The Effect of Leukocyte Removal and Matrix Metalloproteinase Inhibition on Platelet Storage Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Blood Count Analysis
2.2.2. Aggregation of Platelets
2.2.3. Cytofluorimetric Analysis
2.2.4. Isolation of Platelets
2.2.5. Media Preparation
2.2.6. RNA Isolation, Reverse Transcription and Real-Time PCR
2.2.7. Gelatin Zymography
2.2.8. Immunoenzymatic Measurement of the MMP-2 and MMP-9 Concentrations
2.3. Statistical Analysis
3. Results
3.1. Morphological Changes
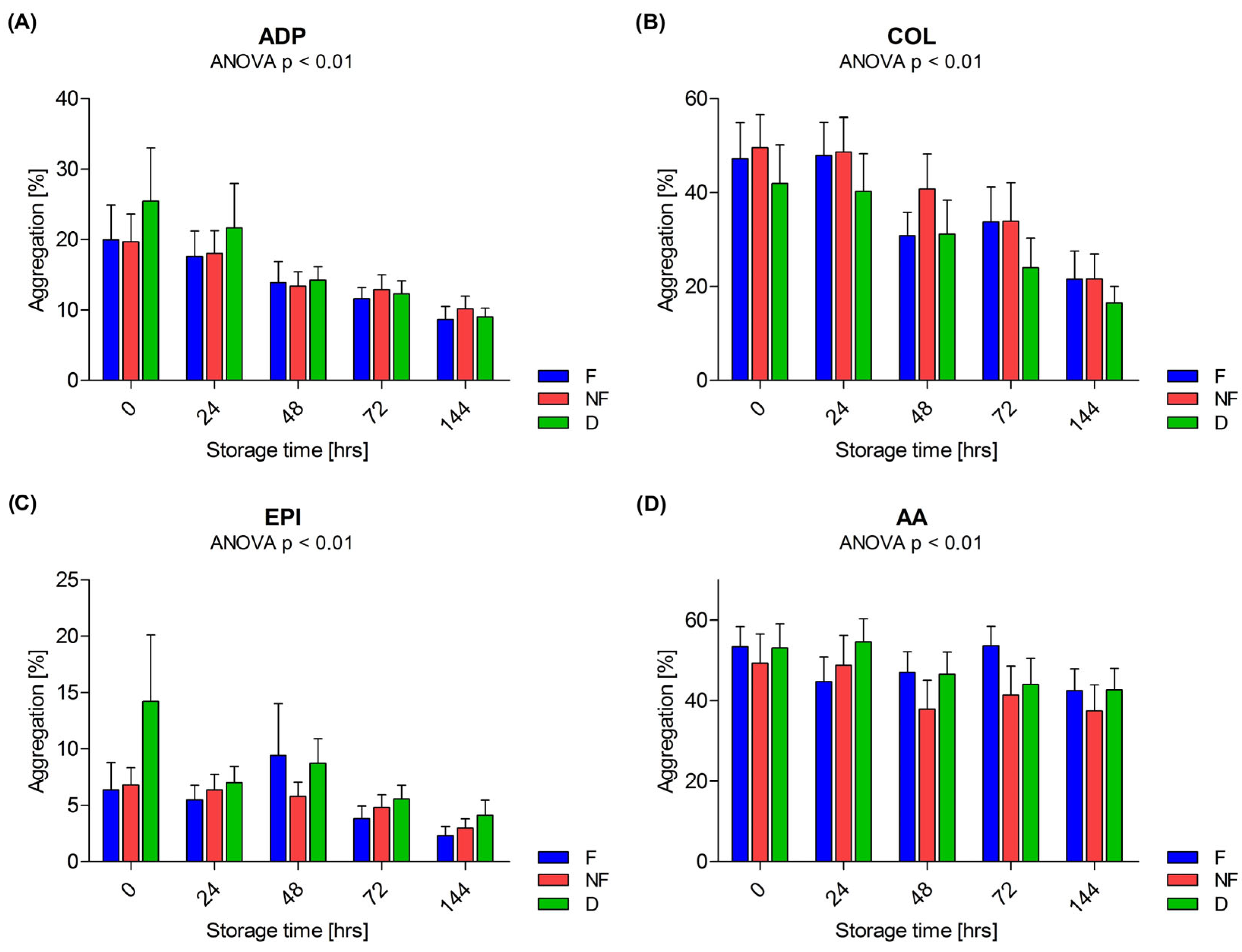
3.2. Aggregation
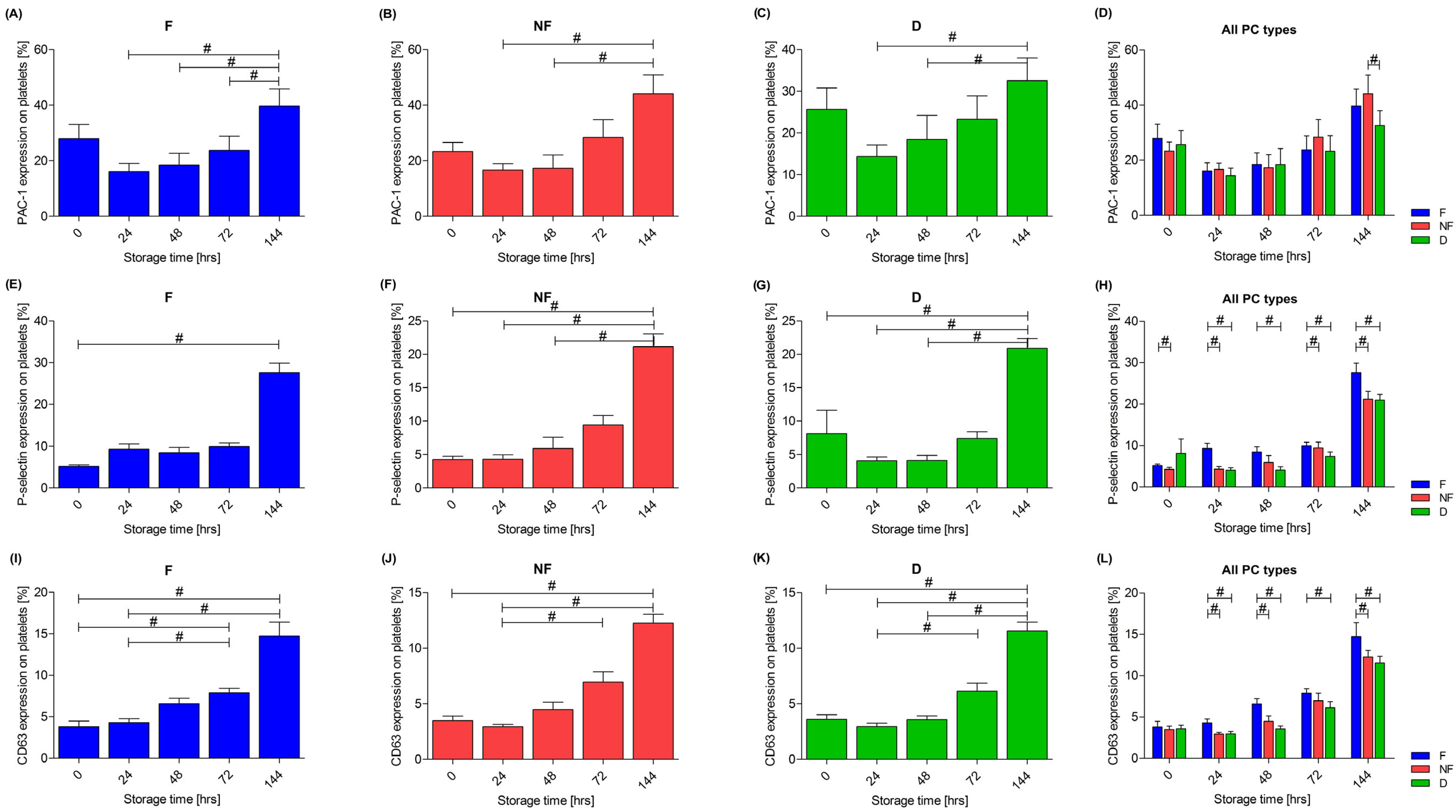
3.3. Platelet Activation
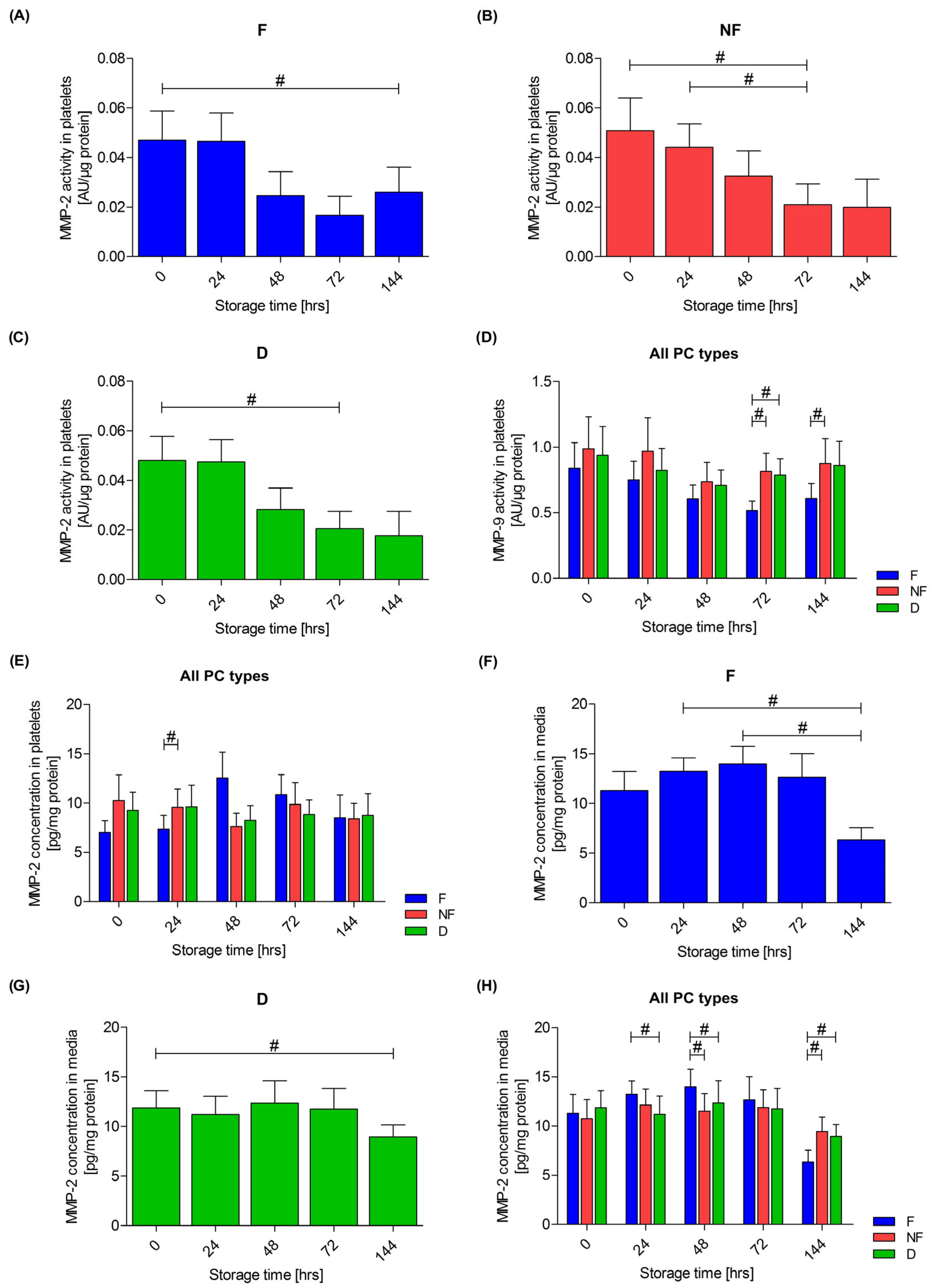
3.4. Extracellular Matrix Metalloproteinases
3.5. Relationship of Metalloproteinases with Platelet Activation and Leukocyte Count
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naghadeh, H.T.; A Badlou, B.; Ferizhandy, A.S.; Mohammadreza, T.S.; Shahram, V. Six hours of resting platelet concentrates stored at 22–24 °C for 48 hours in permeable bags preserved pH, swirling and lactate dehydrogenase better and caused less platelet activation. Blood Transfus. 2013, 11, 400–404. [Google Scholar] [CrossRef]
- Thomas, S. Platelets: Handle with care. Transfus. Med. 2016, 26, 330–338. [Google Scholar] [CrossRef]
- Thon, J.N.; Schubert, P.; Devine, D.V. Platelet Storage Lesion: A New Understanding from a Proteomic Perspective. Transfus. Med. Rev. 2008, 22, 268–279. [Google Scholar] [CrossRef]
- Black, A.; Orsó, E.; Kelsch, R.; Pereira, M.; Kamhieh-Milz, J.; Salama, A.; Fischer, M.B.; Meyer, E.; Frey, B.M.; Schmitz, G. Analysis of platelet-derived extracellular vesicles in plateletpheresis concentrates: A multicenter study. Transfusion 2017, 57, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.J.; Guimarães, T.M.P.D.; de Almeida, N.C.; de Toledo, V.d.P.C.P. Comparison of cytokine levels and metabolic parameters of stored platelet concentrates of the Fundação Hemominas, Belo Horizonte. Brazil Rev. Bras. Hematol. E Hemoter. 2012, 34, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Egidi, M.G.; D’Alessandro, A.; Mandarello, G.; Zolla, L. Troubleshooting in platelet storage temperature and new perspectives through proteomics. Blood Transfus. 2010, 8, 73–81. [Google Scholar] [CrossRef]
