DPPA2/4 Promote the Pluripotency and Proliferation of Bovine Extended Pluripotent Stem Cells by Upregulating the PI3K/AKT/GSK3β/β-Catenin Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Public Data Sources
2.3. RNA-Seq and ATAC-Seq Data Analysis
2.4. Reprogramming of Bovine Fetal Fibroblasts (BFFs)
2.5. Bovine EPSC Culture
2.6. Knockout and Overexpression of DPPA2/4
2.7. Small Interfering RNA (siRNA) Transfection
2.8. AP Activity Assay
2.9. Chromosome Number Analysis
2.10. In Vitro Differentiation
2.11. Teratoma Formation
2.12. Immunofluorescence Staining
2.13. Quantitative RT-PCR
2.14. Western Blotting and Antibodies
2.15. Cell Viability Assay
2.16. EdU Cell Proliferation Assay
2.17. Flow Cytometry Assay
2.18. ChIP-qPCR
2.19. RNA Sequencing
2.20. Statistical Analysis
3. Results
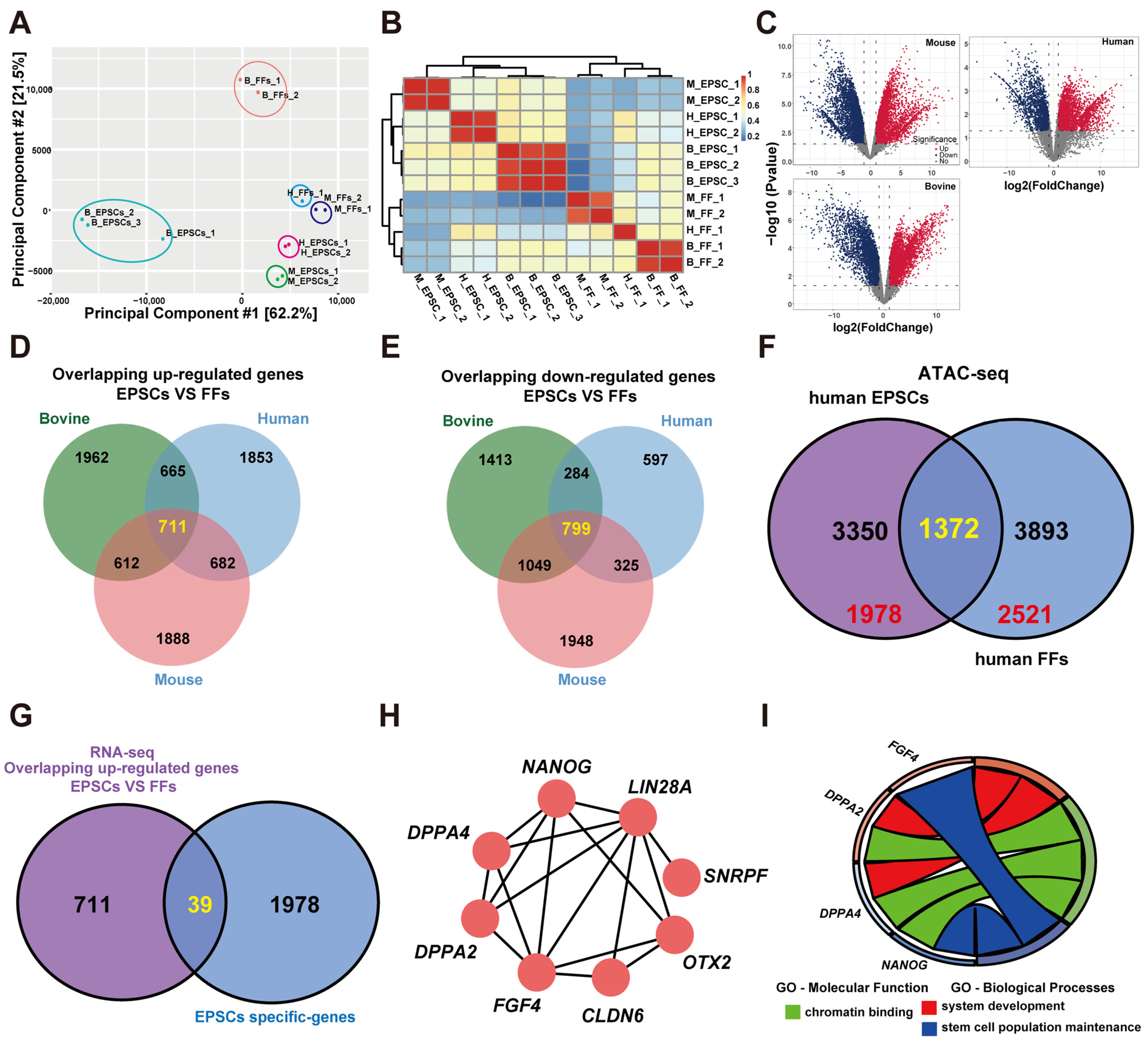
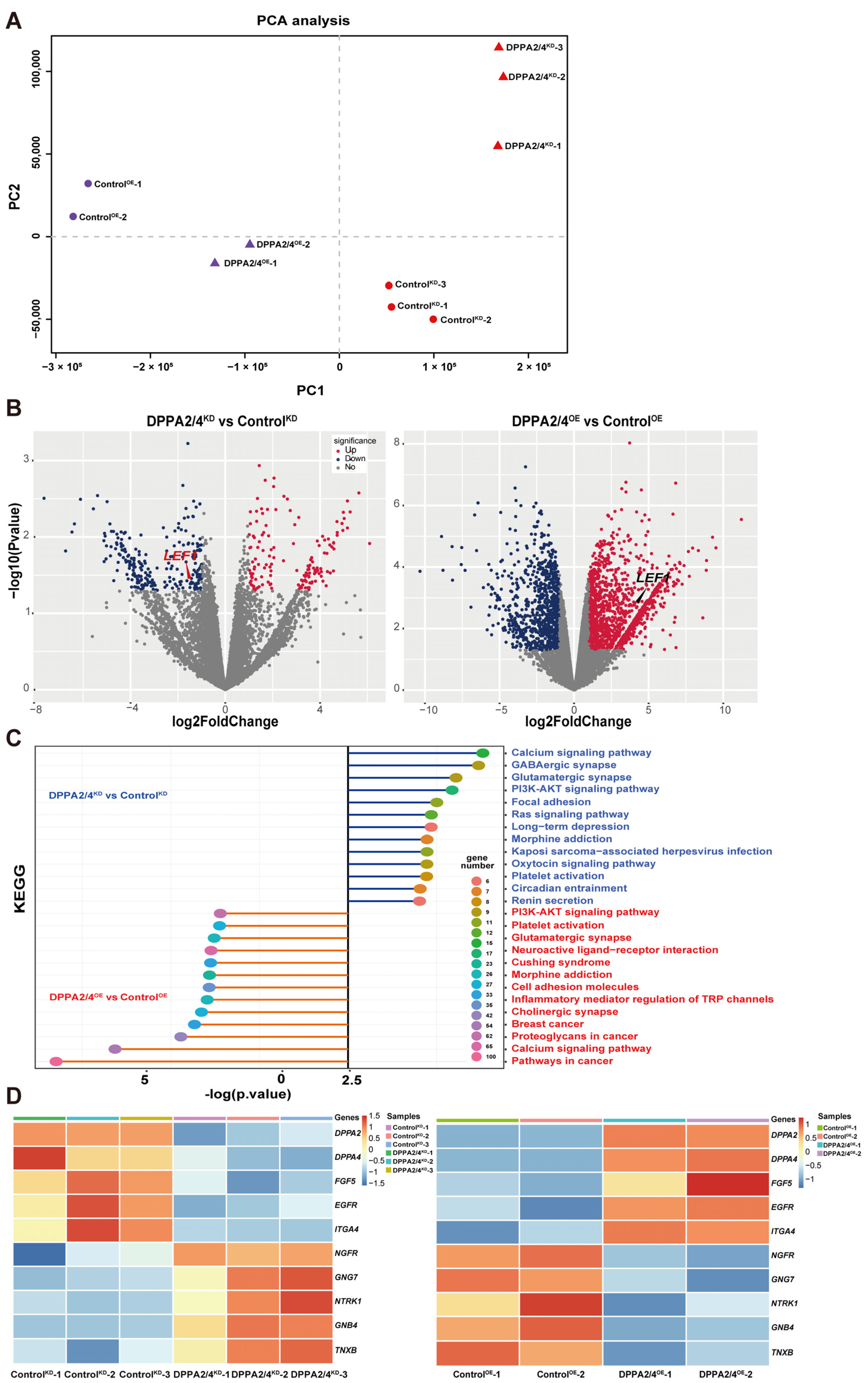
3.1. Using RNA-Seq and ATAC-Seq Data Identify DPPA2 and DPPA4 as Hub Genes Involved in EPSC Reprogramming
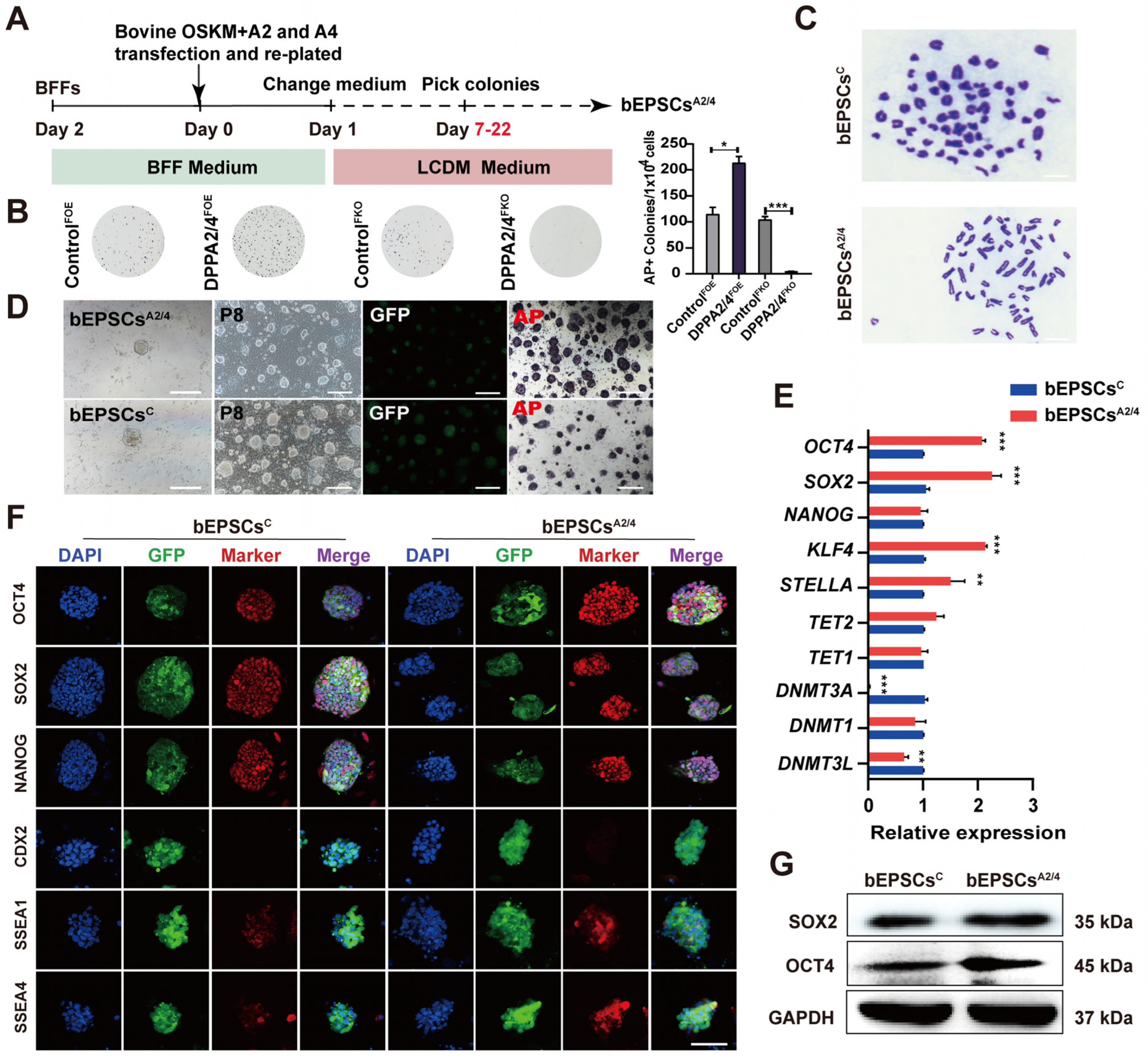
3.2. DPPA2 and DPPA4 Are Essential for Establishing Bovine Extended Potential Stem Cells by Reprogramming
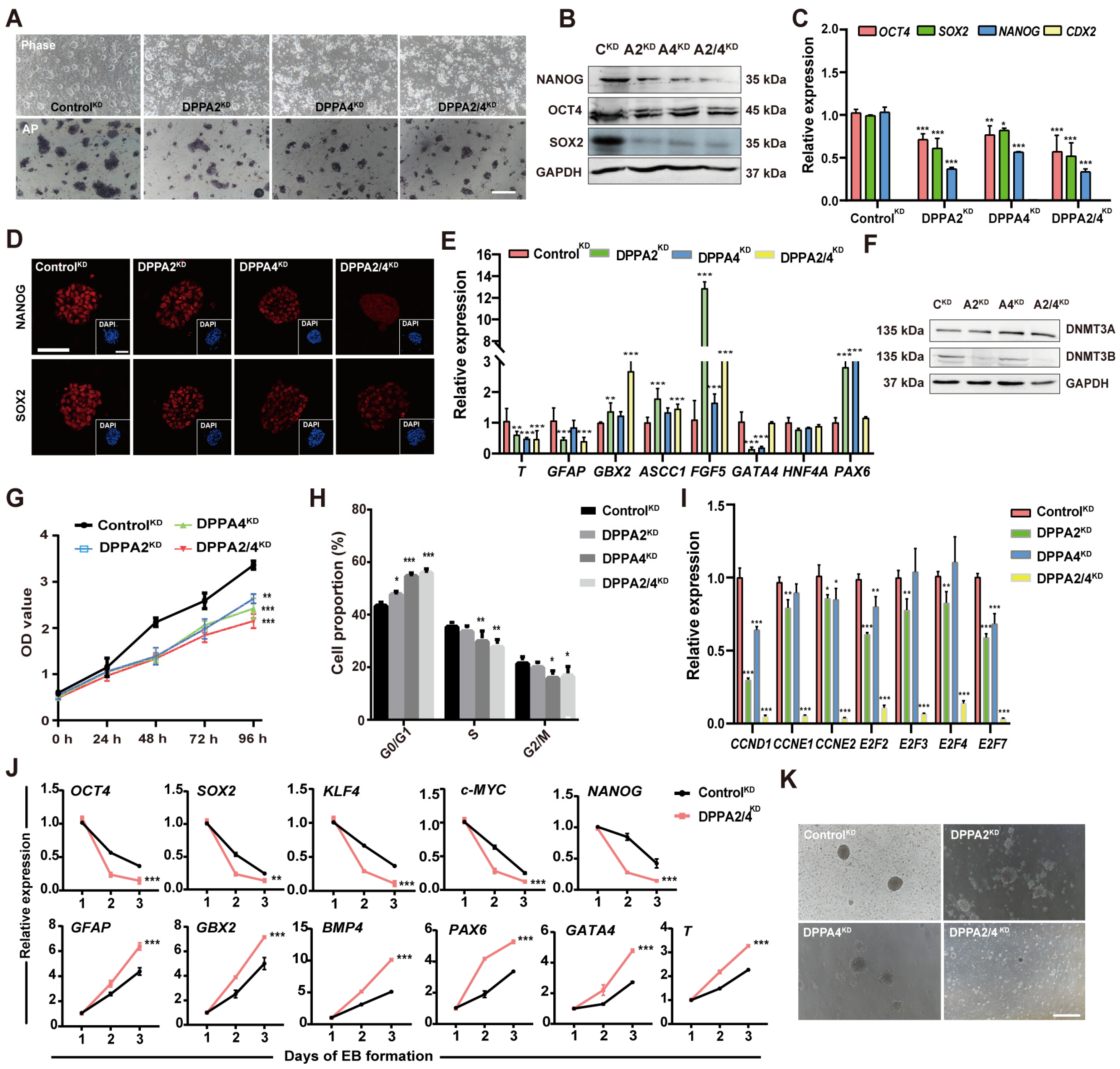
3.3. DPPA2/4 Knockdown Reduces Pluripotency and Accelerates Early Differentiation of Established bEPSCs
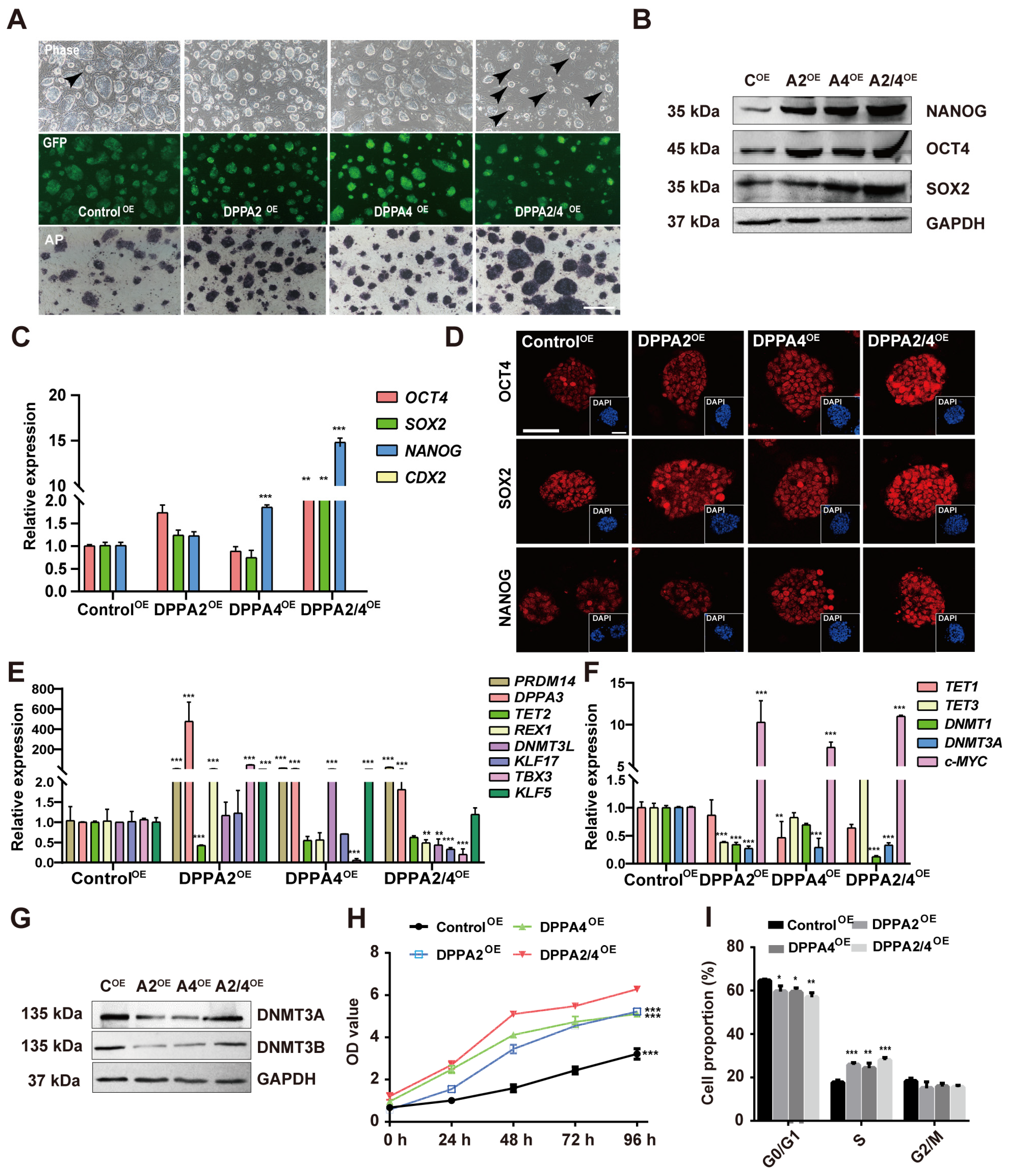
3.4. Overexpression of DPPA2 and DPPA4 Increases the Pluripotency of Established bEPSCs and Promotes Their Proliferation
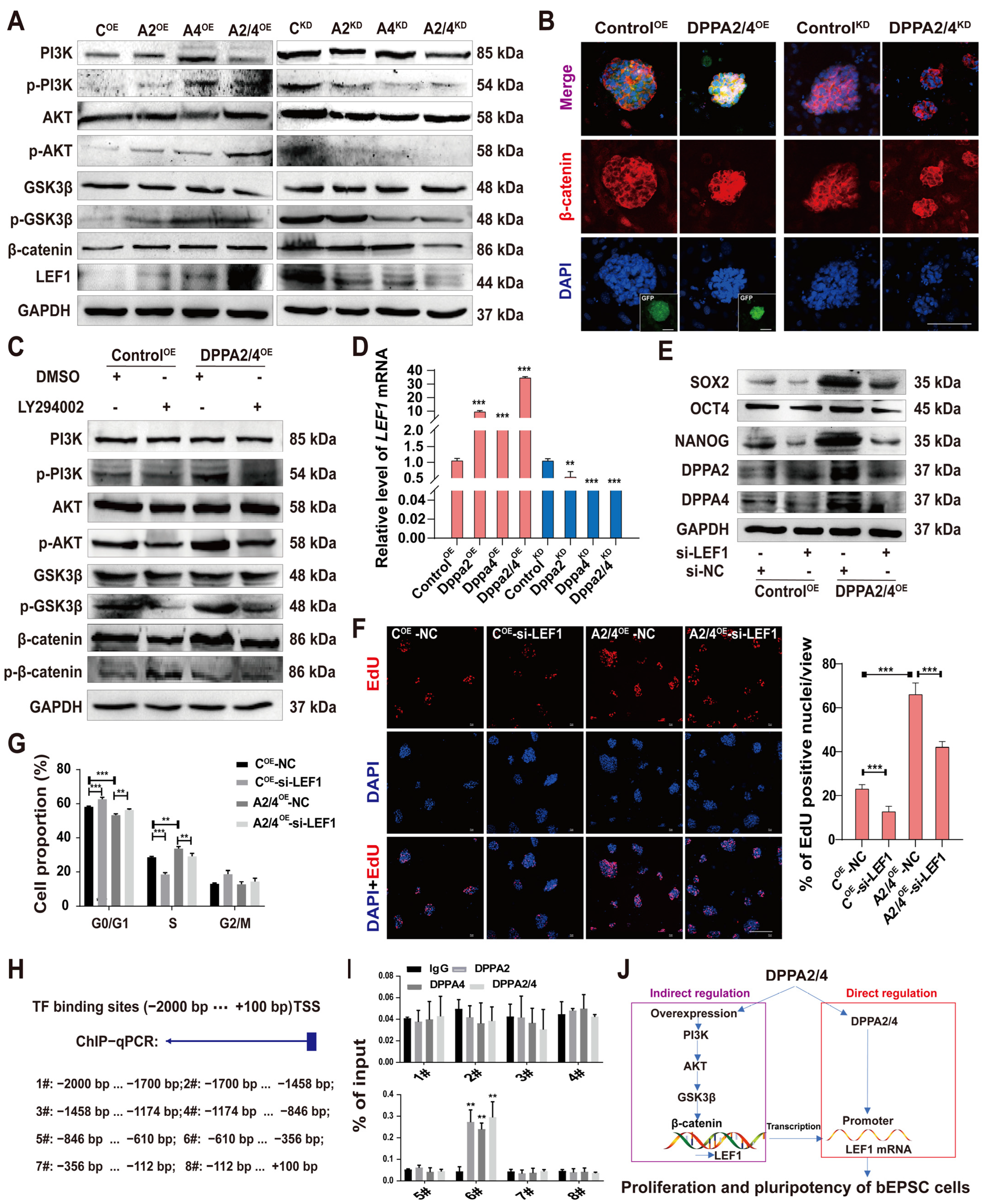
3.5. DPPA2/4 Activate the PI3K/AKT/GSK3β/β-Catenin Signaling Pathway to Affect the Pluripotency of bEPSCs
3.6. DPPA2/4 Exert Their Effects through LEF1 via the PI3K/AKT/GSK3β/β-Catenin Pathway and through Direct Binding to the LEF1 Promoter
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DPPA2/4 | Developmental pluripotency-associated 2/4 |
| LEF1 | Lymphoid enhancer-binding factor 1 |
| bEPSCs | Bovine expanded potential pluripotent stem cells |
