Exploiting Leishmania—Primed Dendritic Cells as Potential Immunomodulators of Canine Immune Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Peripheral Blood Monocyte-Derived Dendritic Cells
2.2. moDC Immunophenotyping
2.3. Leishmania Parasites
2.4. Purification of Extracellular Vesicles Shed by L. infantum and L. amazonensis-Cultured Promastigotes
2.5. moDC Activation
2.6. Microscopic Images of moDCs
2.7. moDC Viability
2.8. Gene Expression of PRRs, Cytokines, and Co-Stimulatory Molecules
2.9. NF-κB Activation
2.10. Chemokines
2.11. Surface Expression of MHC Molecules
2.12. Data Analysis
3. Results
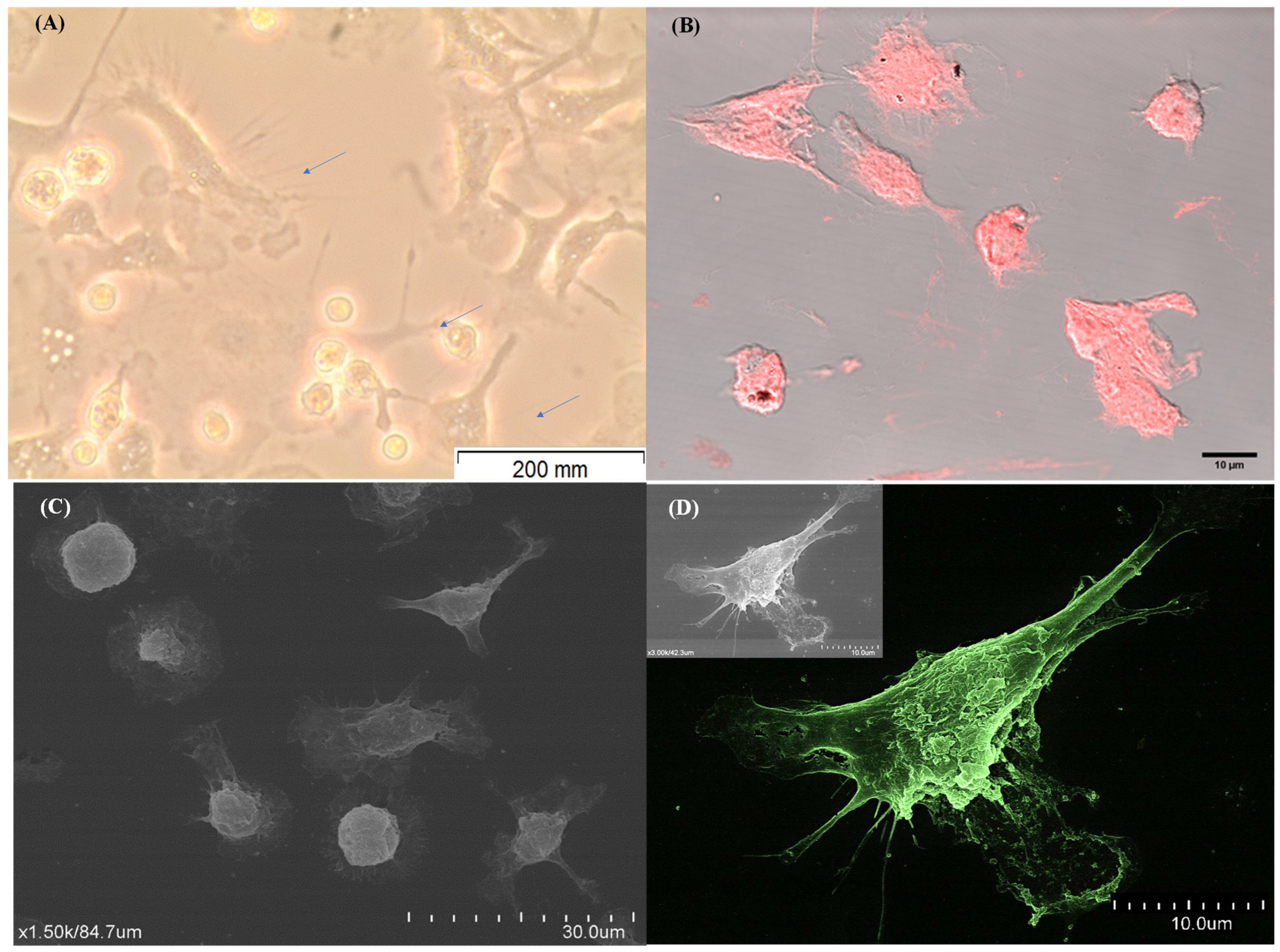
3.1. Monocyte-Differentiated Dendritic Cells Exhibit Characteristic Morphology
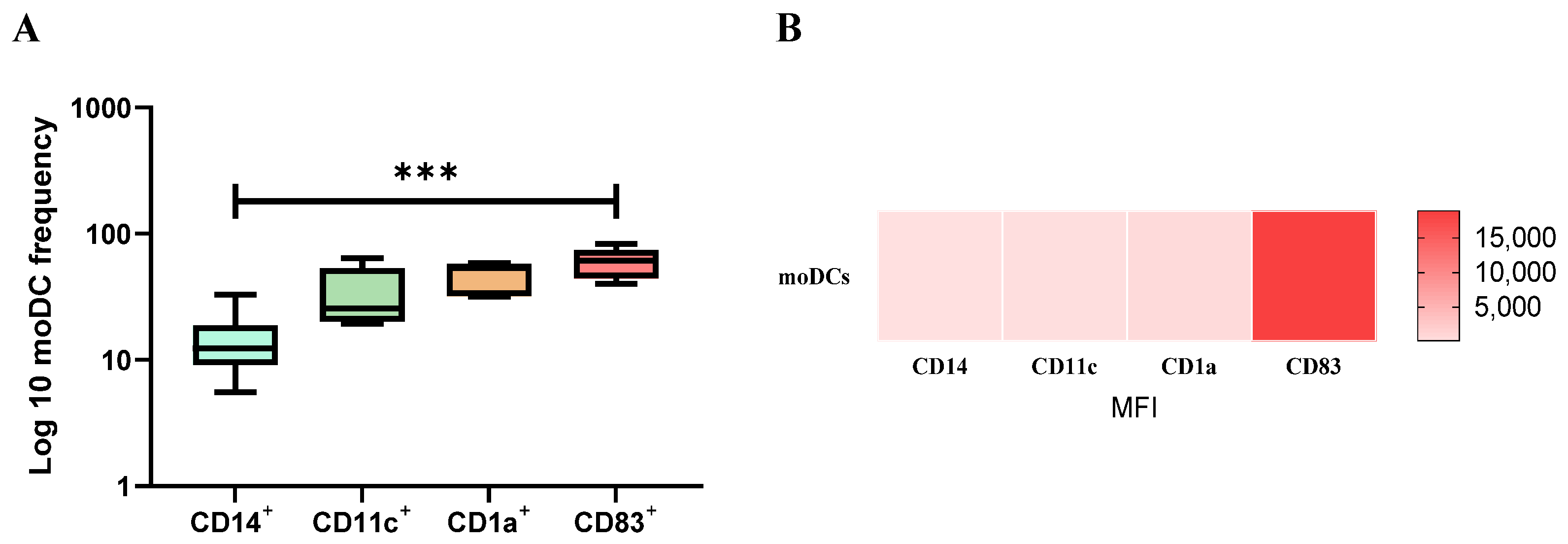
3.2. Monocyte-Differentiated Cells Exhibit a Molecular Signature Compatible with DCs
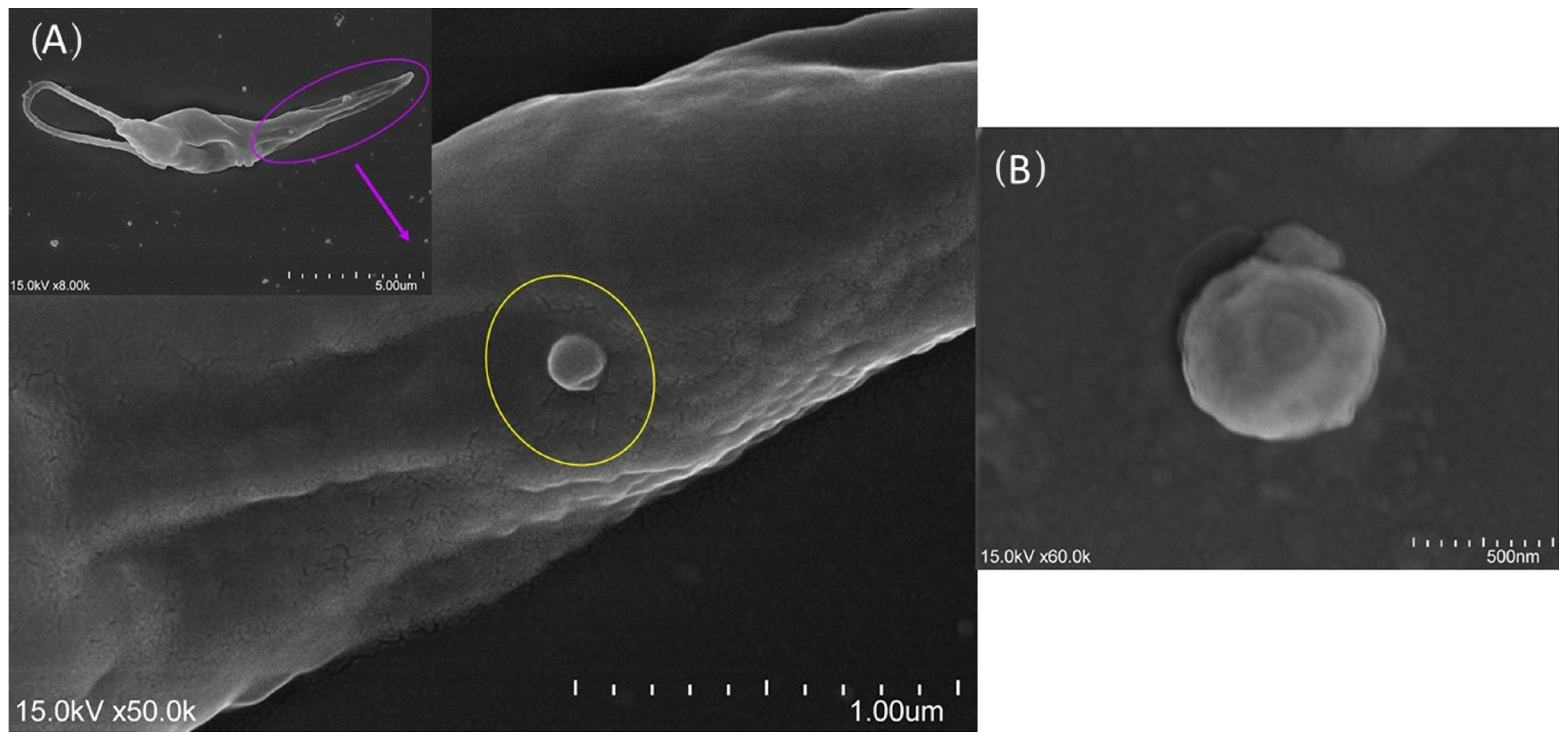
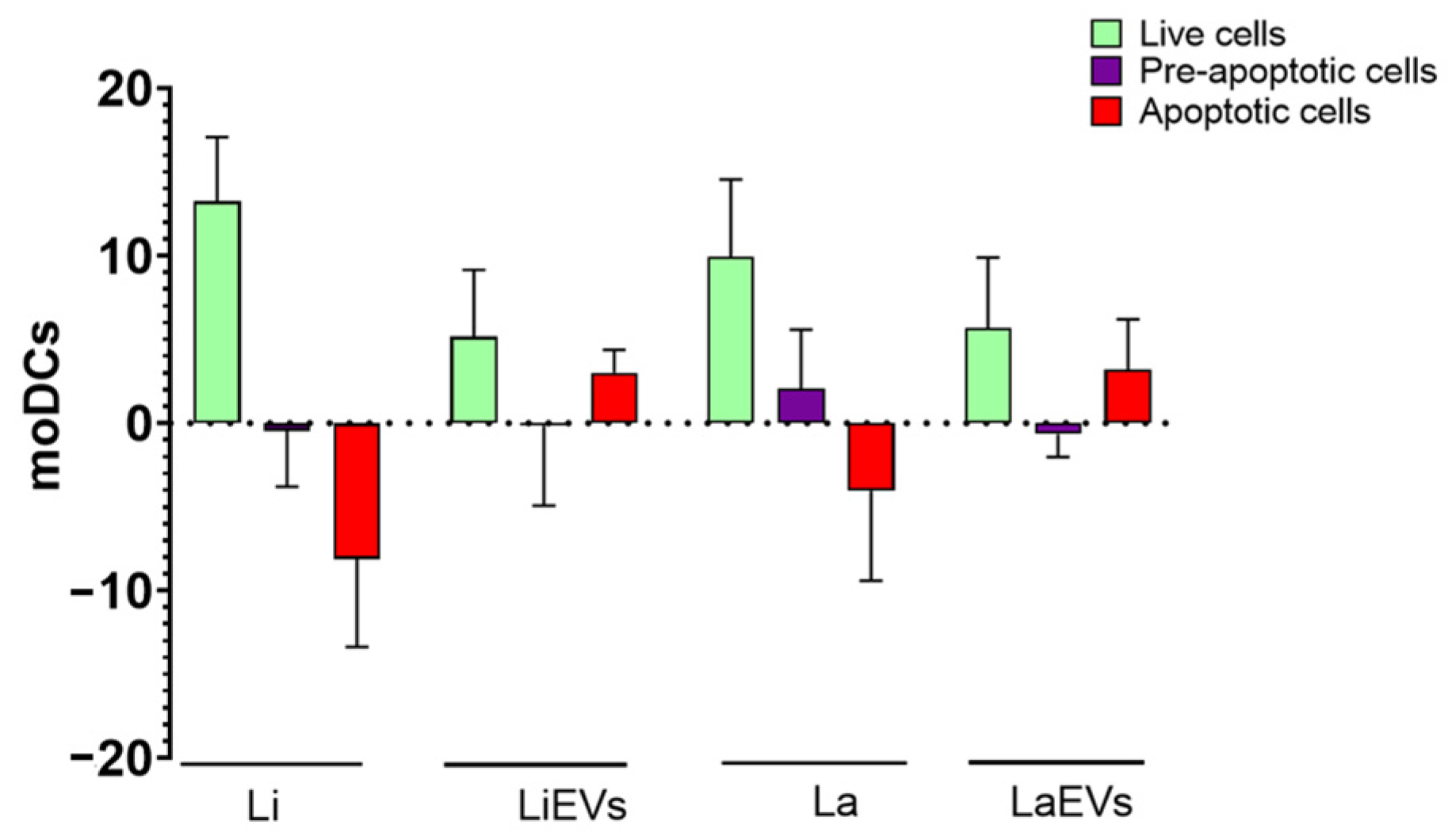
3.3. moDCs Bind and Internalize L. infantum and L. amazonensis Parasites
3.4. L. infantum Parasites Promote TLR4 Gene Expression and Activate NF-κB
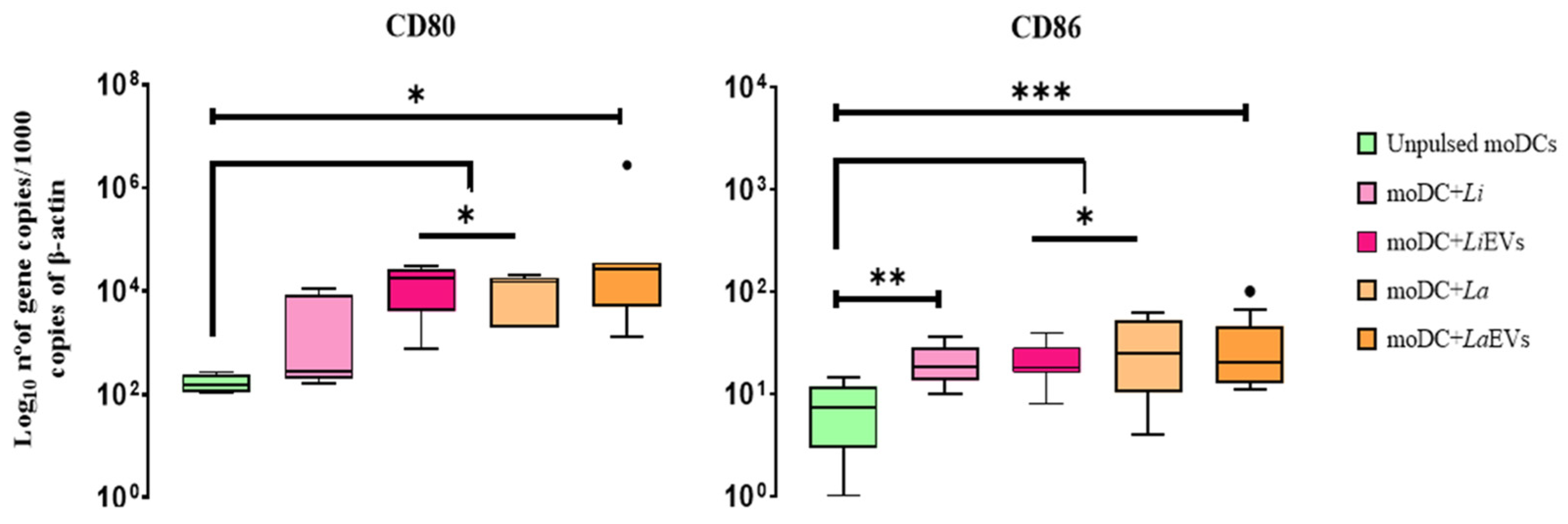
3.5. L. infantum and L. amazonensis EVs Promote Surface Expression of MHCI and Upregulate CD80 and CD86 Co-Stimulatory Molecules
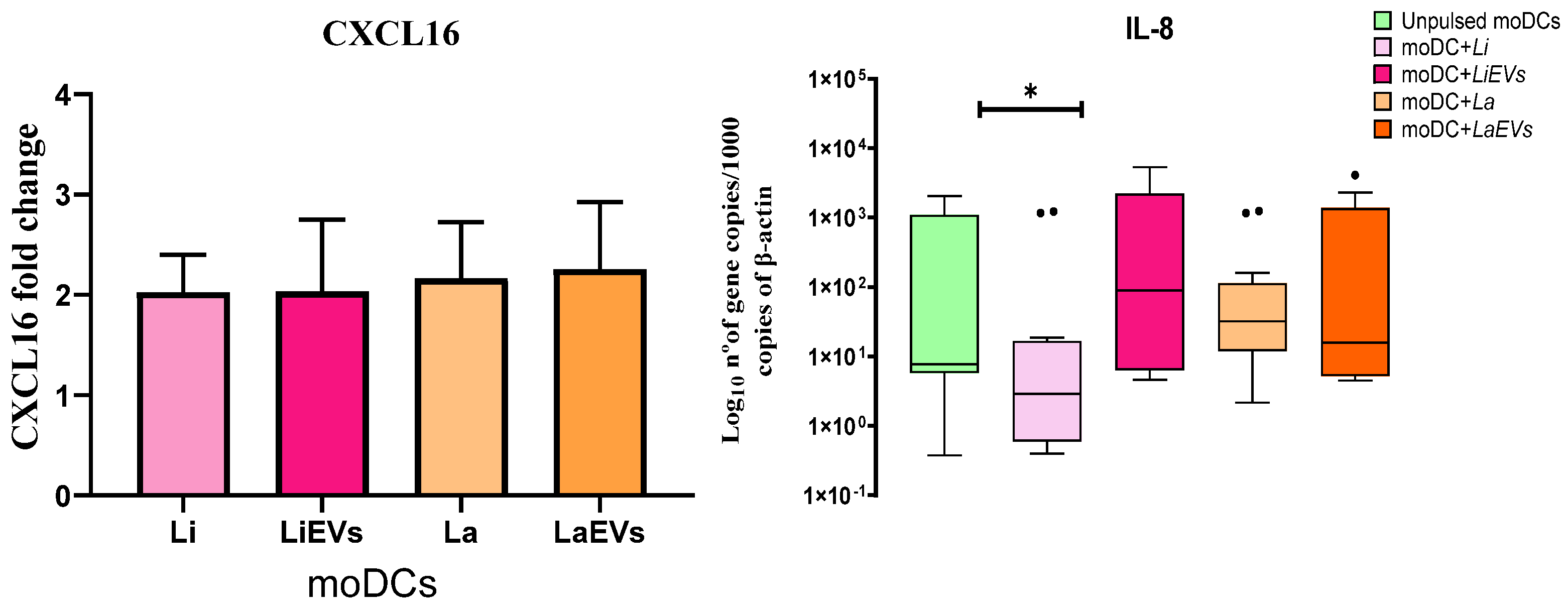
3.6. L. infantum and L. amazonensis Trigger moDCs to Release the Chemokine CXCL16
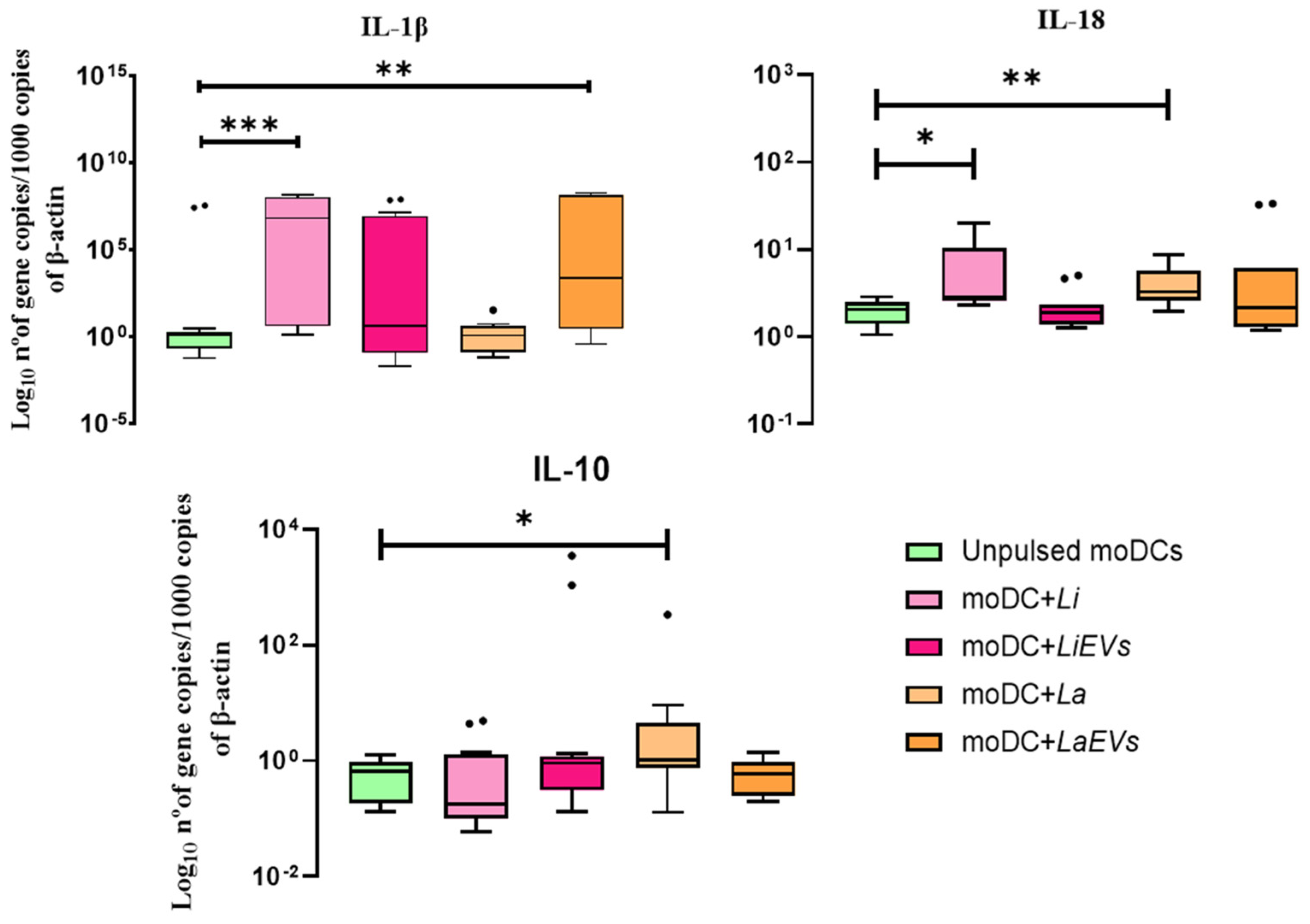
3.7. L. infantum-Infected moDCs Can Generate IL-1β and IL-18
4. Discussion
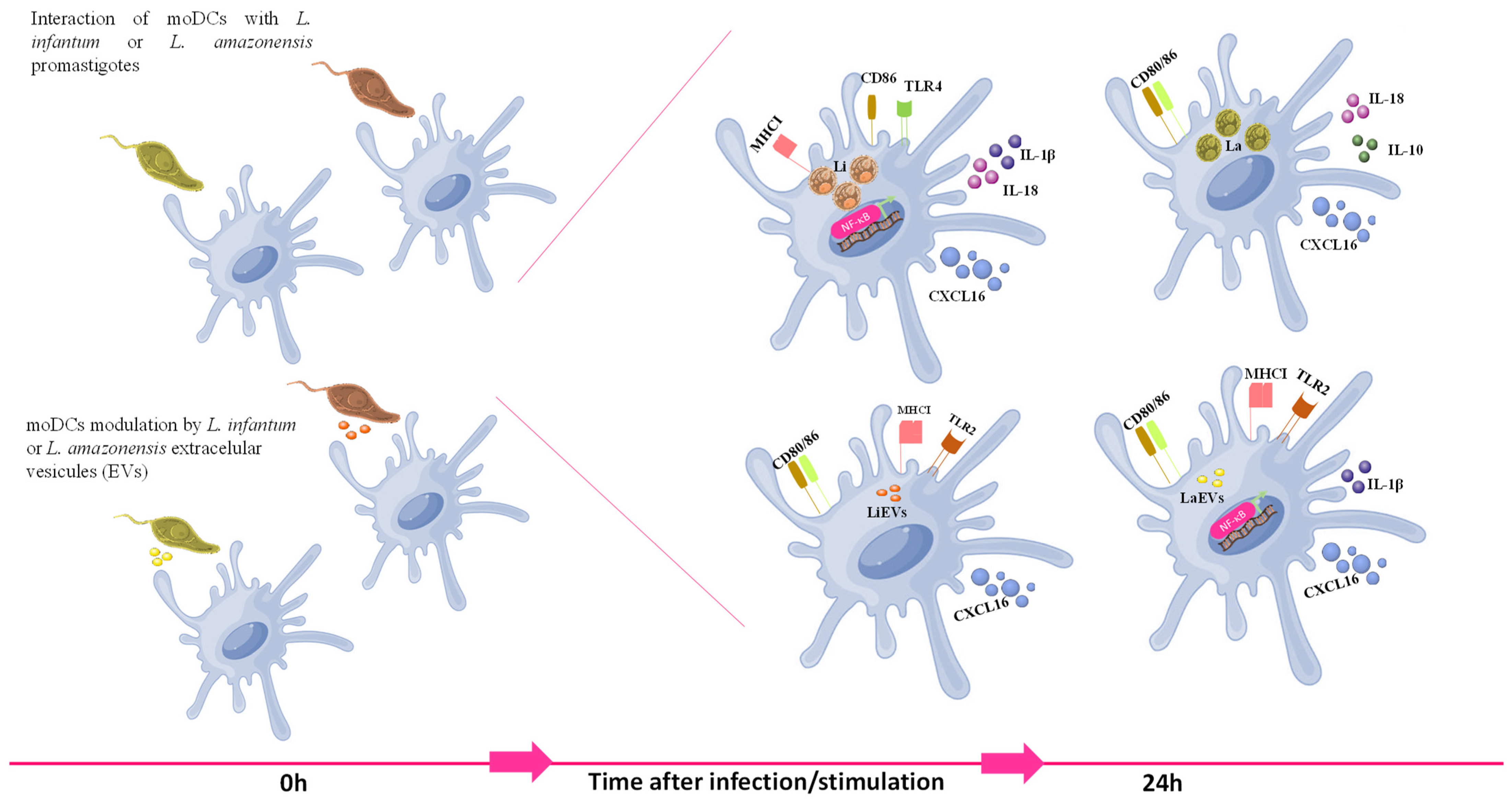
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estevan, L.G.T.M.; Veloso, L.B.; Silva, G.G.; Mori, C.C.; Franco, P.F.; Lima, A.C.V.M.R.; Ássimos, G.R.; Reis, I.A.; Andrade-Filho, J.D.; Araújo, M.S.S.; et al. Leishmania infantum infection rate in dogs housed in open-admission shelters is higher than of domiciled dogs in an endemic area of canine visceral leishmaniasis. Epidemiological implications. Acta Trop. 2022, 232, 106492. [Google Scholar] [CrossRef]
- Lopes, M.F.; Costa-da-Silva, A.C.; DosReis, G.A. Innate immunity to Leishmania infection: Within phagocytes. Mediat. Inflamm. 2014, 2014, 754965. [Google Scholar] [CrossRef]
- Steinman, R.M.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973, 137, 1142–1162. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994, 179, 1109–1118. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, N.; Riol-Blanco, L.; Rodríguez-Fernández, J.L. The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J. Immunol. 2006, 176, 5153–5159. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, S.C.; Williams, A.; Colegio, O.R.; Meng, H.; Strowig, T.; Rongvaux, A.; Henao-Mejia, J.; Thaiss, C.A.; Joly, S.; Gonzalez, D.G.; et al. NLRP10 is a NOD-like receptor essential to initiate adaptive immunity by dendritic cells. Nature 2012, 484, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Gorak, P.M.; Engwerda, C.R.; Kaye, P.M. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 1998, 28, 687–695. [Google Scholar] [CrossRef]
- von Stebut, E.; Belkaid, Y.; Jakob, T.; Sacks, D.L.; Udey, M.C. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: Implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 1998, 188, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- León, B.; López-Bravo, M.; Ardavín, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 2007, 26, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Komai-Koma, M.; Li, D.; Wang, E.; Vaughan, D.; Xu, D. Anti-Toll-like receptor 2 and 4 antibodies suppress inflammatory response in mice. Immunology 2014, 143, 354–362. [Google Scholar] [CrossRef]
