011, 42, 381–390. [Google Scholar] [CrossRef][Green Version]
- Klivenyi, P.; Ferrante, R.J.; Matthews, R.T.; Bogdanov, M.B.; Klein, A.M.; Andreassen, O.A.; Mueller, G.; Wermer, M.; Kaddurah-Daouk, R.; Beal, M.F. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat. Med. 1999, 5, 347–350. [Google Scholar] [CrossRef]
- Martin, L.J.; Fancelli, D.; Wong, M.; Niedzwiecki, M.; Ballarini, M.; Plyte, S.; Chang, Q. GNX-4728, a novel small molecule drug inhibitor of mitochondrial permeability transition, is therapeutic in a mouse model of amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2014, 8, 433. [Google Scholar] [CrossRef]
- Bordet, T.; Berna, P.; Abitbol, J.L.; Pruss, R.M. Olesoxime (TRO19622): A Novel Mitochondrial-Targeted Neuroprotective Compound. Pharmaceuticals 2010, 3, 345–368. [Google Scholar] [CrossRef]
- Baines, C.P.; Kaiser, R.A.; Sheiko, T.; Craigen, W.J.; Molkentin, J.D. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007, 9, 550–555. [Google Scholar] [CrossRef]
- Sileikyte, J.; Blachly-Dyson, E.; Sewell, R.; Carpi, A.; Menabo, R.; Di Lisa, F.; Ricchelli, F.; Bernardi, P.; Forte, M. Regulation of the mitochondrial permeability transition pore by the outer membrane does not involve the peripheral benzodiazepine receptor (Translocator Protein of 18 kDa (TSPO)). J. Biol. Chem. 2014, 289, 13769–13781. [Google Scholar] [CrossRef]
- Leroy, M.; Bertoux, M.; Skrobala, E.; Mode, E.; Adnet-Bonte, C.; Le Ber, I.; Bombois, S.; Cassagnaud, P.; Chen, Y.H.; Deramecourt, V.; et al. Characteristics and progression of patients with frontotemporal dementia in a regional memory clinic network. Alzheimers Res. Ther. 2021, 13, 19. [Google Scholar] [CrossRef]
- Mackenzie, I.R.A.; Neumann, M. Molecular neuropathology of frontotemporal dementia: Insights into disease mechanisms from postmortem studies. J. Neurochem. 2016, 138, 54–70. [Google Scholar] [CrossRef]
- Antonioni, A.; Raho, E.M.; Lopriore, P.; Pace, A.P.; Latino, R.R.; Assogna, M.; Mancuso, M.; Gragnaniello, D.; Granieri, E.; Pugliatti, M.; et al. Frontotemporal Dementia, Where Do We Stand? A Narrative Review. Int. J. Mol. Sci. 2023, 24, 11732. [Google Scholar] [CrossRef]
- Chu, M.; Liu, L.; Nan, H.T.; Jiang, D.M.; Wang, Y.H.; Rosa-Neto, P.; Piao, Y.S.; Wu, L.Y. Extremely Early-Onset Frontotemporal Dementia: A Case Report and Literature Review. J. Alzheimers Dis. 2022, 90, 1139–1151. [Google Scholar] [CrossRef]
- Onyike, C.U.; Diehl-Schmid, J. The epidemiology of frontotemporal dementia. Int. Rev. Psychiatr. 2013, 25, 130–137. [Google Scholar] [CrossRef]
- Anoar, S.; Woodling, N.S.; Niccoli, T. Mitochondria Dysfunction in Frontotemporal Dementia/Amyotrophic Lateral Sclerosis: Lessons From Models. Front. Neurosci. 2021, 15, 6076. [Google Scholar] [CrossRef]
- Benson, B.C.; Shaw, P.J.; Azzouz, M.; Highley, J.R.; Hautbergue, G.M. Proteinopathies as Hallmarks of Impaired Gene Expression, Proteostasis and Mitochondrial Function in Amyotrophic Lateral Sclerosis. Front Neurosci. 2021, 15, 3624. [Google Scholar] [CrossRef]
