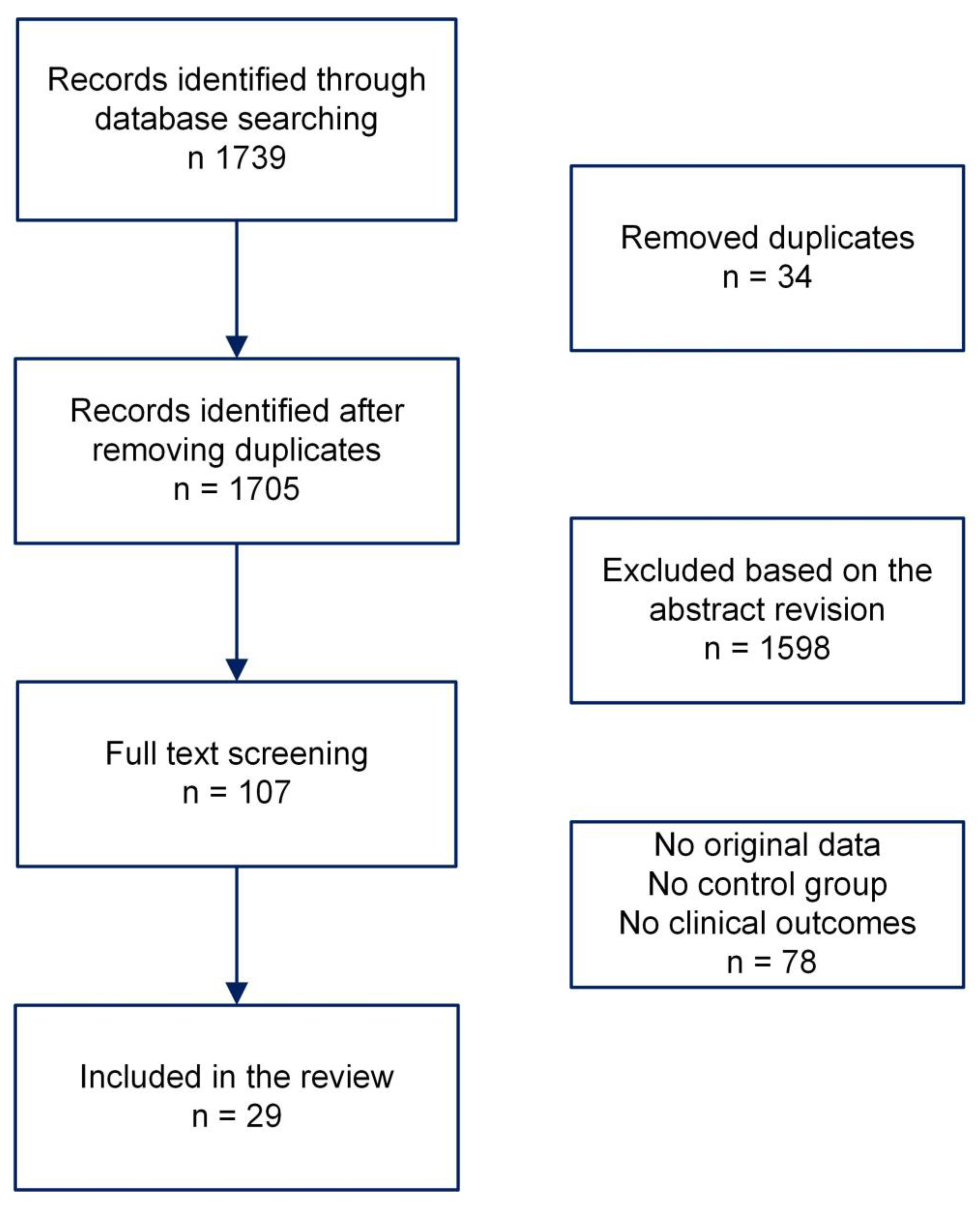
Pros and Cons of Cryopreserving Allogeneic Stem Cell Products
Abstract
1. Introduction
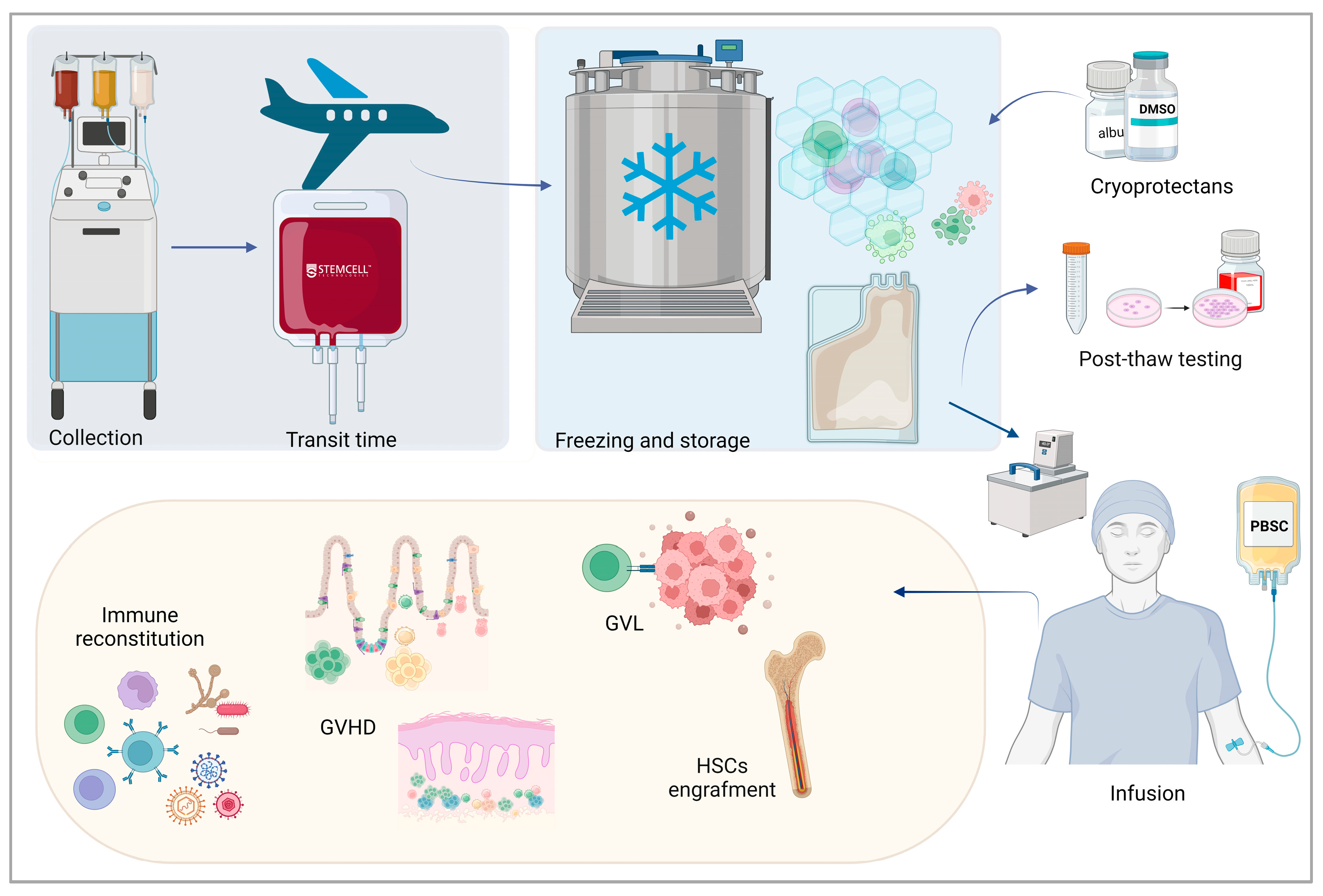
2. Basic Principles of Cryopreservation of Cells and Tissues
3. Cryopreservation of Hematopoietic Stem Cell Grafts
4. Clinical Impact of Allogenic Peripheral Blood Stem Cell Cryopreservation
| Authors [Ref] | Study Type | Disease/Donor Types | Patients (Graft Source) * | Controls (Graft Source) ** | aGvHD Cumulative Incidence (%) | cGvHD Cumulative Incidence (%) | Overall Survival (%) | Relapse Rate (%) | Other Findings |
|---|---|---|---|---|---|---|---|---|---|
| Kim DH et al., 2007 [86] | Single center | Malignant diseases/related donors | 105 (PBSCs) | 106 (PBSCs) | Grade II–IV Cryo 78.2 ± 4.3 Fresh 81.2 ± 4.5 | 1 year Cryo 83.8 ± 5.1 Fresh 90.6 ± 3.4 | 1 year Cryo 64.3 ± 5.1 Fresh 65.1 ± 4.6 2 year Cryo 52.7 ± 6.5 Fresh 59.4 ± 4.8 | 2 years Cryo 26.6 ± 5.8 Fresh 19.4 ± 4.3 | Lymphocyte recovery >0.5 × 109/L Cryo 22 days Fresh 22 days >1 × 109/L Cryo 33 days Fresh 33 days % 1 year NRM Cryo 24.6 ± 4.6 Fresh 20.4 ± 4.2 |
| Lioznov M et al., 2008 [87] | Single center | NR/related and unrelated donors | 39 (31 PBSCs, 8 BM) | 493 (PBSCs) | NR | NR | NR | NR | % GF in PBSCs Cryo 19 Fresh 1.4 |
| Medd P et al., 2013 [88] | Multicenter | Malignant diseases/related and unrelated donors | 76 (PBSCs) | 123 (PBSCs) | Day +100 Grade II–IV Cryo 31.7 Fresh 36.9 | 1 year Extensive Cryo 40.3 Fresh 28.3 | 2 years Cryo 45.3 Fresh 60.3 | 2 years Cryo 35.3 Fresh 36.9 | % 1-year TRM Cryo 14.6 Fresh 17.9 % 2 year RFS Cryo 41.9 Fresh 51.2 |
| Parody R et al., 2013 [89] | Multicenter | Malignant diseases/matched related donors | 224 (PBSCs) | 107 (PBSCs) | Day +100 Cryo 61.5 Fresh 44% p < 0.001 Grade II–IV Cryo 44 Fresh 30 | 3 years extensive Cryo 50 Fresh 42 | 3 years Cryo 58 Fresh 46 | Cryo 35% Fresh 40% | % Day +100 NRM Cryo 15 Fresh 9 % 1-year NRM Cryo 24 Fresh 16 |
| Eapen M et al., 2020 [90] | Multicenter | Aplastic anemia/related and unrelated donors | 52 (19 PBSCs, 33 BM) | 195 (63 PBSCs, 132 BM) | Day +100 Cryo 12 Fresh 13 | 1 year Cryo 23 Fresh 28 | 1 year Cryo 73 Fresh 91 p = 0.0008 Confirmed in PBSCs but not BM | NR | % 1-year GF Cryo 19 Fresh 10 p < 0.001 Confirmed in PBSCs but not BM |
| Hamadani M et al., 2020 [91] | Multicenter, CIBMTR database/propensity score matched | Malignant diseases with ptCy/related HLA- or haploidentical, and unrelated donors | 274 (256 PBSCs, 18 BM) | 1080 (1009 PBSCs, 71 BM) | Day +100 Grade II–IV Cryo 34 Fresh 31.3 | 1 year Cryo 26.8 Fresh 30.7 | 2 years Cryo 58.7 Fresh 60.6 p = 0.04 at regression analysis | 2 years relapse/ progression rate Cryo 36.3 Fresh 30.7 | % 2-year NRM Cryo 22.0 Fresh 19.0 % 2-year DFS Cryo 41.7 Fresh 50.4 p = 0.03 |
| Hsu JW et al., 2021 [92] | Multicenter, CIBMTR database | Malignant diseases/related and unrelated donors | 7397 (1051 related PBSCs; 678 unrelated PBSCs; 154 BM) | 5514 (3030 related PBSCs; 2028 unrelated PBSCs; 456BM) | Day +100 Grade II–IV Related PBSCs Cryo 35 Fresh 30 p = 0.01 Unrelated PBSCs Cryo 39 Fresh 40 BM Cryo 31 Fresh 33 | NR | No difference for BM and related PBSCs Unrelated PBSCs Cryo 57 Fresh 46 p < 0.001 | 2 years BM Cryo 31 Fresh 25 Related PBSCs Cryo 30 Fresh 31 Unelated PBSCs Cryo 28 Fresh 25 | In multivariate analysis Lower aGVHD in related cryo PBSCs similar TRM, OS, and PFS between cryo- and fresh grafts in BM and related PBSCs. In unrelated PBSC multivariate analysis confirmed lower OS (p < 0.001), lower PFS (p < 0.001), higher TRM (p < 0.001), and higher relapse rate (p = 0.002) |
| Dagdas S et al., 2020 [93] | Single-center | Malignant diseases/full-match sibling donors | 30 (PBSCs) | 42 (PBSCs) | Cryo 33.3 Fresh 28.6 | Cryo 40 Fresh 38.1 Less liver cGvHD in cryo p = 0.046 | 1 year Cryo 59 Fresh 60 3 years Cryo 54 Fresh 57 | Cryo 30 Fresh 16.7 | % Day +100 NRM Cryo 7 Fresh 19 % 1-year NRM Cryo 13 Fresh 22 % 3-year NRM Cryo 26 Fresh 30 |
| Authors [Ref] | Study Type | Disease/ Donor Types | Patients (Graft Source) * | Controls (Graft Source) ** | aGvHD Cumulative Incidence (%) | cGvHD Cumulative Incidence (%) | Overall Survival (%) | Relapse Rate (%) | Other Findings |
|---|---|---|---|---|---|---|---|---|---|
| Maurer K et al., 2021 [94] | Multicentric | Malignant diseases/matched related and unrelated and haploidentical donors | COVID-19 Cohort A 64 32 cryo 32 fresh (14 BM) | Pre-COVID-19 cohort B 68 4 cryo 64 fresh (12 BM) Pre-COVID-19 cohort C 76 4 cryo 72 fresh (21 BM) | Day +100 Grade II–IV Cohort A 10.9 Cohort B 16.2 Cohort C 9.2 Day 100 Grade III–IV Cohort A 6.2 Cohort B 1.5 Cohort C 1.3 | NR | Cohort A 92 Cohort B 94 Cohort C 95 | Day +100 Cohort A 9.4 Cohort B 11.8 Cohort C 17.1 | % day +30 WBC chimerism cryo 98 fresh 99 p < 0.001 % day +30 CD3 chimerism cryo 67 fresh 95 p = 0.01 % day +100 CD3 chimerism cryo 80 fresh 97 p = 0.03 |
| Maurer K et al., 2021 [54] | Single-center | Malignant diseases/unrelated donors | 101 (PBSCs) | 203 (PBSCs) | Day +100 Grade II–IV Cryo 17 Fresh 9 p = 0.014 Day +100 Grade III–IV Cryo 6 Fresh 4.5 | NR | Day +100 Cryo 96 Fresh 96.5 6 months Cryo 89 Fresh 89 | Day +100 Cryo 16 Fresh 12 6 months Cryo 22 Fresh 20 | % day +100 NRM Cryo 2 Fresh 2 % 6-month NRM Cryo 2 Fresh 4.6 More GF if infusion or cryopreservation > 48 h |
| Maurer K et al., 2023 [95] | Single-center | Malignant diseases/unrelated donors | 136 (PBSCs) | 251 (PBSCs) | 6 months Grade II–IV Cryo 25 Fresh 20 6-month Grade III–IV Cryo 12 Fresh 8 | 2 years Cryo 39 Fresh 57 p < 0.001 2 years Moderate/ severe Cryo 18 Fresh 31 p < 0.001 | 2 years Cryo 60 Fresh 65 | 2 years Cryo 34 Fresh 29 | % 2-year NRM Cryo 11 Fresh 12 |
| Valentini CG et al., 2022 [96] | Single-center | Malignant Diseases, related and unrelated donors | 32 (PBSCs) | 106 (PBSCs) | Day +100 Grade II–IV Cryo 23.8 Fresh 19.5 | NR | NR | NR | % 1-year NRM Cryo 7.7 Fresh 16.1 |