- Vargas-Inchaustegui, D.A.; Tai, W.; Xin, L.; Hogg, A.E.; Corry, D.B.; Soong, L. Distinct roles for MyD88 and Toll-like receptor 2 during Leishmania braziliensis infection in mice. Infect. Immun. 2009, 77, 2948–2956. [Google Scholar] [CrossRef]
- de Veer, M.J.; Curtis, J.M.; Baldwin, T.M.; DiDonato, J.A.; Sexton, A.; McConville, M.J.; Handman, E.; Schofield, L. MyD88 is essential for clearance of Leishmania major: Possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 2003, 33, 2822–2831. [Google Scholar] [CrossRef]
- Kropf, P.; Freudenberg, M.A.; Modolell, M.; Price, H.P.; Herath, S.; Antoniazi, S.; Galanos, C.; Smith, D.F.; Müller, I. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect. Immun. 2004, 72, 1920–1928. [Google Scholar] [CrossRef]
- Liese, J.; Schleicher, U.; Bogdan, C. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur. J. Immunol. 2007, 37, 3424–3434. [Google Scholar] [CrossRef]
- Schleicher, U.; Liese, J.; Knippertz, I.; Kurzmann, C.; Hesse, A.; Heit, A.; Fischer, J.A.; Weiss, S.; Kalinke, U.; Kunz, S.; et al. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J. Exp. Med. 2007, 204, 893–906. [Google Scholar] [CrossRef]
- Abou Fakher, F.H.; Rachinel, N.; Klimczak, M.; Louis, J.; Doyen, N. TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions. J. Immunol. 2009, 182, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Berberich, C.; Ramírez-Pineda, J.R.; Hambrecht, C.; Alber, G.; Skeiky, Y.A.; Moll, H. Dendritic cell (DC)-based protection against an intracellular pathogen is dependent upon DC-derived IL-12 and can be induced by molecularly defined antigens. J. Immunol. 2003, 170, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- Bertholet, S.; Goldszmid, R.; Morrot, A.; Debrabant, A.; Afrin, F.; Collazo-Custodio, C.; Houde, M.; Desjardins, M.; Sher, A.; Sacks, D. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J. Immunol. 2006, 177, 3525–3533. [Google Scholar] [CrossRef] [PubMed]
- Quinones, M.; Ahuja, S.K.; Melby, P.C.; Pate, L.; Reddick, R.L.; Ahuja, S.S. Preformed membrane-associated stores of interleukin (IL)-12 are a previously unrecognized source of bioactive IL-12 that is mobilized within minutes of contact with an intracellular parasite. J. Exp. Med. 2000, 192, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Misslitz, A.; Colledge, L.; Aebischer, T.; Blackburn, C.C. Silent infection of bone marrow-derived dendritic cells by Leishmania mexicana amastigotes. Eur. J. Immunol. 2001, 31, 876–883. [Google Scholar] [CrossRef]
- Silverman, J.M.; Clos, J.; Horakova, E.; Wang, A.Y.; Wiesgigl, M.; Kelly, I.; Lynn, M.A.; McMaster, W.R.; Foster, L.J.; Levings, M.K.; et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J. Immunol. 2010, 185, 5011–5022. [Google Scholar] [CrossRef]
- Hassani, K.; Antoniak, E.; Jardim, A.; Olivier, M. Temperature-induced protein secretion by Leishmania mexicana modulates macrophage signalling and function. PLoS ONE 2011, 6, e18724. [Google Scholar] [CrossRef]
- Olivier, M.; Atayde, V.D.; Isnard, A.; Hassani, K.; Shio, M.T. Leishmania virulence factors: Focus on the metalloprotease GP. Microbes Infect. 2012, 14, 1377–1389. [Google Scholar] [CrossRef]