- Wagner, M.; Lorenz, G.; Volk, A.E.; Brunet, T.; Edbauer, D.; Berutti, R.; Zhao, C.; Anderl-Straub, S.; Bertram, L.; Danek, A.; et al. Clinico-genetic findings in 509 frontotemporal dementia patients. Mol. Psychiatr. 2021, 26, 5824–5832. [Google Scholar] [CrossRef]
- Rademakers, R.; Neumann, M.; Mackenzie, I.R. Advances in understanding the molecular basis of frontotemporal dementia. Nat. Rev. Neurol. 2012, 8, 423–434. [Google Scholar] [CrossRef]
- Jara, C.; Aranguiz, A.; Cerpa, W.; Tapia-Rojas, C.; Quintanilla, R.A. Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus. Redox Biol. 2018, 18, 279–294. [Google Scholar] [CrossRef]
- Gendron, T.F.; Petrucelli, L. The role of tau in neurodegeneration. Mol. Neurodegener. 2009, 4, 13. [Google Scholar] [CrossRef]
- Esteras, N.; Abramov, A.Y. Mitochondrial Calcium Deregulation in the Mechanism of Beta-Amyloid and Tau Pathology. Cells 2020, 9, 2135. [Google Scholar] [CrossRef]
- Esteras, N.; Kopach, O.; Maiolino, M.; Lariccia, V.; Amoroso, S.; Qamar, S.; Wray, S.; Rusakov, D.A.; Jaganjac, M.; Abramov, A.Y. Mitochondrial ROS control neuronal excitability and cell fate in frontotemporal dementia. Alzheimers Dement. 2022, 18, 318–338. [Google Scholar] [CrossRef]
- Decker, J.M.; Krüger, L.; Sydow, A.; Dennissen, F.J.A.; Siskova, Z.; Mandelkow, E.; Mandelkow, E.M. The Tau/A152T mutation, a risk factor for frontotemporal-spectrum disorders, leads to NR2B receptor-mediated excitotoxicity. EMBO Rep. 2016, 17, 552–569. [Google Scholar] [CrossRef] [PubMed]
- Imamura, K.; Sahara, N.; Kanaan, N.M.; Tsukita, K.; Kondo, T.; Kutoku, Y.; Ohsawa, Y.; Sunada, Y.; Kawakami, K.; Hotta, A.; et al. Calcium dysregulation contributes to neurodegeneration in FTLD patient iPSC-derived neurons. Sci. Rep. 2016, 6, 904. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; D’Souza, I.; Crudder, C.H.; Onodera, H.; Itoyama, Y.; Poorkaj, P.; Bird, T.D.; Schellenberg, G.D. Pro-apoptotic effects of tau mutations in chromosome 17 frontotemporal dementia and parkinsonism. Neuroreport 2000, 11, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Wang, Y.; Yao, P.J.; Fu, W.M.; Mattson, M.P.; Itoyama, Y.; Onodera, H.; D’Souza, I.; Poorkaj, P.H.; Bird, T.D.; et al. Alteration in calcium channel properties is responsible for the neurotoxic action of a familial frontotemporal dementia tau mutation. J. Neurochem. 2003, 87, 427–436. [Google Scholar] [CrossRef]
- Adante, A.; Amadoro, G.; Bobba, A.; de Bari, L.; Corsetti, V.; Pappalardo, G.; Marra, E.; Calissano, P.; Passarella, S. A peptide containing residues 26–44 of tau protein impairs mitochondrial oxidative phosphorylation acting at the level of the adenine nucleotide translocator. BBA-Bioenergetics 2008, 1777, 1289–1300. [Google Scholar] [CrossRef]
- Camilleri, A.; Ghio, S.; Caruana, M.; Weckbecker, D.; Schmidt, F.; Kamp, F.; Leonov, A.; Ryazanov, S.; Griesinger, C.; Giese, A.; et al. Tau-induced mitochondrial membrane perturbation is dependent upon cardiolipin. BBA-Biomembranes 2020, 1862, 64. [Google Scholar] [CrossRef]
- MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.A.; James, M.; Groot, N.; et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef]
- Kumar, A.; Ratan, R.R. Oxidative Stress and Huntington’s Disease: The Good, The Bad, and The Ugly. J. Huntingt. Dis. 2016, 5, 217–237. [Google Scholar] [CrossRef]