- Estebanell, E.; Diaz-Ricart, M.; Lozano, M.; Mazzara, R.; Escolar, G.; Ordinas, A. Cytoskeletal reorganization after preparation of platelet concentrates, using the buffy coat method, and during their storage. Haematologica 1998, 83, 112–117. [Google Scholar]
- Hoareau, G.L.; Jandrey, K.E.; Burges, J.; Bremer, D.; Tablin, F. Comparison of the platelet-rich plasma and buffy coat protocols for preparation of canine platelet concentrates. Vet. Clin. Pathol. 2014, 43, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, L.; Taaning, E.; Dreier, B.; Christensen, L.D.; Mynster, T.; Nielsen, H.J. Extracellular accumulation of bioactive sub-stances during preparation and storage of various platelet concentrates. Am. J. Hematol. 2001, 67, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Sharma, R.R.; Marwaha, N. Defining an appropriate leucoreduction strategy by serial assessment of cytokine levels in platelet concentrates prepared by different methods. Asian J. Transfus. Sci. 2015, 9, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, F.; Rivera, J.; Lozano, M.L.; Corral, J.; Garcia, V.V. Effect of cold-storage on the accumulation of bioreactive substances in platelet concentrates treated with second messenger effectors. Haematologica 2001, 86, 530–536. [Google Scholar]
- Kanter, J.; Khan, S.Y.; Kelher, M.; Gore, L.; Silliman, C.C. Oncogenic and angiogenic growth factors accumulate during routine storage of apheresis platelet concentrates. Clin. Cancer Res. 2008, 14, 3942–3947. [Google Scholar] [CrossRef]
- Cognasse, F.; Boussoulade, F.; Chavarin, P.; Acquart, S.; Fabrigli, P.; Lamy, B.; Garraud, O. Release of potential immunomodulatory factors during platelet storage. Transfusion 2006, 46, 1184–1189. [Google Scholar] [CrossRef]
- Allan, H.E.; Vadgama, A.; Armstrong, P.C.; Warner, T.D. What can we learn from senescent platelets, their transcriptomes and proteomes? Platelets 2023, 34, 2200838. [Google Scholar] [CrossRef]
- Ghezelbash, B.; Kafibad, S.A.; Hojjati, M.T.; Hamidpoor, M.; Vaeli, S.; Tabtabae, M.R.; Gharehbaghian, A. In Vitro Assessment of Platelet Lesions during 5-day Storage in Iranian Blood Transfusion Organization (IBTO) centers. Arch. Iran. Med. 2015, 18, 114–116. [Google Scholar]
