1. Introduction
Neurotrophic keratitis is a rare degenerative eye disorder characterized by decreased corneal sensation, usually due to damage to the corneal nerve fibers. It can cause severe pain, decreased vision, and infection. Symptoms include redness and swelling of the cornea, blurred vision, eye pain, light sensitivity, and a watery discharge [
1]. The prevalence of neurotrophic keratitis is estimated to be between 1 in 10,000 and 1 in 50,000 people. It is more common in elderly individuals and those with a history of eye trauma or certain medical conditions, such as diabetes and chronic inflammatory diseases [
2]. It is also more common in certain parts of the world, such as South and Southeast Asia. The incidence of neurotrophic keratitis in the United States is estimated to be around 1.5 cases per 100,000 people [
3]. In Europe, the incidence is estimated to be around 0.5 cases per 100,000 people. In India, the incidence is estimated to be around 5 cases per 100,000 people [
4].
The evaluation of neurotrophic keratitis typically involves a combination of clinical examination, diagnostic imaging, and laboratory testing. Clinical examination typically includes an eye exam to look for signs of decreased corneal sensation, such as the inability to feel a cotton applicator or sensitivity to touch or light. Additionally, a slit lamp examination can be performed to look for signs of inflammation. Diagnostic imaging, such as corneal confocal microscopy, can be used to examine the cornea in greater detail [
5]. It can help identify areas of nerve damage and measure corneal sensitivity. Laboratory tests, such as a tear film analysis, can be used to measure tear production and evaluate signs of infection. IL6 is a known pro-inflammatory cytokine and plays a very important role in persistent epithelial defect, which is a key feature of NK [
6]. MMP9 is important during tissue remodeling, wound healing, and fibrosis [
7]. Additionally, a lab test for neurotrophic keratitis can measure levels of nerve growth factor in the tears.
Currently, the primary treatment for neurotrophic keratitis is to reduce symptoms and limit further damage to the cornea. This includes using artificial tears, ointments, and steroid drops to reduce inflammation and lubricate the cornea [
8]. Additionally, specific procedures such as tarsorrhaphy or amniotic membrane can be used to attempt to restore corneal epithelialization and limit further damage to the eye. In more severe cases, procedural interventions such as corneal neurotisation may be necessary [
9,
10,
11,
12]. The limitations of the current treatments for neurotrophic keratitis are that they are often only partially successful at restoring corneal sensation and may not be effective in preventing further damage to the eye. Additionally, the risk of infection increases with these treatments, and the potential for complications is higher [
13]. In emergency cases, a corneal graft may be required, which further complicates corneal wound healing.
There are a few animal models of neurotrophic keratitis, including the rat, mouse, and rabbit models. These models are used to study the effects of neurotrophic keratitis on the cornea and evaluate potential treatments. The mouse model involves the injection of capsaicin into the cornea to induce a decrease in corneal sensation. In the rabbit model, various treatments are used to induce corneal neurotrophic keratitis, including contact lens wear, chemical ablation of corneal nerves, and chemical or mechanical stimulation of the cornea [
14,
15].
The current animal models of neurotrophic keratitis have several limitations. First, the models vary in the type of injury-induced and the severity of the corneal damage. Second, the models may not accurately represent the human condition, as corneal nerve damage in humans is often caused by a variety of factors, such as contact lens wear or eye trauma. Third, the models may not accurately represent the natural course of the disease in humans, as corneal nerve damage in humans is often progressive and worsens over time. Finally, the models may not be able to accurately assess the efficacy of potential treatments, as the treatments may vary in their efficacy based on the type and severity of the corneal nerve damage. Therefore, it is important to evaluate the clinical features of NK as per the human standard using imaging techniques closest possible to humans, making the translational aspect of the NK model possible.
In our current study, we generated two types of alkali burn-induced NK models: (i) direct burning of the rabbit cornea with alkali (mild form of NK) and (ii) trephination followed by alkali burn (severe form of NK). We characterized both models here through (i) clinical evaluation—decreased corneal sensation, pain, redness, and blurred vision; (ii) ophthalmic evaluation/imaging using OCT, slit lamp, pentacam, and IVCM; (iii) histology and inflammation. It can lead to serious complications such as corneal ulcers, scarring, and vision loss in severe cases.