| Fernandez-Sojo J et al., 2021 [98] | Single-center | Malignant diseases/unrelated donors | 32 (PBSCs) | 32 (PBSCs) | Day +100 Cryo 41 Fresh 31 | NR | Day +100 Cryo 90 Fresh 81 | NR | Day +100 PFS Cryo 88 Fresh 81 |
| Alotaibi A et al., 2021 [99] | Single-center | Malignant diseases/related, unrelated donors | 310 (PBSCs) | 648 (PBSCs) | Grade II–IV Cryo 49 Fresh 50 | Moderate /severe Cryo 40 Fresh 27 p < 0.001 | 2 years Cryo 52 Fresh 49 | 2 year Cryo 23 Fresh 18 | % 2-year NRM Cryo 29 Fresh 36 p = 0.03 In patients without cGVHD, lower relapse incidence in fresh (HR = 0.67, p = 0.01) |
| Novitzy-Basso I et al., 2021 [100] | Single-center | Malignant diseases/ related and unrelated donors | 135 (PBSCs) | 348 (PBSCs) | Grade II–IV Cryo 47.0 Fresh 34.8 | Moderate/severe Cryo 12.6 Fresh 18.4 | 2 years Cryo 47.6 Fresh 79.4 p = 0.04 In ATG-PTCy cryo 51.9 fresh 65.5 p < 0.05 | 2 years Cryo 28.5 Fresh 23.2 | % 1-year NRM Cryo 20.0 Fresh 17.8 % 2-year GRFS Cryo 41.2 Fresh 51.4 p = 0.04 Higher NMR (p = 0.005) and lower GRFS in MRD cryo (p < 0.001) |
| Guo M et al., 2023 [101] | Single-center | Malignant diseases/related and unrelated donors | 34 (PBSCs) | 21 (PBSCs) | Grade II–IV Cryo 58.8 Fresh 42.9 | Moderate/severe Cryo 49.4 Fresh 9.5 | Day +100 Cryo 94.1 Fresh 100 p = 0.02 1 year Cryo 67.6 Fresh 90.4 | 15 months Cryo 44.1 Fresh 28.6 | % 1-year NRM Cryo 12.8% Fresh 6.3% |
| Facchin G et al., 2022 [102] | Single-center | Malignant diseases, matched unrelated donors | 31 (PBSCs) | 23 (PBSCs) | Grade II–IV aGVHD Cryo 56.5 Fresh 60.0 | NR | 1 year Cryo 80.7 Fresh 78.3 | NR | % 1-year TRM Cryo 13.0 Fresh 13.5 % 1-year PFS Cryo 71.0 Fresh 65.2 |
| Giammarco S et al., 2023 [103] | Single-center | Malignant diseases/ related and unrelated donors | 33 (28 PBSCs, 2 BM, 3 CBU) | 34 (17 PBSCs, 14 BM, 3 CBU) | NR | NR | Cryo 79 Fresh 82 | Cryo 29 Fresh 24 | % GF Cryo 6% Fresh 6% |
| Ersal T et al., 2023 [104] | Single center | Malignant diseases/full-match sibling donors | 37 (PBSCs) | 56 (PBSCs) | Cryo 37.8 Fresh 28.6 | Cryo 10.8 Fresh 10.7 | Day + 100 Cryo 75.7 Fresh 96.4 2 year Cryo 57.3 Fresh 67.9 | NR | % Day +100 PFS Cryo 94.6 Fresh 100 % 2-year PFS Cryo 82.8 Fresh 80.4 |
| Keyzner A et al., 2023 [105] | Single-center | Malignant diseases/ related and unrelated donors | 44 (31 PBSCs, 13 BM) | 37 (27 PBSCs, 10 BM) | NR | NR | NR | NR | No impact on chimerism |
| Laroye C et al., 2023 [106] | Single-center | Malignant diseases/related unrelated | 57 (PBSCs) | 19 (PBSCs) | Cryo 54 Fresh 20 | Cryo 79 Fresh 10 | Cryo 8 Fresh 5 | Cryo 12 Fresh 10 | Median CD34+ cell recovery in cryo 69.0% |
| Bankova A et al., 2022 [107] | Single-center | Malignant diseases/ related and unrelated donors | 30 (PBSCs) | 60 (PBSCs) | NR | NR | Lower in cryo (HR 2.16, 95% CI 1.00–4.67) p = 0.050 | NR | Higher NRM in cryo (HR 1.90, 95% CI 0.95–3.79) p = 0.071 |
| Connelly-Smith L et al., 2023 [109] | Single-center | Malignant diseases/ related and unrelated donors | 213 (PBSCs) | 167 (PBSCs) | Grade II–IV Cryo 55.9 Fresh 61.7 | 1 year Cryo 45 Fresh 40 | Similar OS in multivariate analysis in cryo- and fresh grafts | Similar RR in multivariate analysis in cryo- and fresh grafts | Similar NRM in multivariate analysis in cryo- and fresh grafts |
| Devine SM et al., 2023 [110] | Multicenter CIBMTR dataset | Malignant diseases/ related and unrelated donors | 1543 (1361 PBSCs, 182 BM) | 2499 (1834 PBSCs, 665 BM) | Day +100 Grade II–IV Cryo 36.0 Fresh 32.7 p = 0.042 | 1 year Moderate/severe Cryo 16.9 Fresh 19.8 p = 0.023 | 1 year Cryo 74.6 Fresh 76.9 | 1 year Cryo 22.2 Fresh 19.2% p = 0.042 | % 1-year DFS Cryo 63.2 Fresh 66.9 % 1-year NRM Cryo 14.7 Fresh 13.9 p = 0.027 |
5. Overview on the Evidence Provided by Clinical Studies
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kulkarni, U.; Devasia, A.J.; Korula, A.; Fouzia, N.A.; Nisham, P.N.; Samoon, Y.J.; Lakshmi, K.M.; Abraham, A.; Srivastava, A.; Mathews, V.; et al. Use of Non-Cryopreserved Peripheral Blood Stem Cells Is Associated with Adequate Engraftment in Patients with Multiple Myeloma Undergoing an Autologous Transplant. Biol. Blood Marrow Transplant. 2018, 24, e31–e35. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.B.; Salton, G.D.; Angeli, M.H.; Furlan, J.M.; Schmalfuss, T.; Röhsig, L.M. Effects of Cell Concentration, Time of Fresh Storage, and Cryopreservation on Peripheral Blood Stem Cells PBSC Fresh Storage and Cryopreservation. Transfus. Apher. Sci. 2022, 61, 103298. [Google Scholar] [CrossRef]
- Simpson, R.J.; Merli, P.; Gesù, B.; Manabe, A.; Gupta, A.O.; Wagner, J.E. Umbilical Cord Blood Transplants: Current Status and Evolving Therapies. Front. Pediatr. 2020, 8, 570282. [Google Scholar] [CrossRef]
- Liedtke, S.; Többen, S.; Gressmann, H.; Meyer, A.; Verde, P.E.; Gluckman, E.; Kogler, G. Long-Term Stability of Cord Blood Units After 29 Years of Cryopreservation: Follow-Up Data From the José Carreras Cord Blood Bank. Stem Cells Transl. Med. 2024, 13, 30–42. [Google Scholar] [CrossRef]
- Ljungman, P.; Mikulska, M.; de la Camara, R.; Basak, G.W.; Chabannon, C.; Corbacioglu, S.; Duarte, R.; Dolstra, H.; Lankester, A.C.; Mohty, M.; et al. The Challenge of COVID-19 and Hematopoietic Cell Transplantation; EBMT Recommendations for Management of Hematopoietic Cell Transplant Recipients, Their Donors, and Patients Undergoing CAR T-Cell Therapy. Bone Marrow Transplant. 2020, 55, 2071–2076. [Google Scholar] [CrossRef]
