Pharmacological Stimulation of Soluble Guanylate Cyclase Counteracts the Profibrotic Activation of Human Conjunctival Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Culture of Human Conjunctival Fibroblasts
2.2. Stimulation of Human Conjunctival Fibroblasts
2.3. Cyclic Guanosine Monophosphate Assay
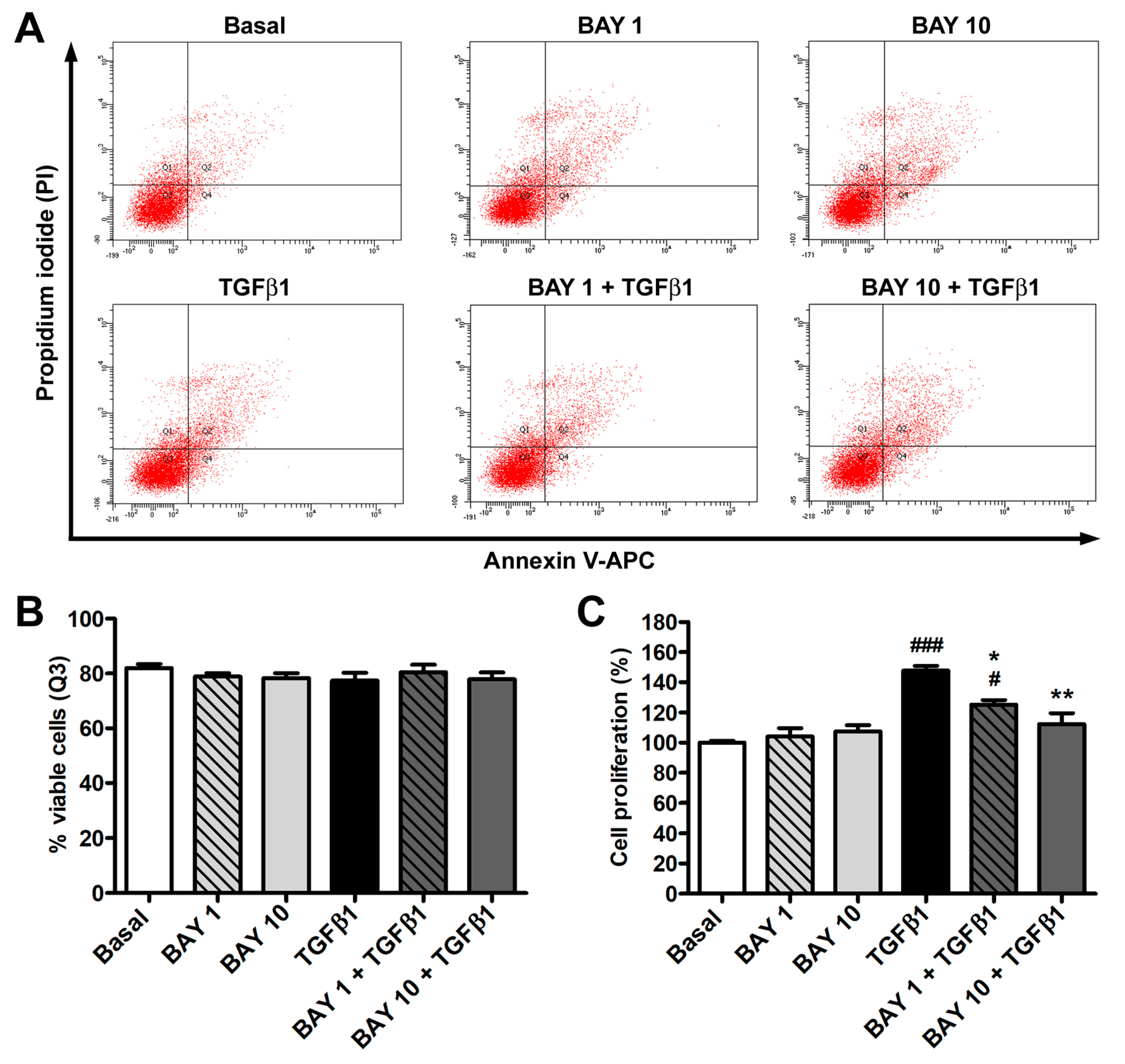
2.4. Annexin V/Propidium Iodide Flow Cytometer Assay
2.5. Cell Proliferation Assay
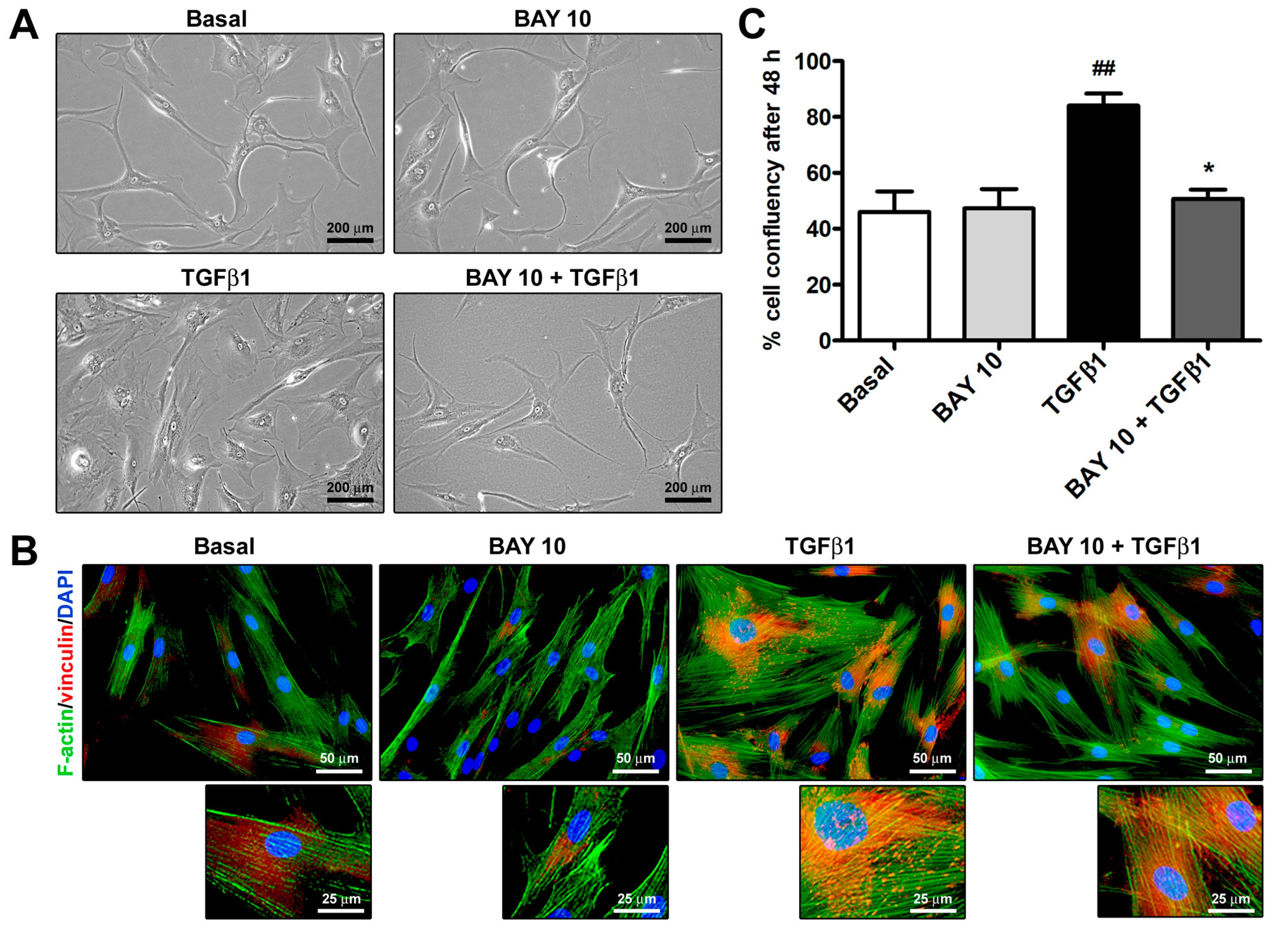
2.6. Cell Morphology and Confluency Assessment
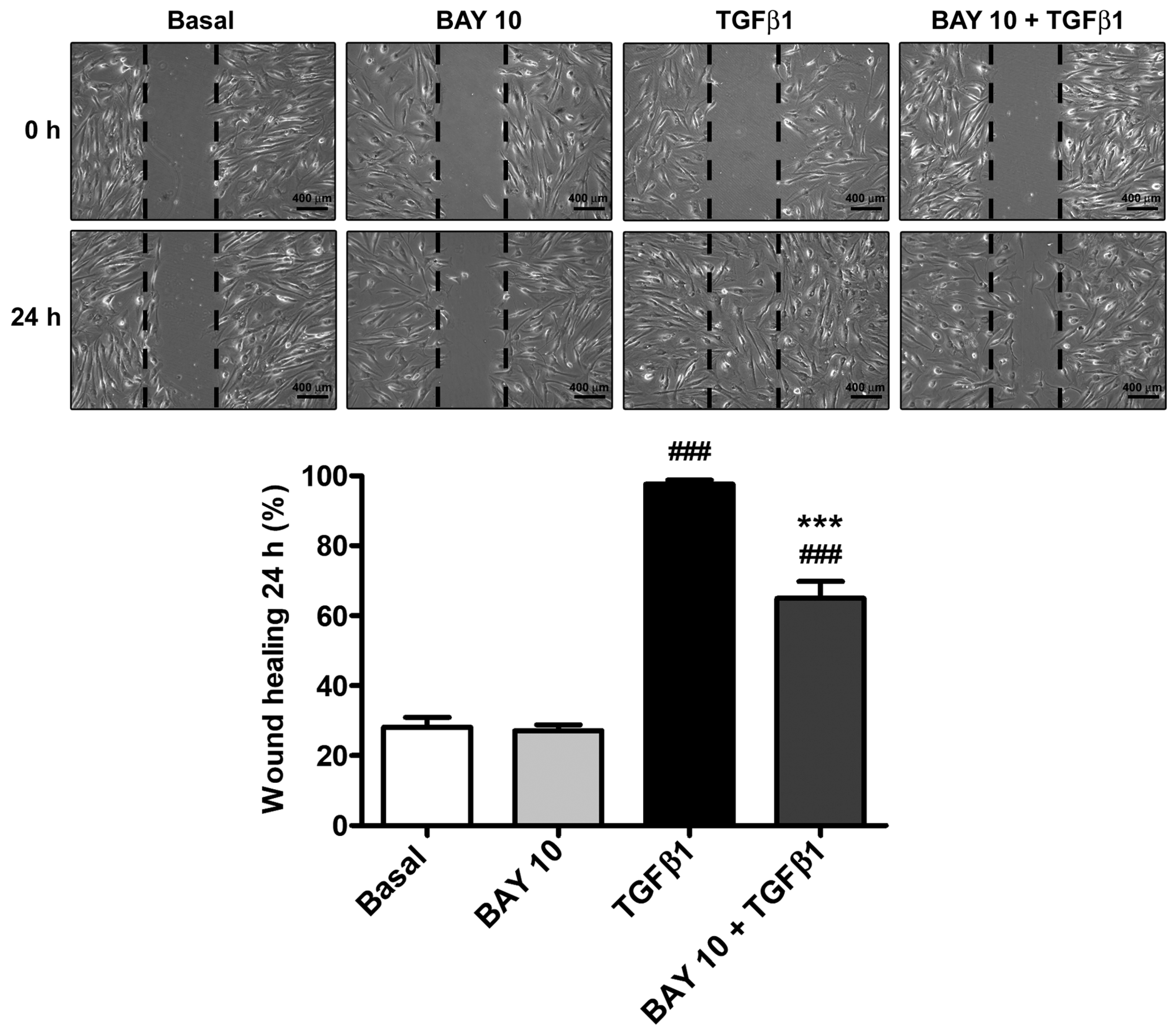
2.7. In Vitro Wound Healing Assay
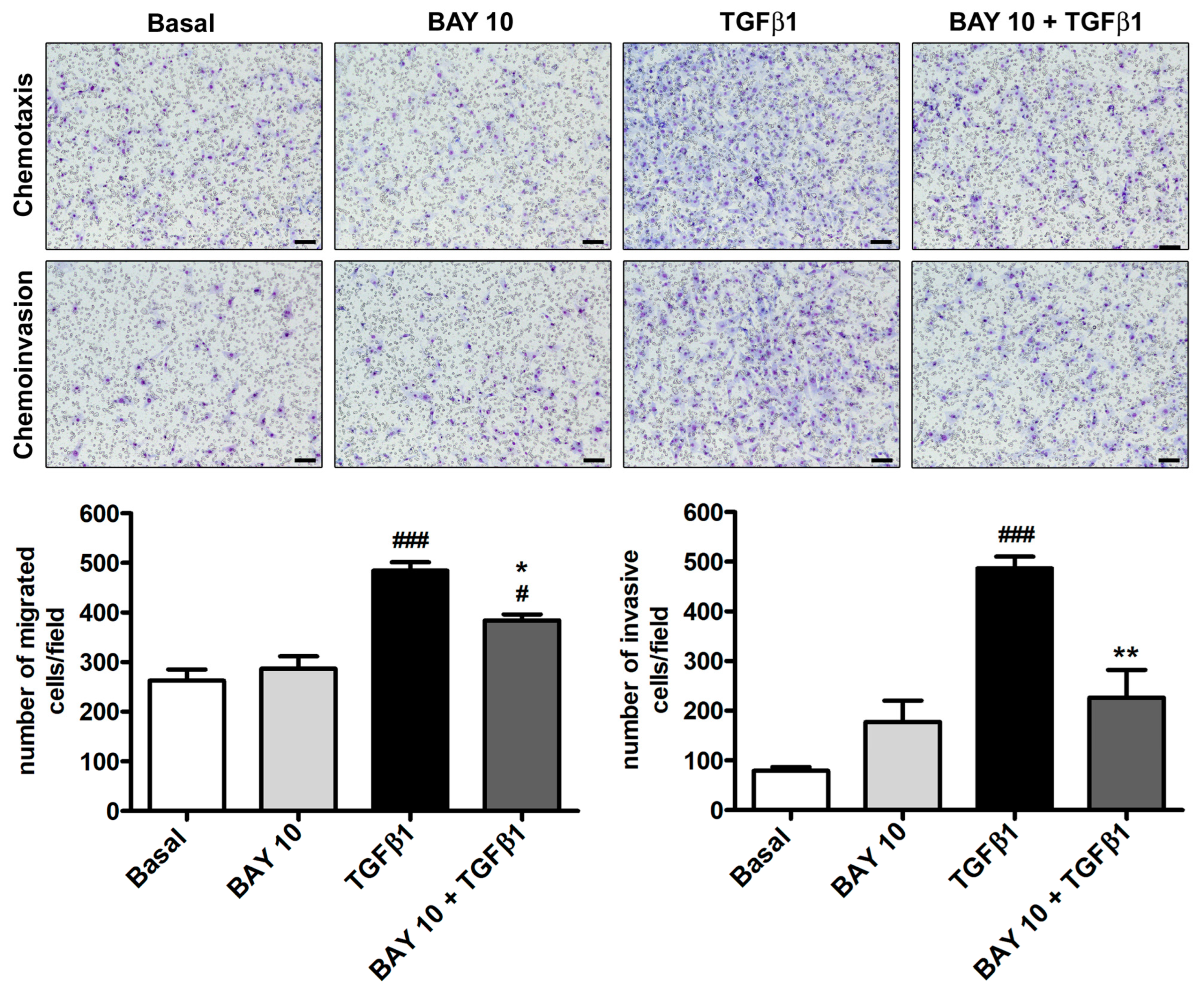
2.8. Chemotaxis and Chemoinvasion Assays
2.9. Quantitative Real-Time PCR
2.10. Western Blotting
2.11. Fluorescence Immunocytochemistry
2.12. Collagen Gel Matrix Contraction Assay
2.13. Enzyme-Linked Immunosorbent Assay
2.14. Statistical Analysis
3. Results
3.1. The sGC Stimulator BAY 41-2272 Has No Effect on Cell Viability and Inhibits TGFβ1-Induced Proliferation of Human Conjunctival Fibroblasts
3.2. Cell Morphology and Confluency Assessment
3.3. TGFβ1-Induced Wound Healing Ability of Human Conjunctival Fibroblasts Is Reduced by Administration of the SGC Stimulator BAY 41-2272
3.4. TGFβ1-Induced Migration and Invasiveness of Human Conjunctival Fibroblasts Is Reduced by Treatment with BAY 41-2272
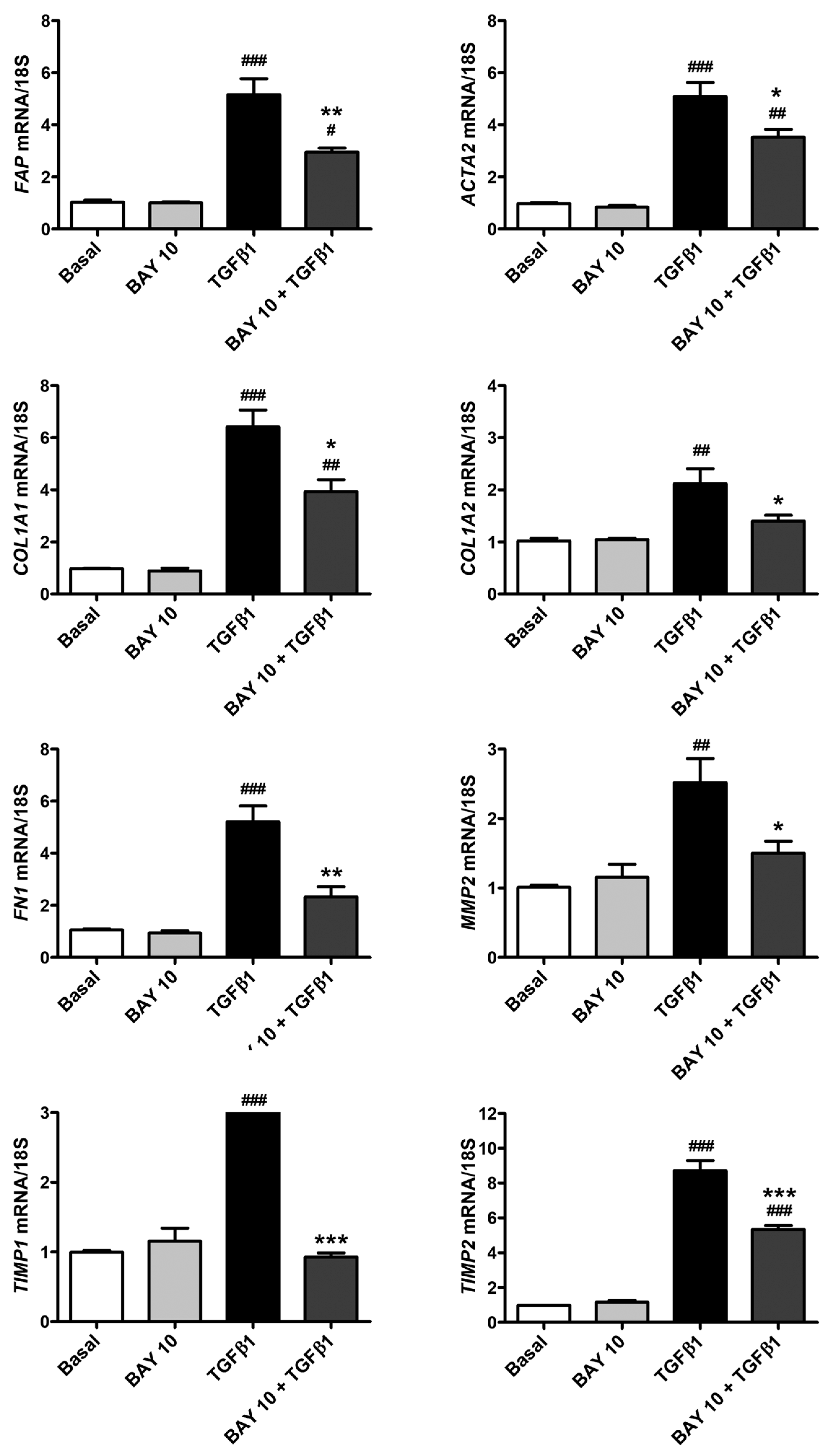
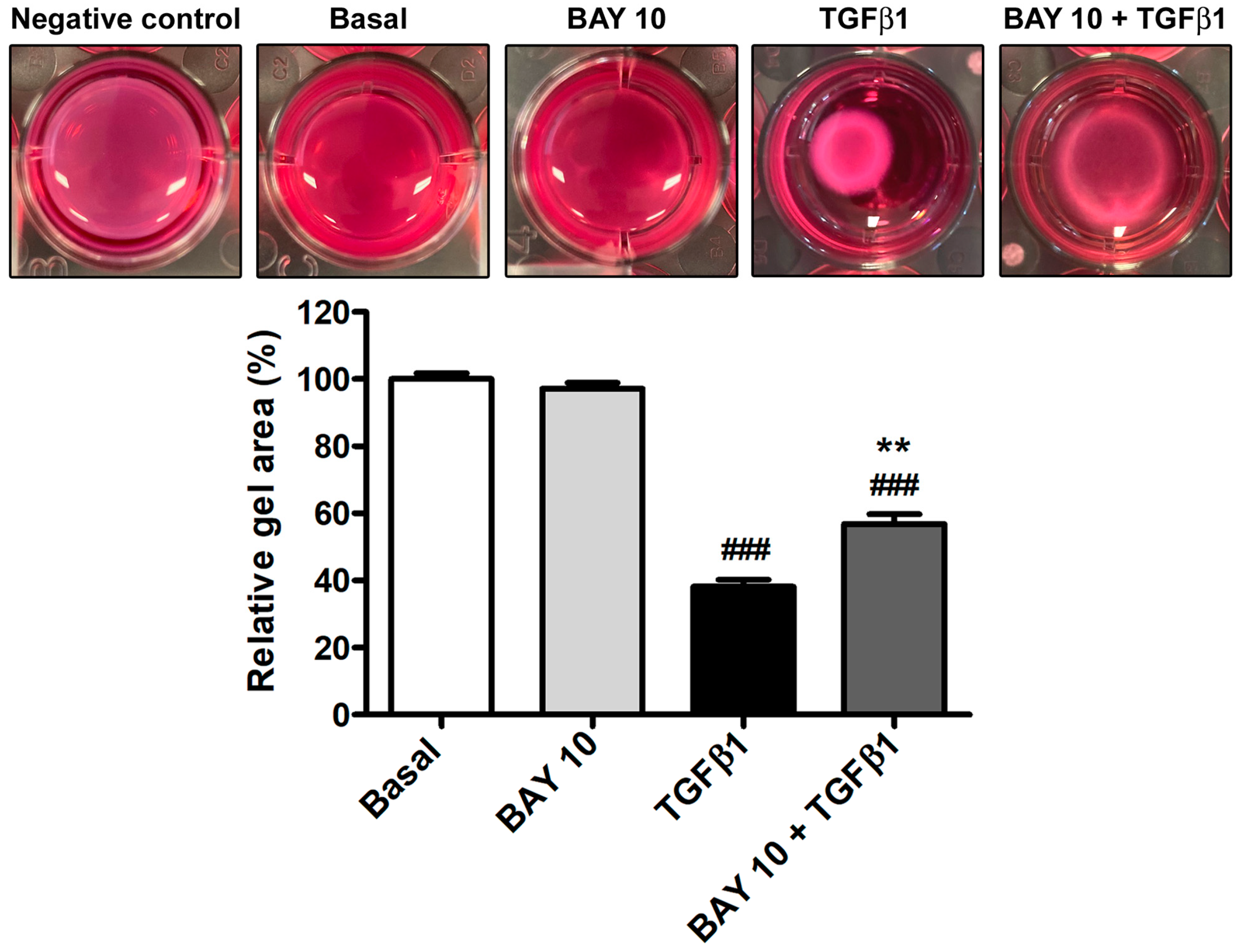
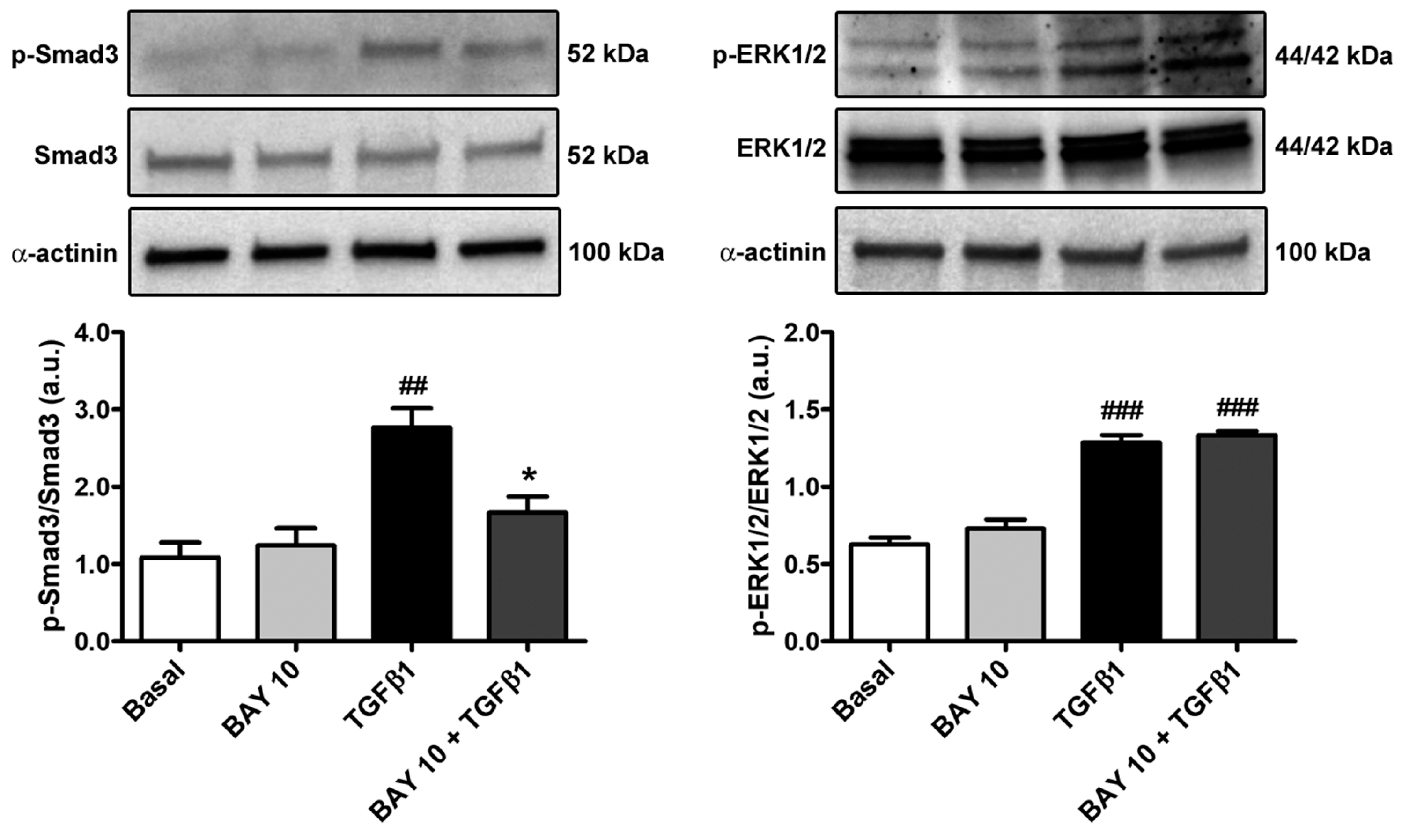
3.5. TGFβ1-Mediated Acquisition of a Myofibroblast-Like Profibrotic and Contractile Phenotype by Human Conjunctival Fibroblasts Is Attenuated by Pretreatment with BAY 41-2272
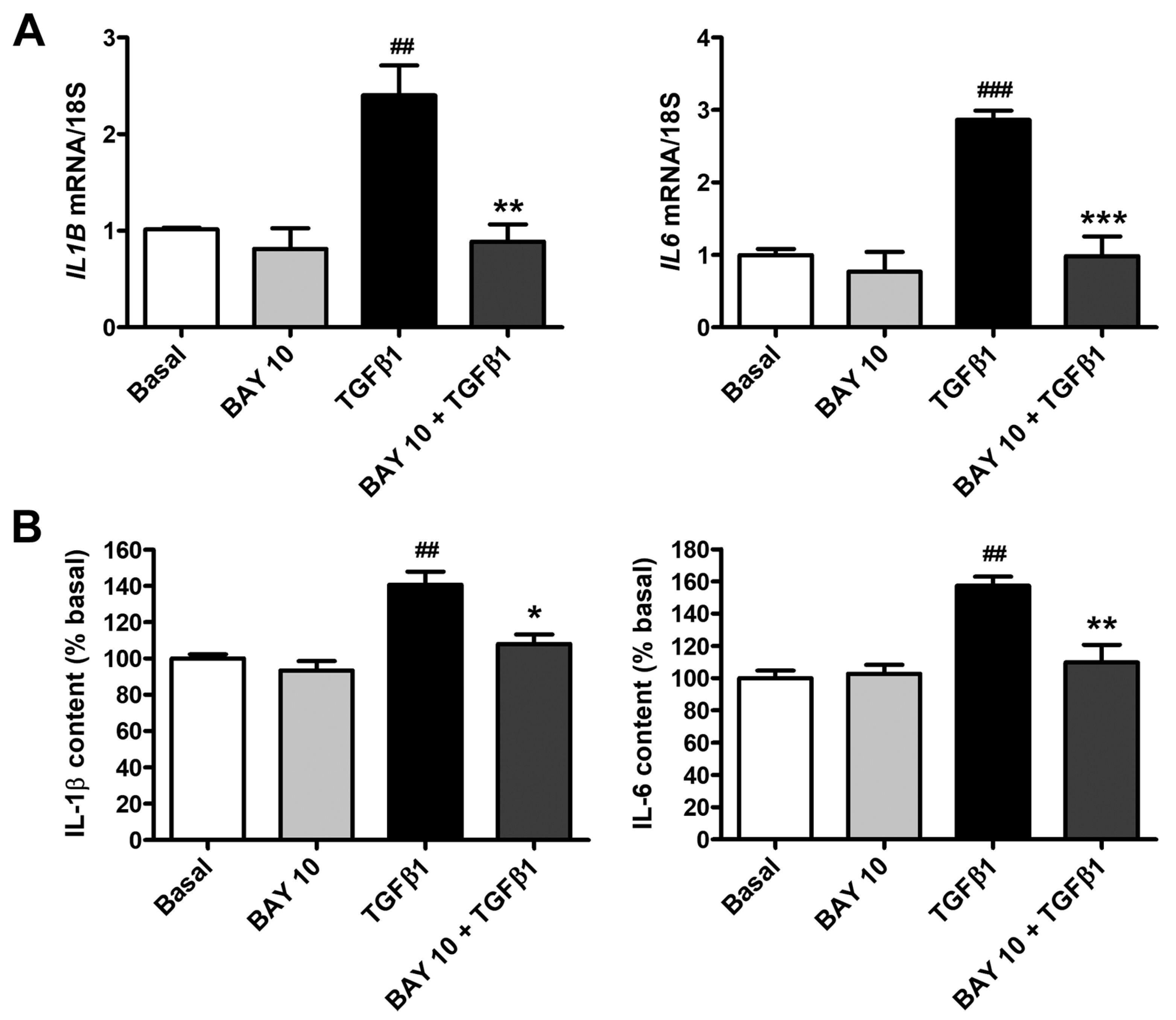
3.6. BAY 41-2272 Administration Lessens TGFβ1-Induced Secretion of Proinflammatory Cytokines by Human Conjunctival Fibroblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shumway, C.L.; Motlagh, M.; Wade, M. Anatomy, Head and Neck, Eye Conjunctiva. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Khaw, P.T.; Bouremel, Y.; Brocchini, S.; Henein, C. The Control of Conjunctival Fibrosis as a Paradigm for the Prevention of Ocular Fibrosis-Related Blindness. “Fibrosis Has Many Friends”. Eye 2020, 34, 2163–2174. [Google Scholar] [CrossRef]