- Hosseini, H.M.; Fooladi, A.A.; Nourani, M.R.; Ghanezadeh, F. The role of exosomes in infectious diseases. Inflamm. Allergy Drug Targets 2013, 12, 29–37. [Google Scholar] [CrossRef]
- Helhazar, M.; Leitão, J.; Duarte, A.; Tavares, L.; da Fonseca, I.P. Natural infection of synathropic rodent species Mus musculus and Rattus norvegicus by Leishmania infantum in Sesimbra and Sintra-Portugal. Parasit. Vectors 2013, 6, 88. [Google Scholar] [CrossRef]
- Pereira, M.A.; Alexandre-Pires, G.; Câmara, M.; Santos, M.; Martins, C.; Rodrigues, A.; Adriana, J.; Passero, L.F.D.; Pereira da Fonseca, I.; Santos-Gomes, G. Canine neutrophils cooperate with macrophages in the early stages of Leishmania infantum in vitro infection. Parasite Immunol. 2019, 41, e12617. [Google Scholar] [CrossRef]
- Ibisch, C.; Pradal, G.; Bach, J.M.; Lieubeau, B. Functional canine dendritic cells can be generated in vitro from peripheral blood mononuclear cells and contain a cytoplasmic ultrastructural marker. J. Immunol. Methods 2005, 298, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Eaton, M.; Mayer, M.; Li, H.; He, D.; Nelson, E.; Christopher-Hennings, J. Porcine reproductive and respiratory syndrome virus productively infects monocyte-derived dendritic cells and compromises their antigen-presenting ability. Arch. Virol. 2007, 152, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.S.; Passero, L.F.; Vale-Gato, I.; Rodrigues, A.; Rodrigues, O.R.; Martins, C.; Correia, I.; Tomás, A.M.; Alexandre-Pires, G.; Ferronha, M.H.; et al. New insights into neutrophil and Leishmania infantum in vitro immune interactions. Comp. Immunol. Microbiol. Infect. Dis. 2015, 40, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.I.; Rodrigues, A.V.; Valério-Bolas, A.; Nunes, T.; Carvalheiro, M.C.; Antunes, W.; Alexandre-Pires, G.; Pereira-da-Fonseca, I.; Santos-Gomes, G. Insights on host-parasite immunomodulation mediated by extracellular vesicles of cutaneous Leishmania shawi and Leishmania guyanensis. Cells 2023, 12, 1101. [Google Scholar] [CrossRef]
- Rodrigues, A.; Claro, M.; Alexandre-Pires, G.; Santos-Mateus, D.; Martins, C.; Valério-Bolas, A.; Rafael-Fernandes, M.; Pereira, M.A.; Pereira da Fonseca, I.; Tomás, A.M.; et al. Leishmania infantum antigens modulate memory cell subsets of liver resident T lymphocytes. Immunobiology 2017, 222, 409–422. [Google Scholar] [CrossRef]
- Argyle, D.J.; McGillivery, C.; Nicolson, L.; Onions, D.E. Cloning, sequencing, and characterization of dog interleukin-18. Immunogenetics 1999, 49, 541–543. [Google Scholar] [CrossRef]
- Yasunaga, S.; Masuda, K.; Ohno, K.; Tsujimoto, H. Antigen-specific enhancements of CD80 mRNA expression in experimentally sensitized dogs with Japanese cedar pollen. J. Vet. Med. Sci. 2003, 65, 295–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peters, I.R.; Helps, C.R.; Calvert, E.L.; Hall, E.J.; Day, M.J. Cytokine mRNA quantification in histologically normal canine duodenal mucosa by real-time RT-PCR. Vet. Immunol. Immunopathol. 2005, 103, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Sauter, S.N.; Allenspach, K.; Gaschen, F.; Gröne, A.; Ontsouka, E.; Blum, J.W. Cytokine expression in an ex vivo culture system of duodenal samples from dogs with chronic enteropathies: Modulation by probiotic bacteria. Domest. Anim. Endocrinol. 