Progressive Irreversible Proprioceptive Piezo2 Channelopathy-Induced Lost Forced Peripheral Oscillatory Synchronization to the Hippocampal Oscillator May Explain the Onset of Amyotrophic Lateral Sclerosis Pathomechanism
Abstract
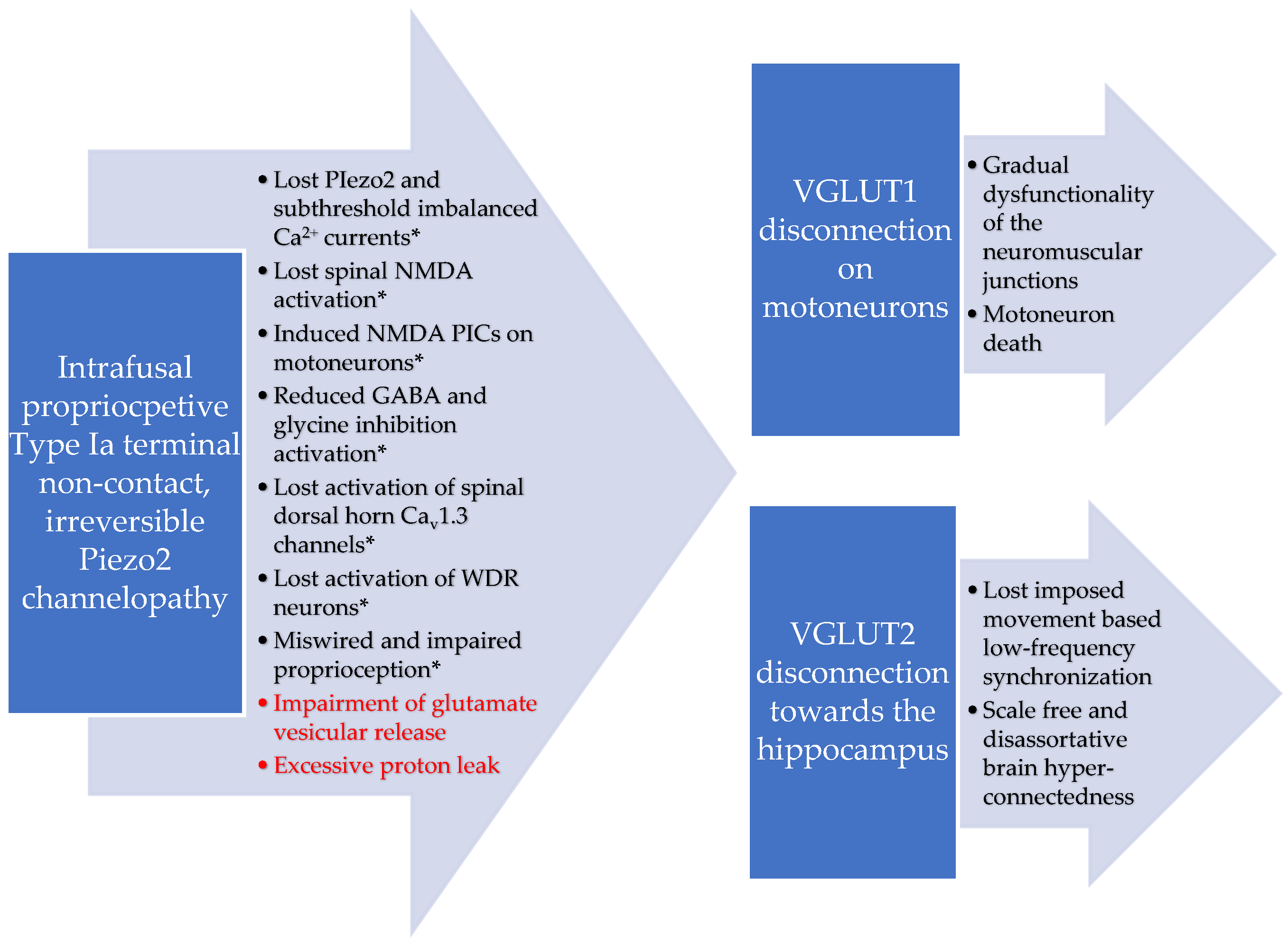
1. Introduction
2. Piezo2 Channelopathy, Piezo2–Piezo1 Crosstalk, and Syndecans
3. The Metabolic Switch
4. Additional Likely Role of Snydecan-3 and MyoD in ALS
5. Conclusions
6. Limitations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pasinelli, P.; Brown, R.H. Molecular biology of amyotrophic lateral sclerosis: Insights from genetics. Nat. Rev. Neurosci. 2006, 7, 710–723. [Google Scholar] [CrossRef]
- Chen, S.; Sayana, P.; Zhang, X.; Le, W. Genetics of amyotrophic lateral sclerosis: An update. Mol. Neurodegener. 2013, 8, 28. [Google Scholar] [CrossRef]
- Kurland, L.T.; Mulder, D.W. Epidemiologic Investigations of Amyotrophic Lateral Sclerosis. 2. Familial aggregations indicative of dominant inheritance. I. Neurology 1955, 5, 182–196. [Google Scholar] [CrossRef]
- Ryan, M.; Heverin, M.; McLaughlin, R.L.; Hardiman, O. Lifetime Risk and Heritability of Amyotrophic Lateral Sclerosis. JAMA Neurol. 2019, 76, 1367–1374. [Google Scholar] [CrossRef]
- van Rheenen, W.; Shatunov, A.; Dekker, A.M.; McLaughlin, R.L.; Diekstra, F.P.; Pulit, S.L.; van der Spek, R.A.A.; Võsa, U.; de Jong, S.; Robinson, M.R.; et al. Genome-wide association analyses identify new risk variants and the genetic architecture of amyotrophic lateral sclerosis. Nat. Genet. 2016, 48, 1043–1048. [Google Scholar] [CrossRef]
- Nicolas, A.; Kenna, K.P.; Renton, A.E.; Ticozzi, N.; Faghri, F.; Chia, R.; Dominov, J.A.; Kenna, B.J.; Nalls, M.A.; Keagle, P.; et al. Genome-Wide Analyses Identify KIF5A as a Novel ALS Gene. Neuron 2018, 97, 1268–1283.e1226. [Google Scholar] [CrossRef]
- Zhang, S.; Cooper-Knock, J.; Weimer, A.K.; Shi, M.; Moll, T.; Marshall, J.N.; Harvey, C.; Nezhad, H.G.; Franklin, J.; Souza, C.d.S.; et al. Genome-wide identification of the genetic basis of amyotrophic lateral sclerosis. Neuron 2022, 110, 992–1008.e1011. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, R.F.; Ogawa, K.; Beck, G.; Ikenaka, K.; Takeuchi, E.; Yasumizu, Y.; Jinno, J.; Kimura, Y.; Baba, K.; Nagai, Y.; et al. piRNA/PIWI Protein Complex as a Potential Biomarker in Sporadic Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2022, 59, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, S.K.; Kemp, Z.; Hatzipetros, T.; Vieira, F.; Valdez, G. Degeneration of proprioceptive sensory nerve endings in mice harboring amyotrophic lateral sclerosis-causing mutations. J. Comp. Neurol. 2015, 523, 2477–2494. [Google Scholar] [CrossRef] [PubMed]
- Held, A.; Major, P.; Sahin, A.; Reenan, R.A.; Lipscombe, D.; Wharton, K.A. Circuit Dysfunction in SOD1-ALS Model First Detected in Sensory Feedback Prior to Motor Neuron Degeneration Is Alleviated by BMP Signaling. J. Neurosci. 2019, 39, 2347–2364. [Google Scholar] [CrossRef] [PubMed]
- Brownstone, R.M.; Lancelin, C. Escape from homeostasis: Spinal microcircuits and progression of amyotrophic lateral sclerosis. J. Neurophysiol. 2018, 119, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Kitaoka, Y.; Kawata, S.; Nishiura, A.; Uchihashi, T.; Hiraoka, S.-I.; Yokota, Y.; Isomura, E.T.; Kogo, M.; Tanaka, S. Characteristics of Sensory Neuron Dysfunction in Amyotrophic Lateral Sclerosis (ALS): Potential for ALS Therapy. Biomedicines 2023, 11, 2967. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B. Delayed Onset Muscle Soreness (DOMS): The Repeated Bout Effect and Chemotherapy-Induced Axonopathy May Help Explain the Dying-Back Mechanism in Amyotrophic Lateral Sclerosis and Other Neurodegenerative Diseases. Brain Sci. 2021, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B. Miswired Proprioception in Amyotrophic Lateral Sclerosis in Relation to Pain Sensation (and in Delayed Onset Muscle Soreness)—Is Piezo2 Channelopathy a Principal Transcription Activator in Proprioceptive Terminals Besides Being the Potential Primary Damage? Life 2023, 13, 657. [Google Scholar] [CrossRef]