- Algwaiz, G.; Aljurf, M.; Koh, M.; Horowitz, M.M.; Ljungman, P.; Weisdorf, D.; Saber, W.; Kodera, Y.; Szer, J.; Jawdat, D.; et al. Real-World Issues and Potential Solutions in Hematopoietic Cell Transplantation during the COVID-19 Pandemic: Perspectives from the Worldwide Network for Blood and Marrow Transplantation and Center for International Blood and Marrow Transplant Research Health Services and International Studies Committee. Biol. Blood Marrow Transplant. 2020, 26, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.M. Transplantation of Allogeneic Cryopreserved Hematopoietic Cell Grafts during the COVID-19 Pandemic: A National Marrow Donor Program Perspective. Am. J. Hematol. 2021, 96, 169–171. [Google Scholar] [CrossRef]
- Murray, K.A.; Gibson, M.I. Chemical Approaches to Cryopreservation. Nat. Rev. Chem. 2022, 6, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P. Cryobiology: The Freezing of Biological Systems. Science 1970, 168, 939–949. [Google Scholar] [CrossRef]
- Wowk, B. Thermodynamic Aspects of Vitrification. Cryobiology 2010, 60, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Shinose, M.; Watanabe, H.; Yamada, S.; Kanda, Y. Cryoprotectant-free cryopreservation of mammalian cells by superflash freezing. Proc. Natl. Acad. Sci. USA 2019, 116, 7738–7743. [Google Scholar] [CrossRef]
- Manuchehrabadi, N.; Gao, Z.; Zhang, J.; Ring, H.L.; Shao, Q.; Liu, F.; McDermott, M.; Fok, A.; Rabin, Y.; Brockbank, K.G.M.; et al. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci. Transl. Med. 2017, 9, eaah4586. [Google Scholar] [CrossRef]
- Ito, A.; Yoshioka, K.; Masumoto, S.; Sato, K.; Hatae, Y.; Nakai, T.; Yamazaki, T.; Takahashi, M.; Tanoue, S.; Horie, M. Magnetic heating of nanoparticles as a scalable cryopreservation technology for human induced pluripotent stem cells. Sci. Rep. 2020, 10, 13605. [Google Scholar] [CrossRef]
- Rajan, R.; Matsumura, K. Development and Application of Cryoprotectants. Adv. Exp. Med. Biol. 2018, 1081, 339–354. [Google Scholar] [CrossRef]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A Review of the Actions and Applications of Cryoprotective Solutes That Modulate Cell Recovery from Ultra-Low Temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.M.; Van Buskirk Baust, J.G. Cell viability improves following inhibition of cryopreservation-induced apoptosis. In Vitro Cell Dev. Biol. Anim. 2000, 36, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.G.; Snyder, K.K.; Van Buskirk, R.; Baust, J.M. Integrating Molecular Control to Improve Cryopreservation Outcome. Biopreserv. Biobank. 2017, 15, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.M.; Snyder, K.K.; Van Buskirk, R.G.; Baust, J.G. Assessment of the Impact of Post-Thaw Stress Pathway Modulation on Cell Recovery Following Cryopreservation in a Hematopoietic Progenitor Cell Model. Cells 2022, 11, 278. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, N.; Gibson, M.I. Post-Thaw Application of ROCK-Inhibitors Increases Cryopreserved T-Cell Yield. RSC Med. Chem. 2023, 14, 2058–2067. [Google Scholar] [CrossRef]
- Karimi-Busheri, F.; Rasouli-Nia, A.; Weinfeld, M. Key Issues Related to Cryopreservation and Storage of Stem Cells and Cancer Stem Cells: Protecting Biological Integrity. Adv. Exp. Med. Biol. 2016, 951, 1–12. [Google Scholar] [CrossRef]
- Yang, J.; Diaz, N.; Adelsberger, J.; Zhou, X.; Stevens, R.; Rupert, A.; Metcalf, J.A.; Baseler, M.; Barbon, C.; Imamichi, T.; et al. The Effects of Storage Temperature on PBMC Gene Expression. BMC Immunol. 2016, 17, 6. [Google Scholar] [CrossRef]
- Baust, J.M.; Campbell, L.H.; Harbell, J.W. Best Practices for Cryopreserving, Thawing, Recovering, and Assessing Cells. Vitr. Cell. Dev. Biol. Anim. 2017, 53, 855–871. [Google Scholar] [CrossRef]
- Berz, D.; McCormack, E.M.; Winer, E.S.; Colvin, G.A.; Quesenberry, P.J. Cryopreservation of Hematopoietic Stem Cells. Am. J. Hematol. 2007, 82, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Wuchter, P. Processing, Cryopreserving and Controlling the Quality of HSCs. In The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies, 7th ed.; Carreras, E., Dufour, C., Mohty, M., Kröger, N., Eds.; Springer: Cham, Switzerland, 2019; Chapter 17. [Google Scholar]
- Cilloni, D.; Garau, D.; Regazzi, E.; Sammarelli, G.; Savoldo, B.; Caramatti, C.; Mangoni, L.; Rizzoli, V.; Carlo-Stella, C. Primitive Hematopoietic Progenitors within Mobilized Blood Are Spared by Uncontrolled Rate Freezing. Bone Marrow Transplant. 1999, 23, 497–503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Detry, G.; Calvet, L.; Straetmans, N.; Cabrespine, A.; Ravoet, C.; Bay, J.O.; Petre, H.; Paillard, C.; Husson, B.; Merlin, E.; et al. Impact of Uncontrolled Freezing and Long-Term Storage of Peripheral Blood Stem Cells at −80 °C on Haematopoietic Recovery after Autologous Transplantation. Report from Two Centres. Bone Marrow Transplant. 2014, 49, 780–785. [Google Scholar] [CrossRef]
- Montanari, M.; Capelli, D.; Poloni, A.; Massidda, D.; Brunori, M.; Spitaleri, L.; Offidani, M.; Lucesole, M.; Masia, M.C.; Balducci, F.; et al. Long-Term Hematologic Reconstitution after Autologous Peripheral Blood Progenitor Cell Transplantation: A Comparison between Controlled-Rate Freezing and Uncontrolled-Rate Freezing at 80 °C. Transfusion 2003, 43, 42–49. [Google Scholar] [CrossRef]
- Röllig, C.; Babatz, J.; Wagner, I.; Maiwald, A.; Schwarze, V.; Ehninger, G.; Bornhäuser, M. Thawing of Cryopreserved Mobilized Peripheral Blood—Comparison between Waterbath and Dry Warming Device. Cytotherapy 2002, 4, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Windrum, P.; Morris, T.; Drake, M.B.; Niederwieser, D.; Ruutu, T. Variation in Dimethyl Sulfoxide Use in Stem Cell Transplantation: A Survey of EBMT Centres. Bone Marrow Transplant. 2005, 36, 601–603. [Google Scholar] [CrossRef]
- Awan, M.; Buriak, I.; Fleck, R.; Fuller, B.; Goltsev, A.; Kerby, J.; Lowdell, M.; Mericka, P.; Petrenko, A.; Petrenko, Y.; et al. Dimethyl Sulfoxide: A Central Player since the Dawn of Cryobiology, Is Efficacy Balanced by Toxicity? Regen. Med. 2020, 15, 1463–1491. [Google Scholar] [CrossRef] [PubMed]