| KSR | Knockout serum replacement |
| LIF | Leukemia inhibitory factor |
| MEF | Mouse embryonic fibroblasts |
| CDK | Cyclin-dependent kinases |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| ChIP | Chromatin immunoprecipitation |
| CDS | Coding sequences |
| TSS | Transcription start site |
References
- Yang, Y.; Liu, B.; Xu, J.; Wang, J.; Wu, J.; Shi, C.; Xu, Y.; Dong, J.; Wang, C.; Lai, W.; et al. Derivation of Pluripotent Stem Cells with In Vivo Embryonic and Extraembryonic Potency. Cell 2017, 169, 243–257.e225. [Google Scholar] [CrossRef]
- Yang, J.; Ryan, D.J.; Wang, W.; Tsang, J.C.; Lan, G.; Masaki, H.; Gao, X.; Antunes, L.; Yu, Y.; Zhu, Z.; et al. Establishment of mouse expanded potential stem cells. Nature 2017, 550, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Nowak-Imialek, M.; Chen, X.; Chen, D.; Herrmann, D.; Ruan, D.; Chen, A.C.H.; Eckersley-Maslin, M.A.; Ahmad, S.; Lee, Y.L.; et al. Establishment of porcine and human expanded potential stem cells. Nat. Cell Biol. 2019, 21, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ryan, D.J.; Lan, G.; Zou, X.; Liu, P. In vitro establishment of expanded-potential stem cells from mouse pre-implantation embryos or embryonic stem cells. Nat. Protoc. 2019, 14, 350–378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gao, X.; Zheng, Y.; Wang, Z.; Zhao, G.; Ren, J.; Zhang, J.; Wu, J.; Wu, B.; Chen, Y.; et al. Establishment of bovine expanded potential stem cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2018505118. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, H.; Zhang, Y.; Wang, J.; Liu, F.; Han, X.; Lu, Z.; Li, C.; Li, Z.; Gao, Y.; et al. LCDM medium supports the derivation of bovine extended pluripotent stem cells with embryonic and extraembryonic potency in bovine-mouse chimeras from iPSCs and bovine fetal fibroblasts. FEBS J. 2021, 288, 4394–4411. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, J.; Yue, Y.; Li, C.; Zhang, X.; Xiang, J.; Wang, H.; Li, X. Derivation of Arbas Cashmere Goat Induced Pluripotent Stem Cells in LCDM with Trophectoderm Lineage Differentiation and Interspecies Chimeric Abilities. Int. J. Mol. Sci. 2023, 24, 14728. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zhou, Y.; Tan, S.; Zhou, G.; Aagaard, L.; Xie, L.; Bünger, C.; Bolund, L.; Luo, Y. Mesenchymal stem cells derived from human induced pluripotent stem cells retain adequate osteogenicity and chondrogenicity but less adipogenicity. Stem Cell Res. Ther. 2015, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Saldivia, J.; van den Bergen, J.; Krouskos, M.; Gilchrist, M.; Lee, C.; Li, R.; Sinclair, A.H.; Surani, M.A.; Western, P.S. Dppa2 and Dppa4 are closely linked SAP motif genes restricted to pluripotent cells and the germ line. Stem Cells 2007, 25, 19–28. [Google Scholar] [CrossRef]
- Bortvin, A.; Eggan, K.; Skaletsky, H.; Akutsu, H.; Berry, D.L.; Yanagimachi, R.; Page, D.C.; Jaenisch, R. Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development 2003, 130, 1673–1680. [Google Scholar] [CrossRef]
- Madan, B.; Madan, V.; Weber, O.; Tropel, P.; Blum, C.; Kieffer, E.; Viville, S.; Fehling, H.J. The pluripotency-associated gene Dppa4 is dispensable for embryonic stem cell identity and germ cell development but essential for embryogenesis. Mol. Cell. Biol. 2009, 29, 3186–3203. [Google Scholar] [CrossRef]
- Aravind, L.; Koonin, E.V. SAP—A putative DNA-binding motif involved in chromosomal organization. Trends Biochem. Sci. 2000, 25, 112–114. [Google Scholar] [CrossRef]
- Masaki, H.; Nishida, T.; Sakasai, R.; Teraoka, H. DPPA4 modulates chromatin structure via association with DNA and core histone H3 in mouse embryonic stem cells. Genes Cells Devoted Mol. Cell. Mech. 2010, 15, 327–337. [Google Scholar] [CrossRef]