- Nagy, Z.F.; Sonkodi, B.; Pál, M.; Klivényi, P.; Széll, M. Likely Pathogenic Variants of Cav1.3 and Nav1.1 Encoding Genes in Amyotrophic Lateral Sclerosis Could Elucidate the Dysregulated Pain Pathways. Biomedicines 2023, 11, 933. [Google Scholar] [CrossRef]
- McIntosh, J.; Mekrouda, I.; Dashti, M.; Giuraniuc, C.V.; Banks, R.W.; Miles, G.B.; Bewick, G.S. Development of abnormalities at the neuromuscular junction in the SOD1-G93A mouse model of ALS: Dysfunction then disruption of postsynaptic structure precede overt motor symptoms. Front. Mol. Neurosci. 2023, 16, 1169075. [Google Scholar] [CrossRef]
- Sonkodi, B.; Marsovszky, L.; Csorba, A.; Balog, A.; Kopper, B.; Keller-Pintér, A.; Nagy, Z.Z.; Resch, M.D. Disrupted Neural Regeneration in Dry Eye Secondary to Ankylosing Spondylitis—With a Theoretical Link between Piezo2 Channelopathy and Gateway Reflex, WDR Neurons, and Flare-Ups. Int. J. Mol. Sci. 2023, 24, 15455. [Google Scholar] [CrossRef]
- Sonkodi, B. Delayed Onset Muscle Soreness and Critical Neural Microdamage-Derived Neuroinflammation. Biomolecules 2022, 12, 1207. [Google Scholar] [CrossRef]
- Sonkodi, B. Does Proprioception Involve Synchronization with Theta Rhythms by a Novel Piezo2 Initiated Ultrafast VGLUT2 Signaling? Biophysica 2023, 3, 695–710. [Google Scholar] [CrossRef]
- Kim, C.W.; A Goldberger, O.; Gallo, R.L.; Bernfield, M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol. Biol. Cell 1994, 5, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Arokiasamy, S.; Balderstone, M.J.M.; De Rossi, G.; Whiteford, J.R. Syndecan-3 in Inflammation and Angiogenesis. Front. Immunol. 2019, 10, 3031. [Google Scholar] [CrossRef]
- Raulo, E.; Chernousov, M.; Carey, D.; Nolo, R.; Rauvala, H. Isolation of a neuronal cell surface receptor of heparin binding growth-associated molecule (HB-GAM). Identification as N-syndecan (syndecan-3). J. Biol. Chem. 1994, 269, 12999–13004. [Google Scholar] [CrossRef]
- Kaksonen, M.; Pavlov, I.; Võikar, V.; Lauri, S.E.; Hienolaa, A.; Riekkiab, R.; Lakso, M.; Tairab, T.; Rauvala, H. Syndecan-3-deficient mice exhibit enhanced LTP and impaired hippocampus-dependent memory. Mol. Cell. Neurosci. 2002, 21, 158–172. [Google Scholar] [CrossRef]
- Murakami, K.; Tanaka, T.; Bando, Y.; Yoshida, S. Nerve injury induces the expression of syndecan-1 heparan sulfate proteoglycan in primary sensory neurons. Neuroscience 2015, 300, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Hudák, A.; Letoha, A.; Vizler, C.; Letoha, T. Syndecan-3 as a Novel Biomarker in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 3407. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B.; Kopa, Z.; Nyirády, P. Post Orgasmic Illness Syndrome (POIS) and Delayed Onset Muscle Soreness (DOMS): Do They Have Anything in Common? Cells 2021, 10, 1867. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B.; Resch, M.D.; Hortobágyi, T. Is the Sex Difference a Clue to the Pathomechanism of Dry Eye Disease? Watch out for the NGF-TrkA-Piezo2 Signaling Axis and the Piezo2 Channelopathy. J. Mol. Neurosci. 2022, 72, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.L.; Allen, D.G. Early events in stretch-induced muscle damage. J. Appl. Physiol. 1999, 87, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Hody, S.; Croisier, J.-L.; Bury, T.; Rogister, B.; Leprince, P. Eccentric Muscle Contractions: Risks and Benefits. Front. Physiol. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B.; Berkes, I.; Koltai, E. Have We Looked in the Wrong Direction for More Than 100 Years? Delayed Onset Muscle Soreness Is, in Fact, Neural Microdamage Rather Than Muscle Damage. Antioxidants 2020, 9, 212. [Google Scholar] [CrossRef]
- Murase, S.; Terazawa, E.; Hirate, K.; Yamanaka, H.; Kanda, H.; Noguchi, K.; Ota, H.; Queme, F.; Taguchi, T.; Mizumura, K. Upregulated glial cell line-derived neurotrophic factor through cyclooxygenase-2 activation in the muscle is required for mechanical hyperalgesia after exercise in rats. J. Physiol. 2013, 591, 3035–3048. [Google Scholar] [CrossRef]
