Mechanobiology of Dental Pulp Cells
Abstract
1. Introduction
2. Mechanical Cues in the Physiology and Pathology of Dental Pulp
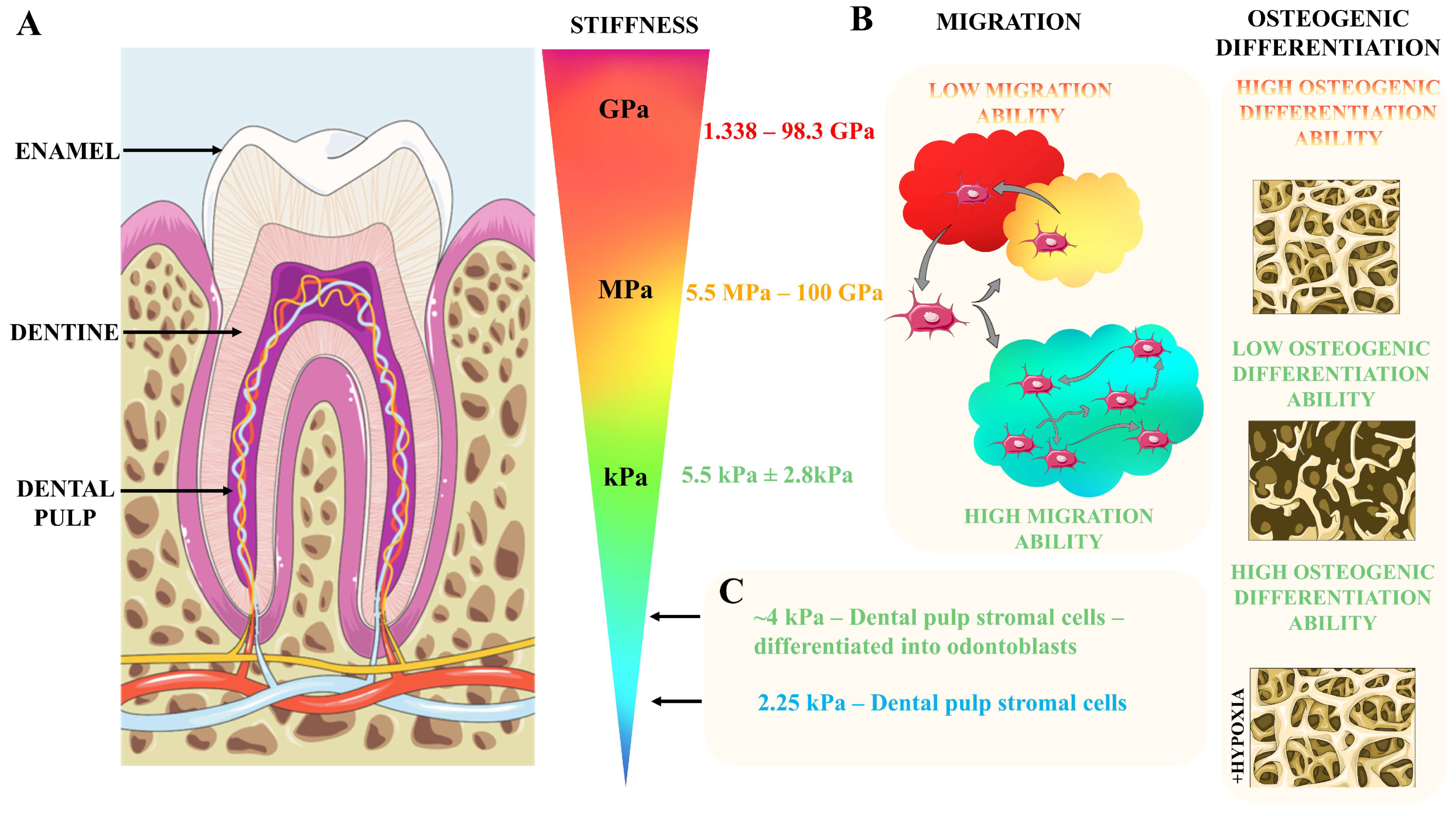
2.1. Biomechanics of the Dental Pulp and Surrounding Tissues
2.2. Biomechanical Cues in Dental Pain Propagation
3. Importance of Mechanical Cues in Bioengineering of Dental Pulp and Tooth
3.1. Mechanical Factors in Dental Pulp Regeneration
3.2. Biomechanical Cues in the Regeneration of Vasculature and Nervous Fibers within the Dental Pulp
3.3. Mechanosensitive Receptors Piezo1 and Piezo2 and Dental Pulp
3.4. Molecular Pathways Regulating DPSCs Mechanosensitivity
4. Concluding Remarks and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
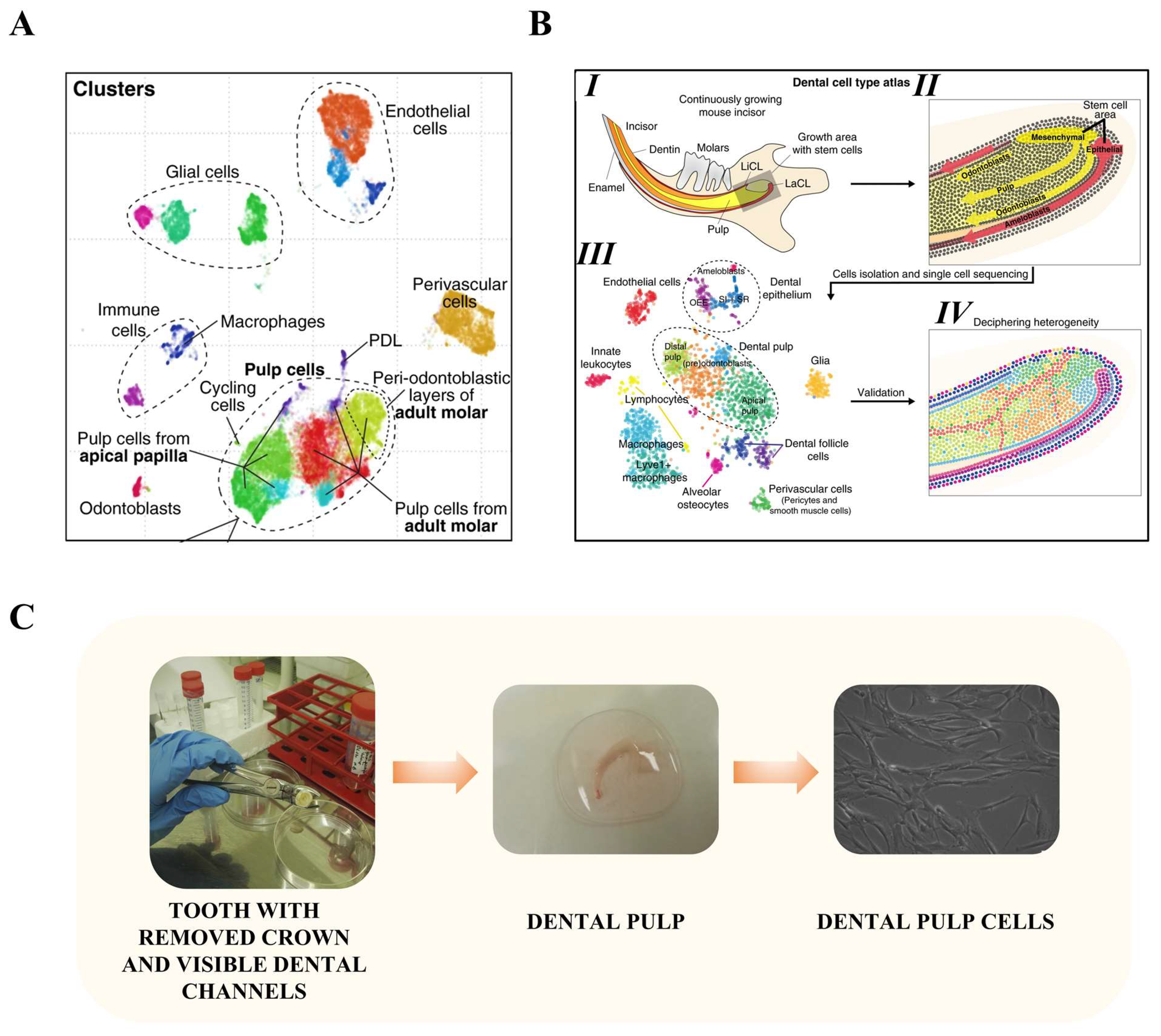
- Krivanek, J.; Soldatov, R.A.; Kastriti, M.E.; Chontorotzea, T.; Herdina, A.N.; Petersen, J.; Szarowska, B.; Landova, M.; Matejova, V.K.; Holla, L.I.; et al. Dental Cell Type Atlas Reveals Stem and Differentiated Cell Types in Mouse and Human Teeth. Nat. Commun. 2020, 11, 4816. [Google Scholar] [CrossRef] [PubMed]
- França, C.M.; Riggers, R.; Muschler, J.L.; Widbiller, M.; Lococo, P.M.; Diogenes, A.; Bertassoni, L.E. 3D-Imaging of Whole Neuronal and Vascular Networks of the Human Dental Pulp via CLARITY and Light Sheet Microscopy. Sci. Rep. 2019, 9, 10860. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Krivanek, J.; Buchtova, M.; Fried, K.; Adameyko, I. Plasticity of Dental Cell Types in Development, Regeneration, and Evolution. J. Dent. Res. 2023, 102, 589–598. [Google Scholar] [CrossRef]
- Miletich, I.; Sharpe, P.T. Neural Crest Contribution to Mammalian Tooth Formation. Birth Defects Res. Part C Embryo Today Rev. 2004, 72, 200–212. [Google Scholar] [CrossRef]
- Al-Maswary, A.A.; O’Reilly, M.; Holmes, A.P.; Walmsley, A.D.; Cooper, P.R.; Scheven, B.A. Exploring the Neurogenic Differentiation of Human Dental Pulp Stem Cells. PLoS ONE 2022, 17, e0277134. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chang, K.-C.; Tsai, S.-J.; Chang, H.-H.; Lin, C.-P. Neurogenic Differentiation of Dental Pulp Stem Cells to Neuron-like Cells in Dopaminergic and Motor Neuronal Inductive Media. J. Formos. Med. Assoc. 2014, 113, 956–965. [Google Scholar] [CrossRef]
- Darvishi, M.; Hamidabadi, H.G.; Bojnordi, M.N.; Saeednia, S.; Zahiri, M.; Niapour, A.; Alizadeh, R. Differentiation of Human Dental Pulp Stem Cells into Functional Motor Neuron: In Vitro and Ex Vivo Study. Tissue Cell 2021, 72, 101542. [Google Scholar] [CrossRef]
- Ellis, K.M.; O’Carroll, D.C.; Lewis, M.D.; Rychkov, G.Y.; Koblar, S.A. Neurogenic Potential of Dental Pulp Stem Cells Isolated from Murine Incisors. Stem Cell Res. Ther. 2014, 5, 30. [Google Scholar] [CrossRef]
- Gervois, P.; Struys, T.; Hilkens, P.; Bronckaers, A.; Ratajczak, J.; Politis, C.; Brône, B.; Lambrichts, I.; Martens, W. Neurogenic Maturation of Human Dental Pulp Stem Cells Following Neurosphere Generation Induces Morphological and Electrophysiological Characteristics of Functional Neurons. Stem Cells Dev. 2015, 24, 296–311. [Google Scholar] [CrossRef]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C.-Y. The Meaning, the Sense and the Significance: Translating the Science of Mesenchymal Stem Cells into Medicine. Nat. Med. 2013, 19, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ananth, H.; Kundapur, V.; Mohammed, H.S.; Anand, M.; Amarnath, G.S.; Mankar, S. A Review on Biomaterials in Dental Implantology. Int. J. Biomed. Sci. 2015, 11, 113–120. [Google Scholar]
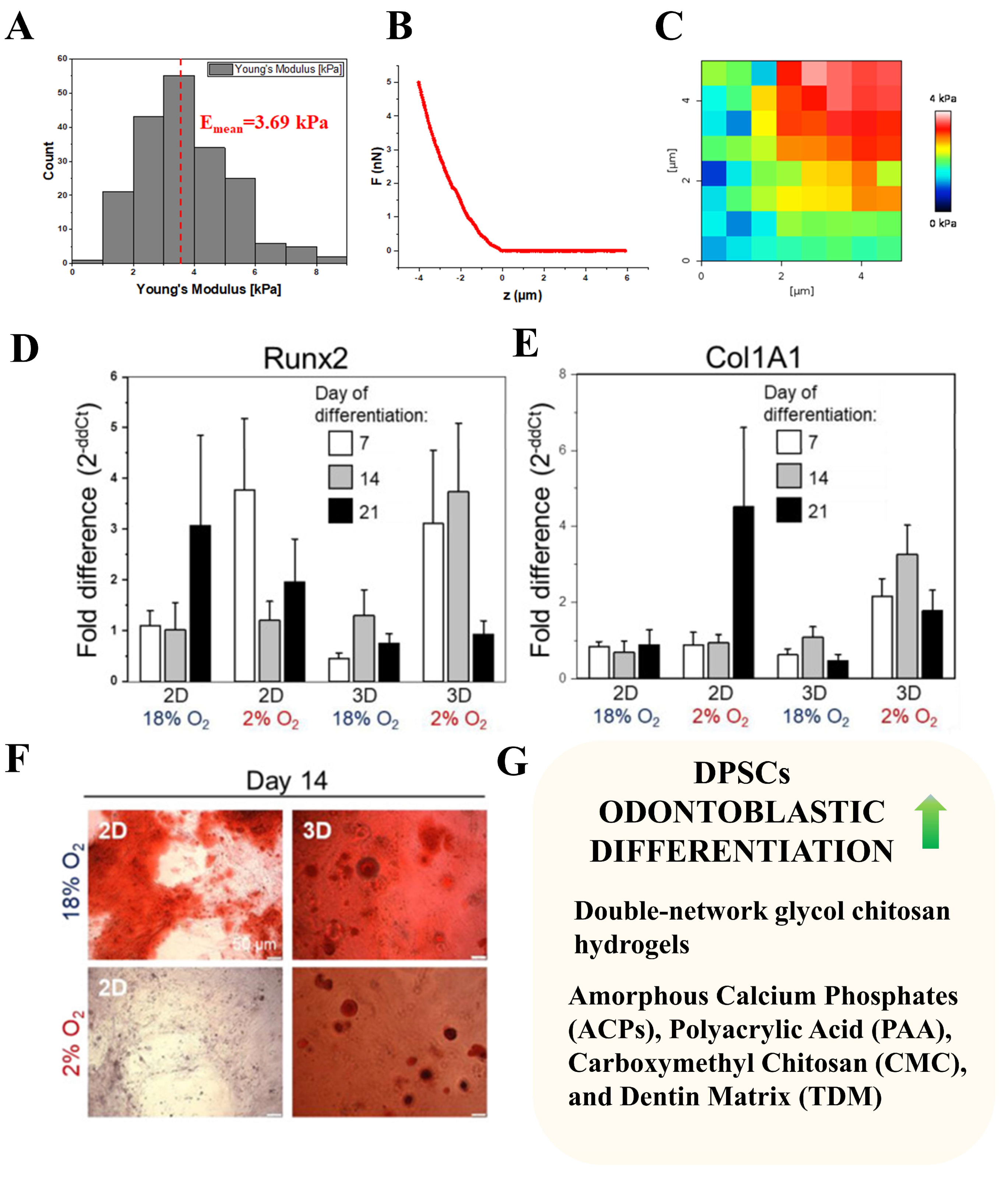