- Nakamura, T.; Nakagawa, M.; Ichisaka, T.; Shiota, A.; Yamanaka, S. Essential roles of ECAT15-2/Dppa2 in functional lung development. Mol. Cell. Biol. 2011, 31, 4366–4378. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Wang, Z.; Ramazanov, B.; Tang, Y.; Mehta, S.; Dambrot, C.; Lee, Y.W.; Tessema, K.; Kumar, I.; Astudillo, M.; et al. Dppa2/4 Facilitate Epigenetic Remodeling during Reprogramming to Pluripotency. Cell Stem Cell 2018, 23, 396–411.e398. [Google Scholar] [CrossRef] [PubMed]
- Eckersley-Maslin, M.A.; Parry, A.; Blotenburg, M.; Krueger, C.; Ito, Y.; Franklin, V.N.R.; Narita, M.; D’Santos, C.S.; Reik, W. Epigenetic priming by Dppa2 and 4 in pluripotency facilitates multi-lineage commitment. Nat. Struct. Mol. Biol. 2020, 27, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Gretarsson, K.H.; Hackett, J.A. Dppa2 and Dppa4 counteract de novo methylation to establish a permissive epigenome for development. Nat. Struct. Mol. Biol. 2020, 27, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; He, Q.Y. ChIPseeker: An R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 2015, 31, 2382–2383. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Z.; Zhang, J.; Yang, J.; Gao, X.; Wu, B.; Zhao, G.; Bao, S.; Hu, S.; Liu, P.; et al. Characterization of the single-cell derived bovine induced pluripotent stem cells. Tissue Cell 2017, 49, 521–527. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Liu, H.; Lu, D.; Chen, X.; Zenonos, Z.; Campos, L.S.; Rad, R.; Guo, G.; Zhang, S.; et al. Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1. Proc. Natl. Acad. Sci. USA 2011, 108, 18283–18288. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, F.; Cao, S.; Zhang, J.; Wang, H.; Wu, B.; Song, Y.; Duo, S.; Li, X.; Bao, S. Bdh2 Deficiency Promotes Endoderm-Biased Early Differentiation of Mouse Embryonic Stem Cells. Front. Cell Dev. Biol. 2021, 9, 655145. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Rainosek, S.W.; Sadovova, N.; Fogle, C.M.; Patterson, T.A.; Hanig, J.P.; Paule, M.G.; Slikker, W., Jr.; Wang, C. Protective effect of acetyl-L-carnitine on propofol-induced toxicity in embryonic neural stem cells. Neurotoxicology 2014, 42, 49–57. [Google Scholar] [CrossRef]