- Perrotta, P.L.; Perrotta, C.L.; Snyder, E.L. Apoptotic activity in stored human platelets. Transfusion 2003, 43, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Vučetić, D.; Ilić, V.; Vojvodić, D.; Subota, V.; Todorović, M.; Balint, B. Flow cytometry analysis of platelet populations: Usefulness for monitoring the storage lesion in pooled buffy-coat platelet concentrates. Blood Transfus. 2018, 16, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Leheta, O.; Younes, S. In vitro assessment of platelet storage lesion in leukoreduced random donor platelet concentrates. Blood Transfus. 2010, 8, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Abonnenc, M.; Sonego, G.; Kaiser-Guignard, J.; Crettaz, D.; Prudent, M.; Tissot, J.-D.; Lion, N. In vitro evaluation of pathogen-inactivated buffy coat-derived platelet concentrates during storage: Psoralen-based photochemical treatment step-by-step. Blood Transfus. 2015, 13, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Jurisic, V.; Radenkovic, S.; Konjevic, G. The Actual Role of LDH as Tumor Marker, Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metallo-proteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Serra, R. Matrix Metalloproteinases in Health and Disease. Biomolecules 2020, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, C.; Vaidya, S.; Wadhwan, V.; Hitesh; Kaur, G.; Pathak, A. Seesaw of matrix metalloproteinases (MMPs). J. Cancer Res. Ther. 2016, 12, 28–35. [Google Scholar] [CrossRef]
- Radenkovic, S.; Konjevic, G.; Jurisic, V.; Karadzic, K.; Nikitovic, M.; Gopcevic, K. Values of MMP-2 and MMP-9 in tumor tissue of basal-like breast cancer patients. Cell Biochem. Biophys. 2014, 68, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Seizer, P.; May, A.E. Platelets and matrix metalloproteinases. Thromb. Haemost. 2013, 110, 903–909. [Google Scholar] [CrossRef]
- Falcinelli, E.; Guglielmini, G.; Torti, M.; Gresele, P. Intraplatelet signaling mechanisms of the priming effect of matrix metalloproteinase-2 on platelet aggregation. J. Thromb. Haemost. 2005, 3, 2526–2535. [Google Scholar] [CrossRef] [PubMed]
- Momi, S.; Falcinelli, E.; Giannini, S.; Ruggeri, L.; Cecchetti, L.; Corazzi, T.; Libert, C.; Gresele, P. Loss of matrix metalloproteinase 2 in platelets reduces arterial thrombosis in vivo. J. Exp. Med. 2009, 206, 2365–2379. [Google Scholar] [CrossRef]