- Donmez, A.; Tombuloglu, M.; Gungor, A.; Soyer, N.; Saydam, G.; Cagirgan, S. Clinical Side Effects during Peripheral Blood Progenitor Cell Infusion. Transfus. Apher. Sci. 2007, 36, 95–101. [Google Scholar] [CrossRef]
- Madsen, B.K.; Hilscher, M.; Zetner, D.; Rosenberg, J. Adverse Reactions of Dimethyl Sulfoxide in Humans: A Systematic Review. F1000Research 2019, 7, 1746. [Google Scholar] [CrossRef]
- Shu, Z.; Heimfeld, S.; Gao, D. Hematopoietic SCT with Cryopreserved Grafts: Adverse Reactions after Transplantation and Cryoprotectant Removal before Infusion. Bone Marrow Transplant. 2014, 49, 469–476. [Google Scholar] [CrossRef]
- Kligman, A.M. Topical pharmacology and toxicology of dimethyl sulfoxide. 1. JAMA 1965, 193, 796–804. [Google Scholar] [CrossRef]
- Morris, C.; De Wreede, L.; Scholten, M.; Brand, R.; Van Biezen, A.; Sureda, A.; Dickmeiss, E.; Trneny, M.; Apperley, J.; Chiusolo, P.; et al. Should the Standard Dimethyl Sulfoxide Concentration Be Reduced? Results of a European Group for Blood and Marrow Transplantation Prospective Noninterventional Study on Usage and Side Effects of Dimethyl Sulfoxide. Transfusion 2014, 54, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Akkök, Ç.A.; Holte, M.R.; Tangen, J.M.; Østenstad, B.; Bruserud, Ø. Hematopoietic Engraftment of Dimethyl Sulfoxide-Depleted Autologous Peripheral Blood Progenitor Cells. Transfusion 2009, 49, 354–361. [Google Scholar] [CrossRef]
- Syme, R.; Bewick, M.; Stewart, D.; Porter, K.; Chadderton, T.; Glück, S. The Role of Depletion of Dimethyl Sulfoxide before Autografting: On Hematologic Recovery, Side Effects, and Toxicity. Biol. Blood Marrow Transplant. 2004, 10, 135–141. [Google Scholar] [CrossRef]
- Alencar, S.; Garnica, M.; Luiz, R.R.; Nogueira, C.M.; Borojevic, R.; Maiolino, A.; Dutra, H.S. Cryopreservation of Peripheral Blood Stem Cell: The Influence of Cell Concentration on Cellular and Hematopoietic Recovery. Transfusion 2010, 50, 2402–2412. [Google Scholar] [CrossRef]
- Mitrus, I.; Smagur, A.; Fidyk, W.; Czech, M.; Prokop, M.; Chwieduk, A.; Glowala-Kosinska, M.; Czerw, T.; Sobczyk-Kruszelnicka, M.; Mendrek, W.; et al. Reduction of DMSO Concentration in Cryopreservation Mixture from 10% to 7.5% and 5% Has No Impact on Engraftment after Autologous Peripheral Blood Stem Cell Transplantation: Results of a Prospective, Randomized Study. Bone Marrow Transplant. 2018, 53, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, R.; Jahan, S.; McGregor, C.; Pineault, N. Dimethyl Sulfoxide-Free Cryopreservation Solutions for Hematopoietic Stem Cell Grafts. Cytotherapy 2022, 24, 272–281. [Google Scholar] [CrossRef]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO Induces Drastic Changes in Human Cellular Processes and Epigenetic Landscape in Vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, K.; Yu, G.; McKenna, D.; Hubel, A. Cryopreservation of Hematopoietic Stem Cells: Emerging Assays, Cryoprotectant Agents, and Technology to Improve Outcomes. Transfus. Med. Hemotherapy 2019, 46, 188. [Google Scholar] [CrossRef]
- Rowley, S.; Anderson, G. Effect of DMSO Exposure without Cryopreservation on Hematopoietic Progenitor Cells. Bone Marrow Transplant. 1993, 11, 389–393. [Google Scholar]
- Calderwood, S.; Cecuiti, M.A.; Herst, R.; Solh, H. Hematopoietic Progenitor Cells Are Resistant to Dimethyl Sulfoxide Toxicity. Transfusion 1994, 34, 887–890. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, H.; Acker, J.P.; Liu, J.Z.; Akabutu, J.; McGann, L.E. Effect of Dimethyl Sulfoxide on Post-Thaw Viability Assessment of CD45+ and CD34+ Cells of Umbilical Cord Blood and Mobilized Peripheral Blood. Cryobiology 2005, 51, 165–175. [Google Scholar] [CrossRef]
- Hattori, Y.; Kato, H.; Nitta, M.; Takamoto, S. Decrease of L-Selectin Expression on Human CD34+ Cells on Freeze-Thawing and Rapid Recovery with Short-Term Incubation. Exp. Hematol. 2001, 29, 114–122. [Google Scholar] [CrossRef]
- Zyuz’kov, G.N.; Gur’yantseva, L.A.; Simanina, E.V.; Zhdanov, V.V.; Dygai, A.M.; Goldberg, E.D. Effect of Dimethylsulfoxide on the Functions of Mesenchymal and Hemopoietic Precursors. Bull. Exp. Biol. Med. 2007, 143, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Jarocha, D.; Zuba-Surma, E.; Majka, M. Dimethyl Sulfoxide (DMSO) Increases Percentage of CXCR4+ Hematopoietic Stem/Progenitor Cells, Their Responsiveness to an SDF-1 Gradient, Homing Capacities, and Survival. Cell Transplant. 2016, 25, 1247–1257. [Google Scholar] [CrossRef]
- Humpe, A.; Beck, C.; Schoch, R.; Kneba, M.; Horst, H.A. Establishment and Optimization of a Flow Cytometric Method for Evaluation of Viability of CD34+ Cells after Cryopreservation and Comparison with Trypan Blue Exclusion Staining. Transfusion 2005, 45, 1208–1213. [Google Scholar] [CrossRef]
- Rosskopf, K.; Ragg, S.J.; Worel, N.; Grommé, M.; Preijers, F.W.M.B.; Braakman, E.; Schuurhuis, G.J.; van Riet, I.; Wendel, S.; Fontão-Wendel, R.; et al. Quality Controls of Cryopreserved Haematopoietic Progenitor Cells (Peripheral Blood, Cord Blood, Bone Marrow). Vox Sang. 2011, 101, 255–275. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Kim, H.; Baek, E.J.; Jin, H.; Kim, J.; Kim, H.O. Post-Thaw Viable CD34+ Cell Count Is a Valuable Predictor of Haematopoietic Stem Cell Engraftment in Autologous Peripheral Blood Stem Cell Transplantation. Vox Sang. 2008, 94, 146–152. [Google Scholar] [CrossRef]
- Reich-Slotky, R.; Colovai, A.I.; Semidei-Pomales, M.; Patel, N.; Cairo, M.; Jhang, J.; Schwartz, J. Determining Post-Thaw CD34+ Cell Dose of Cryopreserved Haematopoietic Progenitor Cells Demonstrates High Recovery and Confirms Their Integrity. Vox Sang. 2008, 94, 351–357. [Google Scholar] [CrossRef]
- Lysak, D.; Brychtová, M.; Leba, M.; Čedíková, M.; Georgiev, D.; Jindra, P.; Vlas, T.; Holubova, M. Long-Term Cryopreservation Does Not Affect Quality of Peripheral Blood Stem Cell Grafts: A Comparative Study of Native, Short-Term and Long-Term Cryopreserved Haematopoietic Stem Cells. Cell Transplant. 2021, 30, 9636897211036004. [Google Scholar] [CrossRef]