- De Iaco, A.; Coudray, A.; Duc, J.; Trono, D. DPPA2 and DPPA4 are necessary to establish a 2C-like state in mouse embryonic stem cells. EMBO Rep. 2019, 20, e47382. [Google Scholar] [CrossRef]
- Eckersley-Maslin, M.; Alda-Catalinas, C.; Blotenburg, M.; Kreibich, E.; Krueger, C.; Reik, W. Dppa2 and Dppa4 directly regulate the Dux-driven zygotic transcriptional program. Genes Dev. 2019, 33, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Hossini, A.M.; Quast, A.S.; Plötz, M.; Grauel, K.; Exner, T.; Küchler, J.; Stachelscheid, H.; Eberle, J.; Rabien, A.; Makrantonaki, E.; et al. PI3K/AKT Signaling Pathway Is Essential for Survival of Induced Pluripotent Stem Cells. PLoS ONE 2016, 11, e0154770. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Lim, H.W.; Lee, S.H.; Han, H.J. Smad, PI3K/Akt, and Wnt-dependent signaling pathways are involved in BMP-4-induced ESC self-renewal. Stem Cells 2009, 27, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Tan, A.; Song, Y.; Lee, H.; Ying, Q.L.; Jho, E.H. The Distinct Role of Tcfs and Lef1 in the Self-Renewal or Differentiation of Mouse Embryonic Stem Cells. Int. J. Stem Cells 2020, 13, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, Y.; Liu, D.; Ying, Q.L.; Ye, S. TFCP2L1 represses multiple lineage commitment of mouse embryonic stem cells through MTA1 and LEF1. J. Cell Sci. 2017, 130, 3809–3817. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qin, D. Role of Lef1 in sustaining self-renewal in mouse embryonic stem cells. J. Genet. Genom. Yi Chuan Xue Bao 2010, 37, 441–449. [Google Scholar] [CrossRef]