- Sebastiano, M.; Momi, S.; Falcinelli, E.; Bury, L.; Hoylaerts, M.F.; Gresele, P. A novel mechanism regulating human platelet activation by MMP-2–mediated PAR1 biased signaling. Blood 2017, 129, 883–895. [Google Scholar] [CrossRef]
- Kälvegren, H.; Jönsson, S.; Jonasson, L. Release of matrix metalloproteinases-1 and -2, but not -9, from activated platelets measured by enzyme-linked immunosorbent assay. Platelets 2011, 22, 572–578. [Google Scholar] [CrossRef]
- Wrzyszcz, A.; Woźniak, M. On the origin of matrix metalloproteinase-2 and -9 in blood platelets. Platelets 2012, 23, 467–474. [Google Scholar] [CrossRef]
- Mannello, F.; Medda, V. Differential expression of MMP-2 and MMP-9 activity in megakaryocytes and platelets. Blood 2011, 118, 6470–6471. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Patron, C.; Martinez-Cuesta, M.A.; Salas, E.; Sawicki, G.; Wozniak, M.; Radomski, M.W.; Davidge, S.T. Differential Regulation of Platelet Aggregation by Matrix Metalloproteinases-9 and -2. Thromb. Haemost. 1999, 82, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.R.; Fong, T.H.; Liu, C.M.; Shen, M.Y.; Chen, T.L.; Chang, Y.; Lu, M.S.; Hsiao, G. Expression of matrix metalloproteinase-9 in human platelets: Regulation of platelet activation in in vitro and in vivo studies. Br. J. Pharmacol. 2004, 143, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.S.Y.; Tung, J.-P.; Fraser, J.F. Platelet Storage Lesions: What More Do We Know Now? Transfus. Med. Rev. 2018, 32, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Wrzyszcz, A.; Urbaniak, J.; Sapa, A.; Woźniak, M. An efficient method for isolation of representative and contamination-free population of blood platelets for proteomic studies. Platelets 2017, 28, 43–53. [Google Scholar] [CrossRef]
- Zsóri, K.; Muszbek, L.; Csiki, Z.; Shemirani, A. Validation of reference genes for the determination of platelet transcript level in healthy individuals and in patients with the history of myocardial infarction. Int. J. Mol. Sci. 2013, 14, 3456–3466. [Google Scholar] [CrossRef]
- Makowski, G.S.; Ramsby, M.L. Calibrating Gelatin Zymograms with Human Gelatinase Standards. Anal. Biochem. 1996, 236, 353–356. [Google Scholar] [CrossRef]
- Seghatchian, J.; Krailadsiri, P. The Platelet Storage Lesion. Transfus. Med. Rev. 1997, 11, 130–144. [Google Scholar] [CrossRef]