- Zheng, J.; Winderickx, J.; Franssens, V.; Liu, B. A Mitochondria-Associated Oxidative Stress Perspective on Huntington’s Disease. Front. Mol. Neurosci. 2018, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Carmo, C.; Naia, L.; Lopes, C.; Rego, A.C. Mitochondrial Dysfunction in Huntington’s Disease. Adv. Exp. Med. Biol. 2018, 1049, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, M.; Oliveros, J.C.; Naranjo, J.R.; Carafoli, E. Neuronal Ca(2+) dyshomeostasis in Huntington disease. Prion 2013, 7, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.V.; Gutekunst, C.A.; Leavitt, B.R.; Hayden, M.R.; Burke, J.R.; Strittmatter, W.J.; Greenamyre, J.T. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat. Neurosci. 2002, 5, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.S.; Johnson, G.V.; MacDonald, M.; Detloff, P.J.; Lesort, M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum. Mol. Genet. 2004, 13, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.S.; Slow, E.; Lupu, V.; Stavrovskaya, I.G.; Sugimori, M.; Llinas, R.; Kristal, B.S.; Hayden, M.R.; Bezprozvanny, I. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 2602–2607. [Google Scholar] [CrossRef]
- Gellerich, F.N.; Gizatullina, Z.; Nguyen, H.P.; Trumbeckaite, S.; Vielhaber, S.; Seppet, E.; Zierz, S.; Landwehrmeyer, B.; Riess, O.; von Horsten, S.; et al. Impaired regulation of brain mitochondria by extramitochondrial Ca2+ in transgenic Huntington disease rats. J. Biol. Chem. 2008, 283, 30715–30724. [Google Scholar] [CrossRef] [PubMed]
- Milakovic, T.; Quintanilla, R.A.; Johnson, G.V. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: Functional consequences. J. Biol. Chem. 2006, 281, 34785–34795. [Google Scholar] [CrossRef] [PubMed]
- Gizatullina, Z.Z.; Lindenberg, K.S.; Harjes, P.; Chen, Y.; Kosinski, C.M.; Landwehrmeyer, B.G.; Ludolph, A.C.; Striggow, F.; Zierz, S.; Gellerich, F.N. Low stability of Huntington muscle mitochondria against Ca2+ in R6/2 mice. Ann. Neurol. 2006, 59, 407–411. [Google Scholar] [CrossRef]
- Fernandes, H.B.; Baimbridge, K.G.; Church, J.; Hayden, M.R.; Raymond, L.A. Mitochondrial sensitivity and altered calcium handling underlie enhanced NMDA-induced apoptosis in YAC128 model of Huntington’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 13614–13623. [Google Scholar] [CrossRef]
- Quintanilla, R.A.; Jin, Y.N.; von Bernhardi, R.; Johnson, G.V. Mitochondrial permeability transition pore induces mitochondria injury in Huntington disease. Mol. Neurodegener. 2013, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Shirendeb, U.; Reddy, A.P.; Manczak, M.; Calkins, M.J.; Mao, P.; Tagle, D.A.; Reddy, P.H. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington’s disease: Implications for selective neuronal damage. Hum. Mol. Genet. 2011, 20, 1438–1455. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A. Neuroprotective effect of cyclosporine and FK506 against 3-nitropropionic acid induced cognitive dysfunction and glutathione redox in rat: Possible role of nitric oxide. Neurosci. Res. 2009, 63, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Amico, E.; Martinello, K.; Draghi, F.; Cavalletti, E.; Fucile, S.; Maglione, V.; Pardo, A.D. L14 Inhibition of mitochondrial permeability transition pore (MPTP), by GNX-4728, is beneficial in an in-vitro huntington’s disease model. J. Neurol. Neurosurg. Psychiatry 2016, 87, A94–A952016. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Jekabsons, M.B.; Chen, S.; Lin, A.; Rego, A.C.; Goncalves, J.; Ellerby, L.M.; Nicholls, D.G. Mitochondrial dysfunction in Huntington’s disease: The bioenergetics of isolated and in situ mitochondria from transgenic mice. J. Neurochem. 2007, 101, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Brustovetsky, N.; LaFrance, R.; Purl, K.J.; Brustovetsky, T.; Keene, C.D.; Low, W.C.; Dubinsky, J.M. Age-dependent changes in the calcium sensitivity of striatal mitochondria in mouse models of Huntington’s Disease. J. Neurochem. 2005, 93, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- De Mario, A.; Scarlatti, C.; Costiniti, V.; Primerano, S.; Lopreiato, R.; Cali, T.; Brini, M.; Giacomello, M.; Carafoli, E. Calcium Handling by Endoplasmic Reticulum and Mitochondria in a Cell Model of Huntington’s Disease. PLoS Curr. 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Pellman, J.J.; Hamilton, J.; Brustovetsky, T.; Brustovetsky, N. Ca(2+) handling in isolated brain mitochondria and cultured neurons derived from the YAC128 mouse model of Huntington’s disease. J. Neurochem. 2015, 134, 652–667. [Google Scholar] [CrossRef]
- Brustovetsky, N. Mutant Huntingtin and Elusive Defects in Oxidative Metabolism and Mitochondrial Calcium Handling. Mol. Neurobiol. 2016, 53, 2944–2953. [Google Scholar] [CrossRef]
- Lopes, C.; Ferreira, I.L.; Maranga, C.; Beatriz, M.; Mota, S.I.; Sereno, J.; Castelhano, J.; Abrunhosa, A.; Oliveira, F.; De Rosa, M.; et al. Mitochondrial and redox modifications in early stages of Huntington’s disease. Redox Biol. 2022, 56, 102424. [Google Scholar] [CrossRef]
- Tashakori-Miyanroudi, M.; Souresrafil, A.; Hashemi, P.; Jafar Ehsanzadeh, S.; Farrahizadeh, M.; Behroozi, Z. Prevalence of depression, anxiety, and psychological distress in patients with epilepsy during COVID-19: A systematic review. Epilepsy Behav. 2021, 125, 108410. [Google Scholar] [CrossRef] [PubMed]
- Madireddy, S.; Madireddy, S. Therapeutic Strategies to Ameliorate Neuronal Damage in Epilepsy by Regulating Oxidative Stress, Mitochondrial Dysfunction, and Neuroinflammation. Brain Sci. 2023, 13, 784. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Smith, J.N.; Patel, M. Metabolic Dysfunction and Oxidative Stress in Epilepsy. Int. J. Mol. Sci. 2017, 18, 2365. [Google Scholar] [CrossRef]
- Kovac, S.; Domijan, A.M.; Walker, M.C.; Abramov, A.Y. Prolonged seizure activity impairs mitochondrial bioenergetics and induces cell death. J. Cell Sci. 2012, 125, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Simeone, K.A.; Simeone, T.A.; Pandya, J.D.; Wilke, J.C.; Ahn, Y.; Geddes, J.W.; Sullivan, P.G.; Rho, J.M. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann. Neurol. 2015, 78, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kudin, A.P.; Debska-Vielhaber, G.; Vielhaber, S.; Elger, C.E.; Kunz, W.S. The Mechanism of Neuroprotection by Topiramate in an Animal Model of Epilepsy. Epilepsy 2004, 45, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Kovács, R.; Kardos, J.; Heinemann, U.; Kann, O. Mitochondrial Calcium Ion and Membrane Potential Transients Follow the Pattern of Epileptiform Discharges in Hippocampal Slice Cultures. J. Neurosci. 2005, 25, 4260–4269. [Google Scholar] [CrossRef]