- Bespalov, M.M.; Sidorova, Y.A.; Tumova, S.; Ahonen-Bishopp, A.; Magalhães, A.C.; Kulesskiy, E.; Paveliev, M.; Rivera, C.; Rauvala, H.; Saarma, M. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J. Cell Biol. 2011, 192, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Murase, S.; Kobayashi, K.; Nasu, T.; Kihara, C.; Taguchi, T.; Mizumura, K. Synergistic interaction of nerve growth factor and glial cell-line derived neurotrophic factor in muscular mechanical hyperalgesia in rats. J. Physiol. 2021, 599, 1783–1798. [Google Scholar] [CrossRef] [PubMed]
- Murase, S.; Terazawa, E.; Queme, F.; Ota, H.; Matsuda, T.; Hirate, K.; Kozaki, Y.; Katanosaka, K.; Taguchi, T.; Urai, H.; et al. Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness). J. Neurosci. 2010, 30, 3752–3761. [Google Scholar] [CrossRef]
- Tegeder, I.; Zimmermann, J.; Meller, S.; Geisslinger, G. Release of algesic substances in human experimental muscle pain. Inflamm. Res. 2002, 51, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Lambaerts, K.; Wilcox-Adelman, S.A.; Zimmermann, P. The signaling mechanisms of syndecan heparan sulfate proteoglycans. Curr. Opin. Cell Biol. 2009, 21, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.O.; Caires, R.; Kaitlyn Victor, A.; Ramirez, J.; Sierra-Valdez, F.J.; Walsh, P.; Truong, V.; Lee, J.; Mayor, U.; Reiter, L.T.; et al. Linoleic acid improves PIEZO2 dysfunction in a mouse model of Angelman Syndrome. Nat. Commun. 2023, 14, 1167. [Google Scholar] [CrossRef]
- Hartmann, H.; Ho, W.Y.; Chang, J.; Ling, S. Cholesterol dyshomeostasis in amyotrophic lateral sclerosis: Cause, consequence, or epiphenomenon? FEBS J. 2022, 289, 7688–7709. [Google Scholar] [CrossRef]
- Thompson, K.J.; Watson, S.; Zanato, C.; Dall’Angelo, S.; De Nooij, J.C.; Pace-Bonello, B.; Shenton, F.C.; Sanger, H.E.; Heinz, B.A.; Broad, L.M.; et al. The atypical ‘hippocampal’ glutamate receptor coupled to phospholipase D that controls stretch-sensitivity in primary mechanosensory nerve endings is homomeric purely metabotropic GluK2. Exp. Physiol. 2023, 109, 81–99. [Google Scholar] [CrossRef]
- Sonkodi, B.; Pállinger, É.; Radovits, T.; Csulak, E.; Shenker-Horváth, K.; Kopper, B.; Buzás, E.I.; Sydó, N.; Merkely, B. CD3+/CD56+ NKT-like Cells Show Imbalanced Control Immediately after Exercise in Delayed-Onset Muscle Soreness. Int. J. Mol. Sci. 2022, 23, 11117. [Google Scholar] [CrossRef]
- Sonkodi, B.; Hortobágyi, T. Amyotrophic lateral sclerosis and delayed onset muscle soreness in light of the impaired blink and stretch reflexes—Watch out for Piezo2. Open Med. 2022, 17, 397–402. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Sadasivam, M.; A Hamad, A.R. Unexpected alliance between syndecan-1 and innate-like T cells to protect host from autoimmune effects of interleukin-17. World J. Diabetes 2018, 9, 220–225. [Google Scholar] [CrossRef]
- Rentzos, M.; Evangelopoulos, E.; Sereti, E.; Zouvelou, V.; Marmara, S.; Alexakis, T.; Evdokimidis, I. Alterations of T cell subsets in ALS: A systemic immune activation? Acta Neurol. Scand. 2012, 125, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Nógrádi, A.; Domoki, F.; Dégi, R.; Borda, S.; Pákáski, M.; Szabó, A.; Bari, F. Up-regulation of cerebral carbonic anhydrase by anoxic stress in piglets. J. Neurochem. 2003, 85, 843–850. [Google Scholar] [CrossRef]
- Liu, X.; Lu, D.; Bowser, R.; Liu, J. Expression of Carbonic Anhydrase I in Motor Neurons and Alterations in ALS. Int. J. Mol. Sci. 2016, 17, 1820. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Mondal, U.K.; Supuran, C.T.; Ilies, M.A.; McKenna, R. Crystal Structure of Carbonic Anhydrase II in Complex with an Activating Ligand: Implications in Neuronal Function. Mol. Neurobiol. 2018, 55, 7431–7437. [Google Scholar] [CrossRef]
- Behan, A.T.; Breen, B.; Hogg, M.; Woods, I.; Coughlan, K.; Mitchem, M.; Prehn, J.H. Acidotoxicity and acid-sensing ion channels contribute to motoneuron degeneration. Cell Death Differ. 2013, 20, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Shenton, F.; Hunter, I.; Banks, R.W.; Bewick, G.S. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J. Physiol. 2010, 588, 171–185. [Google Scholar] [CrossRef]