2. Material and Methods
2.1. Study Design and Approval
The experimental design involved the use of Male New White Zealand (NWZ) rabbits that were subjected to ocular surface alkali burn in the right eye to study the development and progression of alkali injury in the cornea leading to NK. All the animals were 8–10 weeks of age and 1.8–2.0 kg body weight. The animals were followed up for a period of one month, and their ophthalmic and histopathological parameters were monitored during the course of the experiment. The study was approved by the Institutional Animal Ethics Committee. All surgeries were performed with controlled temperature and humidity under the supervision of the veterinarian and animal care team. All the animals were handled in agreement with the Association for Research in Vision and Ophthalmology (ARVO) statement for animal use in Ophthalmic and Vision Research.
2.2. Corneal Alkali Burn in Rabbits
Before the operation, the rabbits were anesthetized using ketamine hydrochloride (30 mg/kg) and xylazine (5 mg/kg) through intramuscular injection, and a topical drop of proparacaine was used in the right eye. A fresh 0.75 N NaOH solution was made, and a single circular Whatman filter paper with a diameter of 5 mm was soaked with 10 µL of 0.75 N sodium hydroxide (NaOH) solution.
Two kinds of injury models were made. For the first model (mild form of NK), 10 µL of 0.75 N NaOH, soaked in 5 mm diameter Whatman paper for 10 s, was directly applied and washed with 10 mL normal saline for 30 s. For the second model (severe form of NK), corneal tissue was excavated using a guarded trephine with a 5 mm diameter and 150 μm depth. A single circular filter paper with a diameter of 5 mm was cut, and 10 µL of 0.75 N sodium hydroxide (NaOH) solution was applied on the Whatman filter paper, subsequently on the trephined site for 10 s. After 10 s, the eyes were rinsed with 10 mL of normal saline to wash off excess alkali. The rabbit corneas from both models (N = 4 for the mild form of NK and N = 8 for the severe form of NK) were evaluated at regular intervals using ophthalmic imaging techniques. Antibiotic eye drops (Moxifloxacin) were administered in the eyes four times a day immediately after surgery for a minimum of 7–10 days until re-epithelization occurred. The maximal fibrotic response to epithelium-stroma post-alkali injury occurs at approximately 25–30 days after injury. Hence, the rabbit corneas were evaluated for 30 days.
2.3. Ophthalmic Imaging Techniques
After the ocular surface alkali injury, animals were followed up for the progression of the disease by obtaining clinical photographs at regular time intervals using the following techniques:
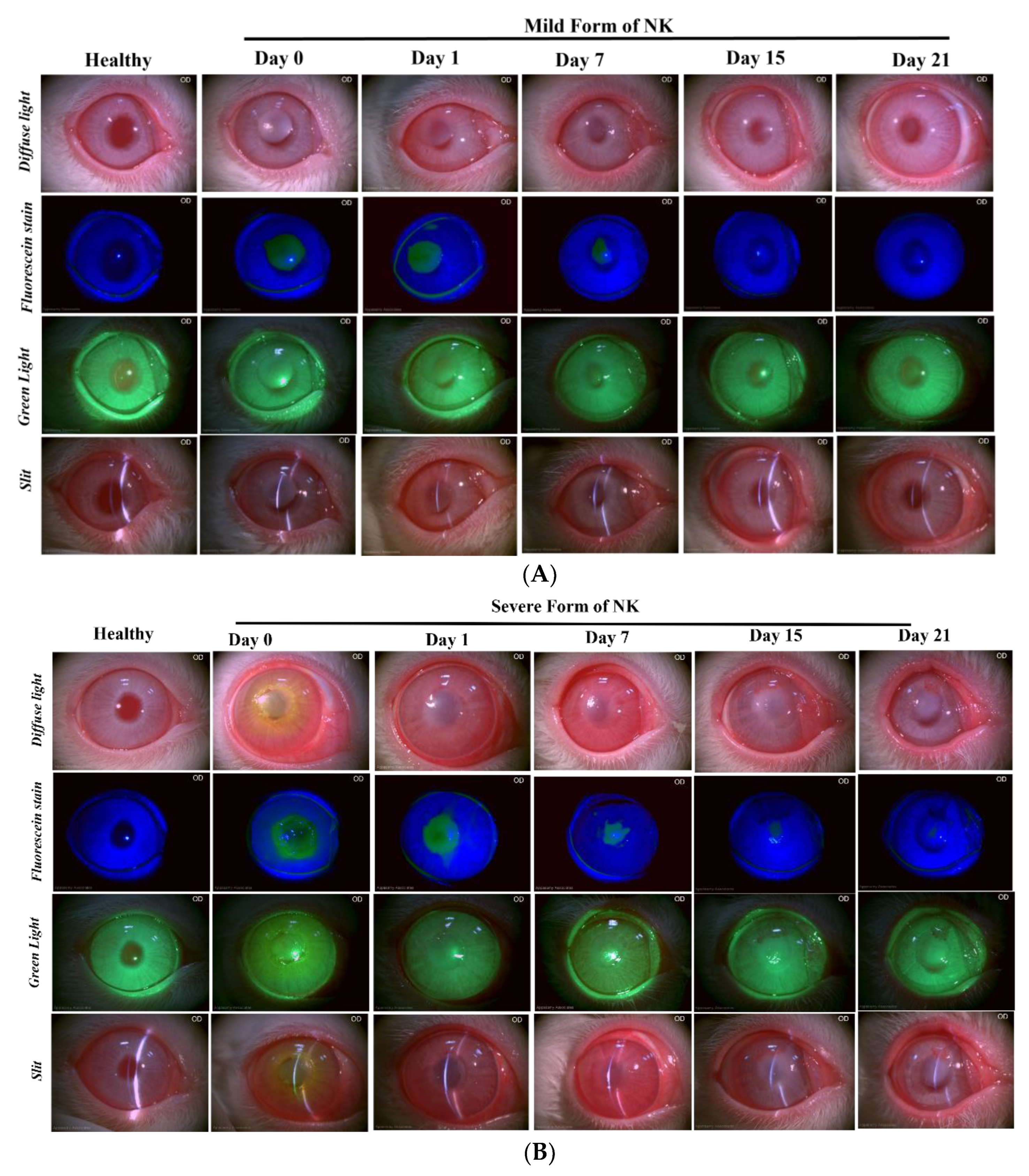
Slit Lamp: The rabbits were held in the correct position such that the entire frame of the cornea was visible, and images were acquired. Multiple images at 10X magnification were quickly taken to avoid eye drying using a slit-lamp biomicroscope (SL-CAM ICM 7, Appasamy Associates, Tamil Nadu, India) under