- Li, H.; Long, C.; Xiang, J.; Liang, P.; Li, X.; Zuo, Y. Dppa2/4 as a trigger of signaling pathways to promote zygote genome activation by binding to CG-rich region. Brief. Bioinform. 2021, 22, bbaa342. [Google Scholar] [CrossRef]
- Hu, J.; Wang, F.; Yuan, Y.; Zhu, X.; Wang, Y.; Zhang, Y.; Kou, Z.; Wang, S.; Gao, S. Novel importin-alpha family member Kpna7 is required for normal fertility and fecundity in the mouse. J. Biol. Chem. 2010, 285, 33113–33122. [Google Scholar] [CrossRef]
- Zhu, L.F.; Chen, Q.R.; Chen, S.Z.; Wang, L.Y.; Luo, X.F.; Ren, J.H.; Yuan, X.H.; Wu, X.Q.; Zeng, Y.L.; Xiao, M.; et al. The Construction and Identification of Induced Pluripotent Stem Cells Derived from Acute Myelogenous Leukemia Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 41, 1661–1674. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Song, L.; Di, A.; Su, G.; Bai, C.; Wei, Z.; Li, G. Transient Dux expression facilitates nuclear transfer and induced pluripotent stem cell reprogramming. EMBO Rep. 2020, 21, e50054. [Google Scholar] [CrossRef]
- Kubinyecz, O.; Santos, F.; Drage, D.; Reik, W.; Eckersley-Maslin, M.A. Maternal Dppa2 and Dppa4 are dispensable for zygotic genome activation but important for offspring survival. Development 2021, 148, dev200191. [Google Scholar] [CrossRef]
- Guttula, P.K.; Agarwal, A.; Maharana, U.; Gupta, M.K. Prediction of novel pluripotent proteins involved in reprogramming of male Germline stem cells (GSCs) into multipotent adult Germline stem cells (maGSCs) by network analysis. Comput. Biol. Chem. 2018, 76, 302–309. [Google Scholar] [CrossRef]
- Watabe, T. Roles of Dppa2 in the regulation of the present status and future of pluripotent stem cells. J. Biochem. 2012, 152, 1–3. [Google Scholar] [CrossRef]
- Paling, N.R.; Wheadon, H.; Bone, H.K.; Welham, M.J. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J. Biol. Chem. 2004, 279, 48063–48070. [Google Scholar] [CrossRef] [PubMed]
- Jirmanova, L.; Afanassieff, M.; Gobert-Gosse, S.; Markossian, S.; Savatier, P. Differential contributions of ERK and PI3-kinase to the regulation of cyclin D1 expression and to the control of the G1/S transition in mouse embryonic stem cells. Oncogene 2002, 21, 5515–5528. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Liu, J.; Ma, X.; Zhou, Z.; Song, Y.; Cao, B. miR-26a promoted endometrial epithelium cells (EECs) proliferation and induced stromal cells (ESCs) apoptosis via the PTEN-PI3K/AKT pathway in dairy goats. J. Cell. Physiol. 2018, 233, 4688–4706. [Google Scholar] [CrossRef] [PubMed]
- Muise-Helmericks, R.C.; Grimes, H.L.; Bellacosa, A.; Malstrom, S.E.; Tsichlis, P.N.; Rosen, N. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 1998, 273, 29864–29872. [Google Scholar] [CrossRef] [PubMed]
- Storm, M.P.; Bone, H.K.; Beck, C.G.; Bourillot, P.Y.; Schreiber, V.; Damiano, T.; Nelson, A.; Savatier, P.; Welham, M.J. Regulation of Nanog expression by phosphoinositide 3-kinase-dependent signaling in murine embryonic stem cells. J. Biol. Chem. 2007, 282, 6265–6273. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.S.; Kim, J.; Park, C.S.; Chung, I.Y. The phosphoinositide-3-kinase/Akt pathway mediates the transient increase in Nanog expression during differentiation of F9 cells. Arch. Pharmacal Res. 2010, 33, 1117–1125. [Google Scholar] [CrossRef]
- Ding, V.M.; Ling, L.; Natarajan, S.; Yap, M.G.; Cool, S.M.; Choo, A.B. FGF-2 modulates Wnt signaling in undifferentiated hESC and iPS cells through activated PI3-K/GSK3beta signaling. J. Cell. Physiol. 2010, 225, 417–428. [Google Scholar] [CrossRef]
- Hao, Y.H.; Lafita-Navarro, M.C.; Zacharias, L.; Borenstein-Auerbach, N.; Kim, M.; Barnes, S.; Kim, J.; Shay, J.; DeBerardinis, R.J.; Conacci-Sorrell, M. Induction of LEF1 by MYC activates the WNT pathway and maintains cell proliferation. Cell Commun. Signal. CCS 2019, 17, 129. [Google Scholar] [CrossRef]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and apoptosis: Size matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef]