- Seghatchian, J. A new platelet storage lesion index based on paired samples, without and with EDTA and cell counting: Comparison of three types of leukoreduced preparations. Transfus. Apher. Sci. 2006, 35, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Kicken, C.H.; Roest, M.; Henskens, Y.M.C.; de Laat, B.; Huskens, D. Application of an optimized flow cytometry-based quantification of Platelet Activation (PACT): Monitoring platelet activation in platelet concentrates. PLoS ONE 2017, 12, e0172265. [Google Scholar] [CrossRef] [PubMed]
- Slichter, S.J.; Bolgiano, D.; Corson, J.; Jones, M.K.; Christoffel, T.; Bailey, S.L.; Pellham, E. Extended storage of buffy coat platelet concentrates in plasma or a platelet additive solution. Transfusion 2014, 54, 2283–2291. [Google Scholar] [CrossRef]
- Krailadsiri, P.; Seghatchian, J.; Williamson, L.M. Platelet storage lesion of WBC-reduced, pooled, buffy coat-derived platelet concentrates prepared in three in-process filter/storage bag combinations. Transfusion 2001, 41, 243–250. [Google Scholar] [CrossRef]
- Black, A.; Pienimaeki-Roemer, A.; Kenyon, O.; Orsó, E.; Schmitz, G. Platelet-derived extracellular vesicles in plateletpheresis concentrates as a quality control approach. Transfusion 2015, 55, 2184–2196. [Google Scholar] [CrossRef]
- Amorini, A.M.; Tuttobene, M.; Tamasello, F.M.; Biazzo, F. Glucose ameliorates the metabolic profile and mitochondrial function of platelet concentrates during storage in autologous plasma. Blood Transfus. 2013, 11, 61–70. [Google Scholar] [CrossRef]
- Amorini, A.M.; Tuttobene, M.; Lazzarino, G.; Denti, G. Evaluation of biochemical parameters in platelet concentrates stored in glucose solution. Blood Transfus. 2007, 5, 24–32. [Google Scholar] [CrossRef]
- Plaza, E.M.; Lozano, M.L.; Guiu, I.S.; Egea, J.M.; Vicente, V.; De Terán, L.C.; Rivera, J. Evaluation of platelet function during extended storage in additive solution, prepared in a new container that allows manual buffy-coat platelet pooling and leucoreduction in the same system. Blood Transfus. 2012, 10, 480–489. [Google Scholar] [CrossRef]
- Canault, M.; Duerschmied, D.; Brill, A.; Stefanini, L.; Schatzberg, D.; Cifuni, S.M.; Bergmeier, W.; Wagner, D.D. p38 mitogen-activated protein kinase activation during platelet storage: Consequences for platelet recovery and hemostatic function in vivo. Blood 2010, 115, 1835–1842. [Google Scholar] [CrossRef]