different lighting conditions to examine corneal status and neovascularization. Diffuse light and oblique slit images indicated corneal clarity and a 3D view of the cornea, while fluorescein dye images taken with a blue light filter examined corneal abrasion by staining the underlying layer of collagen or hydrophilic stroma, which was exposed due to the corneal epithelium damage. Corneal neovascularisation was examined by acquiring the images in the green light filter.
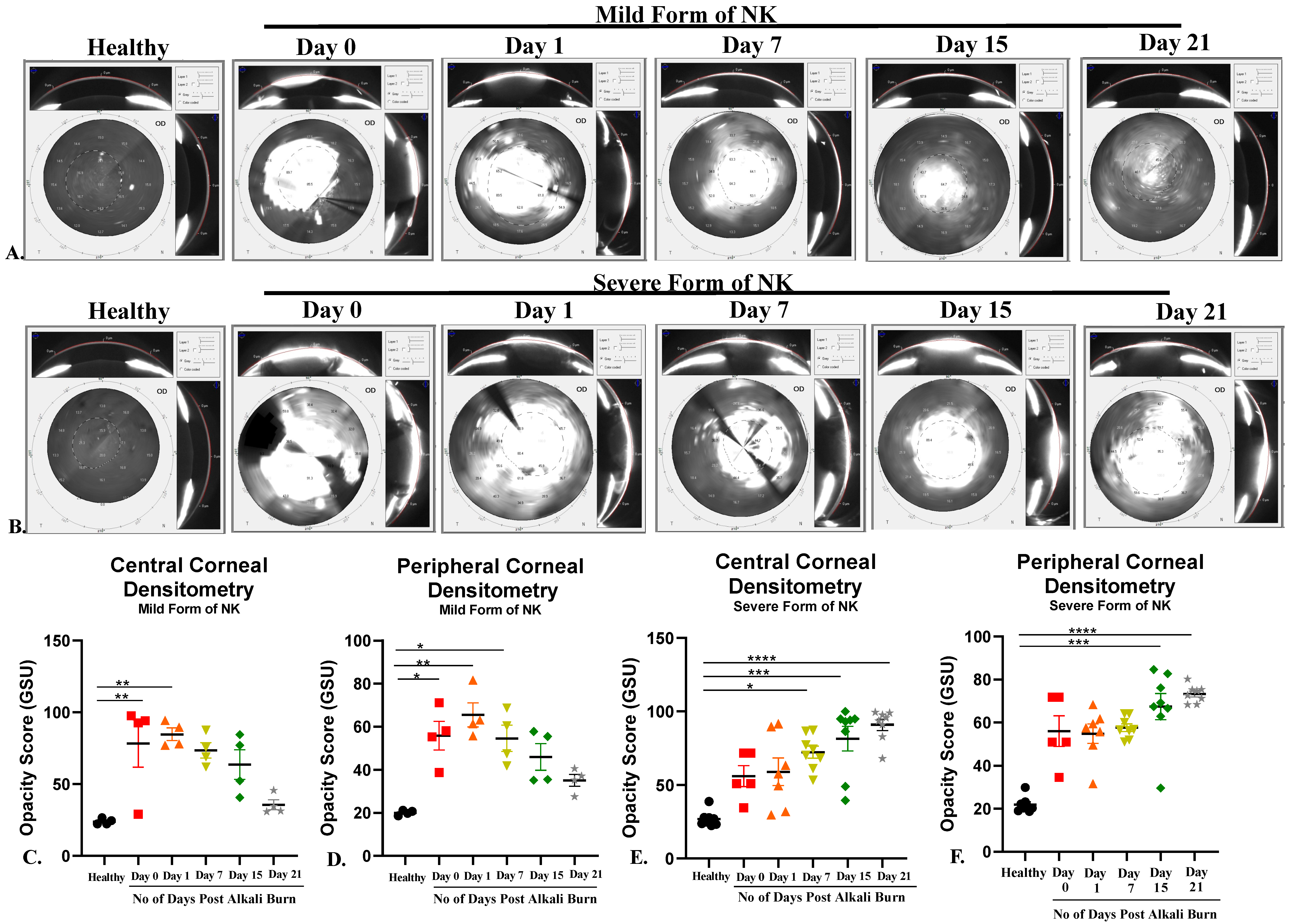
Pentacam: Pentacam (Oculus, Wetzlar, Germany) is a rotating scheimpflug camera that helps us understand corneal biomechanics (Densitometry and Corneal Curvature) at different diameters of the cornea. This was a dark room procedure, so lights were switched off to image the rabbit cornea, and scans were taken from the center. The images were processed and analyzed for corneal curvature (keratometry values) and opacity (densitometry values) using built-in software (Pentacam HR, V1.21r65) in the machine.
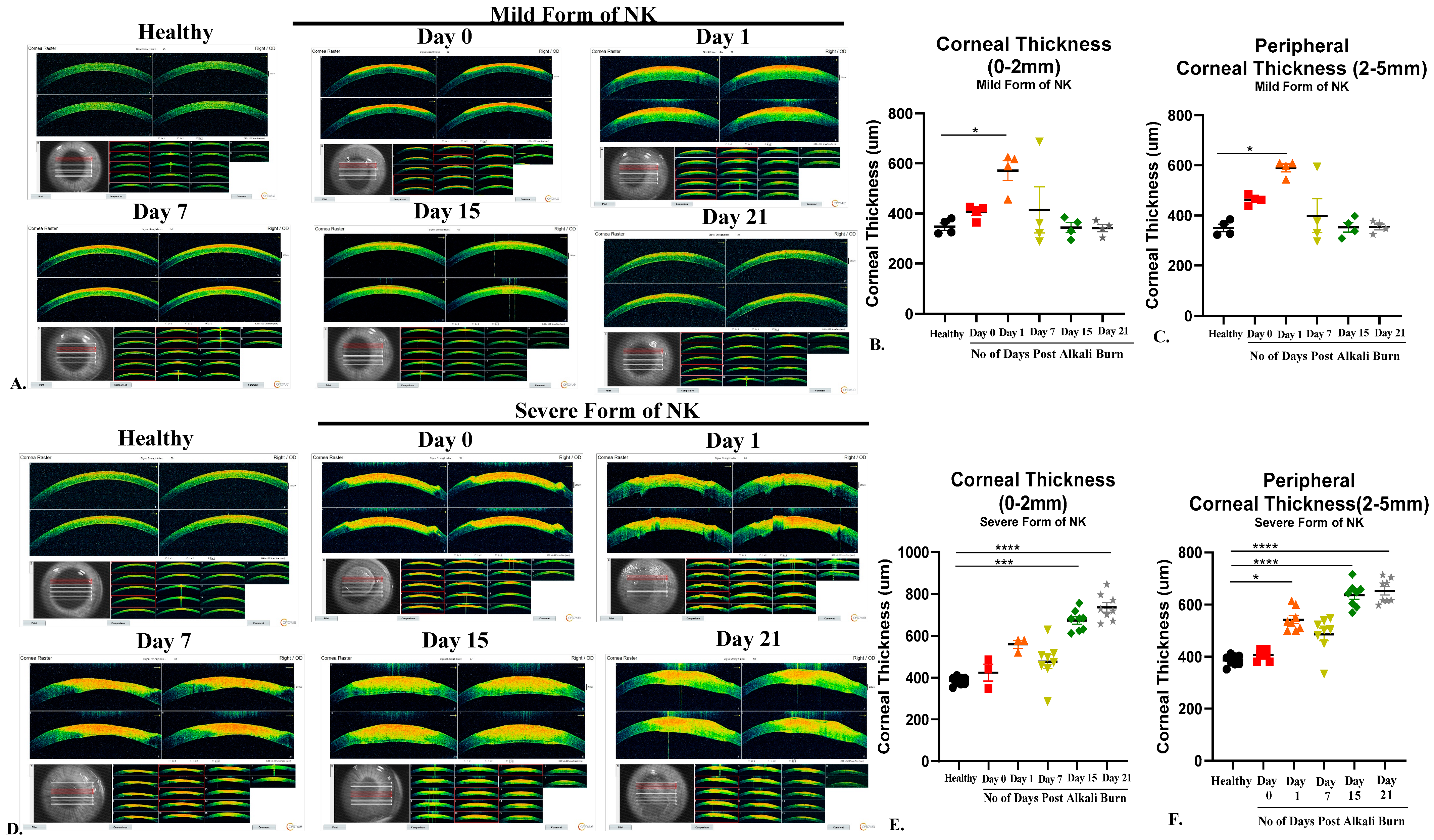
Anterior Segment Optical Coherence Tomography (AS-OCT): AS-OCT is widely used to measure corneal thickness and epithelial and stromal hyperreflectivity. An anterior segment scan of the rabbit cornea was performed using the AS-OCT (Optovue, Avanti RTVUE-XR, Fremont, CA, USA). For imaging the cornea, the rabbits were held by a trained technician, an OCT probe was brought closer to the eye, and a scan was taken from the center. A single-trained optometrist obtained all images. The images were processed and analyzed for corneal thickness using the machine’s built-in software (Avanti XR, version 2016.1.0.26).