- Maurer, K.; Kim, H.T.; Kuczmarski, T.M.; Garrity, H.M.; Weber, A.; Reynolds, C.G.; Liney, D.; Cutler, C.; Antin, J.H.; Koreth, J.; et al. Impact of Cryopreservation and Transit Times of Allogeneic Grafts on Hematopoietic and Immune Reconstitution. Blood Adv. 2021, 5, 5140–5149. [Google Scholar] [CrossRef]
- Purtill, D.; Antonenas, V.; Chiappini, P.; Tong, D.; O’flaherty, E.; Bajel, A.; Kabani, K.; Larsen, S.; Tan, S.; Hutchins, C.; et al. Variable CD34 1 Recovery of Cryopreserved Allogeneic HPC Products: Transplant Implications during the COVID-19 Pandemic. Blood Adv. 2020, 4, 4147–4150. [Google Scholar] [CrossRef]
- Fernandez-Sojo, J.; Horton, R.; Cid, J.; Azqueta, C.; Garcia-Buendia, A.; Valdivia, E.; Martorell, L.; Rubio-Lopez, N.; Codinach, M.; Aran, G.; et al. Leukocytapheresis Variables and Transit Time for Allogeneic Cryopreserved Hpc: Better Safe than Sorry. Bone Marrow Transplant. 2022, 57, 1531–1538. [Google Scholar] [CrossRef]
- Foïs, E.; Desmartin, M.; Benhamida, S.; Xavier, F.; Vanneaux, V.; Rea, D.; Fermand, J.P.; Arnulf, B.; Mounier, N.; Ertault, M.; et al. Recovery, Viability and Clinical Toxicity of Thawed and Washed Haematopoietic Progenitor Cells: Analysis of 952 Autologous Peripheral Blood Stem Cell Transplantations. Bone Marrow Transplant. 2007, 40, 831–835. [Google Scholar] [CrossRef]
- Reddy, O.L.; Sall, M.T.; Dinh, A.; Cai, Y.; Ongkeko, M.; Arya, N.; Wilder, J.; Tran, M.; Jin, P.; Stroncek, D.F.; et al. Effects of Extended Transport on Cryopreserved Allogeneic Hematopoietic Progenitor Cell (HPC) Product Quality and Optimal Methods to Assess HPC Stability. Transfusion 2023, 63, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Saraceni, F.; Shem-Tov, N.; Olivieri, A.; Nagler, A. Mobilized Peripheral Blood Grafts Include More than Hematopoietic Stem Cells: The Immunological Perspective. Bone Marrow Transplant. 2015, 50, 886–891. [Google Scholar] [CrossRef]
- Gu, G.; Yang, J.Z.; Sun, L.X. Correlation of Graft Immune Composition with Outcomes after Allogeneic Stem Cell Transplantation: Moving towards a Perfect Transplant. Cell. Immunol. 2018, 323, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pi, C.H.; Hornberger, K.; Dosa, P.; Hubel, A. Understanding the Freezing Responses of T Cells and Other Subsets of Human Peripheral Blood Mononuclear Cells Using DSMO-Free Cryoprotectants. Cytotherapy 2020, 22, 291–300. [Google Scholar] [CrossRef]
- Berens, C.; Heine, A.; Müller, J.; Held, S.A.E.; Mayer, K.; Brossart, P.; Oldenburg, J.; Pötzsch, B.; Wolf, D.; Rühl, H. Variable Resistance to Freezing and Thawing of CD34-Positive Stem Cells and Lymphocyte Subpopulations in Leukapheresis Products. Cytotherapy 2016, 18, 1325–1331. [Google Scholar] [CrossRef]
- Worsham, D.N.; Reems, J.A.; Szczepiorkowski, Z.M.; McKenna, D.H.; Leemhuis, T.; Mathew, A.J.; Cancelas, J.A. Clinical Methods of Cryopreservation for Donor Lymphocyte Infusions Vary in Their Ability to Preserve Functional T-Cell Subpopulations. Transfusion 2017, 57, 1555–1565. [Google Scholar] [CrossRef]
- Weinberg, A.; Song, L.Y.; Wilkening, C.; Sevin, A.; Blais, B.; Louzao, R.; Stein, D.; Defechereux, P.; Durand, D.; Riedel, E.; et al. Optimization and Limitations of Use of Cryopreserved Peripheral Blood Mononuclear Cells for Functional and Phenotypic T-Cell Characterization. Clin. Vaccine Immunol. 2009, 16, 1176–1186. [Google Scholar] [CrossRef]
- Owen, R.E.; Sinclair, E.; Emu, B.; Heitman, J.W.; Hirschkorn, D.F.; Epling, C.L.; Tan, Q.X.; Custer, B.; Harris, J.M.; Jacobson, M.A.; et al. Loss of T Cell Responses Following Long-Term Cryopreservation. J. Immunol. Methods 2007, 326, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cao, W.; Huang, L.; Xiao, M.; Cao, Y.; Zhao, L.; Wang, N.; Zhou, J. Effects of Cryopreservation on Chimeric Antigen Receptor T Cell Functions. Cryobiology 2018, 83, 40–47. [Google Scholar] [CrossRef]
- Panch, S.R.; Srivastava, S.K.; Elavia, N.; McManus, A.; Liu, S.; Jin, P.; Highfill, S.L.; Li, X.; Dagur, P.; Kochenderfer, J.N.; et al. Effect of Cryopreservation on Autologous Chimeric Antigen Receptor T Cell Characteristics. Mol. Ther. 2019, 27, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Castagna, L.; Sarina, B.; Bramanti, S.; Perseghin, P.; Mariotti, J.; Morabito, L. Donor lymphocyte infusion after allogeneic stem cell transplantation. Transfus. Apher. Sci. 2016, 54, 345–355. [Google Scholar] [CrossRef]
- Costantini, A.; Mancini, S.; Giuliodoro, S.; Butini, L.; Regnery, C.M.; Silvestri, G.; Montroni, M. Effects of Cryopreservation on Lymphocyte Immunophenotype and Function. J. Immunol. Methods 2003, 278, 145–155. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, P.; Liu, H.; Zhu, Z.; Li, C.; Gao, Y. The State of T Cells before Cryopreservation: Effects on Post-Thaw Proliferation and Function. Cryobiology 2017, 79, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Golab, K.; Leveson-Gower, D.; Wang, X.J.; Grzanka, J.; Marek-Trzonkowska, N.; Krzystyniak, A.; Millis, J.M.; Trzonkowski, P.; Witkowski, P. Challenges in Cryopreservation of Regulatory T Cells (Tregs) for Clinical Therapeutic Applications. Int. Immunopharmacol. 2013, 16, 371–375. [Google Scholar] [CrossRef]
- Florek, M.; Schneidawind, D.; Pierini, A.; Baker, J.; Armstrong, R.; Pan, Y.; Leveson-Gower, D.; Negrin, R.; Meyer, E. Freeze and Thaw of CD4 + CD25 + Foxp3 + Regulatory T Cells Results in Loss of CD62L Expression and a Reduced Capacity to Protect against Graft-versus-Host Disease. PLoS ONE 2015, 10, e0145763. [Google Scholar] [CrossRef]
- Mata, M.M.; Mahmood, F.; Sowell, R.T.; Baum, L.L. Effects of Cryopreservation on Effector Cells for Antibody Dependent Cell-Mediated Cytotoxicity (ADCC) and Natural Killer (NK) Cell Activity in 51Cr-Release and CD107a Assays. J. Immunol. Methods 2014, 406, 1–9. [Google Scholar] [CrossRef]