2005, 29, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Hashimoto, M.; Oguma, K.; Kano, R.; Moritomo, T.; Hasegawa, A. Molecular cloning and tissue expression of canine Toll-like receptor 2 (TLR2). Vet. Immunol. Immunopathol. 2006, 110, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Hung, S.W.; Jan, T.R.; Liao, K.W.; Cheng, C.H.; Wang, Y.S.; Chu, R.M. CD5-low expression lymphocytes in canine peripheral blood show characteristics of natural killer cells. J. Leukoc. Biol. 2008, 84, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.H.; Noh, D.H.; Song, R.H.; Park, J. Ethyl pyruvate downregulates tumor necrosis factor alpha and interleukin (IL)-6 and upregulates IL-10 in lipopolysaccharide-stimulated canine peripheral blood mononuclear cells. J. Vet. Med. Sci. 2010, 72, 1379–1381. [Google Scholar] [CrossRef]
- Harman, R.M.; Bussche, L.; Ledbetter, E.C.; Van de Walle, G.R. Establishment and characterization of an air-liquid canine corneal organ culture model to study acute herpes keratitis. J. Virol. 2014, 88, 13669–13677. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3- new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, O.R.; Moura, R.A.; Gomes-Pereira, S.; Santos-Gomes, G.M. H-2 complex influences cytokine gene expression in Leishmania infantum-infected macrophages. Cell. Immunol. 2006, 243, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Rojas-Canales, D.M.; Divito, S.J.; Shufesky, W.J.; Stolz, D.B.; Erdos, G.; Sullivan, M.L.; Gibson, G.A.; Watkins, S.C.; Larregina, A.T.; et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J. Clin. Investig. 2016, 126, 2805–2820. [Google Scholar] [CrossRef]
- Giza, H.M.; Bozzacco, L. Unboxing dendritic cells: Tales of multi-faceted biology and function. Immunology 2021, 164, 433–449. [Google Scholar] [CrossRef]
- Carrasco, C.P.; Rigden, R.C.; Schaffner, R.; Gerber, H.; Neuhaus, V.; Inumaru, S.; Takamatsu, H.; Bertoni, G.; McCullough, K.C.; Summerfield, A. Porcine dendritic cells generated in vitro: Morphological, phenotypic and functional properties. Immunology 2001, 104, 175–184. [Google Scholar] [CrossRef]
- Wijewardana, V.; Sugiura, K.; Oichi, T.; Fujimoto, M.; Akazawa, T.; Hatoya, S.; Inaba, M.; Ikehara, S.; Jayaweera, T.S.; Inaba, T. Generation of canine dendritic cells from peripheral blood monocytes without using purified cytokines. Vet. Immunol. Immunopathol. 2006, 114, 37–48. [Google Scholar] [CrossRef]
- Bienzle, D.; Reggeti, F.; Clark, M.E.; Chow, C. Immunophenotype and functional properties of feline dendritic cells derived from blood and bone marrow. Vet. Immunol. Immunopathol. 2003, 96, 19–30. [Google Scholar] [CrossRef]
- Gutzwiller, M.E.R.; Moulin, H.R.; Zurbriggen, A.; Roosje, P.; Summerfield, A. Comparative analysis of canine monocyte- and bone-marrow-derived dendritic cells. Vet. Res. 2010, 41, 40. [Google Scholar] [CrossRef]
- Cao, W.; Lee, S.H.; Lu, J. CD83 is performed inside monocytes, macrophages and dendritic cells, but it is only stably expressed on activated dendritic cells. Biochem. J. 2005, 385, 85–93. [Google Scholar] [CrossRef]
- Affolter, V.K.; Moore, P.F. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet. Pathol. 2002, 39, 74–83. [Google Scholar] [CrossRef]