- Neganova, M.E.; Blik, V.A.; Klochkov, S.G.; Chepurnova, N.E.; Shevtsova, E.F. Investigation of the Antioxidant Characteristics of a New Tryptamine Derivative of Securinine and its Influence on Seizure Activity in the Brain in Experimental Epilepsy. Neurochem. J. 2011, 5, 208–214. [Google Scholar] [CrossRef]
- Barrientos, S.A.; Martinez, N.W.; Yoo, S.; Jara, J.S.; Zamorano, S.; Hetz, C.; Twiss, J.L.; Alvarez, J.; Court, F.A. Axonal Degeneration Is Mediated by the Mitochondrial Permeability Transition Pore. J. Neuroscience. 2011, 31, 966–978. [Google Scholar] [CrossRef]
- Forte, M.; Gold, B.G.; Marracci, G.; Chaudhary, P.; Basso, E.; Johnsen, D.; Yu, X.; Fowlkes, J.; Rahder, M.; Stem, K.; et al. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2007, 104, 7558–7563. [Google Scholar] [CrossRef]
- Migliaccio, E.; Giorgio, M.; Mele, S.; Pelicci, G.; Reboidl, P.; Pandolfi, P.P.; Lanfrancone, L.; Pelicci, P.G. The p66 adaptor protein controls oxidative stress response and life span in mammals. Nature 1999, 402, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Su, K.G.; Savino, C.; Marracci, G.; Chaudhary, P.; Yu, X.L.; Morris, B.; Galipeau, D.; Giorgio, M.; Forte, M.; Bourdette, D. Genetic inactivation of the p66 isoform of ShcA is neuroprotective in a murine model of multiple sclerosis. Eur. J. Neurosci. 2012, 35, 562–571. [Google Scholar] [CrossRef]
- Warne, J.; Pryce, G.; Hill, J.M.; Shi, X.; Lennerås, F.; Puentes, F.; Kip, M.; Hilditch, L.; Walker, P.; Simone, M.I.; et al. Selective Inhibition of the Mitochondrial Permeability Transition Pore Protects against Neurodegeneration in Experimental Multiple Sclerosis. J. Biol. Chem. 2016, 291, 4356–4373. [Google Scholar] [CrossRef] [PubMed]
- Keogh, M.J.; Chinnery, P.F. Mitochondrial DNA mutations in neurodegeneration. BBA-Bioenergetics 2015, 1847, 1401–1411. [Google Scholar] [CrossRef]
- Bazzani, V.; Redin, M.E.; McHale, J.; Perrone, L.; Vascotto, C. Mitochondrial DNA Repair in Neurodegenerative Diseases and Ageing. Int. J. Mol. Sci. 2022, 23, 1391. [Google Scholar] [CrossRef]
- Coskun, P.E.; Beal, M.F.; Wallace, D.C. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc. Natl. Acad. Sci. USA 2004, 101, 10726–10731. [Google Scholar] [CrossRef]
- Bender, A.; Krishnan, K.J.; Morris, C.M.; Taylor, G.A.; Reeve, A.K.; Perry, R.H.; Jaros, E.; Hersheson, J.S.; Betts, J.; Klopstock, T.; et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006, 38, 515–517. [Google Scholar] [CrossRef]
- Cha, M.-Y.; Kim, D.K.; Mook-Jung, I. The role of mitochondrial DNA mutation on neurodegenerative diseases. Exp. Mol. Med. 2015, 47, e150. [Google Scholar] [CrossRef]
- Turnbull, H.E.; Lax, N.Z.; Diodato, D.; Ansorge, O.; Turnbull, D.M. The mitochondrial brain: From mitochondrial genome to neurodegeneration. BBA-Mol. Basis Dis. 2010, 1802, 111–121. [Google Scholar] [CrossRef]