- Seghatchian, J. Platelet storage lesion: An update on the impact of various leukoreduction processes on the biological response modifiers. Transfus. Apher. Sci. 2006, 34, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Vucic, M.; Stanojkovic, Z.; Antic, A.; Vucic, J.; Pavlovic, V. Evaluation of platelet activation in leukocyte-depleted platelet concentrates during storage. Bosn. J. Basic Med. Sci. 2018, 18, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Lei, P.; Waters, L.; Padula, M.P.; Marks, D.C. Identification of platelet subpopulations in cryopreserved platelet components using multi-colour imaging flow cytometry. Sci. Rep. 2023, 13, 1221. [Google Scholar] [CrossRef] [PubMed]
- Lesyk, G.; Jurasz, P. Advances in Platelet Subpopulation Research. Front. Cardiovasc. Med. 2019, 6, 138. [Google Scholar] [CrossRef] [PubMed]
- Handtke, S.; Steil, L.; Greinacher, A.; Thiele, T. Toward the Relevance of Platelet Subpopulations for Transfusion Medicine. Front. Med. 2018, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Prudova, A.; Serrano, K.; Eckhard, U.; Fortelny, N.; Devine, D.V.; Overall, C.M. TAILS N-terminomics of human platelets reveals pervasive metalloproteinase-dependent proteolytic processing in storage. Blood 2014, 124, e49–e60. [Google Scholar] [CrossRef]
- Bartoli, C.R.; Kang, J.; Restle, D.J.; Zhang, D.M.; Shabahang, C.; Acker, M.A.; Atluri, P. Inhibition of ADAMTS-13 by Doxycycline Reduces von Willebrand Factor Degradation During Supraphysiological Shear Stress: Therapeutic Implications for Left Ventricular Assist Device-Associated Bleeding. JACC Heart Fail. 2015, 3, 860–869. [Google Scholar] [CrossRef]
- Bergmeier, W.; Burger, P.C.; Piffath, C.L.; Hoffmeister, K.M.; Hartwig, J.H.; Nieswandt, B.; Wagner, D.D. Metalloproteinase inhibitors improve the recovery and hemostatic function of in vitro–aged or–injured mouse platelets. Blood 2003, 102, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, J.; Zhang, Q.; Chen, Y.; Zhu, X.; Xia, R. The role of microRNAs in platelet biology during storage. Transfus. Apher. Sci. 2017, 56, 147–150. [Google Scholar] [CrossRef]
- Nilsson, R.J.A.; Balaj, L.; Hulleman, E.; Van Rijn, S.; Pegtel, D.M.; Walraven, M.; Widmark, A.; Gerritsen, W.R.; Verheul, H.M.; Vandertop, W.P.; et al. Blood platelets contain tumor-derived RNA biomarkers. Blood 2011, 118, 3680–3683. [Google Scholar] [CrossRef]