- Goto-Koshino, Y.; Ohno, K.; Nakajima, M.; Mochizuki, H.; Kanemoto, H.; Tsujimoto, H. A rapid and simple method to obtain canine peripheral blood-derived macrophages. J. Vet. Med. Sci. 2011, 73, 773–778. [Google Scholar] [CrossRef]
- Woelbing, F.; Kostka, S.L.; Moelle, K.; Belkaid, Y.; Sunderkoetter, C.; Verbeek, S.; Waisman, A.; Nigg, A.P.; Knop, J.; Udey, M.C.; et al. Uptake of Leishmania major by dendritic cells is mediated by Fc gamma receptors and facilitates acquisition of protective immunity. J. Exp. Med. 2006, 203, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Neves, B.M.; Silvestre, R.; Resende, M.; Ouaissi, A.; Cunha, J.; Tavares, J.; Loureiro, I.; Santarém, N.; Silva, A.M.; Lopes, M.C.; et al. Activation of phosphatidylinositol 3-kinase/Akt and impairment of nuclear factor-kappaB: Molecular mechanisms behind the arrested maturation/activation state of Leishmania infantum-infected dendritic cells. Am. J. Pathol. 2010, 177, 2898–2911. [Google Scholar] [CrossRef]
- Falcão, S.A.; Jaramillo, T.M.; Ferreira, L.G.; Bernardes, D.M.; Santana, J.M.; Favali, C.B. Leishmania infantum and Leishmania braziliensis: Differences and similarities to evade the innate immune system. Front. Immunol. 2016, 7, 287. [Google Scholar] [CrossRef] [PubMed]
- Jaumouillé, V.; Grinstein, S. Molecular mechanisms of phagosome formation. In Myeloid Cells in Health and Disease: A Synthesis; Gordon, S., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Argueta-Donohué, J.; Wilkins-Rodríguez, A.A.; Aguirre-García, M.; Gutiérrez-Kobeh, L. Differential phagocytosis of Leishmania mexicana promastigotes and amastigotes by monocyte-derived dendritic cells. Microbiol. Immunol. 2016, 60, 369–381. [Google Scholar] [CrossRef]
- von Stebut, E.; Belkaid, Y.; Nguyen, B.V.; Cushing, M.; Sacks, D.L.; Udey, M.C. Leishmania major-infected murine Langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous leishmaniasis. Eur. J. Immunol. 2000, 30, 3498–3506. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Popov, V.; Soong, L. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4(+) T cells in vivo. J. Immunol. 2001, 167, 4534–4542. [Google Scholar] [CrossRef]
- Xin, L.; Li, Y.; Soong, L. Role of interleukin-1beta in activating the CD11c (high) CD45RB-dendritic cell subset and priming Leishmania amazonensis-specific CD4+ T cells in vitro and in vivo. Infect. Immun. 2007, 75, 5018–5026. [Google Scholar] [CrossRef]
- Soong, L. Modulation of dendritic cell function by Leishmania parasites. J. Immunol. 2008, 180, 4355–4360. [Google Scholar] [CrossRef]
- Margaroni, M.; Agallou, M.; Vasilakaki, A.; Karagkouni, D.S.; Koufos, G.; Hatzigeorgiou, A.G.; Karagouni, E. Transcriptional profiling of Leishmania infantum infected dendritic cells: Insights into the role of immunometabolism in host-parasite interaction. Microorganisms 2022, 10, 1271. [Google Scholar] [CrossRef] [PubMed]
- Prina, E.; Abdi, S.Z.; Lebastard, M.; Perret, E.; Winter, N.; Antoine, J.C. Dendritic cells as host cells for the promastigote and amastigote stages of Leishmania amazonensis: The role of opsonins in parasite uptake and dendritic cell maturation. J. Cell. Sci. 2004, 117, 315–325. [Google Scholar] [CrossRef]
- von Stebut, E.; Tenzer, S. Cutaneous leishmaniasis: Distinct functions of dendritic cells and macrophages in the interaction of the host immune system with Leishmania major. Int. J. Med. Microbiol. 2018, 308, 206–214. [Google Scholar] [CrossRef]