- Ding, V.W.; Chen, R.H.; McCormick, F. Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J. Biol. Chem. 2000, 275, 32475–32481. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xuan, W.; Yan, R.; Tropak, M.B.; Jean-St-Michel, E.; Liang, W.; Gladstone, R.; Backx, P.H.; Kharbanda, R.K.; Redington, A.N. Remote preconditioning provides potent cardioprotection via PI3K/Akt activation and is associated with nuclear accumulation of β-catenin. Clin. Sci. 2011, 120, 451–462. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, S.; Wang, J.; Liu, G.; Qu, B.; Chunyu, J.; Xu, W.; Xiang, J.; Li, X. DPPA2/4 Promote the Pluripotency and Proliferation of Bovine Extended Pluripotent Stem Cells by Upregulating the PI3K/AKT/GSK3β/β-Catenin Signaling Pathway. Cells 2024, 13, 382. https://doi.org/10.3390/cells13050382
Fang S, Wang J, Liu G, Qu B, Chunyu J, Xu W, Xiang J, Li X. DPPA2/4 Promote the Pluripotency and Proliferation of Bovine Extended Pluripotent Stem Cells by Upregulating the PI3K/AKT/GSK3β/β-Catenin Signaling Pathway. Cells. 2024; 13(5):382. https://doi.org/10.3390/cells13050382
Chicago/Turabian StyleFang, Shu, Jing Wang, Guangbo Liu, Burong Qu, Jian Chunyu, Wenqiang Xu, Jinzhu Xiang, and Xueling Li. 2024. "DPPA2/4 Promote the Pluripotency and Proliferation of Bovine Extended Pluripotent Stem Cells by Upregulating the PI3K/AKT/GSK3β/β-Catenin Signaling Pathway" Cells 13, no. 5: 382. https://doi.org/10.3390/cells13050382
APA StyleFang, S., Wang, J., Liu, G., Qu, B., Chunyu, J., Xu, W., Xiang, J., & Li, X. (2024). DPPA2/4 Promote the Pluripotency and Proliferation of Bovine Extended Pluripotent Stem Cells by Upregulating the PI3K/AKT/GSK3β/β-Catenin Signaling Pathway. Cells, 13(5), 382. https://doi.org/10.3390/cells13050382