- El Assal, R.; Abou-Elkacem, L.; Tocchio, A.; Pasley, S.; Matosevic, S.; Kaplan, D.L.; Zylberberg, C.; Demirci, U. Bioinspired Preservation of Natural Killer Cells for Cancer Immunotherapy. Adv. Sci. 2019, 6, 1802045. [Google Scholar] [CrossRef]
- Lugthart, G.; Van Ostaijen-Ten Dam, M.M.; Van Tol, M.J.D.; Lankester, A.C.; Schilham, M.W. CD56dimCD16− NK Cell Phenotype Can Be Induced by Cryopreservation. Blood 2015, 125, 1842–1843. [Google Scholar] [CrossRef]
- Berg, M.; Lundqvist, A.; McCoy, P.; Samsel, L.; Fan, Y.; Tawab, A.; Childs, R. Clinical-Grade Ex Vivo-Expanded Human Natural Killer Cells up-Regulate Activating Receptors and Death Receptor Ligands and Have Enhanced Cytolytic Activity against Tumor Cells. Cytotherapy 2009, 11, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Mark, C.; Czerwinski, T.; Roessner, S.; Mainka, A.; Hörsch, F.; Heublein, L.; Winterl, A.; Sanokowski, S.; Richter, S.; Bauer, N.; et al. Cryopreservation Impairs 3-D Migration and Cytotoxicity of Natural Killer Cells. Nat. Commun. 2020, 11, 5224. [Google Scholar] [CrossRef]
- Lewalle, P.; Rouas, R.; Lehmann, F.; Martiat, P. Freezing of Dendritic Cells, Generated from Cryopreserved Leukaphereses, Does Not Influence Their Ability to Induce Antigen-Specific Immune Responses or Functionally React to Maturation Stimuli. J. Immunol. Methods 2000, 240, 69–78. [Google Scholar] [CrossRef]
- Waller, E.K.; Logan, B.R.; Harris, W.A.C.; Devine, S.M.; Porter, D.L.; Mineishi, S.; McCarty, J.M.; Gonzalez, C.E.; Spitzer, T.R.; Krijanovski, O.I.; et al. Improved Survival after Transplantation of More Donor Plasmacytoid Dendritic or Naïve T Cells from Unrelated-Donor Marrow Grafts: Results from BMTCTN 0201. J. Clin. Oncol. 2014, 32, 2365–2372. [Google Scholar] [CrossRef]
- Weng, L.; Beauchesne, P.R. Dimethyl Sulfoxide-Free Cryopreservation for Cell Therapy: A Review. Cryobiology 2020, 94, 9–17. [Google Scholar] [CrossRef]
- Yao, X.; Matosevic, S. Cryopreservation of NK and T Cells Without DMSO for Adoptive Cell-Based Immunotherapy. BioDrugs 2021, 35, 529–545. [Google Scholar] [CrossRef]
- Das, S.; Niemeyer, E.; Leung, Z.A.; Fritsch, T.; Matosevic, S. Human Natural Killer Cells Cryopreserved without DMSO Sustain Robust Effector Responses. Mol. Pharm. 2024, 21, 651–660. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Stockschläder, M.; Hassan, H.T.; Krog, C.; Krüger, W.; Löliger, C.; Horstman, M.; Altnöder, M.; Clausen, J.; Grimm, J.; Kabisch, H.; et al. Long-Term Follow-up of Leukaemia Patients after Related Cryopreserved Allogeneic Bone Marrow Transplantation. Br. J. Haematol. 1997, 96, 382–386. [Google Scholar] [CrossRef]
- Eckardt, J.R.; Roodman, G.D.; Boldt, D.H.; Clark, G.M.; Alvarez, R.; Page, C.; Gaskill, H.; LeMaistre, C.F. Comparison of Engraftment and Acute GVHD in Patients Undergoing Cryopreserved or Fresh Allogeneic BMT. Bone Marrow Transplant. 1993, 11, 125–131. [Google Scholar] [PubMed]
- Kim, D.H.; Jamal, N.; Saragosa, R.; Loach, D.; Wright, J.; Gupta, V.; Kuruvilla, J.; Lipton, J.H.; Minden, M.; Messner, H.A. Similar Outcomes of Cryopreserved Allogeneic Peripheral Stem Cell Transplants (PBSCT) Compared to Fresh Allografts. Biol. Blood Marrow Transplant. 2007, 13, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Lioznov, M.; Dellbrügger, C.; Sputtek, A.; Fehse, B.; Kröger, N.; Zander, A.R. Transportation and Cryopreservation May Impair Haematopoietic Stem Cell Function and Engraftment of Allogeneic PBSCs, but Not BM. Bone Marrow Transplant. 2008, 42, 121–128. [Google Scholar] [CrossRef]
- Medd, P.; Nagra, S.; Hollyman, D.; Craddock, C.; Malladi, R. Cryopreservation of Allogeneic PBSC from Related and Unrelated Donors Is Associated with Delayed Platelet Engraftment but Has No Impact on Survival. Bone Marrow Transplant. 2013, 48, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Parody, R.; Caballero, D.; Márquez-Malaver, F.J.; Vázquez, L.; Saldaña, R.; Madrigal, M.D.; Calderón, C.; Carrillo, E.; Lopez-Corral, L.; Espigado, I.; et al. To freeze or not to freeze peripheral blood stem cells prior to allogeneic transplantation from matched related donors. Eur. J. Haematol. 2013, 91, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Eapen, M.; Zhang, M.J.; Tang, X.Y.; Lee, S.J.; Fei, M.W.; Wang, H.L.; Hebert, K.M.; Arora, M.; Chhabra, S.; Devine, S.M.; et al. Hematopoietic Cell Transplantation with Cryopreserved Grafts for Severe Aplastic Anemia. Biol. Blood Marrow Transplant. 2020, 26, e161–e166. [Google Scholar] [CrossRef] [PubMed]
- Hamadani, M.; Zhang, M.J.; Tang, X.Y.; Fei, M.; Brunstein, C.; Chhabra, S.; D’Souza, A.; Milano, F.; Phelan, R.; Saber, W.; et al. Graft Cryopreservation Does Not Impact Overall Survival after Allogeneic Hematopoietic Cell Transplantation Using Post-Transplantation Cyclophosphamide for Graft-versus-Host Disease Prophylaxis. Biol. Blood Marrow Transplant. 2020, 26, 1312–1317. [Google Scholar] [CrossRef]
- Hsu, J.W.; Farhadfar, N.; Murthy, H.; Logan, B.R.; Bo-Subait, S.; Frey, N.; Goldstein, S.C.; Horowitz, M.M.; Lazarus, H.; Schwanke, J.D.; et al. The Effect of Donor Graft Cryopreservation on Allogeneic Hematopoietic Cell Transplantation Outcomes: A Center for International Blood and Marrow Transplant Research Analysis. Implications during the COVID-19 Pandemic. Transplant. Cell. Ther. 2021, 27, 507–516. [Google Scholar] [CrossRef]
- Dagdas, S.; Ucar, M.A.; Ceran, F.; Gunes, A.K.; Falay, M.; Ozet, G. Comparison of Allogenic Stem Cell Transplantations Performed with Frozen or Fresh Stem Cell Products with Regard to GVHD and Mortality. Transfus. Apher. Sci. 2020, 59, 102742. [Google Scholar] [CrossRef]