- Rodrigues, A.V.; Valério-Bolas, A.; Alexandre-Pires, G.; Pereira, M.A.; Nunes, T.; Ligeiro, D.; Pereira da Fonseca, I.; Santos-Gomes, G. Zoonotic Visceral Leishmaniasis: New insights on innate immune response by blood macrophages and liver Kupffer cells to Leishmania infantum parasites. Biology 2022, 11, 100. [Google Scholar] [CrossRef]
- Lecoeur, H.; Rosazza, T.; Kokou, K.; Varet, H.; Coppée, J.Y.; Lari, A.; Commère, P.H.; Weil, R.; Meng, G.; Milon, G.; et al. Leishmania amazonensis subverts the transcription factor landscape in dendritic cells to avoid inflammasome activation and stall maturation. Front. Immunol. 2020, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, L.A.; da Costa, J.L.; de Lima, M.H.; Sampaio, P.A.; Almeida, R.P.; Cunha, F.Q.; Silva, J.S.; Carregaro, V. Toll-Like Receptor 2 is required for inflammatory process development during Leishmania infantum infection. Front. Microbiol. 2017, 8, 262. [Google Scholar] [CrossRef]
- Bamigbola, I.E.; Ali, S. Paradoxical immune response in leishmaniasis: The role of toll-like receptors in disease progression. Parasite Immunol. 2022, 44, e12910. [Google Scholar] [CrossRef] [PubMed]
- Muxel, S.M.; Acuña, S.M.; Aoki, J.I.; Zampieri, R.A.; Floeter-Winter, L.M. Toll-Like Receptor and miRNA-let-7e expression alter the inflammatory response in Leishmania amazonensis-infected macrophages. Front. Immunol. 2018, 9, 2792. [Google Scholar] [CrossRef]
- Baska, P.; Norbury, L.J. The role of nuclear factor kappa B (NF-κB) in the immune response against parasites. Pathogens 2022, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, P.M.; de Menezes-Neto, A.; Borges, V.M.; Descoteaux, A.; Torrecilhas, A.C.; Xander, P.; Revach, O.Y.; Regev-Rudzki, N.; Soares, R.P. Immunomodulatory properties of Leishmania extracellular vesicles during host-parasite interaction: Differential activation of TLRs and NF-κB translocation by dermotropic and viscerotropic species. Front. Cell. Infect. Microbiol. 2020, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.B.; Souza-Testasicca, M.C.; Mineo, T.W.P.; Afonso, L.C.C. Leishmania amazonensis-induced cAMP triggered by adenosine A2B receptor is important to inhibit dendritic cell activation and evade immune response in infected mice. Front. Immunol. 2017, 8, 849. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Moroi, Y.; Uchi, H.; Furue, M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J. Dermatol. Sci. 2006, 42, 1–11. [Google Scholar] [CrossRef]
- Tibúrcio, R.; Nunes, S.; Nunes, I.; Ampuero, R.M.; Silva, I.B.; Lima, R.; Machado Tavares, N.; Brodskyn, C. Molecular aspects of dendritic cell activation in Leishmaniasis: An immunobiological view. Front. Immunol. 2019, 10, 227. [Google Scholar] [CrossRef]
- Ribeiro-de-Jesus, A.; Almeida, R.P.; Lessa, H.; Bacellar, O.; Carvalho, E.M. Cytokine profile and pathology in human leishmaniasis. Braz. J. Med. Biol. Res. 1998, 31, 143–148. [Google Scholar] [CrossRef]
- Alexander, J.; Bryson, K. T helper (h) 1/Th2 and Leishmania: Paradox rather than paradigm. Immunol. Lett. 2005, 99, 17–23. [Google Scholar] [CrossRef]
- Lima-Junior, S.; Costa, D.L.; Carregaro, V.; Cunha, L.D.; Silva, A.L.; Mineo, T.W.; Gutierrez, F.R.; Bellio, M.; Bortoluci, K.R.; Flavell, R.A.; et al. Inflammasome-derived IL-1β production induces nitric oxide-mediated resistance to Leishmania. Nat. Med. 2013, 19, 909–915. [Google Scholar] [CrossRef]