- Nilsson, R.J.A.; Karachaliou, N.; Berenguer, J.; Gimenez-Capitan, A.; Schellen, P.; Teixido, C.; Tannous, J.; Kuiper, J.L.; Drees, E.; Grabowska, M.; et al. Rearranged EML4-ALK fusion transcripts sequester in circulating blood platelets and enable blood-based crizotinib response monitoring in non-small-cell lung cancer. Oncotarget 2015, 7, 1066–1075. [Google Scholar] [CrossRef]




| Correlation between MMP Activity in Isolated Platelets and Activation Marker | |||
|---|---|---|---|
| PC Type | PAC-1 Expression | P-Selectin Expression | CD63 Expression |
| MMP-2 | |||
| F | r = −0.218; p = 0.15 | r = −0.270; p = 0.08 | r = −0.269; p = 0.07 |
| NF | r = −0.383; p < 0.01 | r = −0.330; p = 0.03 | r = −0.336; p = 0.02 |
| D | r = −0.239; p = 0.11 | r = 0.013; p = 0.93 | r = −0.060; p = 0.69 |
| MMP-9 | |||
| F | r = 0.060; p = 0.70 | r = 0.026; p = 0.87 | r = −0.038; p = 0.80 |
| NF | r = −0.059; p = 0.70 | r = 0.138; p = 0.37 | r = 0.033; p = 0.83 |
| D | r = 0.046; p = 0.77 | r = 0.173; p = 0.26 | r = 0.225; p = 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rak-Pasikowska, A.; Hałucha, K.; Sapa-Wojciechowska, A.; Wrzyszcz, A.; Gałuszka, W.; Pęcak-Solińska, A.; Bil-Lula, I. The Effect of Leukocyte Removal and Matrix Metalloproteinase Inhibition on Platelet Storage Lesions. Cells 2024, 13, 506. https://doi.org/10.3390/cells13060506
Rak-Pasikowska A, Hałucha K, Sapa-Wojciechowska A, Wrzyszcz A, Gałuszka W, Pęcak-Solińska A, Bil-Lula I. The Effect of Leukocyte Removal and Matrix Metalloproteinase Inhibition on Platelet Storage Lesions. Cells. 2024; 13(6):506. https://doi.org/10.3390/cells13060506
Chicago/Turabian StyleRak-Pasikowska, Alina, Kornela Hałucha, Agnieszka Sapa-Wojciechowska, Aneta Wrzyszcz, Wioletta Gałuszka, Anna Pęcak-Solińska, and Iwona Bil-Lula. 2024. "The Effect of Leukocyte Removal and Matrix Metalloproteinase Inhibition on Platelet Storage Lesions" Cells 13, no. 6: 506. https://doi.org/10.3390/cells13060506
APA StyleRak-Pasikowska, A., Hałucha, K., Sapa-Wojciechowska, A., Wrzyszcz, A., Gałuszka, W., Pęcak-Solińska, A., & Bil-Lula, I. (2024). The Effect of Leukocyte Removal and Matrix Metalloproteinase Inhibition on Platelet Storage Lesions. Cells, 13(6), 506. https://doi.org/10.3390/cells13060506