- Labedz-Maslowska, A.; Bryniarska, N.; Kubiak, A.; Kaczmarzyk, T.; Sekula-Stryjewska, M.; Noga, S.; Boruczkowski, D.; Madeja, Z.; Zuba-Surma, E. Multilineage Differentiation Potential of Human Dental Pulp Stem Cells—Impact of 3D and Hypoxic Environment on Osteogenesis In Vitro. Int. J. Mol. Sci. 2020, 21, 6172. [Google Scholar] [CrossRef] [PubMed]
- Bryniarska, N.; Kubiak, A.; Łabędź-Masłowska, A.; Zuba-Surma, E. Impact of Developmental Origin, Niche Mechanics and Oxygen Availability on Osteogenic Differentiation Capacity of Mesenchymal Stem/Stromal Cells. Acta Biochim. Pol. 2019, 66, 491–498. [Google Scholar] [CrossRef]
- Vining, K.H.; Mooney, D.J. Mechanical Forces Direct Stem Cell Behaviour in Development and Regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- Kaukua, N.; Shahidi, M.K.; Konstantinidou, C.; Dyachuk, V.; Kaucka, M.; Furlan, A.; An, Z.; Wang, L.; Hultman, I.; Ährlund-Richter, L.; et al. Glial Origin of Mesenchymal Stem Cells in a Tooth Model System. Nature 2014, 513, 551–554. [Google Scholar] [CrossRef]
- Coutiño, B.C.; Mayor, R. Neural Crest Mechanosensors: Seeing Old Proteins in a New Light. Dev. Cell 2022, 57, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, R.; Tu, X.; Janairo, R.R.R.; Kwong, G.; Wang, D.; Zhu, Y.; Li, S. Differentiation of Neural Crest Stem Cells in Response to Matrix Stiffness and TGF-Β1 in Vascular Regeneration. Stem Cells Dev. 2020, 29, 249–256. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Smith, D.T.; Jahanmir, S.; Romberg, E.; Kelly, J.R.; Thompson, V.P.; Rekow, E.D. Indentation Damage and Mechanical Properties of Human Enamel and Dentin. J. Dent. Res. 1998, 77, 472–480. [Google Scholar] [CrossRef]
- Stanford, J.W.; Paffenbarger, G.C.; Kumpula, J.W.; Sweeney, W.T. Determination of Some Compressive Properties of Human Enamel and Dentin. J. Am. Dent. Assoc. 1958, 57, 487–495. [Google Scholar] [CrossRef]
- Craig, R.G.; Peyton, F.A.; Johnson, D.W. Compressive Properties of Enamel, Dental Cements, and Gold. J. Dent. Res. 1961, 40, 936–945. [Google Scholar] [CrossRef]
- Fong, H.; Sarikaya, M.; White, S.N.; Snead, M.L. Nano-Mechanical Properties Profiles across Dentin–Enamel Junction of Human Incisor Teeth. Mater. Sci. Eng. C 1999, 7, 119–128. [Google Scholar] [CrossRef]
- He, L.H.; Fujisawa, N.; Swain, M.V. Elastic Modulus and Stress-Strain Response of Human Enamel by Nano-Indentation. Biomaterials 2006, 27, 4388–4398. [Google Scholar] [CrossRef] [PubMed]
- Habelitz, S.; Marshall, S.J.; Marshall, G.W.; Balooch, M. Mechanical Properties of Human Dental Enamel on the Nanometre Scale. Arch. Oral Biol. 2001, 46, 173–183. [Google Scholar] [CrossRef]
- Trengrove, H.G.; Carter, G.M.; Hood, J.A. Stress Relaxation Properties of Human Dentin. Dent. Mater. 1995, 11, 305–310. [Google Scholar] [CrossRef]
- Jantarat, J.; Palamara, J.E.A.; Lindner, C.; Messer, H.H. Time-Dependent Properties of Human Root Dentin. Dent. Mater. 2002, 18, 486–493. [Google Scholar] [CrossRef]
- Haines, D.J. Physical Properties of Human Tooth Enamel and Enamel Sheath Material under Load. J. Biomech. 1968, 1, 117–125. [Google Scholar] [CrossRef]
- Marshall, G.W.; Balooch, M.; Gallagher, R.R.; Gansky, S.A.; Marshall, S.J. Mechanical Properties of the Dentinoenamel Junction: AFM Studies of Nanohardness, Elastic Modulus, and Fracture. J. Biomed. Mater. Res. 2001, 54, 87–95. [Google Scholar] [CrossRef]
- Arcís, R.W.; López-Macipe, A.; Toledano, M.; Osorio, E.; Rodríguez-Clemente, R.; Murtra, J.; Fanovich, M.A.; Pascual, C.D. Mechanical Properties of Visible Light-Cured Resins Reinforced with Hydroxyapatite for Dental Restoration. Dent. Mater. 2002, 18, 49–57. [Google Scholar] [CrossRef]
- Senawongse, P.; Otsuki, M.; Tagami, J.; Mjör, I. Age-Related Changes in Hardness and Modulus of Elasticity of Dentine. Arch. Oral Biol. 2006, 51, 457–463. [Google Scholar] [CrossRef]
- Matsubara, T.; Iga, T.; Sugiura, Y.; Kusumoto, D.; Sanosaka, T.; Tai-Nagara, I.; Takeda, N.; Fong, G.-H.; Ito, K.; Ema, M.; et al. Coupling of Angiogenesis and Odontogenesis Orchestrates Tooth Mineralization in Mice. J. Exp. Med. 2022, 219, e20211789. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, B.; Bayrak, E.; Erisken, C. Characterization of Human Dental Pulp Tissue under Oscillatory Shear and Compression. J. Biomech. Eng. 2016, 138, 061006. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.D.; Naimipour, H.; Sun, S.; Cho, M.; Alapati, S.B. Mechanical Changes in Human Dental Pulp Stem Cells during Early Odontogenic Differentiation. J. Endod. 2015, 41, 50–55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ehlinger, C.; Mathieu, E.; Rabineau, M.; Ball, V.; Lavalle, P.; Haikel, Y.; Vautier, D.; Kocgozlu, L. Insensitivity of Dental Pulp Stem Cells Migration to Substrate Stiffness. Biomaterials 2021, 275, 120969. [Google Scholar] [CrossRef] [PubMed]
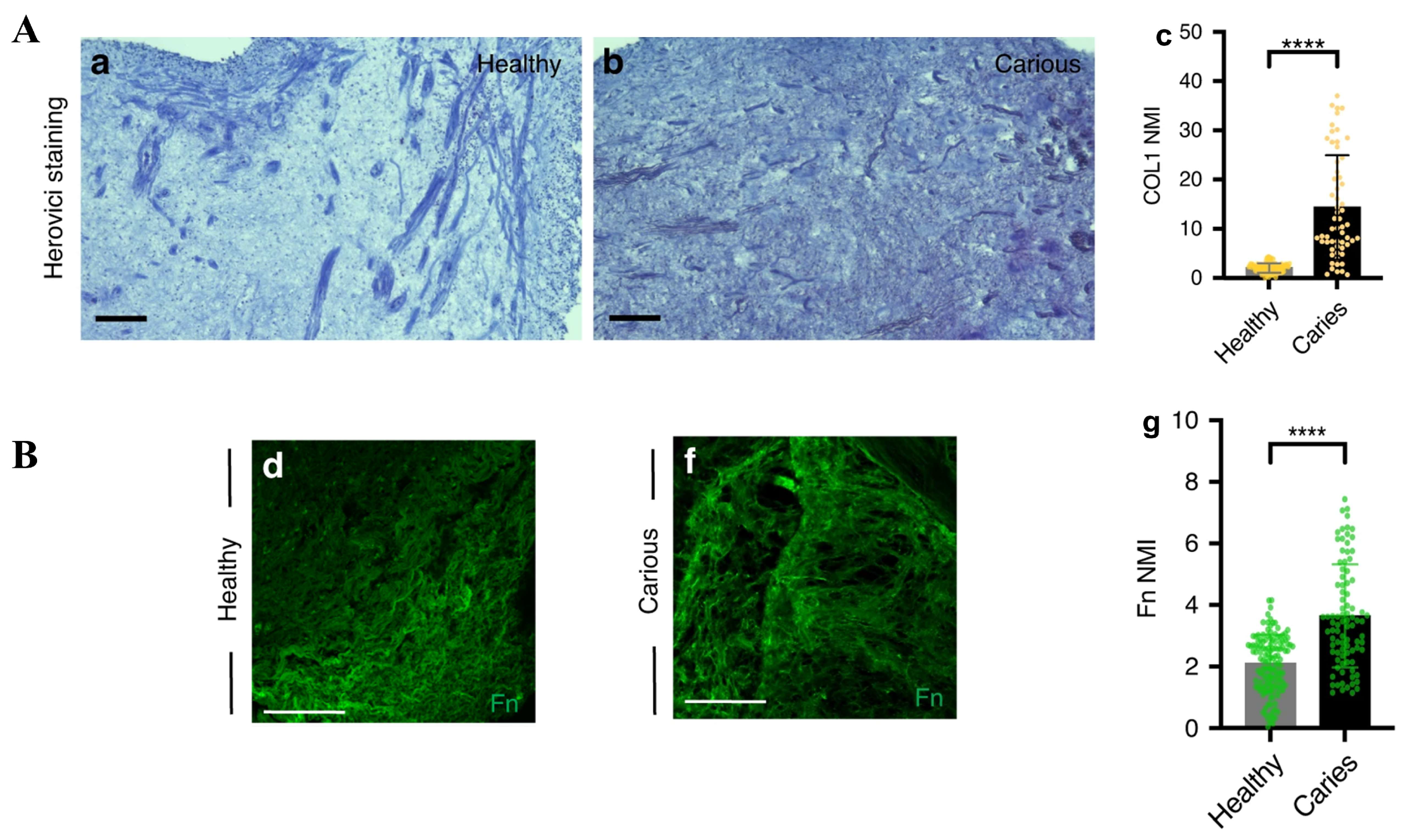
- Balic, A.; Perver, D.; Pagella, P.; Rehrauer, H.; Stadlinger, B.; Moor, A.E.; Vogel, V.; Mitsiadis, T.A. Extracellular Matrix Remodelling in Dental Pulp Tissue of Carious Human Teeth through the Prism of Single-Cell RNA Sequencing. Int. J. Oral Sci. 2023, 15, 30. [Google Scholar] [CrossRef]
- Bryniarska-Kubiak, N.; Kubiak, A.; Trojan, E.; Wesołowska, J.; Lekka, M.; Basta-Kaim, A. Oxygen-Glucose Deprivation in Organotypic Hippocampal Cultures Leads to Cytoskeleton Rearrangement and Immune Activation: Link to the Potential Pathomechanism of Ischaemic Stroke. Cells 2023, 12, 1465. [Google Scholar] [CrossRef]
- Assen, F.P.; Abe, J.; Hons, M.; Hauschild, R.; Shamipour, S.; Kaufmann, W.A.; Costanzo, T.; Krens, G.; Brown, M.; Ludewig, B.; et al. Multitier Mechanics Control Stromal Adaptations in the Swelling Lymph Node. Nat. Immunol. 2022, 23, 1246–1255. [Google Scholar] [CrossRef]
- Giblin, M.J.; Ontko, C.D.; Penn, J.S. Effect of Cytokine-Induced Alterations in Extracellular Matrix Composition on Diabetic Retinopathy-Relevant Endothelial Cell Behaviors. Sci. Rep. 2022, 12, 12955. [Google Scholar] [CrossRef]
- Shi, L.; Fu, S.; Fahim, S.; Pan, S.; Lina, H.; Mu, X.; Niu, Y. TNF-Alpha Stimulation Increases Dental Pulp Stem Cell Migration in Vitro through Integrin Alpha-6 Subunit Upregulation. Arch. Oral Biol. 2017, 75, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, C.; Jeanneau, C.; Bakopoulou, A.; About, I. Dental Pulp Stem Cell Recruitment Signals within Injured Dental Pulp Tissue. Dent. J. 2016, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Bahuguna, R. Role of Matrix Metalloproteinases in Dental Caries, Pulp and Periapical Inflammation: An Overview. J. Oral Biol. Craniofacial Res. 2015, 5, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Amano, K.; Iohara, K.; Ito, M.; Imabayashi, K.; Into, T.; Matsushita, K.; Nakamura, H.; Nakashima, M. Matrix Metalloproteinase-3 Accelerates Wound Healing Following Dental Pulp Injury. Am. J. Pathol. 2009, 175, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Colombo, J.S.; Ayre, W.N.; Sloan, A.J.; Waddington, R.J. Elucidating the Cellular Actions of Demineralised Dentine Matrix Extract on a Clonal Dental Pulp Stem Cell Population in Orchestrating Dental Tissue Repair. J. Tissue Eng. 2015, 6, 2041731415586318. [Google Scholar] [CrossRef] [PubMed]
- Datko Williams, L.; Farley, A.; Cupelli, M.; Alapati, S.; Kennedy, M.S.; Dean, D. Effects of Substrate Stiffness on Dental Pulp Stromal Cells in Culture. J. Biomed. Mater. Res. Part A 2018, 106, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Renton, T. Dental (Odontogenic) Pain. Rev. Pain. 2011, 5, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.W. Dentin: Microstructure and Characterization. Quintessence Int. 1993, 24, 606–617. [Google Scholar] [PubMed]
- Lin, M.; Liu, S.; Niu, L.; Xu, F.; Lu, T.J. Analysis of Thermal-Induced Dentinal Fluid Flow and Its Implications in Dental Thermal Pain. Arch. Oral Biol. 2011, 56, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Charoenlarp, P.; Wanachantararak, S.; Vongsavan, N.; Matthews, B. Pain and the Rate of Dentinal Fluid Flow Produced by Hydrostatic Pressure Stimulation of Exposed Dentine in Man. Arch. Oral Biol. 2007, 52, 625–631. [Google Scholar] [CrossRef]
- Davari, A.; Ataei, E.; Assarzadeh, H. Dentin Hypersensitivity: Etiology, Diagnosis and Treatment; A Literature Review. J. Dent. 2013, 14, 136–145. [Google Scholar]
- Bender, I.B. Pulpal Pain Diagnosis—A Review. J. Endod. 2000, 26, 175–179. [Google Scholar] [CrossRef]
- Yu, C.; Abbott, P.V. An Overview of the Dental Pulp: Its Functions and Responses to Injury. Aust. Dent. J. 2007, 52, S4–S16. [Google Scholar] [CrossRef] [PubMed]
- Ngassapa, D. Correlation of Clinical Pain Symptoms with Histopathological Changes of the Dental Pulp: A Review. East Afr. Med. J. 1996, 73, 779–781. [Google Scholar]
- Tønder, K.J.H.; Kvinnsland, I. Micropuncture Measurements of Interstitial Fluid Pressure in Normal and Inflamed Dental Pulp in Cats. J. Endod. 1983, 9, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.; Goodis, H. Seltzer and Bender’s Dental Pulp; Franklin, R., Ed.; Quintessence: Chicago, IL, USA, 2012. [Google Scholar]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant Biomaterials: A Comprehensive Review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Gomes, K.U.; Carlini, J.L.; Biron, C.; Rapoport, A.; Dedivitis, R.A. Use of Allogeneic Bone Graft in Maxillary Reconstruction for Installation of Dental Implants. J. Oral Maxillofac. Surg. 2008, 66, 2335–2338. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Cao, C.; Wang, A.; Zhao, Y.; Jin, M.; Wang, Y.; Chen, S.; Yu, M.; Yang, Z.; Qu, X.; et al. Injectable Double-Network Hydrogel-Based Three-Dimensional Cell Culture Systems for Regenerating Dental Pulp. ACS Appl. Mater. Interfaces 2023, 15, 7821–7832. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Sasaki, J.I.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-Dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef]
- Miyashita, S.; Ahmed, N.E.M.B.; Murakami, M.; Iohara, K.; Yamamoto, T.; Horibe, H.; Kurita, K.; Takano-Yamamoto, T.; Nakashima, M. Mechanical Forces Induce Odontoblastic Differentiation of Mesenchymal Stem Cells on Three-Dimensional Biomimetic Scaffolds: Mechanical Forces Induce Odontoblastic Differentiation of MSCs. J. Tissue Eng. Regen. Med. 2014, 11, 434–446. [Google Scholar] [CrossRef]
- Yu, V.; Damek-Poprawa, M.; Nicoll, S.B.; Akintoye, S.O. Dynamic Hydrostatic Pressure Promotes Differentiation of Human Dental Pulp Stem Cells. Biochem. Biophys. Res. Commun. 2009, 386, 661–665. [Google Scholar] [CrossRef]
- Wen, B.; Dai, Y.; Han, X.; Huo, F.; Xie, L.; Yu, M.; Wang, Y.; An, N.; Li, Z.; Guo, W. Biomineralization-Inspired Mineralized Hydrogel Promotes the Repair and Regeneration of Dentin/Bone Hard Tissue. NPJ Regen. Med. 2023, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Paino, F.; d’Aquino, R.; De Rosa, A.; Iezzi, G.; Piattelli, A.; Laino, L.; Mitsiadis, T.; Desiderio, V.; Mangano, F.; et al. Human Dental Pulp Stem Cells Hook into Biocoral Scaffold Forming an Engineered Biocomplex. PLoS ONE 2011, 6, e18721. [Google Scholar] [CrossRef]
- Woloszyk, A.; Dircksen, S.H.; Bostanci, N.; Müller, R.; Hofmann, S.; Mitsiadis, T.A. Influence of the Mechanical Environment on the Engineering of Mineralised Tissues Using Human Dental Pulp Stem Cells and Silk Fibroin Scaffolds. PLoS ONE 2014, 9, e111010. [Google Scholar] [CrossRef]
- Monterubbianesi, R.; Bencun, M.; Pagella, P.; Woloszyk, A.; Orsini, G.; Mitsiadis, T.A. A Comparative in Vitro Study of the Osteogenic and Adipogenic Potential of Human Dental Pulp Stem Cells, Gingival Fibroblasts and Foreskin Fibroblasts. Sci. Rep. 2019, 9, 1761. [Google Scholar] [CrossRef] [PubMed]
- Woloszyk, A.; Buschmann, J.; Waschkies, C.; Stadlinger, B.; Mitsiadis, T.A. Human Dental Pulp Stem Cells and Gingival Fibroblasts Seeded into Silk Fibroin Scaffolds Have the Same Ability in Attracting Vessels. Front. Physiol. 2016, 7, 140. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Sarkar, B.; Kim, K.-K.; Kadincesme, N.; Paul, R.; Kumar, A.; Kobayashi, Y.; Roy, A.; Choudhury, M.; Yang, J.; et al. Angiogenic Hydrogels for Dental Pulp Revascularization. Acta Biomater. 2021, 126, 109–118. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Hargreaves, K.M.; Jin, L.; Samaranayake, L.P.; Zhang, C. The Interplay of Dental Pulp Stem Cells and Endothelial Cells in an Injectable Peptide Hydrogel on Angiogenesis and Pulp Regeneration In Vivo. Tissue Eng. Part A 2015, 21, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Paracrine-Mediated Neuroprotection and Neuritogenesis of Axotomised Retinal Ganglion Cells by Human Dental Pulp Stem Cells: Comparison with Human Bone Marrow and Adipose-Derived Mesenchymal Stem Cells. PLoS ONE 2014, 9, e109305. [Google Scholar] [CrossRef]
- Sultan, N.; Amin, L.E.; Zaher, A.R.; Grawish, M.E.; Scheven, B.A. Dental Pulp Stem Cells Stimulate Neuronal Differentiation of PC12 Cells. Neural Regen. Res. 2021, 16, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Sultan, N.; Amin, L.E.; Zaher, A.R.; Grawish, M.E.; Scheven, B.A. Neurotrophic Effects of Dental Pulp Stem Cells on Trigeminal Neuronal Cells. Sci. Rep. 2020, 10, 19694. [Google Scholar] [CrossRef]
- Morrison, S.J.; Scadden, D.T. The Bone Marrow Niche for Haematopoietic Stem Cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef]
- Anthony, B.A.; Link, D.C. Regulation of Hematopoietic Stem Cells by Bone Marrow Stromal Cells. Trends Immunol. 2014, 35, 32–37. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Venugopal, C.; Parveen, S.; Shobha, K.; Rai, K.S.; Kutty, B.M.; Dhanushkodi, A. Remarkable Migration Propensity of Dental Pulp Stem Cells towards Neurodegenerative Milieu: An in Vitro Analysis. NeuroToxicology 2020, 81, 89–100. [Google Scholar] [CrossRef]
- Pagella, P.; Miran, S.; Neto, E.; Martin, I.; Lamghari, M.; Mitsiadis, T.A. Human Dental Pulp Stem Cells Exhibit Enhanced Properties in Comparison to Human Bone Marrow Stem Cells on Neurites Outgrowth. FASEB J. 2020, 34, 5499–5511. [Google Scholar] [CrossRef] [PubMed]
- Mitsiadis, T.A.; Magloire, H.; Pagella, P. Nerve Growth Factor Signalling in Pathology and Regeneration of Human Teeth. Sci. Rep. 2017, 7, 1327. [Google Scholar] [CrossRef] [PubMed]
- Bryniarska-Kubiak, N.; Kubiak, A.; Lekka, M.; Basta-Kaim, A. The Emerging Role of Mechanical and Topographical Factors in the Development and Treatment of Nervous System Disorders: Dark and Light Sides of the Force. Pharmacol. Rep. 2021, 73, 1626–1641. [Google Scholar] [CrossRef] [PubMed]
- Previtera, M.L.; Langhammer, C.G.; Firestein, B.L. Effects of Substrate Stiffness and Cell Density on Primary Hippocampal Cultures. J. Biosci. Bioeng. 2010, 110, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Cheng, S.-J.; Tzen, J.T.C.; Cheng, C.-M.; Lin, Y.-W. Probing Relevant Molecules in Modulating the Neurite Outgrowth of Hippocampal Neurons on Substrates of Different Stiffness. PLoS ONE 2013, 8, e83394. [Google Scholar] [CrossRef] [PubMed]
- Leipzig, N.D.; Shoichet, M.S. The Effect of Substrate Stiffness on Adult Neural Stem Cell Behavior. Biomaterials 2009, 30, 6867–6878. [Google Scholar] [CrossRef]
- Colleen, T.C.; Fanale, K.; Sabrina, S.J. Characterizing the Effect of Substrate Stiffness on Neural Stem Cell Differentiation. MRS Online Proc. Libr. 2012, 1498, 47–52. [Google Scholar] [CrossRef]
- Budday, S.; Nay, R.; de Rooij, R.; Steinmann, P.; Wyrobek, T.; Ovaert, T.C.; Kuhl, E. Mechanical Properties of Gray and White Matter Brain Tissue by Indentation. J. Mech. Behav. Biomed. Mater. 2015, 46, 318–330. [Google Scholar] [CrossRef]
- Bryniarska-Kubiak, N.; Kubiak, A.; Basta-Kaim, A. Mechanotransductive Receptor Piezo1 as a Promising Target in the Treatment of Neurological Diseases. Curr. Neuropharmacol. 2023, 21, 2030–2035. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, J.; McGrath, C. Mechanosensitive Piezo1 and Piezo2 Ion Channels in Craniofacial Development and Dentistry: Recent Advances and Prospects. Front. Physiol. 2022, 13, 2263. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, A.; Sugimoto, A.; Yoshizaki, K.; Kawarabayashi, K.; Iwata, K.; Kurogoushi, R.; Kitamura, T.; Otsuka, K.; Hasegawa, T.; Akazawa, Y.; et al. Coordination of WNT Signaling and Ciliogenesis during Odontogenesis by Piezo Type Mechanosensitive Ion Channel Component 1. Sci. Rep. 2019, 9, 14762. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, A.; Miyazaki, A.; Kawarabayashi, K.; Shono, M.; Akazawa, Y.; Hasegawa, T.; Ueda-Yamaguchi, K.; Kitamura, T.; Yoshizaki, K.; Fukumoto, S.; et al. Piezo Type Mechanosensitive Ion Channel Component 1 Functions as a Regulator of the Cell Fate Determination of Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 17696. [Google Scholar] [CrossRef] [PubMed]
- Mousawi, F.; Peng, H.; Li, J.; Ponnambalam, S.; Roger, S.; Zhao, H.; Yang, X.; Jiang, L.-H. Chemical Activation of the Piezo1 Channel Drives Mesenchymal Stem Cell Migration via Inducing ATP Release and Activation of P2 Receptor Purinergic Signaling. Stem Cells 2020, 38, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Yang, B.; He, Y.; Xie, B.; Zhao, T.; Chen, J. Effects of Mechanosensitive Ion Channel Piezo1 on Proliferation and Osteogenic Differentiation of Human Dental Follicle Cells. Ann. Anat. Anat. Anz. 2022, 239, 151847. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Cooper, P.R.; Walmsley, A.D.; Scheven, B.A. Role of Piezo Channels in Ultrasound-Stimulated Dental Stem Cells. J. Endod. 2017, 43, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, M.; Kimura, M.; Ouchi, T.; Nakamura, T.; Ohyama, S.; Ando, M.; Nomura, S.; Azuma, T.; Ichinohe, T.; Shibukawa, Y. Mechanical Stimulation-Induced Calcium Signaling by Piezo1 Channel Activation in Human Odontoblast Reduces Dentin Mineralization. Front. Physiol. 2021, 12, 704518. [Google Scholar] [CrossRef]
- Khatibi Shahidi, M.; Krivanek, J.; Kaukua, N.; Ernfors, P.; Hladik, L.; Kostal, V.; Masich, S.; Hampl, A.; Chubanov, V.; Gudermann, T.; et al. Three-Dimensional Imaging Reveals New Compartments and Structural Adaptations in Odontoblasts. J. Dent. Res. 2015, 94, 945–954. [Google Scholar] [CrossRef]
- Sun, X.-F.; Qiao, W.-W.; Meng, L.-Y.; Bian, Z. PIEZO1 Ion Channels Mediate Mechanotransduction in Odontoblasts. J. Endod. 2022, 48, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Han, H.M.; Jeong, S.Y.; Kim, T.H.; Choi, S.Y.; Kim, Y.S.; Bae, Y.C. Expression of Piezo1 in the Trigeminal Neurons and in the Axons That Innervate the Dental Pulp. Front. Cell. Neurosci. 2022, 16, 945948. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Vang, H.; Lee, P.R.; Kim, Y.H.; Kim, H.W.; Kang, Y.; Oh, S.B. Piezo2 Expression in Mechanosensitive Dental Primary Afferent Neurons. J. Dent. Res. 2017, 96, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Ruan, N.; Tribble, J.; Peterson, A.M.; Jiang, Q.; Wang, J.Q.; Chu, X.-P. Acid-Sensing Ion Channels and Mechanosensation. Int. J. Mol. Sci. 2021, 22, 4810. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, B.-M.; Park, C.-K.; Kim, Y.H.; Chung, G. Ion Channels Involved in Tooth Pain. Int. J. Mol. Sci. 2019, 20, 2266. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Du, W.; Guo, D.; Wang, S.; Du, W. Mechanical Signaling in Dental Pulp Stem Cells. FBL 2023, 28, 274. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, L.; Chen, L.; Jiang, J.; Zhou, X.; Wang, M.; Fan, Y. Static Magnetic Field Regulates Proliferation, Migration, Differentiation, and YAP/TAZ Activation of Human Dental Pulp Stem Cells. J. Tissue Eng. Regen. Med. 2018, 12, 2029–2040. [Google Scholar] [CrossRef]
- Du, Y.; Montoya, C.; Orrego, S.; Wei, X.; Ling, J.; Lelkes, P.I.; Yang, M. Topographic Cues of a Novel Bilayered Scaffold Modulate Dental Pulp Stem Cells Differentiation by Regulating YAP Signalling through Cytoskeleton Adjustments. Cell Prolif. 2019, 52, e12676. [Google Scholar] [CrossRef]
- Li, G.; Xu, Z.; Yang, M.; Ning, Y.; Ye, L.; Jiang, H.; Du, Y. Topographic Cues of a PLGA Scaffold Promote Odontogenic Differentiation of Dental Pulp Stem Cells through the YAP/β-Catenin Signaling Axis. ACS Biomater. Sci. Eng. 2023, 9, 1598–1607. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, M.; Zhang, Q.; Zhang, T.; Tian, T.; Ma, Q.; Xue, C.; Lin, S.; Cai, X. Stiffness Regulates the Proliferation and Osteogenic/Odontogenic Differentiation of Human Dental Pulp Stem Cells via the WNT Signalling Pathway. Cell Prolif. 2018, 51, e12435. [Google Scholar] [CrossRef]
- He, W.; Wang, Z.; Luo, Z.; Yu, Q.; Jiang, Y.; Zhang, Y.; Zhou, Z.; Smith, A.J.; Cooper, P.R. LPS Promote the Odontoblastic Differentiation of Human Dental Pulp Stem Cells via MAPK Signaling Pathway. J. Cell. Physiol. 2015, 230, 554–561. [Google Scholar] [CrossRef]
- Puech, P.-H.; Taubenberger, A.; Ulrich, F.; Krieg, M.; Muller, D.J.; Heisenberg, C.-P. Measuring Cell Adhesion Forces of Primary Gastrulating Cells from Zebrafish Using Atomic Force Microscopy. J. Cell Sci. 2005, 118, 4199–4206. [Google Scholar] [CrossRef]
- Krieg, M.; Arboleda-Estudillo, Y.; Puech, P.-H.; Käfer, J.; Graner, F.; Müller, D.J.; Heisenberg, C.-P. Tensile Forces Govern Germ-Layer Organization in Zebrafish. Nat. Cell Biol. 2008, 10, 429–436. [Google Scholar] [CrossRef]
- Yanagida, A.; Corujo-Simon, E.; Revell, C.K.; Sahu, P.; Stirparo, G.G.; Aspalter, I.M.; Winkel, A.K.; Peters, R.; De Belly, H.; Cassani, D.A.D.; et al. Cell Surface Fluctuations Regulate Early Embryonic Lineage Sorting. Cell 2022, 185, 777–793.e20. [Google Scholar] [CrossRef]
- Zak, A.; Merino-Cortés, S.V.; Sadoun, A.; Mustapha, F.; Babataheri, A.; Dogniaux, S.; Dupré-Crochet, S.; Hudik, E.; He, H.-T.; Barakat, A.I.; et al. Rapid Viscoelastic Changes Are a Hallmark of Early Leukocyte Activation. Biophys. J. 2021, 120, 1692–1704. [Google Scholar] [CrossRef]
- Sadoun, A.; Biarnes-Pelicot, M.; Ghesquiere-Dierickx, L.; Wu, A.; Théodoly, O.; Limozin, L.; Hamon, Y.; Puech, P.-H. Controlling T Cells Spreading, Mechanics and Activation by Micropatterning. Sci. Rep. 2021, 11, 6783. [Google Scholar] [CrossRef]
- Horsnell, H.L.; Tetley, R.J.; De Belly, H.; Makris, S.; Millward, L.J.; Benjamin, A.C.; Heeringa, L.A.; de Winde, C.M.; Paluch, E.K.; Mao, Y.; et al. Lymph Node Homeostasis and Adaptation to Immune Challenge Resolved by Fibroblast Network Mechanics. Nat. Immunol. 2022, 23, 1169–1182. [Google Scholar] [CrossRef]
- Wirtz, D.; Konstantopoulos, K.; Searson, P.C. The Physics of Cancer: The Role of Physical Interactions and Mechanical Forces in Metastasis. Nat. Rev. Cancer 2011, 11, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Chighizola, M.; Schulte, C.; Bryniarska, N.; Wesołowska, J.; Pudełek, M.; Lasota, M.; Ryszawy, D.; Basta-Kaim, A.; Laidler, P.; et al. Stiffening of DU145 Prostate Cancer Cells Driven by Actin Filaments—Microtubule Crosstalk Conferring Resistance to Microtubule-Targeting Drugs. Nanoscale 2021, 13, 6212–6226. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Zieliński, T.; Pabijan, J.; Lekka, M. Nanomechanics in Monitoring the Effectiveness of Drugs Targeting the Cancer Cell Cytoskeleton. Int. J. Mol. Sci. 2020, 21, 8786. [Google Scholar] [CrossRef] [PubMed]
- Raudenska, M.; Kratochvilova, M.; Vicar, T.; Gumulec, J.; Balvan, J.; Polanska, H.; Pribyl, J.; Masarik, M. Cisplatin Enhances Cell Stiffness and Decreases Invasiveness Rate in Prostate Cancer Cells by Actin Accumulation. Sci. Rep. 2019, 9, 1660. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.H.; Bush, B.; Cam, M.; Sevcik, E.; DelRio, F.W.; Nandy, K.; Schneider, J.P. Identification of a Mechanogenetic Link between Substrate Stiffness and Chemotherapeutic Response in Breast Cancer. Biomaterials 2019, 202, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tian, B.; Liang, J.; Yu, S.; Zhou, Y.; Li, S. Matrix Stiffness Regulates the Interactions between Endothelial Cells and Monocytes. Biomaterials 2019, 221, 119362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, D.; Xu, L.; Xu, Y.; Gao, Z.; Pan, Y.; Jiang, M.; Wei, Y.; Wang, L.; Liao, Y.; et al. Harnessing Matrix Stiffness to Engineer a Bone Marrow Niche for Hematopoietic Stem Cell Rejuvenation. Cell Stem Cell 2023, 30, 378–395.e8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryniarska-Kubiak, N.; Basta-Kaim, A.; Kubiak, A. Mechanobiology of Dental Pulp Cells. Cells 2024, 13, 375. https://doi.org/10.3390/cells13050375
Bryniarska-Kubiak N, Basta-Kaim A, Kubiak A. Mechanobiology of Dental Pulp Cells. Cells. 2024; 13(5):375. https://doi.org/10.3390/cells13050375
Chicago/Turabian StyleBryniarska-Kubiak, Natalia, Agnieszka Basta-Kaim, and Andrzej Kubiak. 2024. "Mechanobiology of Dental Pulp Cells" Cells 13, no. 5: 375. https://doi.org/10.3390/cells13050375
APA StyleBryniarska-Kubiak, N., Basta-Kaim, A., & Kubiak, A. (2024). Mechanobiology of Dental Pulp Cells. Cells, 13(5), 375. https://doi.org/10.3390/cells13050375