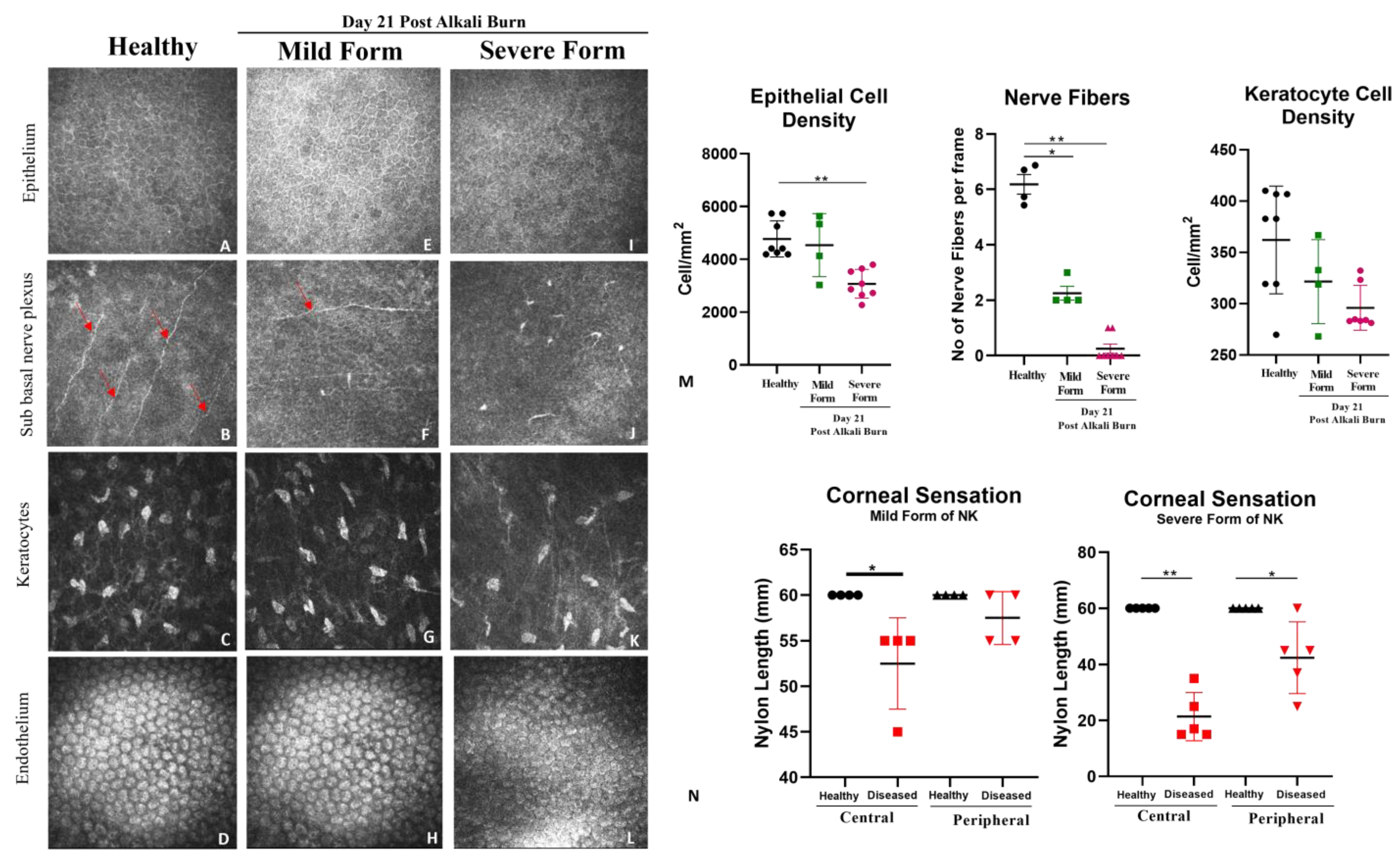
Aesthesiometer: Corneal sensitivity of the rabbit eyes was measured using a Cochet–Bonnet aesthesiometer (Luneau Technology, Pont-de-l’Arche, France). This instrument is typically used clinically to evaluate neurotrophic keratopathy. The handheld aesthesiometer (Cochet–Bonnet) contains a thin, retractable, nylon monofilament extending up to 60 mm in length. The device can apply variable pressure by adjusting the length. The monofilament ranges from 60 mm to 5 mm, and as the length is decreased, the pressure increases. We measured the corneal sensation of both eyes at the center of the cornea (within 5 mm diameter) and at the periphery (more than 5 mm diameter).
IVCM: In vivo, confocal microscopy (RCM 3, Heidelberg, Germany) of the cornea was performed on live animals in real-time. The high contrast of this laser-scanning system was critical for detecting delicate regenerating epithelial basal cells and nerves, while the high axial resolution enabled precise depth localization of individual repopulating keratocytes. The objective lens of the microscope was cleared using lint-free tissue, and a large drop of ophthalmic gel was applied on the front surface of the microscope objective lens. Tomocap was placed securely over the gel-covered objective lens, ensuring that the gel was flattened over the front surface of the objective lens and no air bubbles were present. Once the cornea was aligned with the tomocap, the images of different layers were acquired by adjusting the focal depths. For a full-thickness scan, the acquisition started at the superficial epithelium and was posteriorly adjusted to the focal depth. Post-acquisition, the images were analyzed for epithelial, keratocyte, and endothelium cellular density along the sub-basal nerve plexus.
2.4. Histological Analysis
After 30 days, rabbits were sacrificed with an overdose of ketamine and xylazine. Tissues of the cornea were collected from NK-diseased and healthy rabbits to evaluate the differences between the two forms of the NK model generation (i.e., Mild and Severe Form). The tissues were briefly washed with saline and then cut into two halves; the first half was used for histological analysis, and the second half was used for real time PCR.
For histology, sections were fixed in formalin, paraffin blocks were made, and sections were stained with Haematoxylin for 10 min and further differentiated with 1% acid alcohol for 30 s. They were further washed in running tap water for 10 min and then rinsed in 95% alcohol for 20 s. The sections were later counterstained with Eosin Y for 30 s, after which the sections were dehydrated in 95% alcohol and two changes of absolute alcohol for 5 min each. Sections were cleared with two changes of xylene for 5 min each and mounted with DPX (Dibutyl phthalate Polystyrene Xylene) mounting medium.
ELISA from Tear Samples: Schimer’s strip was used to collect the tear samples. 1x PBS with Protease Inhibitor Cocktail (PIC) was used to extract protein from the tear sample, which was further quantified using the BCA method. Commercially available kits of rabbit ELISA for MMP9, IL-6, and VEGF from CusaBio were used to estimate the level of metalloproteinase, pro-inflammatory cytokine, and angiogenesis factor from the tear samples of the healthy cornea and mild and severe form of NK models post 21 days of alkali injury.
Total RNA was isolated using the TriZol method (Invitrogen, Waltham, MA, USA) from the cornea of the control/healthy eye, mild form of NK, and severe form of NK. Complementary DNA was generated via reverse transcription reaction using the Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA). Real-time quantitative polymerase chain reaction (PCR) was performed using SYBR Green Master Mix (Qiagen, Hilden, Germany) in the Azure Cielo 6 Dx real-time PCR machine (Azure Biosystems Inc., Dublin, CA, USA).
PCR reaction setup comprised 5 µL of 1:10 diluted cDNA, 7.5 µL of Power Track SYBR Green Master Mix (SYBR-Green; Applied Biosystems Life Technologies, Waltham, MA, USA), 1 µL of each primer with a concentration of 10 µM, and 1.5 µL of ddH20 in a total volume of 15 µL. Real-time PCR was performed with an initial denaturation step of 3 min at 95 °C, followed by 40 cycles of 10 s at 95 °C, 40 s at an annealing temperature of 60 °C, and 45 s at 72 °C. The relative quantitative expression levels of the alpha-SMA (
Supplementary Table S1) were evaluated after normalizing to levels of GAPDH as endogenous control and compared to a normal cornea sample as a reference for the calculation of ∆∆Ct values, and fold change was calculated with the 2
−ΔΔCT method. The results are presented as significant increases or decreases in mRNA detected in the experimental groups (cornea of mild and severe form of NK) compared with their respective controls (healthy cornea).
2.5. Statistical Analysis
Data were represented as mean values with standard error, obtained using Graph Pad Prism version 8.0.2. Appropriate non-parametric one-way ANOVA tests were used to obtain significance between multiple time points post-alkali burn. A comparison of two different groups was made using the Mann–Whitney test. The threshold for statistical significance was defined as p < 0.05.
4. Discussion
This is the first study, to the best of our knowledge, that demonstrates a severe form of NK model generation using a combinatorial approach of trephination and alkali burn compared to direct alkali burns for studying the underlying disease pathology of neurotrophic keratitis in rabbits. The most widely used laboratory animals for studying the model systems are mice, rats, guinea pigs, and rabbits. Among the reasons for the increasing use of rodents, such as mice, instead of rabbits are reduced maintenance costs, small size, availability of inbred strains, ease of breeding, short reproductive cycle, high numbers of progeny, and the wide availability of many knockouts (KO) and transgenic models [
16]. However, rabbits have the advantage of an intermediate size between rodents and primates, longer life span than that of rodents, almost similar shape and size of the cornea as humans, and the immune system genes of rabbits are apparently more similar to those of the human immune system compared to rodent genes [
17]. Hence, our study used rabbits as models to generate neurotrophic keratitis and, for the first time, examined many ophthalmic parameters in a single study in complete depth.
The severe form of the model system generated in rabbit corneas mimics grade 3 neurotrophic keratitis in humans with the clinical features of non-healing epithelial defect with surrounding edema, scalped margins of epithelial defects, reduced corneal sensation, stromal ulceration, and angiogenesis, while the mild form of NK depicts grade 1 neurotrophic keratitis, which is majorly associated with corneal haze [
3]. Information gathered here from this study will help in the following two ways: first, for understanding the cascade of events leading to corneal scarring and loss of nerve sensation in neurotrophic keratitis; second, many emerging therapies targeting to prevent or reverse NK are being explored by various research groups. Thus, developing these animal models will also help in testing therapies for both their safety and efficacy [
18,
19].
In the current study, an array of ophthalmic and molecular parameters, like corneal thickness, opacification, nerve innervation, nerve sensation, and inflammation, was explored to investigate the detrimental effects of alkali burn in two model systems. With the aid of AS-OCT and Pentacam, we were able to demonstrate that both corneal thickness and opacity decrease over time in the direct alkali burn model, while a significant increase in thickness, opacity, persistent epithelial defect up to 15 days, and loss of corneal sensation occurs in the severe form of the NK model in rabbits. With the use of non-invasive techniques like in vivo confocal microscopy (IVCM) and Cochet–Bonnet aesthesiometer, we found that nerve sensation and the number of trigeminal nerves decreased significantly in the severe form of NK compared to the mild form. IVCM is a powerful instrument that clearly shows layer-by-layer changes occurring in the healthy and diseased cornea. As the results show, typically in the severe form of NK, the epithelial and stromal cell densities are significantly reduced along with no nerve fibers. The stroma is hyper-reflective with the presence of activated dendritic cells in the severe form compared to the mild form. As a consequence of corneal sensitivity loss or reduction, dry-eye disease can develop, causing neuropathic pain, corneal ulcers, and delayed corneal wound healing [
20,
21,
22]. All these clinical features of the persistent epithelial defect and stromal ulceration were evident in the grade 3 model of NK in rabbits. Although these imaging tools have been around for more than 20 years, adapting them to detect and relate to clinical features has not been explored to the full extent. With this study, we are trying to demonstrate the power of this non-invasive imaging tool in assessing the microscopic pathology of the disease.
Additional clinical features, like corneal neovascularization, were observed as a late-stage result of chemical burns. Usually, the growth of new blood vessels is facilitated by increased expression of angiogenic cytokines, which were evaluated in the tears of rabbits. An increase in the levels of IL-6 and VEGF was observed in the severe form compared to the direct form, which clinically correlated with 360-degree angiogenesis captured in the diffuse light images of the slit lamp, suggesting underlying dynamic processes of inflammation-mediated angiogenesis taking place. Hence, when ocular inflammation occurs, corneal epithelial and endothelial cells, macrophages, and certain inflammatory cells produce angiogenic growth factors, namely VEGF and fibroblast growth factors. This inflammatory milieu, along with nerve damage created in the severe form of NK, leads to delayed corneal re-epithelization compared to the milder form, where we observe complete epithelization post-one-week, as no fluorescein stain was taken up by the rabbit cornea, unlike the severe form where we observed epithelial defect to be persistent till day 15 post alkali injury. In Herpetic stromal keratitis also, researchers have reported that proinflammatory cytokines such as IL-6 and TNF-α provoke angiogenesis through increased expression of VEGF [
5,
23]. IL-6 plays and modulates many important roles in ocular inflammation and angiogenesis in the conjunctiva, cornea, iris, retina, and orbit [
24].
Apart from inflammation and angiogenesis, the level of matrix metalloproteinases (MMPs), which are zinc-dependent proteinases and play a key role in wound healing, were evaluated. In our study, we assessed the expression level of total MMP 9, which is a key metalloproteinase known to degrade extracellular matrix (ECM) proteins, thereby regulating fibrosis. We found levels of MMP9 to be decreased in the severe form compared to direct alkali burn. Our observation of decreased MMP9 in the severe form of NK is in line with Fini’s group observation, where MMP9 knockout showed delayed epithelial wound healing [
6,
7]. Henceforth, clinically relating it to the extent of opacity obtained in the two model systems, with an opaque cornea in trephination with alkali (a severe form of NK) vs. milder opacity in direct alkali burn. We also observed significantly increased expression of alpha-SMA at the transcript level in the severe NK form compared to the control and milder forms of NK (
Supplementary Figure S1). Thus, the data suggest more disorganized extracellular matrix deposition through myofibroblast in the severe form of NK than in the mild form, which produces corneal haze.
In 1982, Schimmelpfennig and R. Beuerman gave a very primitive rabbit animal model of NK using thermocoagulation of the ophthalmic branch of the trigeminal nerve [
25]. This study highlighted the use of a slit lamp, histology, and corneal sensation to establish the base for the animal model [
25]; however, it failed to comment on the corneal opacification, vascularisation, and other cellular changes taking place in the rabbit cornea and are key features to relate it clinically in humans. Attempts have been made to make the model in other animal species, like mice and rats, using either thermocoagulation or capsaicin injection at the neonatal stage [
14]. Unfortunately, the injection of capsaicin at the neonatal stage does not mimic the clinical cause of neurotrophic keratitis in humans.
In summary, this study describes the development of a reliable rabbit animal model of neurotrophic keratitis of both grade 1 and grade 3 of the disease. We evaluated in-vivo longitudinal corneal metrics of swelling, opacification, nerve sensation, and neovascularization resulting from alkali burns in two model systems (
Figure 6) and found trephination followed by alkali burn proved to be a good, stable, and reproducible model that closely resembles the clinical features of NK in human eyes as per the clinician. Hence, this NK model can be used for testing both prophylactic and therapeutic modalities in the near future.