- Manna, L.; Reale, S.; Viola, E.; Vitale, F.; Foglia Manzillo, V.; Pavone, L.M.; Caracappa, S.; Gravino, A.E. Leishmania DNA load and cytokine expression levels in asymptomatic naturally infected dogs. Vet. Parasit. 2006, 142, 271–280. [Google Scholar] [CrossRef]
- Liu, D.; Uzonna, J.E. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front. Cell. Infect. Microbiol. 2012, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hoffmann, K.F.; Mendez, S.; Kamhawi, S.; Udey, M.C.; Wynn, T.A.; Sacks, D.L. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 2001, 194, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.L.; Sauter, B.; Bhardwaj, N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998, 392, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Regnault, A.; Lankar, D.; Lacabanne, V.; Rodriguez, A.; Théry, C.; Rescigno, M.; Saito, T.; Verbeek, S.; Bonnerot, C.; Ricciardi-Castagnoli, P.; et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 1999, 89, 371–380. [Google Scholar] [CrossRef]
- Rodriguez, A.; Regnault, A.; Kleijmeer, M.; Ricciardi-Castagnoli, P.; Amigorena, S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat. Cell. Biol. 1999, 1, 362–368. [Google Scholar] [CrossRef]
- Lindsten, T.; Lee, K.P.; Harris, E.S.; Petryniak, B.; Craighead, N.; Reynolds, P.J.; Lombard, D.B.; Freeman, G.J.; Nadler, L.M.; Gray, G.S. Characterization of CTLA-4 structure and expression on human T cells. J. Immunol. 1993, 151, 3489–3499. [Google Scholar] [CrossRef]
- Inaba, K.; Witmer-Pack, M.; Inaba, M.; Hathcock, K.S.; Sakuta, H.; Azuma, M.; Yagita, H.; Okumura, K.; Linsley, P.S.; Ikehara, S.; et al. The tissue distribution of the B7-2 costimulator in mice: Abundant expression on dendritic cells in situ and during maturation in vitro. J. Exp. Med. 1994, 180, 1849–1860. [Google Scholar] [CrossRef]
- Freedman, A.S.; Freeman, G.J.; Rhynhart, K.; Nadler, L.M. Selective induction of B7/BB-1 on interferon-gamma stimulated monocytes: A potential mechanism for amplification of T cell activation through the CD28 pathway. Cell. Immunol. 1991, 137, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Boussiotis, V.A.; Freeman, G.J.; Gribben, J.G.; Daley, J.; Gray, G.; Nadler, L.M. Activated human B lymphocytes express three CTLA-4 counterreceptors that costimulate T-cell activation. Proc. Natl. Acad. Sci. USA 1993, 90, 11059–11063. [Google Scholar] [CrossRef]
- Lenschow, D.J.; Sperling, A.I.; Cooke, M.P.; Freeman, G.; Rhee, L.; Decker, D.C.; Gray, G.; Nadler, L.M.; Goodnow, C.C.; Bluestone, J.A. Differential up-regulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J. Immunol. 1994, 153, 1990–1997. [Google Scholar] [CrossRef]
- Abel, S.; Hundhausen, C.; Mentlein, R.; Schulte, A.; Berkhout, T.A.; Broadway, N.; Hartmann, D.; Sedlacek, R.; Dietrich, S.; Muetze, B.; et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM. J. Immunol. 2004, 172, 6362–6372. [Google Scholar] [CrossRef]