- Bornstein, B.; Watkins, B.; Passini, F.S.; Blecher, R.; Assaraf, E.; Sui, X.M.; Brumfeld, V.; Tsoory, M.; Kröger, S.; Zelzer, E. The mechanosensitive ion channel ASIC2 mediates both proprioceptive sensing and spinal alignment. Exp. Physiol. 2023, 109, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-H.; Lukacs, V.; de Nooij, J.C.; Zaytseva, D.; Criddle, C.R.; Francisco, A.; Jessell, T.M.; A Wilkinson, K.; Patapoutian, A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 2015, 18, 1756–1762. [Google Scholar] [CrossRef]
- Assaraf, E.; Blecher, R.; Heinemann-Yerushalmi, L.; Krief, S.; Vinestock, R.C.; Biton, I.E.; Brumfeld, V.; Rotkopf, R.; Avisar, E.; Agar, G.; et al. Piezo2 expressed in proprioceptive neurons is essential for skeletal integrity. Nat. Commun. 2020, 11, 3168. [Google Scholar] [CrossRef]
- Sonkodi, B.; Hegedűs, Á.; Kopper, B.; Berkes, I. Significantly Delayed Medium-Latency Response of the Stretch Reflex in Delayed-Onset Muscle Soreness of the Quadriceps Femoris Muscles Is Indicative of Sensory Neuronal Microdamage. J. Funct. Morphol. Kinesiol. 2022, 7, 43. [Google Scholar] [CrossRef]
- Schwane, J.A.; Watrous, B.G.; Johnson, S.R.; Armstrong, R.B. Is Lactic Acid Related to Delayed-Onset Muscle Soreness? Physician Sportsmed. 1983, 11, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B. Should We Void Lactate in the Pathophysiology of Delayed Onset Muscle Soreness? Not So Fast! Let’s See a Neurocentric View! Metabolites 2022, 12, 857. [Google Scholar] [CrossRef] [PubMed]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Schneider, U.; Poole, R.; Halestrap, A.; Grafe, P. Lactate-proton co-transport and its contribution to interstitial acidification during hypoxia in isolated rat spinal roots. Neuroscience 1993, 53, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Ng, C.P.; Jones, O.; Fung, T.S.; Ryu, K.W.; Li, D.; Thompson, C.B. Lactate activates the mitochondrial electron transport chain independently of its metabolism. Mol. Cell 2023, 83, 3904–3920.e3907. [Google Scholar] [CrossRef]
- Brookes, P.S. Mitochondrial H+ leak and ROS generation: An odd couple. Free. Radic. Biol. Med. 2005, 38, 12–23. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Tian, X.Y.; Wong, W.T.; Xu, A.; Lu, Y.; Zhang, Y.; Wang, L.; Cheang, W.S.; Wang, Y.; Yao, X.; Huang, Y.; et al. Uncoupling Protein-2 protects endothelial function in diet-induced obese mice. Circ. Res. 2012, 110, 1211–1216. [Google Scholar] [CrossRef]
- Tian, S.; Cai, Z.; Sen, P.; van Uden, D.; van de Kamp, E.; Thuillet, R.; Tu, L.; Guignabert, C.; Boomars, K.; Van der Heiden, K.; et al. Loss of lung microvascular endothelial Piezo2 expression impairs NO synthesis, induces EndMT, and is associated with pulmonary hypertension. Am. J. Physiol. Circ. Physiol. Heart 2022, 323, H958–H974. [Google Scholar] [CrossRef]
- Karagiannis, A.; Gallopin, T.; Lacroix, A.; Plaisier, F.; Piquet, J.; Geoffroy, H.; Hepp, R.; Naudé, J.; Le Gac, B.; Egger, R.; et al. Lactate is an energy substrate for rodent cortical neurons and enhances their firing activity. eLife 2021, 10, 71424. [Google Scholar] [CrossRef]
- Sada, N.; Lee, S.; Katsu, T.; Otsuki, T.; Inoue, T. Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 2015, 347, 1362–1367. [Google Scholar] [CrossRef]
- Eriksen, J.; Chang, R.; McGregor, M.; Silm, K.; Suzuki, T.; Edwards, R.H. Protons Regulate Vesicular Glutamate Transporters through an Allosteric Mechanism. Neuron 2016, 90, 768–780. [Google Scholar] [CrossRef]
- Takamori, S. VGLUTs: ‘Exciting’ times for glutamatergic research? Neurosci. Res. 2006, 55, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Jamali, S.; Klier, M.; Ames, S.; Barros, L.F.; McKenna, R.; Deitmer, J.W.; Becker, H.M. Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function. Sci. Rep. 2015, 5, 13605. [Google Scholar] [CrossRef]
- Hirano, K.; Tsuchiya, M.; Shiomi, A.; Takabayashi, S.; Suzuki, M.; Ishikawa, Y.; Kawano, Y.; Takabayashi, Y.; Nishikawa, K.; Nagao, K.; et al. The mechanosensitive ion channel PIEZO1 promotes satellite cell function in muscle regeneration. Life Sci. Alliance 2023, 6, e202201783. [Google Scholar] [CrossRef]
- Kumar, A.; Pyaram, K.; Yarosz, E.L.; Hong, H.; Lyssiotis, C.A.; Giri, S.; Chang, C.-H. Enhanced oxidative phosphorylation in NKT cells is essential for their survival and function. Proc. Natl. Acad. Sci. USA 2019, 116, 7439–7448. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Pellerin, L. Astrocytes Couple Synaptic Activity to Glucose Utilization in the Brain. News Physiol. Sci. 1999, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Mason, S. Lactate Shuttles in Neuroenergetics—Homeostasis, Allostasis and Beyond. Front. Neurosci. 2017, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, P.; Rucco, R.; Jacini, F.; Trojsi, F.; Lardone, A.; Baselice, F.; Femiano, C.; Santangelo, G.; Granata, C.; Vettoliere, A.; et al. Brain functional networks become more connected as amyotrophic lateral sclerosis progresses: A source level magnetoencephalographic study. NeuroImage: Clin. 2018, 20, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Cathers, I.; O’dwyer, N.; Neilson, P. Entrainment to extinction of physiological tremor by spindle afferent input. Exp. Brain Res. 2006, 171, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Christidi, F.; Argyropoulos, G.D.; Karavasilis, E.; Velonakis, G.; Zouvelou, V.; Kourtesis, P.; Pantoleon, V.; Tan, E.L.; Daponte, A.; Aristeidou, S.; et al. Hippocampal Metabolic Alterations in Amyotrophic Lateral Sclerosis: A Magnetic Resonance Spectroscopy Study. Life 2023, 13, 571. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, S.; Machts, J.; Kaufmann, J.; Patrick, K.; Kollewe, K.; Dengler, R.; Heinze, H.-J.; Petri, S.; Vielhaber, S.; Nestor, P.J. Hippocampal degeneration in patients with amyotrophic lateral sclerosis. Neurobiol. Aging 2014, 35, 2639–2645. [Google Scholar] [CrossRef]
- Machts, J.; Vielhaber, S.; Kollewe, K.; Petri, S.; Kaufmann, J.; Schoenfeld, M.A. Global Hippocampal Volume Reductions and Local CA1 Shape Deformations in Amyotrophic Lateral Sclerosis. Front. Neurol. 2018, 9, 565. [Google Scholar] [CrossRef]
- Cornelison, D.; Wilcox-Adelman, S.A.; Goetinck, P.F.; Rauvala, H.; Rapraeger, A.C.; Olwin, B.B. Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes Dev. 2004, 18, 2231–2236. [Google Scholar] [CrossRef]
- Abarkan, M.; Gaitan, J.; Lebreton, F.; Perrier, R.; Jaffredo, M.; Mulle, C.; Magnan, C.; Raoux, M.; Lang, J. The glutamate receptor GluK2 contributes to the regulation of glucose homeostasis and its deterioration during aging. Mol. Metab. 2019, 30, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Brice, N.L.; Varadi, A.; Ashcroft, S.J.H.; Molnar, E. Metabotropic glutamate and GABAB receptors contribute to the modulation of glucose-stimulated insulin secretion in pancreatic beta cells. Diabetologia 2002, 45, 242–252. [Google Scholar] [CrossRef]
- Ye, Y.; Barghouth, M.; Dou, H.; Luan, C.; Wang, Y.; Karagiannopoulos, A.; Jiang, X.; Krus, U.; Fex, M.; Zhang, Q.; et al. A critical role of the mechanosensor PIEZO1 in glucose-induced insulin secretion in pancreatic β-cells. Nat. Commun. 2022, 13, 4237. [Google Scholar] [CrossRef] [PubMed]
- Berndt, C.; Casaroli-Marano, R.P.; Vilaró, S.; Reina, M. Cloning and characterization of human syndecan-3. J. Cell. Biochem. 2001, 82, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B.; Radovits, T.; Csulak, E.; Kopper, B.; Sydó, N.; Merkely, B. Orthostasis Is Impaired Due to Fatiguing Intensive Acute Concentric Exercise Succeeded by Isometric Weight-Loaded Wall-Sit in Delayed-Onset Muscle Soreness: A Pilot Study. Sports 2023, 11, 209. [Google Scholar] [CrossRef]
- Dodge, J.C.; Treleaven, C.M.; Fidler, J.A.; Tamsett, T.J.; Bao, C.; Searles, M.; Taksir, T.V.; Misra, K.; Sidman, R.L.; Cheng, S.H.; et al. Metabolic signatures of amyotrophic lateral sclerosis reveal insights into disease pathogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 10812–10817. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, P.; Kolonics, A.; Aczel, D.; Zhou, L.; Mozaffaritabar, S.; Molnár, K.; László, L.; Kutasi, B.; Tanisawa, K.; Park, J.; et al. Voluntary exercise does not increase gastrointestinal motility but increases spatial memory, intestinal eNOS, Akt levels, and Bifidobacteria abundance in the microbiome. Front. Physiol. 2023, 14, 1173636. [Google Scholar] [CrossRef]
- Chi, S.; Cui, Y.; Wang, H.; Jiang, J.; Zhang, T.; Sun, S.; Zhou, Z.; Zhong, Y.; Xiao, B. Astrocytic Piezo1-mediated mechanotransduction determines adult neurogenesis and cognitive functions. Neuron 2022, 110, 2984–2999.e2988. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B.; Csorba, A.; Marsovszky, L.; Balog, A.; Kopper, B.; Nagy, Z.Z.; Resch, M.D. Evidence of Disruption in Neural Regeneration in Dry Eye Secondary to Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 7514. [Google Scholar] [CrossRef] [PubMed]
- Nasu, T.; Hori, A.; Hotta, N.; Kihara, C.; Kubo, A.; Katanosaka, K.; Suzuki, M.; Mizumura, K. Vacuolar-ATPase-mediated muscle acidification caused muscular mechanical nociceptive hypersensitivity after chronic stress in rats, which involved extracellular matrix proteoglycan and ASIC3. Sci. Rep. 2023, 13, 13585. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Warita, H.; Aoki, M.; Itoyama, Y. Accumulation of chondroitin sulfate proteoglycans in the microenvironment of spinal motor neurons in amyotrophic lateral sclerosis transgenic rats. J. Neurosci. Res. 2008, 86, 2512–2523. [Google Scholar] [CrossRef]
- Kirby, J.; Ning, K.; Ferraiuolo, L.; Heath, P.R.; Ismail, A.; Kuo, S.-W.; Valori, C.F.; Cox, L.; Sharrack, B.; Wharton, S.B.; et al. Phosphatase and tensin homologue/protein kinase B pathway linked to motor neuron survival in human superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain 2011, 134, 506–517. [Google Scholar] [CrossRef]
- Efraim, Y.; Chen, F.Y.T.; Cheong, K.N.; Gaylord, E.A.; McNamara, N.A.; Knox, S.M. A synthetic tear protein resolves dry eye through promoting corneal nerve regeneration. Cell Rep. 2022, 40, 111307. [Google Scholar] [CrossRef]
- Reizes, O.; Lincecum, J.; Wang, Z.; Goldberger, O.; Huang, L.; Kaksonen, M.; Ahima, R.; Hinkes, M.T.; Barsh, G.S.; Rauvala, H.; et al. Transgenic expression of syndecan-1 uncovers a physiological control of feeding behavior by syndecan-3. Cell 2001, 106, 105–116. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Min, S.; Oh, Y.; Verma, P.; Whitehead, S.C.; Yapici, N.; Van Vactor, D.; Suh, G.S.; Liberles, S. Control of feeding by Piezo-mediated gut mechanosensation in Drosophila. eLife 2021, 10, 63049. [Google Scholar] [CrossRef] [PubMed]
- Servin-Vences, M.R.; Lam, R.M.; Koolen, A.; Wang, Y.; Saade, D.N.; Loud, M.; Kacmaz, H.; Frausto, S.; Zhang, Y.; Beyder, A.; et al. PIEZO2 in somatosensory neurons controls gastrointestinal transit. Cell 2023, 186, 3386–3399.e3315. [Google Scholar] [CrossRef]
- Toepfer, M.; Folwaczny, C.; Klauser, A.; Riepl, R.L.; Muller-Felber, W.; Pongratz, D. Gastrointestinal dysfunction in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 1999, 1, 15–19. [Google Scholar] [CrossRef]
- Moglia, C.; Calvo, A.; Grassano, M.; Canosa, A.; Manera, U.; D’Ovidio, F.; Bombaci, A.; Bersano, E.; Mazzini, L.; Mora, G.; et al. Early weight loss in amyotrophic lateral sclerosis: Outcome relevance and clinical correlates in a population-based cohort. J. Neurol. Neurosurg. Psychiatry 2019, 90, 666–673. [Google Scholar] [CrossRef]
- Wei, Q.-Q.; Ou, R.; Cao, B.; Chen, Y.; Hou, Y.; Zhang, L.; Wu, F.; Shang, H. Early weight instability is associated with cognitive decline and poor survival in amyotrophic lateral sclerosis. Brain Res. Bull. 2021, 171, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, S.; Chen, L.; Tang, L.; Fan, D. Loss of appetite in patients with amyotrophic lateral sclerosis is associated with weight loss and anxiety/depression. Sci. Rep. 2021, 11, 9119. [Google Scholar] [CrossRef]
- Cornelison, D.; Filla, M.S.; Stanley, H.M.; Rapraeger, A.C.; Olwin, B.B. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev. Biol. 2001, 239, 79–94. [Google Scholar] [CrossRef]
- Kang, H.; Hong, Z.; Zhong, M.; Klomp, J.; Bayless, K.J.; Mehta, D.; Karginov, A.V.; Hu, G.; Malik, A.B. Piezo1 mediates angiogenesis through activation of MT1-MMP signaling. Am. J. Physiol. Cell. Physiol. 2019, 316, C92–C103. [Google Scholar] [CrossRef]
- Maves, L.; Waskiewicz, A.J.; Paul, B.; Cao, Y.; Tyler, A.; Moens, C.B.; Tapscott, S.J. Pbx homeodomain proteins direct Myod activity to promote fast-muscle differentiation. Development 2007, 134, 3371–3382. [Google Scholar] [CrossRef]
- Park, K.H.J.; Franciosi, S.; Leavitt, B.R. Postnatal muscle modification by myogenic factors modulates neuropathology and survival in an ALS mouse model. Nat. Commun. 2013, 4, 2906. [Google Scholar] [CrossRef] [PubMed]
- Scaricamazza, S.; Salvatori, I.; Ferri, A.; Valle, C. Skeletal Muscle in ALS: An Unappreciated Therapeutic Opportunity? Cells 2021, 10, 525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ma, X.; Lin, Y.; Cheng, D.; Bavi, N.; Secker, G.A.; Li, J.V.; Janbandhu, V.; Sutton, D.L.; Scott, H.S.; et al. MyoD-family inhibitor proteins act as auxiliary subunits of Piezo channels. Science 2023, 381, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.; Jørgensen, L.H.; Bech, R.D.; Frandsen, U.; Schrøder, H.D. Skeletal Muscle Remodelling as a Function of Disease Progression in Amyotrophic Lateral Sclerosis. BioMed. Res. Int. 2016, 2016, 5930621. [Google Scholar] [CrossRef]
- Proietti, T.; O’neill, C.; Gerez, L.; Cole, T.; Mendelowitz, S.; Nuckols, K.; Hohimer, C.; Lin, D.; Paganoni, S.; Walsh, C. Restoring arm function with a soft robotic wearable for individuals with amyotrophic lateral sclerosis. Sci. Transl. Med. 2023, 15, eadd1504. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, R.; Sala, R.; Regondi, S.; Beltrami, B.; Lunetta, C. Emerging technologies for management of patients with amyotrophic lateral sclerosis: From telehealth to assistive robotics and neural interfaces. J. Neurol. 2022, 269, 2910–2921. [Google Scholar] [CrossRef] [PubMed]
- Morioka, H.; Hirayama, T.; Sugisawa, T.; Murata, K.; Shibukawa, M.; Ebina, J.; Sawada, M.; Hanashiro, S.; Nagasawa, J.; Yanagihashi, M.; et al. Robot-assisted training using hybrid assistive limb ameliorates gait ability in patients with amyotrophic lateral sclerosis. J. Clin. Neurosci. 2022, 99, 158–163. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, B.-C.; Kulkarni, P.; Andrews, J.; Shneider, N.A.; Agrawal, S. Amyotrophic Lateral Sclerosis Patients Regain Head-Neck Control Using a Powered Neck Exoskeleton. In Proceedings of the 2022 9th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), Seoul, Republic of Korea, 21–24 August 2022; pp. 1–6. [Google Scholar]
- Keriven, H.; Sierra, A.S.; De-La-Flor, G.; García-Arrabé, M.; Bravo-Aguilar, M.; Frutos, M.d.l.P.S.; Garcia-Perez-De-Sevilla, G.; Tornero-Aguilera, J.F.; Clemente-Suarez, V.J.; Domínguez-Balmaseda, D. Effects of combined treatment with transcranial and peripheral electromagnetic stimulation on performance and pain recovery from delayed onset muscle soreness induced by eccentric exercise in young athletes. A randomized clinical trial. Front. Physiol. 2023, 14, 1267315. [Google Scholar] [CrossRef]
- Musarò, A.; Dobrowolny, G.; Cambieri, C.; Onesti, E.; Ceccanti, M.; Frasca, V.; Pisano, A.; Cerbelli, B.; Lepore, E.; Ruffolo, G.; et al. Neuromuscular magnetic stimulation counteracts muscle decline in ALS patients: Results of a randomized, double-blind, controlled study. Sci. Rep. 2019, 9, 2837. [Google Scholar] [CrossRef]
- Floyd, A.G.; Yu, Q.P.; Piboolnurak, P.; Tang, M.X.; Fang, Y.; Smith, W.A.; Yim, J.; Rowland, L.P.; Mitsumoto, H.; Pullman, S.L. Transcranial magnetic stimulation in ALS: Utility of central motor conduction tests. Neurology 2009, 72, 498–504. [Google Scholar] [CrossRef]
- Vucic, S.; Ziemann, U.; Eisen, A.; Hallett, M.; Kiernan, M.C. Transcranial magnetic stimulation and amyotrophic lateral sclerosis: Pathophysiological insights. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1161–1170. [Google Scholar] [CrossRef]
- Kandhavivorn, W.; Glaß, H.; Herrmannsdörfer, T.; Böckers, T.M.; Uhlarz, M.; Gronemann, J.; Funk, R.H.W.; Pietzsch, J.; Pal, A.; Hermann, A. Restoring Axonal Organelle Motility and Regeneration in Cultured FUS-ALS Motoneurons through Magnetic Field Stimulation Suggests an Alternative Therapeutic Approach. Cells 2023, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, M.; Onesti, E.; Rubino, A.; Cambieri, C.; Tartaglia, G.; Miscioscia, A.; Frasca, V.; Inghilleri, M. Modulation of human corticospinal excitability by paired associative stimulation in patients with amyotrophic lateral sclerosis and effects of Riluzole. Brain Stimul. 2018, 11, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Guida, N.; Sanguigno, L.; Mascolo, L.; Calabrese, L.; Serani, A.; Molinaro, P.; Lau, C.G.; Annunziato, L.; Formisano, L. The Transcriptional Complex Sp1/KMT2A by Up-Regulating Restrictive Element 1 Silencing Transcription Factor Accelerates Methylmercury-Induced Cell Death in Motor Neuron-Like NSC34 Cells Overexpressing SOD1-G93A. Front. Neurosci. 2021, 15, 771580. [Google Scholar] [CrossRef] [PubMed]
- Hounoum, B.M.; Vourc’h, P.; Felix, R.; Corcia, P.; Patin, F.; Guéguinou, M.; Potier-Cartereau, M.; Vandier, C.; Raoul, C.; Andres, C.R.; et al. NSC-34 Motor Neuron-Like Cells Are Unsuitable as Experimental Model for Glutamate-Mediated Excitotoxicity. Front. Cell. Neurosci. 2016, 10, 118. [Google Scholar] [CrossRef]
- Laudati, G.; Mascolo, L.; Guida, N.; Sirabella, R.; Pizzorusso, V.; Bruzzaniti, S.; Serani, A.; Di Renzo, G.; Canzoniero, L.M.; Formisano, L. Resveratrol treatment reduces the vulnerability of SH-SY5Y cells and cortical neurons overexpressing SOD1-G93A to Thimerosal toxicity through SIRT1/DREAM/PDYN pathway. NeuroToxicology 2019, 71, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Yang, Q.; Cao, Y.; Dong, Y.; Bi, Y.; Liu, G. Immunoregulatory Role of the Mechanosensitive Ion Channel Piezo1 in Inflammation and Cancer. Molecules 2022, 28, 213. [Google Scholar] [CrossRef]
- Bonifacino, T.; Zerbo, R.A.; Balbi, M.; Torazza, C.; Frumento, G.; Fedele, E.; Bonanno, G.; Milanese, M. Nearly 30 Years of Animal Models to Study Amyotrophic Lateral Sclerosis: A Historical Overview and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 12236. [Google Scholar] [CrossRef]
| Amyotrophic Lateral Sclerosis (ALS) | Delayed-Onset Muscle Soreness (DOMS) | |||
|---|---|---|---|---|
| Primary intrafusal damage phase | YES | Irreversible intrafusal proprioceptive terminal microdamage | YES | Transient intrafusal proprioceptive terminal microdamage |
| YES | Irreversibly lost imposed movement-based low-frequency forcing oscillation from the Type Ia afferent signal due to irreversible Piezo2 channelopathy ** | YES | Transiently lost imposed movement-based low-frequency forcing oscillation from the Type Ia afferent signal due to transient Piezo2 channelopathy ** | |
| NO | Lost NMDA receptor activation on spinal dorsal horn due to irreversibly lost glutamate vesicular release on type Ia proprioceptive neurons | YES | NMDA receptor activation on spinal dorsal horn due to impairment of the glutamate vesicular release on type Ia proprioceptive neurons | |
| NO | Lost L-type calcium currents and nonspecific cationic currents in spinal dorsal horn due to lost Piezo2 functional at peripheral proprioceptive terminals | YES | L-type calcium currents and nonspecific cationic currents in spinal dorsal horn due to peripheral proprioceptive terminal Piezo2 channelopathy induced subthreshold imbalanced calcium currents | |
| NO | Wide dynamic range (WDR) neuron activation | YES | WDR neuron activation | |
| Soreness condition | Considered as a painless disease | DOMS lasting up to 7 days | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonkodi, B. Progressive Irreversible Proprioceptive Piezo2 Channelopathy-Induced Lost Forced Peripheral Oscillatory Synchronization to the Hippocampal Oscillator May Explain the Onset of Amyotrophic Lateral Sclerosis Pathomechanism. Cells 2024, 13, 492. https://doi.org/10.3390/cells13060492
Sonkodi B. Progressive Irreversible Proprioceptive Piezo2 Channelopathy-Induced Lost Forced Peripheral Oscillatory Synchronization to the Hippocampal Oscillator May Explain the Onset of Amyotrophic Lateral Sclerosis Pathomechanism. Cells. 2024; 13(6):492. https://doi.org/10.3390/cells13060492
Chicago/Turabian StyleSonkodi, Balázs. 2024. "Progressive Irreversible Proprioceptive Piezo2 Channelopathy-Induced Lost Forced Peripheral Oscillatory Synchronization to the Hippocampal Oscillator May Explain the Onset of Amyotrophic Lateral Sclerosis Pathomechanism" Cells 13, no. 6: 492. https://doi.org/10.3390/cells13060492
APA StyleSonkodi, B. (2024). Progressive Irreversible Proprioceptive Piezo2 Channelopathy-Induced Lost Forced Peripheral Oscillatory Synchronization to the Hippocampal Oscillator May Explain the Onset of Amyotrophic Lateral Sclerosis Pathomechanism. Cells, 13(6), 492. https://doi.org/10.3390/cells13060492