- Soong, N.W.; Hinton, D.R.; Cortopassi, G.; Arnheim, N. Mosaicism for a Specific Somatic Mitochondrial-DNA Mutation in Adult Human Brain. Nat. Genet. 1992, 2, 318–323. [Google Scholar] [CrossRef]
- Khotina, V.A.; Vinokurov, A.Y.; Ekta, M.B.; Sukhorukov, V.N.; Orekhov, A.N. Creation of Mitochondrial Disease Models Using Mitochondrial DNA Editing. Biomedicines 2023, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Smulders-Srinivasan, T.K.; Kirby, D.M.; Acin-Perez, R.; Enriquez, J.A.; Lightowlers, R.N.; Duchen, M.R.; Turnbull, D.M. Mechanism of neurodegeneration of neurons with mitochondrial DNA mutations. Brain 2010, 133, 797–807. [Google Scholar] [CrossRef]
- Siddiqui, A.; Rivera-Sánchez, S.; Castro, M.d.R.; Acevedo-Torres, K.; Rane, A.; Torres-Ramos, C.A.; Nicholls, D.G.; Andersen, J.K.; Ayala-Torres, S. Mitochondrial DNA damage Is associated with reduced mitochondrial bioenergetics in Huntington’s disease. Free Radic. Biol. Med. 2012, 53, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Chen, Q.; Wang, X.H.; Wang, Q.C.; Wang, Y.; Cheng, H.P.; Guo, C.X.; Sun, Q.M.; Chen, Q.; Tang, T.S. Dysregulation of Mitochondrial Calcium Signaling and Superoxide Flashes Cause Mitochondrial Genomic DNA Damage in Huntington Disease. J. Biol. Chem. 2013, 288, 3070–3084. [Google Scholar] [CrossRef] [PubMed]
- Warita, H.; Hayashi, T.; Murakami, T.; Manabe, Y.; Abe, K. Oxidative damage to mitochondrial DNA in spinal motoneurons of transgenic ALS mice. Mol. Brain Res. 2001, 89, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Mott, J.L.; Zhang, D.K.; Zassenhaus, H.P. Mitochondrial DNA mutations, apoptosis, and the misfolded protein response. Rejuv. Res. 2005, 8, 216–226. [Google Scholar] [CrossRef]
- Uchino, H.; Hatakeyama, K.; Morota, S.; Tanoue, T.; Nishiyama, T.; Usui, D.; Taguchi, C.; Suzuki, M.; Hansson, M.J.; Elmér, E. Cyclophilin-D Inhibition in Neuroprotection: Dawn of a New Era of Mitochondrial Medicine. Brain Edema 2013, 15, 311–315. [Google Scholar]
- Pflugrad, H.; Schrader, A.K.; Tryc, A.B.; Ding, X.; Lanfermann, H.; Jäckel, E.; Schrem, H.; Beneke, J.; Barg-Hock, H.; Klempnauer, J.; et al. Longterm calcineurin inhibitor therapy and brain function in patients after liver transplantation. Liver Transplant. 2018, 24, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Duchen, M.R. Actions of ionomycin, 4-BrA23187 and a novel electrogenic Ca2+ ionophore on mitochondria in intact cells. Cell Calcium 2003, 33, 101–112. [Google Scholar] [CrossRef]
- Macho, A.; Blanco-Molina, M.; Spagliardi, P.; Appendino, G.; Bremner, P.; Heinrich, M.; Fiebich, B.L.; Muñoz, E. Calcium ionophoretic and apoptotic effects of ferutinin in the human Jurkat T-cell line. Biochem. Pharmacol. 2004, 68, 875–883. [Google Scholar] [CrossRef]
- Nazrullaev, S.S.; Khubaktova, Z.A.; Syrov, V.N.; Alieva, D.A.; Akhmetkhodzhaeva Kh, S. Experimental evaluation of the effect of a new medicinal form of tefestrol on reproduction in rats. Eksperimental’naia I Klin. Farmakol. 2006, 69, 35–39. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).