- Murphy, J.E.; Tedbury, P.R.; Homer-Vanniasinkam, S.; Walker, J.H.; Ponnambalam, S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis 2005, 182, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Oghumu, S.; Lezama-Dávila, C.M.; Isaac-Márquez, A.P.; Satoskar, A.R. Role of chemokines in regulation of immunity against leishmaniasis. Exp. Parasitol. 2010, 126, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, B.P.; Beaumann, M.; Heyde, S.; Regli, I.B.; Müller, A.J.; Tacchini-Cottier, F. Frontline Science: Leishmania mexicana amastigotes can replicate within neutrophils. J. Leukoc. Biol. 2017, 102, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Bahr, V.; Stierhof, Y.D.; Ilg, T.; Demar, M.; Quinten, M.; Overath, P. Expression of lipophosphoglycan, high-molecular weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol. Biochem. Parasitol. 1993, 58, 107–121. [Google Scholar] [CrossRef]
- Naderer, T.; McConville, M.J. The Leishmania-macrophage interaction: A metabolic perspective. Cell. Microbiol. 2008, 10, 301–308. [Google Scholar] [CrossRef]
- Carvalho, A.K.; Silveira, F.T.; Passero, L.F.; Gomes, C.M.; Corbett, C.E.; Laurenti, M.D. Leishmania (V.) braziliensis and L. (L.) amazonensis promote differential expression of dendritic cells and cellular immune response in murine model. Parasite Immunol. 2012, 34, 395–403. [Google Scholar] [CrossRef]
- Favali, C.; Tavares, N.; Clarencio, J.; Barral, A.; Barral-Netto, M.; Brodskyn, C. Leishmania amazonensis infection impairs differentiation and function of human dendritic cells. J. Leukoc. Biol. 2007, 82, 1401–1406. [Google Scholar] [CrossRef][Green Version]
- Xin, L.; Li, K.; Soong, L. Down-regulation of dendritic cell signaling pathways by Leishmania amazonensis amastigotes. Mol. Immunol. 2008, 45, 3371–3382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Figueiredo, A.B.; Serafim, T.D.; Marques-da-Silva, E.A.; Meyer-Fernandes, J.R.; Afonso, L.C.C. Leishmania amazonensis impairs DC function by inhibiting CD40 expression via A 2B adenosine receptor activation. Eur. J. Immunol. 2012, 42, 1203–1215. [Google Scholar] [CrossRef] [PubMed]


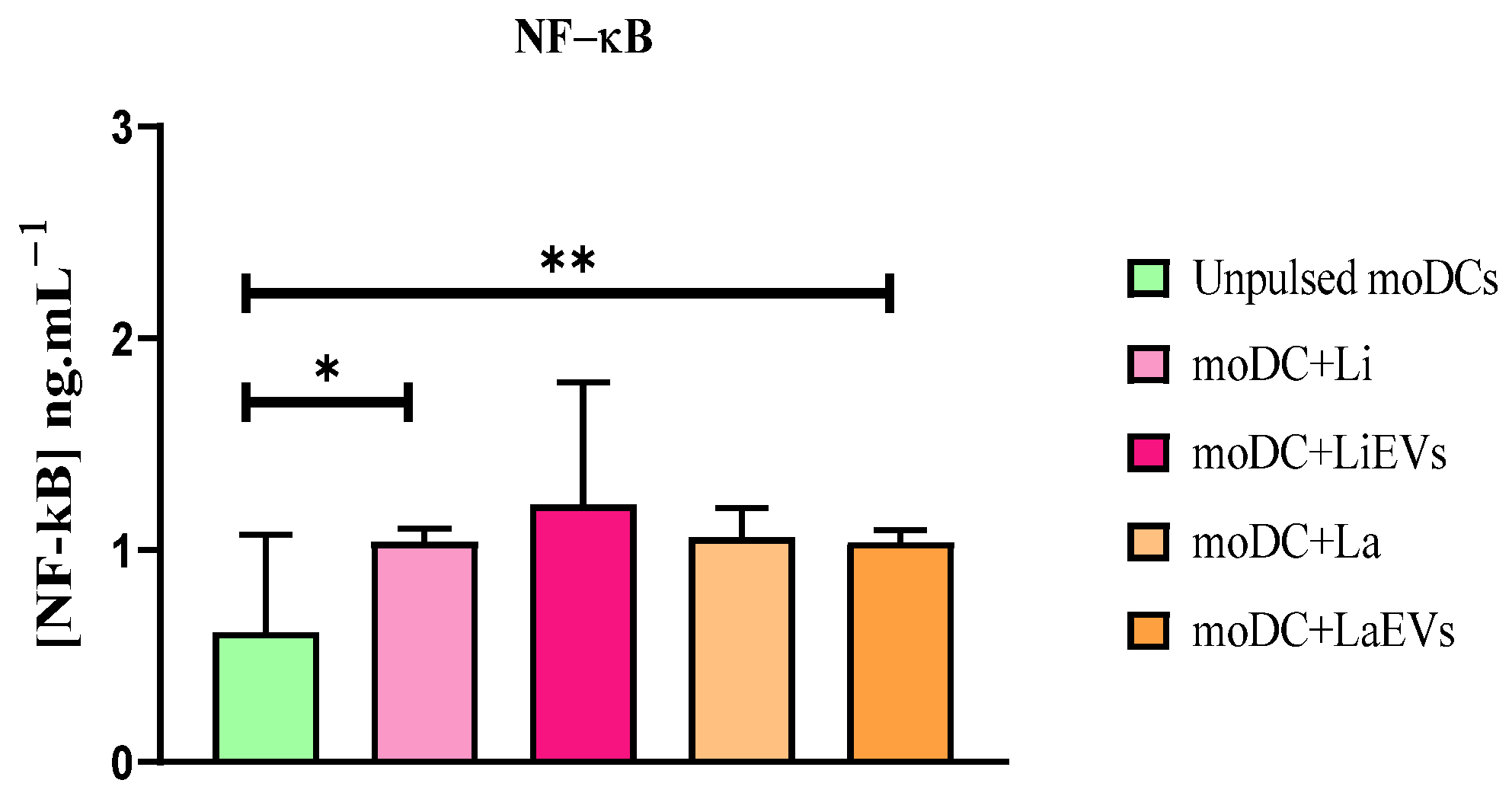
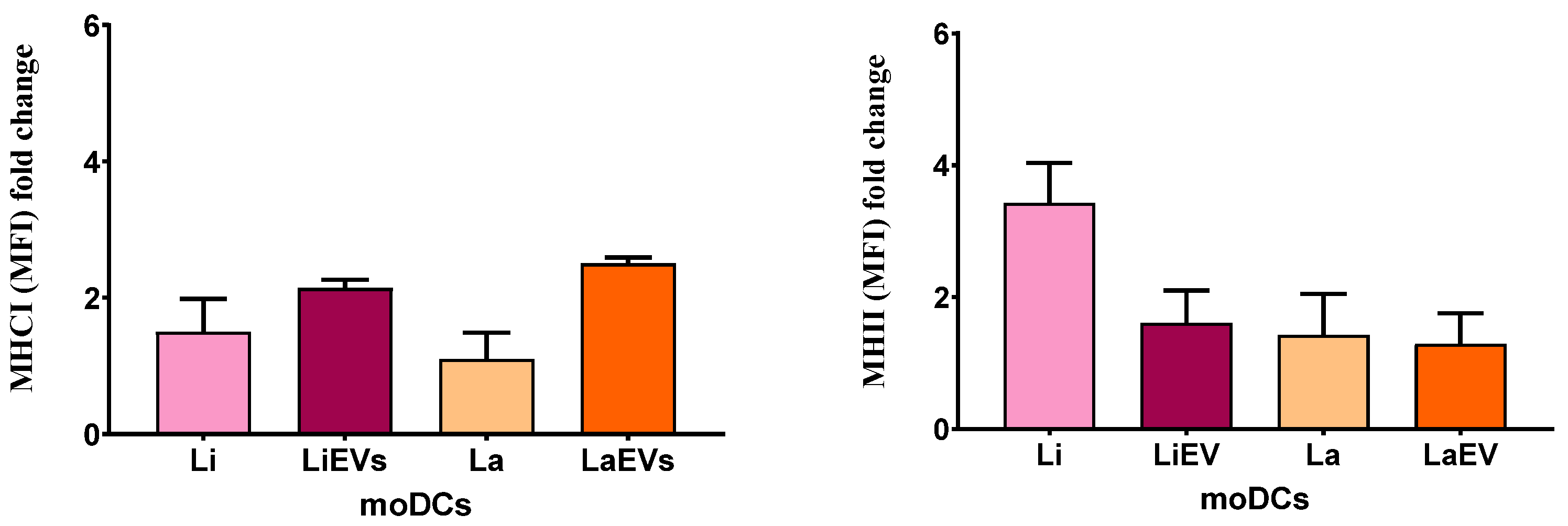
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valério-Bolas, A.; Meunier, M.; Palma-Marques, J.; Rodrigues, A.; Santos, A.M.; Nunes, T.; Ferreira, R.; Armada, A.; Alves, J.C.; Antunes, W.; et al. Exploiting Leishmania—Primed Dendritic Cells as Potential Immunomodulators of Canine Immune Response. Cells 2024, 13, 445. https://doi.org/10.3390/cells13050445
Valério-Bolas A, Meunier M, Palma-Marques J, Rodrigues A, Santos AM, Nunes T, Ferreira R, Armada A, Alves JC, Antunes W, et al. Exploiting Leishmania—Primed Dendritic Cells as Potential Immunomodulators of Canine Immune Response. Cells. 2024; 13(5):445. https://doi.org/10.3390/cells13050445
Chicago/Turabian StyleValério-Bolas, Ana, Mafalda Meunier, Joana Palma-Marques, Armanda Rodrigues, Ana Margarida Santos, Telmo Nunes, Rui Ferreira, Ana Armada, João Carlos Alves, Wilson Antunes, and et al. 2024. "Exploiting Leishmania—Primed Dendritic Cells as Potential Immunomodulators of Canine Immune Response" Cells 13, no. 5: 445. https://doi.org/10.3390/cells13050445
APA StyleValério-Bolas, A., Meunier, M., Palma-Marques, J., Rodrigues, A., Santos, A. M., Nunes, T., Ferreira, R., Armada, A., Alves, J. C., Antunes, W., Cardoso, I., Mesquita-Gabriel, S., Lobo, L., Alexandre-Pires, G., Marques, L., Pereira da Fonseca, I., & Santos-Gomes, G. (2024). Exploiting Leishmania—Primed Dendritic Cells as Potential Immunomodulators of Canine Immune Response. Cells, 13(5), 445. https://doi.org/10.3390/cells13050445