- Mallone, F.; Costi, R.; Marenco, M.; Plateroti, R.; Minni, A.; Attanasio, G.; Artico, M.; Lambiase, A. Understanding Drivers of Ocular Fibrosis: Current and Future Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 11748. [Google Scholar] [CrossRef] [PubMed]
- Posarelli, M.; Romano, D.; Tucci, D.; Giannaccare, G.; Scorcia, V.; Taloni, A.; Pagano, L.; Borgia, A. Ocular-Surface Regeneration Therapies for Eye Disorders: The State of the Art. BioTech 2023, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Gater, R.; Ipek, T.; Sadiq, S.; Nguyen, D.; Jones, L.; El Haj, A.; Yang, Y. Investigation of Conjunctival Fibrosis Response Using a 3D Glaucoma Tenon’s Capsule + Conjunctival Model. Investig. Ophthalmol. Vis. Sci. 2019, 60, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Akpek, E.K.; Ahmad, S. Glaucoma and Ocular Surface Disease: More than Meets the Eye. Clin. Ophthalmol. 2022, 16, 3641–3649. [Google Scholar] [CrossRef]
- Schlunck, G.; Meyer-ter-Vehn, T.; Klink, T.; Grehn, F. Conjunctival Fibrosis Following Filtering Glaucoma Surgery. Exp. Eye Res. 2016, 142, 76–82. [Google Scholar] [CrossRef]
- Cheng, W.-S.; Chen, C.-L.; Chen, J.-T.; Lin, L.-T.; Pao, S.-I.; Chen, Y.-H.; Lu, D.-W. AR12286 Alleviates TGF-β-Related Myofibroblast Transdifferentiation and Reduces Fibrosis after Glaucoma Filtration Surgery. Molecules 2020, 25, 4422. [Google Scholar] [CrossRef]
- Shao, C.G.; Sinha, N.R.; Mohan, R.R.; Webel, A.D. Novel Therapies for the Prevention of Fibrosis in Glaucoma Filtration Surgery. Biomedicines 2023, 11, 657. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Agnifili, L.; Mastropasqua, R.; Fasanella, V. Conjunctival Modifications Induced by Medical and Surgical Therapies in Patients with Glaucoma. Curr. Opin. Pharmacol. 2013, 13, 56–64. [Google Scholar] [CrossRef]
- Wooff, Y.; Man, S.M.; Aggio-Bruce, R.; Natoli, R.; Fernando, N. IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases. Front. Immunol. 2019, 10, 1618. [Google Scholar] [CrossRef]
- Xiao, R.; Lei, C.; Zhang, Y.; Zhang, M. Interleukin-6 in Retinal Diseases: From Pathogenesis to Therapy. Exp. Eye Res. 2023, 233, 109556. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Labbé, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in Eyedrops: The Good, the Bad and the Ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Kong, X.; Xu, J.; Sun, X. Effects of Long-Term Antiglaucoma Eye Drops on Conjunctival Structures: An In Vivo Confocal Microscopy Study. J. Ophthalmol. 2015, 2015, e165475. [Google Scholar] [CrossRef] [PubMed]
- Anumanthan, G.; Wilson, P.J.; Tripathi, R.; Hesemann, N.P.; Mohan, R.R. Blockade of KCa3.1: A Novel Target to Treat TGF-Β1 Induced Conjunctival Fibrosis. Exp. Eye Res. 2018, 167, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Qian, T.; Lu, Y.; Zhou, W.; Xu, X.; Zhang, C.; Zhang, J.; Zhang, Z. SPARC-YAP/TAZ Inhibition Prevents the Fibroblasts-Myofibroblasts Transformation. Exp. Cell Res. 2023, 429, 113649. [Google Scholar] [CrossRef]
- Hachana, S.; Larrivée, B. TGF-β Superfamily Signaling in the Eye: Implications for Ocular Pathologies. Cells 2022, 11, 2336. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Jung, S.-A.; Lyu, J.; Kim, Y.Y.; Lee, J.H. Transforming Growth Factor-Β1-Induced Human Subconjunctival Fibrosis Is Mediated by MicroRNA 143/145 Expression. Investig. Ophthalmol Vis. Sci. 2019, 60, 2064–2071. [Google Scholar] [CrossRef]
- Liang, L.; Wang, X.; Zheng, Y.; Liu, Y. All-trans-retinoic Acid Modulates TGF-β-induced Apoptosis, Proliferation, Migration and Extracellular Matrix Synthesis of Conjunctival Fibroblasts by Inhibiting PI3K/AKT Signaling. Mol. Med. Rep. 2019, 20, 2929–2935. [Google Scholar] [CrossRef]
- Matsumura, T.; Fujimoto, T.; Futakuchi, A.; Takihara, Y.; Watanabe-Kitamura, F.; Takahashi, E.; Inoue-Mochita, M.; