Allopurinol Disrupts Purine Metabolism to Increase Damage in Experimental Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultured Cell Extraction
2.2. Tissue Extraction
2.3. Extracellular Fecal Extraction
2.4. HPLC Analysis
2.5. HPLC-ESI MS Analysis
2.6. Histological and Immunofluorescent Analyses
2.7. Vertebrate Animal Use
2.8. Seahorse XF Assay
2.9. Statistical Analyses
3. Results
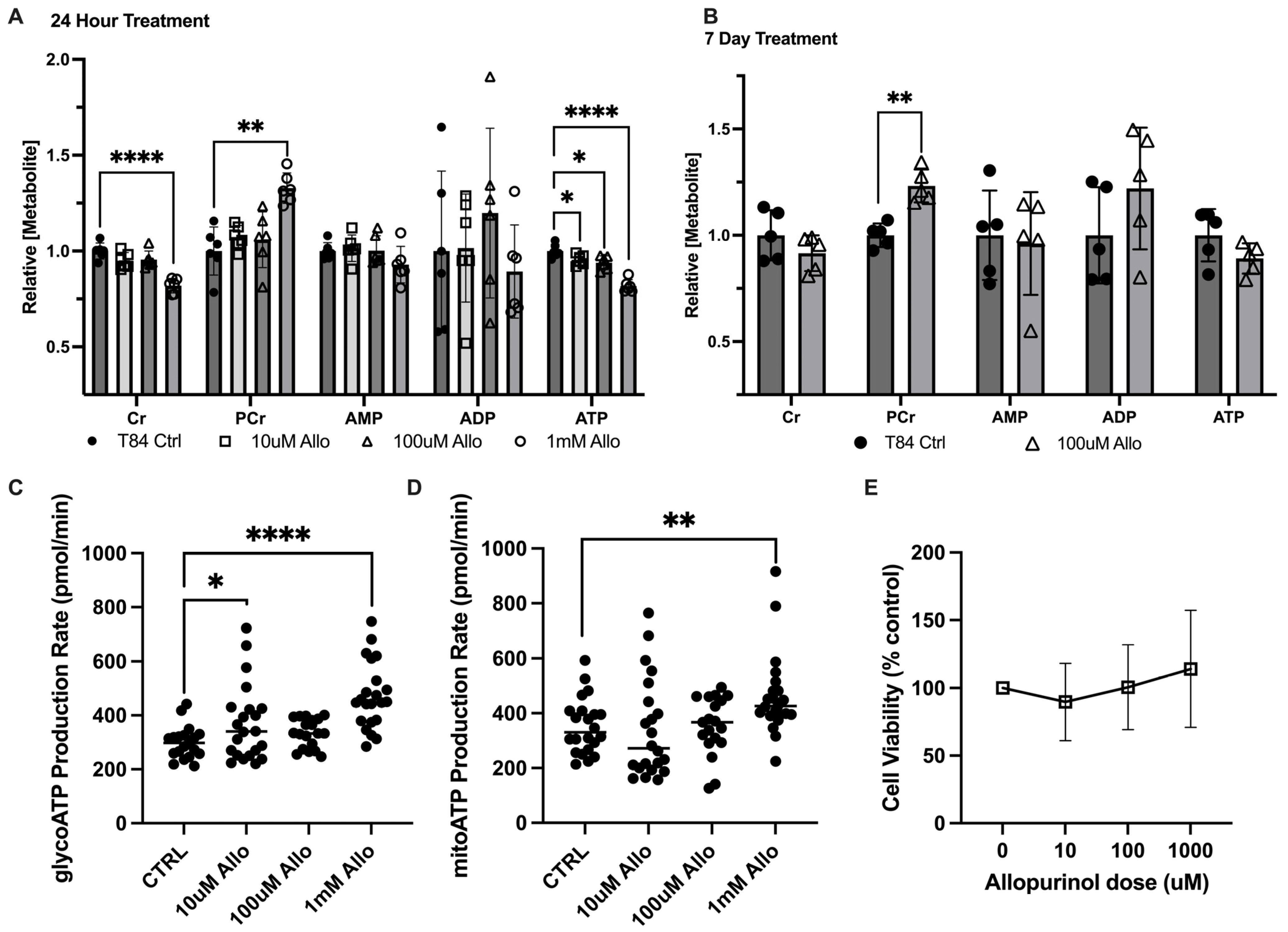
3.1. Allopurinol Modulates Intestinal Epithelial Cell Energy Metabolism
3.2. Allopurinol Riboside Diminishes Cellular and Extracellular Creatine Concentrations
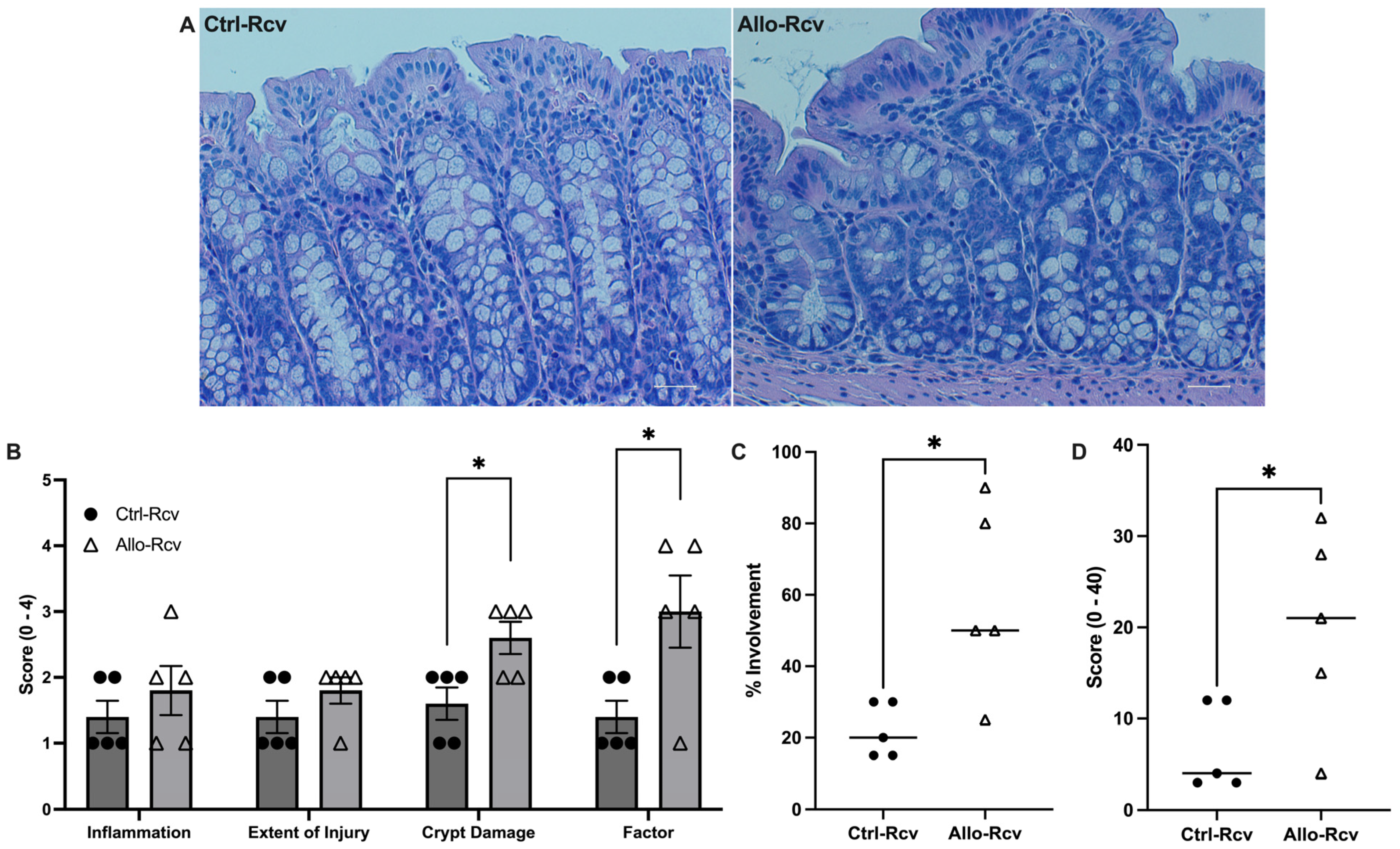
3.3. Allopurinol Sensitizes the Epithelium to Metabolic Insult in Acute Colitis
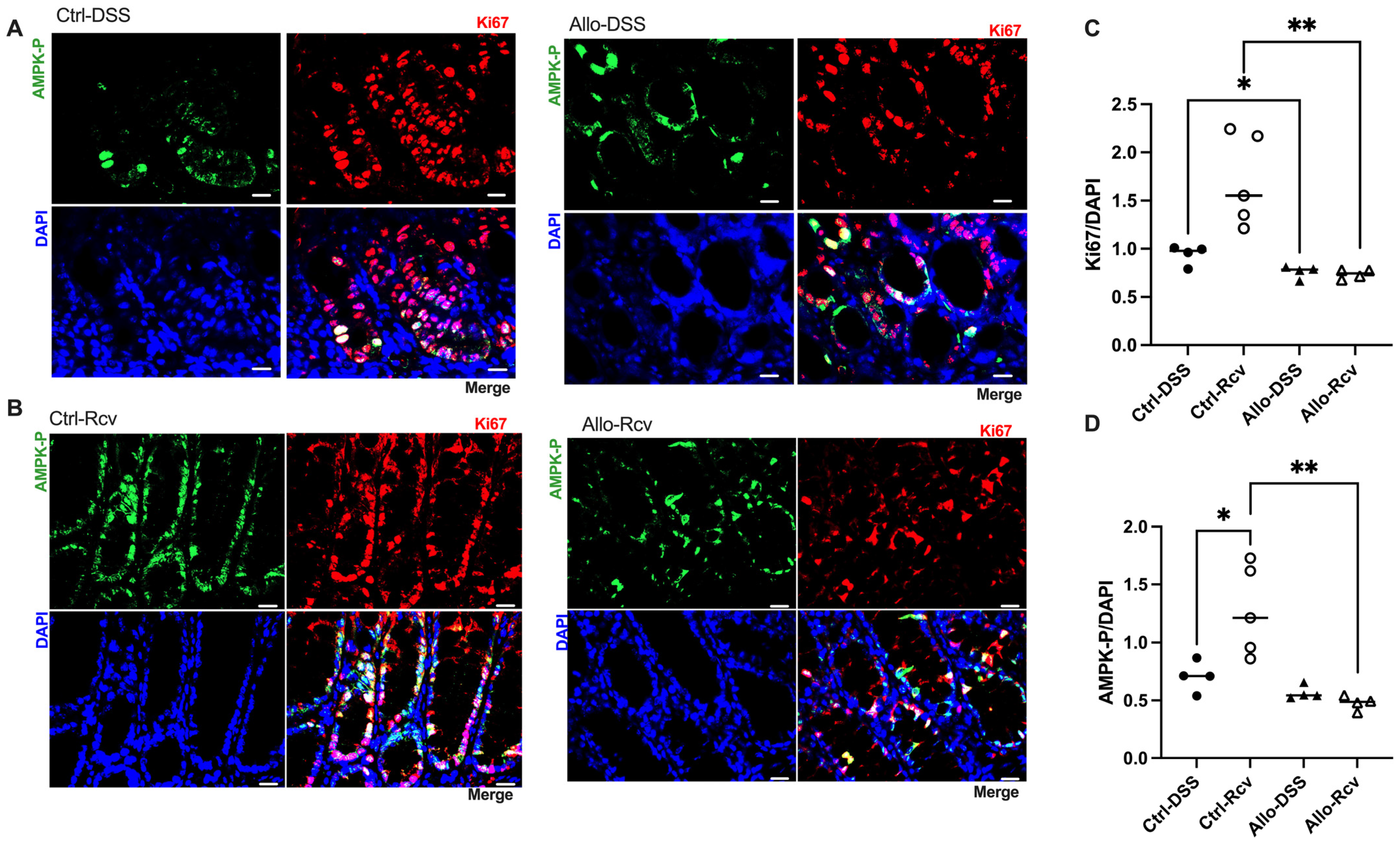
3.4. Allopurinol Inhibits Epithelial Proliferation and Downregulates Activated AMPK during Recovery
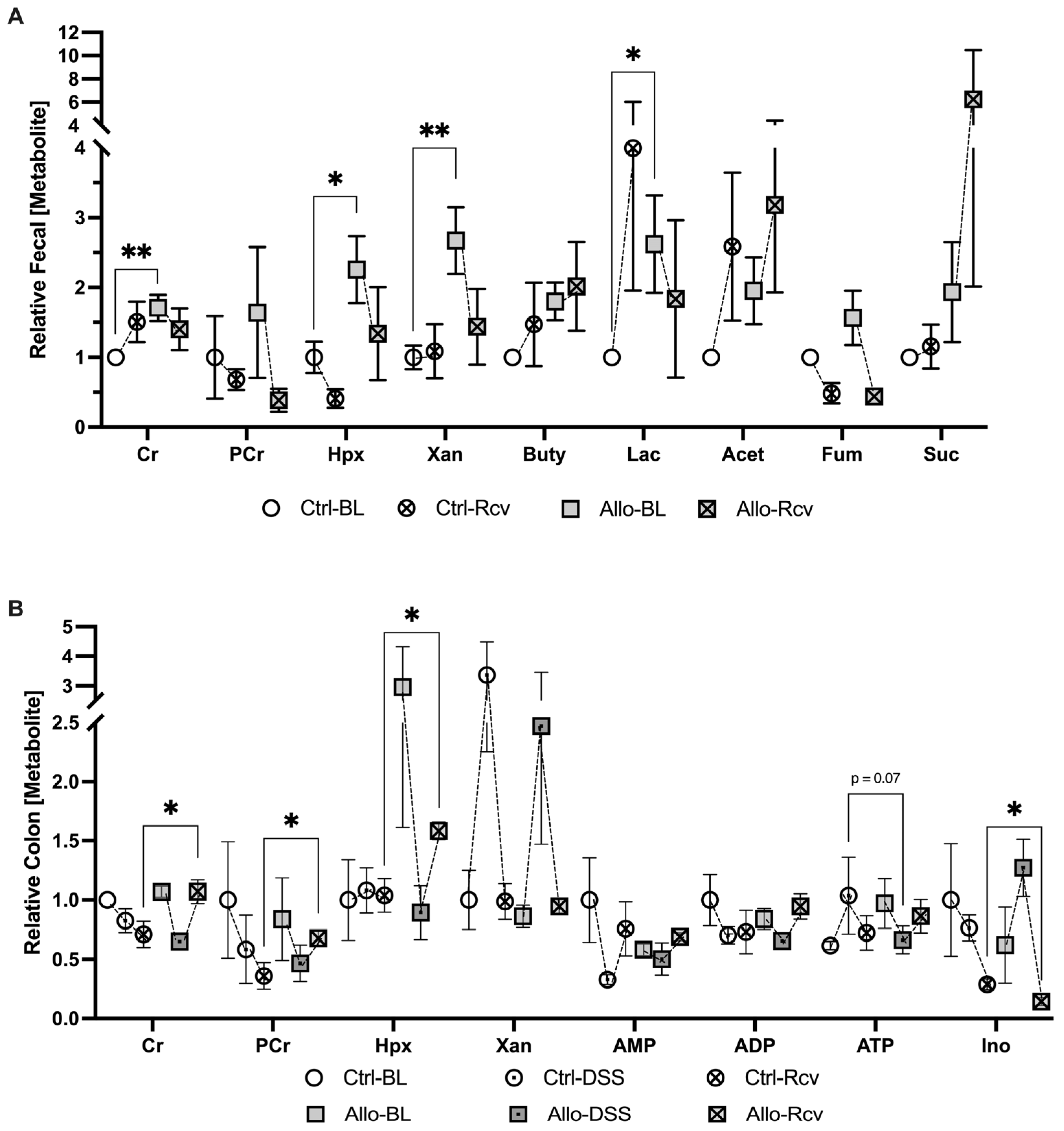
3.5. Disruption of Purine Salvage by Allopurinol Induces Fecal and Colonic Tissue Metabolic Changes
4. Discussion
5. Conclusions
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef]
- Colgan, S.P.; Wang, R.X.; Hall, C.H.T.; Bhagavatula, G.; Lee, J.S. Revisiting the “starved gut” hypothesis in inflammatory bowel disease. Immunometabolism 2023, 5, e0016. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Wang, R.X.; Alexeev, E.E.; Colgan, S.P. Intestinal Inflammation as a Dysbiosis of Energy Procurement: New Insights into an Old Topic. Gut Microbes 2021, 13, 1880241. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Wang, R.X.; Goldberg, M.S.; Clifford, G.P.; Kao, D.J.; Colgan, S.P. Microbiota-Sourced Purines Support Wound Healing and Mucous Barrier Function. iScience 2020, 23, 101226. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Wang, R.X.; Alexeev, E.E.; Lanis, J.M.; Battista, K.D.; Glover, L.E.; Colgan, S.P. Hypoxanthine is a checkpoint stress metabolite in colonic epithelial energy modulation and barrier function. J. Biol. Chem. 2018, 293, 6039–6051. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered defense: How mucus and tight junctions seal the intestinal barrier. J. Mol. Med. 2017, 95, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Chang, S.H.; Ko, Y.F.; Hwang, T.L.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 2022, 33, 247–265. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Grillo, T.G.; De Oliveira, E.C.S.; Di Stasi, L.C.; Sassaki, L.Y. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Rath, E.; Haller, D. Intestinal epithelial cell metabolism at the interface of microbial dysbiosis and tissue injury. Mucosal Immunol. 2022, 15, 595–604. [Google Scholar] [CrossRef] [PubMed]
- van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Kondo, M.; Honda, S.; Iwahana, H.; Moritani, M.; Ii, S.; Yoshimoto, K.; Itakura, M. Amidophosphoribosyltransferase limits the rate of cell growth-linked de novo purine biosynthesis in the presence of constant capacity of salvage purine biosynthesis. J. Biol. Chem. 1997, 272, 17719–17725. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Pedley, A.M.; Benkovic, S.J. Human de novo purine biosynthesis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Weissmüller, T.; Mager, A.; Eckle, T. Nucleotide metabolism and cell-cell interactions. Methods Mol. Biol. 2006, 341, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Qurie, A.; Preuss, C.V.; Musa, R. Allopurinol. 2023 Jun 26. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Furuhashi, M. New insights into purine metabolism in metabolic diseases: Role of xanthine oxidoreductase activity. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E827–E834. [Google Scholar] [CrossRef]
- Reiter, S.; Simmonds, H.A.; Zöllner, N.; Braun, S.L.; Knedel, M. Demonstration of a combined deficiency of xanthine oxidase and aldehyde oxidase in xanthinuric patients not forming oxipurinol. Clin. Chim. Acta 1990, 187, 221–234. [Google Scholar] [CrossRef]
- Shapiro, T.A.; Were, J.B.; Danso, K.; Nelson, D.J.; Desjardins, R.E.; Pamplin, C.L., 3rd. Pharmacokinetics and metabolism of allopurinol riboside. Clin. Pharmacol. Ther. 1991, 49, 506–514. [Google Scholar] [CrossRef]
- Nelson, D.J.; Elion, G.B. Metabolic studies of high doses of allopurinol in humans. Adv. Exp. Med. Biol. 1984, 165 Pt A, 167–170. [Google Scholar] [CrossRef]
- Reiter, S.; Simmonds, H.A.; Webster, D.R.; Watson, A.R. On the metabolism of allopurinol. Formation of allopurinol-1-riboside in purine nucleoside phosphorylase deficiency. Biochem. Pharmacol. 1983, 32, 2167–2174. [Google Scholar] [CrossRef]
- Dharmsathaphorn, K.; Madara, J.L. Established intestinal cell lines as model systems for electrolyte transport studies. Methods Enzymol. 1990, 192, 354–389. [Google Scholar] [CrossRef]
- Ibla, J.C.; Khoury, J.; Kong, T.; Robinson, A.; Colgan, S.P. Transcriptional repression of Na-K-2Cl cotransporter NKCC1 by hypoxia-inducible factor-1. Am. J. Physiol. Cell Physiol. 2006, 291, C282–C289. [Google Scholar] [CrossRef]
- Wang, R.X.; Henen, M.A.; Lee, J.S.; Vögeli, B.; Colgan, S.P. Microbiota-derived butyrate is an endogenous HIF prolyl hydroxylase inhibitor. Gut Microbes 2021, 13, 1938380. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Peña, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Crouch, R.D.; Blobaum, A.L.; Felts, A.S.; Conn, P.J.; Lindsley, C.W. Species-Specific Involvement of Aldehyde Oxidase and Xanthine Oxidase in the Metabolism of the Pyrimidine-Containing mGlu5-Negative Allosteric Modulator VU0424238 (Auglurant). Drug Metab. Dispos. 2017, 45, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Tani, S.; Matsuo, R.; Imatake, K.; Suzuki, Y.; Takahashi, A.; Yagi, T.; Matsumoto, N.; Okumura, Y. The serum uric acid level in females may be a better indicator of metabolic syndrome and its components than in males in a Japanese population. J. Cardiol. 2020, 76, 100–108. [Google Scholar] [CrossRef]
- Meijer, B.; Seinen, M.L.; Hosman, T.; Linskens, R.K.; Kneppelhout, J.K.; Peters, G.J.; Mulder, C.J.; van Bodegraven, A.A.; de Boer, N.K. High inter-individual variability of serum xanthine oxidoreductase activity in IBD patients. Nucleosides Nucleotides Nucleic Acids 2018, 37, 317–323. [Google Scholar] [CrossRef]
- Torres, R.J.; Prior, C.; Puig, J.G. Efficacy and safety of allopurinol in patients with hypoxanthine-guanine phosphoribosyltransferase deficiency. Metabolism 2007, 56, 1179–1186. [Google Scholar] [CrossRef]
- Torres, R.J.; Puig, J.G. Hypoxanthine-guanine phosophoribosyltransferase (HPRT) deficiency: Lesch-Nyhan syndrome. Orphanet J. Rare Dis. 2007, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.L.; Will, A.; Jackson, G.H.; Webb, N.J.; Rule, S. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br. J. Haematol. 2015, 169, 661–671. [Google Scholar] [CrossRef]
- Rath, E.; Berger, E.; Messlik, A.; Nunes, T.; Liu, B.; Kim, S.C.; Hoogenraad, N.; Sans, M.; Sartor, R.B.; Haller, D. Induction of dsRNA-activated protein kinase links mitochondrial unfolded protein response to the pathogenesis of intestinal inflammation. Gut 2012, 61, 1269–1278. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Olivier, S.; Diounou, H.; Pochard, C.; Frechin, L.; Durieu, E.; Foretz, M.; Neunlist, M.; Rolli-Derkinderen, M.; Viollet, B. Intestinal Epithelial AMPK Deficiency Causes Delayed Colonic Epithelial Repair in DSS-Induced Colitis. Cells 2022, 11, 590. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- King, M.E.; Honeysett, J.M.; Howell, S.B. Regulation of de novo purine synthesis in human bone marrow mononuclear cells by hypoxanthine. J. Clin. Investig. 1983, 72, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.J.; Winter, M.G.; Gillis, C.C.; Silva, L.A.d.; Dobbins, A.L.; Muramatsu, M.K.; Jimenez, A.G.; Chanin, R.B.; Spiga, L.; Llano, E.M.; et al. Colonocyte-derived lactate promotes E. coli fitness in the context of inflammation-associated gut microbiota dysbiosis. Microbiome 2022, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Pedley, A.M.; Pareek, V.; Benkovic, S.J. The Purinosome: A Case Study for a Mammalian Metabolon. Annu. Rev. Biochem. 2022, 91, 89–106. [Google Scholar] [CrossRef] [PubMed]
- French, J.B.; Jones, S.A.; Deng, H.; Pedley, A.M.; Kim, D.; Chan, C.Y.; Hu, H.; Pugh, R.J.; Zhao, H.; Zhang, Y.; et al. Spatial colocalization and functional link of purinosomes with mitochondria. Science 2016, 351, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Hove, H.; Mortensen, P.B. Influence of intestinal inflammation (IBD) and small and large bowel length on fecal short-chain fatty acids and lactate. Dig. Dis. Sci. 1995, 40, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, R.; Wang, Z.; Zhang, Z.; Wang, J.; Qiao, Y.; Huang, Y.; Liu, W. Lactate-utilizing bacteria ameliorates DSS-induced colitis in mice. Life Sci. 2022, 288, 120179. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.L.t.; Garvik, B.; Hartwell, L. Principles for the buffering of genetic variation. Science 2001, 291, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A. Robustness, evolvability, and neutrality. FEBS Lett. 2005, 579, 1772–1778. [Google Scholar] [CrossRef]
- Sambamoorthy, G.; Raman, K. Understanding the evolution of functional redundancy in metabolic networks. Bioinformatics 2018, 34, i981–i987. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Caprilli, R.; Latella, G.; Barbetti, F.; Magliocca, F.M.; Cittadini, M. Fecal lactate and ulcerative colitis. Gastroenterology 1988, 95, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.U.; Baumgart, D.C.; Sturm, A. Review article: The aetiopathogenesis of inflammatory bowel disease--immunology and repair mechanisms. Aliment. Pharmacol. Ther. 2004, 20 (Suppl. S4), 9–17. [Google Scholar] [CrossRef]
- Roediger, W.E.W. The starved colon—Diminished mucosal nutrition, diminished absorption, and colitis. Dis. Colon Rectum 1990, 33, 858–862. [Google Scholar] [CrossRef]
- Garcia-Gil, M.; Camici, M.; Allegrini, S.; Pesi, R.; Tozzi, M.G. Metabolic Aspects of Adenosine Functions in the Brain. Front. Pharmacol. 2021, 12, 672182. [Google Scholar] [CrossRef]
- Turbayne, A.K.; Sparrow, M.P. Low-Dose Azathioprine in Combination with Allopurinol: The Past, Present and Future of This Useful Duo. Dig. Dis. Sci. 2022, 67, 5382–5391. [Google Scholar] [CrossRef] [PubMed]
- Moreau, B.; Clement, P.; Theoret, Y.; Seidman, E.G. Allopurinol in combination with thiopurine induces mucosal healing and improves clinical and metabolic outcomes in IBD. Therap Adv. Gastroenterol. 2017, 10, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Warner, B.; Johnston, E.; Arenas-Hernandez, M.; Marinaki, A.; Irving, P.; Sanderson, J. A practical guide to thiopurine prescribing and monitoring in IBD. Frontline Gastroenterol. 2018, 9, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, A.; Con, D.; De Cruz, P.; Sparrow, M.P.; Friedman, A.B.; Garg, M.; Kashkooli, S.; Gibson, P.R.; van Langenberg, D.R. Clinical trial: Combination allopurinol-thiopurine versus standard thiopurine in patients with IBD escalating to immunomodulators (the DECIDER study). Aliment. Pharmacol. Ther. 2024, 59, 504–514. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worledge, C.S.; Kostelecky, R.E.; Zhou, L.; Bhagavatula, G.; Colgan, S.P.; Lee, J.S. Allopurinol Disrupts Purine Metabolism to Increase Damage in Experimental Colitis. Cells 2024, 13, 373. https://doi.org/10.3390/cells13050373
Worledge CS, Kostelecky RE, Zhou L, Bhagavatula G, Colgan SP, Lee JS. Allopurinol Disrupts Purine Metabolism to Increase Damage in Experimental Colitis. Cells. 2024; 13(5):373. https://doi.org/10.3390/cells13050373
Chicago/Turabian StyleWorledge, Corey S., Rachael E. Kostelecky, Liheng Zhou, Geetha Bhagavatula, Sean P. Colgan, and J. Scott Lee. 2024. "Allopurinol Disrupts Purine Metabolism to Increase Damage in Experimental Colitis" Cells 13, no. 5: 373. https://doi.org/10.3390/cells13050373
APA StyleWorledge, C. S., Kostelecky, R. E., Zhou, L., Bhagavatula, G., Colgan, S. P., & Lee, J. S. (2024). Allopurinol Disrupts Purine Metabolism to Increase Damage in Experimental Colitis. Cells, 13(5), 373. https://doi.org/10.3390/cells13050373