- Maurer, K.; Saucier, A.; Kim, H.T.; Acharya, U.; Mo, C.C.; Porter, J.; Albert, C.; Cutler, C.; Antin, J.H.; Koreth, J.; et al. COVID-19 and Hematopoietic Stem Cell Transplantation and Immune Effector Cell Therapy: A US Cancer Center Experience. Blood Adv. 2021, 5, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.; Kim, H.T.; Garrity, H.M.; Liney, D.; Cutler, C.; Antin, J.H.; Koreth, J.; Ritz, J.; Shapiro, R.M.; Romee, R.; et al. Lower Incidence of Chronic GVHD Observed after Transplantation with Cryopreserved Unrelated Allogeneic Stem Cells. Blood Adv. 2023, 7, 2431–2435. [Google Scholar] [CrossRef] [PubMed]
- Valentini, C.G.; Chiusolo, P.; Bianchi, M.; Metafuni, E.; Orlando, N.; Giammarco, S.; Bacigalupo, A.; Sica, S.; Teofili, L. Coronavirus Disease 2019 Pandemic and Allogeneic Hematopoietic Stem Cell Transplantation: A Single Center Reappraisal. Cytotherapy 2021, 23, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Mfarrej, B.; Lemarié, C.; Granata, A.; Pagliardini, T.; Malenfant, C.; Lignée, P.; Fays, M.; Blaise, D.; Chabannon, C.; Calmels, B. Related versus Unrelated Allogeneic HPC Graft Cryopreservation: A Single-Center Experience in the Context of the Global COVID-19 Pandemic. Bone Marrow Transplant. 2021, 56, 2013–2015. [Google Scholar] [CrossRef]
- Fernandez-Sojo, J.; Azqueta, C.; Valdivia, E.; Martorell, L.; Medina-Boronat, L.; Martínez-Llonch, N.; Torrents, S.; Codinach, M.; Canals, C.; Elorza, I.; et al. Cryopreservation of Unrelated Donor Hematopoietic Stem Cells: The Right Answer for Transplantations during the COVID-19 Pandemic? Bone Marrow Transplant. 2021, 56, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.S.; Prem, S.; Chen, S.; Lipton, J.H.; Kim, D.D.; Viswabandya, A.; Kumar, R.; Lam, W.; Law, A.D.; Mattsson, J.; et al. Fresh vs. Frozen Allogeneic Peripheral Blood Stem Cell Grafts: A Successful Timely Option. Am. J. Hematol. 2021, 96, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Novitzky-Basso, I.; Remberger, M.; Chen, C.; Pasić, I.; Lam, W.; Law, A.; Gerbitz, A.; Viswabandya, A.; Lipton, J.H.; Kim, D.D.; et al. Anti-Thymocyte Globulin and Post-Transplant Cyclophosphamide Predisposes to Inferior Outcome When Using Cryopreserved Stem Cell Grafts. Eur. J. Haematol. 2022, 108, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, J.; Clark, P.; Ahmad, S.; Patel, R.; Varela, J.C.; Mori, S. Cryopreserved versus Fresh Peripheral Blood Allogeneic Stem Cell Transplantation Outcomes in Patients Receiving Post-Transplant Cyclophosphamide for Graft-versus-Host Prophylaxis during the COVID-19 Pandemic: A Single Center Experience. Int. J. Hematol. 2023, 117, 428–437. [Google Scholar] [CrossRef]
- Facchin, G.; Savignano, C.; Battista, M.L.; Isola, M.; De Martino, M.; Petruzzellis, G.; Rosignoli, C.; Pizzano, U.; Cerno, M.; De Cecco, G.; et al. Impact of Cryopreservation of Peripheral Blood Stem Cells (PBSC) in Transplantation from Matched Unrelated Donor (MUD). J. Clin. Med. 2022, 11, 4114. [Google Scholar] [CrossRef]
- Giammarco, S.; Sica, S.; Metafuni, E.; Limongiello, M.A.; Valentini, C.G.; Sorà, F.; Marra, J.D.; Bacigalupo, A.; Teofili, L.; Chiusolo, P. Impact of Covid 19 Pandemic on Hematopoietic Stem Cell Transplantation Activities: Report from a Single Center. Transfus. Apher. Sci. 2023, 62, 103708. [Google Scholar] [CrossRef]
- Ersal, T.; Özkocaman, V.; Yalçın, C.; Orhan, B.; Candar, Ö.; Çubukçu, S.; Koca, T.G.; Pınar, İ.E.; Hunutlu, F.Ç.; Özkalemkaş, F. The effect of cryopreservation on engraftment kinetics in fully matched allogeneic stem cell transplantation: Real-life data and literature review. Transfus. Apher. Sci. 2023, 62, 103821. [Google Scholar] [CrossRef]
- Keyzner, A.; Azzi, J.; Jakubowski, R.; Sinitsyn, Y.; Tindle, S.; Shpontak, S.; Kwon, D.; Isola, L.; Iancu-Rubin, C. Cryopreservation of Allogeneic Hematopoietic Cell Products During COVID-19 Pandemic: Graft Characterization and Engraftment Outcomes. Transplant. Proc. 2023, 55, 1799–1809. [Google Scholar] [CrossRef]
- Laroye, C.; Thilly, N.; Gauthier, M.; Luc, A.; Latger-Cannard, V.; Eschwege, V.; Bensoussan, D.; Pochon, C.; Campidelli, A.; Rubio, M.-T.; et al. A French single-center experience on allogeneic stem cell transplant cryopreservation during severe acute respiratory syndrome coronavirus 2 pandemic. Cytotherapy 2023, 25, 877–884. [Google Scholar] [CrossRef]
- Bankova, A.K.; Caveney, J.; Yao, B.; Ramos, T.L.; Bögeholz, J.; Heydari, K.; Diaz, N.; Jackson, M.L.; Lowsky, R.; Brown, J.; et al. Real-World Experience of Cryopreserved Allogeneic Hematopoietic Grafts during the COVID-19 Pandemic: A Single-Center Report. Transplant. Cell. Ther. 2022, 28, 215.e1–215.e10. [Google Scholar] [CrossRef]
- Kanda, Y.; Doki, N.; Kojima, M.; Kako, S.; Inoue, M.; Uchida, N.; Onishi, Y.; Kamata, R.; Kotaki, M.; Kobayashi, R.; et al. Effect of Cryopreservation in Unrelated Bone Marrow and Peripheral Blood Stem Cell Transplantation in the Era of the COVID-19 Pandemic: An Update from the Japan Marrow Donor Program. Transplant. Cell. Ther. 2022, 28, 677.e1–677.e6. [Google Scholar] [CrossRef] [PubMed]
- Connelly-Smith, L.; Gooley, T.; Roberts, L.; Mielcarek, M.; Linenberger, M.; Petersdorf, E.; Sandmaier, B.M.; Milano, F. Cryopreservation of Growth Factor-Mobilized Peripheral Blood Stem Cells Does Not Compromise Major Outcomes after Allogeneic Hematopoietic Cell Transplantation: A Single-Center Experience. Transplant Cell Ther. 2023, 29, 700.e1–700.e8. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.M.; Bo-Subait, S.; Kuxhausen, M.; Spellman, S.R.; Bupp, C.; Ahn, K.W.; Stefanski, H.E.; Auletta, J.J.; Logan, B.R.; Shaw, B.E. Clinical impact of cryopreservation of allogeneic hematopoietic cell grafts during the onset of the COVID-19 pandemic. Blood Adv. 2023, 7, 5982–5993. [Google Scholar] [CrossRef] [PubMed]
- Purtill, D.; Hutchins, C.; Kennedy, G.; McClean, A.; Fraser, C.; Shaw, P.J.; Chiappini, P.; Tao, H.; Ma, D.D.; Kabani, K.; et al. Good Engraftment but Quality and Donor Concerns for Cryopreserved Hemopoietic Progenitor Cell Products Collected During the COVID-19 Pandemic. Transplant. Cell. Ther. 2021, 27, 1022.e1–1022.e6. [Google Scholar] [CrossRef]
- Frey, N.V.; Lazarus, H.M.; Goldstein, S.C. Has Allogeneic Stem Cell Cryopreservation Been given the “Cold Shoulder”? An Analysis of the Pros and Cons of Using Frozen versus Fresh Stem Cell Products in Allogeneic Stem Cell Transplantation. Bone Marrow Transplant. 2006, 38, 399–405. [Google Scholar] [CrossRef]
- Worel, N.; Ljungman, P.; Verheggen, I.C.M.; Hoogenboom, J.D.; Knelange, N.S.; Eikema, D.-J.; Sánchez-Ortega, I.; Riillo, C.; Centorrino, I.; Averbuch, D.; et al. Fresh or frozen grafts for allogeneic stem cell transplantation: Conceptual considerations and a survey on the practice during the COVID-19 pandemic from the EBMT Infectious Diseases Working Party (IDWP) and Cellular Therapy & Immunobiology Working Party (CTIWP). Bone Marrow Transplant. 2023, 58, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H.; Buk, D.; Bernas, S.N.; Mengling, T.; Neujahr, E.; van den Brink, M.R.M. A DKMS (German Bone Marrow Donor Center) view on cryopreservation of unrelated donor stem cell products during the COVID-19 pandemic. Am. J. Hematol. 2021, 96, E91–E92. [Google Scholar] [CrossRef] [PubMed]
- Auletta, J.J.; Novakovich, J.L.; Stritesky, G.L.; Newman, J.; Fridy-Chesser, S.T.; Hailperin, K.; Devine, S.M. Meeting the Demand for Unrelated Donors in the Midst of the COVID-19 Pandemic: Rapid Adaptations by the National Marrow Donor Program and Its Network Partners Ensured a Safe Supply of Donor Products. Transplant. Cell Ther. 2021, 27, 133–141. [Google Scholar] [CrossRef] [PubMed]

| Authors [Ref], Graft Source | Year | Engraftment | aGvHD | cGvHD | OS | DFS/PFS | NRM | Other Findings | |
|---|---|---|---|---|---|---|---|---|---|
| N | PLT | ||||||||
| Eckardt [85], BM | 1993 | similar | similar | lower | NR | NR | NR/NR | NR | |
| Kim [86], PBSCs | 2007 | similar | similar | similar | similar | similar | NR/similar | similar | similar lymphocyte recovery |
| Lioznov [87], BM | 2008 | similar | similar | NR | NR | NR | NR/NR | NR | |
| Lioznov [87], PBSCs | 2008 | more graft failure | NR | NR | NR | NR/NR | NR | ||
| Medd [88], PBSCs | 2013 | delayed | delayed | similar | similar | similar | NR/similar | NR | |
| Parody [89], PBSCs | 2013 | faster | similar | higher | similar | similar | NR/NR | similar | |
| Eapen [90], PBSCs/BM | 2020 | more graft failures in PBSCs | similar | similar | lower in PBSCs | NR/NR | NR | ||
| Hamadani [91], PBSCs/BM | 2020 | delayed | delayed | similar | lower | similar | lower/similar | similar | |
| Hsu [92], PBSCs unrelated | 2021 | delayed | delayed | similar | NR | lower | NR/similar | higher | |
| Hsu [92], PBSCs related | 2021 | similar | delayed | lower | NR | similar | NR/similar | similar | |
| Hsu [92], BM | 2021 | similar | delayed | similar | NR | similar | NR/similar | similar | |
| Dagdas [93], PBSCs | 2020 | delayed | similar | similar | lower (liver) | similar | NR/NR | similar | similar relapse rate |
| Maurer [94], PBSCs/BM | 2021 | similar | similar | similar | NR | similar | NR/similar | similar | |
| Maurer [54], PBSCs | 2021 | delayed | delayed | higher | NR | similar | NR/NR | similar | lower lymphocyte recovery and CD3 chimerism |
| Maurer [95], PBSCs | 2023 | NR | NR | similar | lower | similar | similar/similar | similar | similar 1 y CD3 chimerism |
| Valentini [96], PBSCs | 2022 | similar | similar | similar | NR | NR | NR/NR | similar | similar relapse rate |
| Fernandez-Sojo [98], PBSCs | 2021 | similar | similar | similar | NR | similar | NR/similar | NR | |
| Alotaibi [99], PBSCs | 2021 | similar | similar | similar | higher | similar | NR/NR | similar relapse rate | |
| Novitzy-Basso [100], PBSCs | 2021 | delayed | similar | similar | lower | lower | NR/NR | higher | GRFS lower |
| Guo [101], PBSCs | 2023 | delayed | delayed | similar | similar | lower | similar/NR | similar | |
| Facchin [102], PBSCs | 2022 | similar | similar | similar | NR | similar | NR/similar | NR | |
| Giammarco [103], PBSCs/BM, CB | 2023 | similar graft failure | NR | NR | similar | NR/NR | NR | ||
| Ersal [104], PBSCs | 2023 | similar | delayed | similar | similar | similar | NR/similar | similar | similar 30 d chimerism |
| Keyzner [105], PBSCs/BM | 2023 | similar | similar | NR | NR | NR | NR/NR | NR | similar 30 d/100 d chimerism |
| Laroye [106], PBSCs | 2023 | similar | similar | similar | similar | similar | similar | similar | |
| Bankova [107], PBSCs | 2022 | delayed in RIC | delayed in RIC | NR | NR | lower | lower/NR | higher | |
| Kanda [108], PBSCs | 2022 | delayed | delayed | NR | NR | NR | NR/NR | NR | |
| Kanda [108], BM | 202 | similar | delayed | NR | NR | NR | NR/NR | NR | |
| Connelly-Smith [109], PBSCs | 2023 | similar | delayed | similar | similar | similar | NR/similar | similar | |
| Devine [110], PBSCs/BM | 2023 | delayed | delayed | similar | lower | similar | lower/NR | similar | higher relapse rate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentini, C.G.; Pellegrino, C.; Teofili, L. Pros and Cons of Cryopreserving Allogeneic Stem Cell Products. Cells 2024, 13, 552. https://doi.org/10.3390/cells13060552
Valentini CG, Pellegrino C, Teofili L. Pros and Cons of Cryopreserving Allogeneic Stem Cell Products. Cells. 2024; 13(6):552. https://doi.org/10.3390/cells13060552
Chicago/Turabian StyleValentini, Caterina Giovanna, Claudio Pellegrino, and Luciana Teofili. 2024. "Pros and Cons of Cryopreserving Allogeneic Stem Cell Products" Cells 13, no. 6: 552. https://doi.org/10.3390/cells13060552
APA StyleValentini, C. G., Pellegrino, C., & Teofili, L. (2024). Pros and Cons of Cryopreserving Allogeneic Stem Cell Products. Cells, 13(6), 552. https://doi.org/10.3390/cells13060552






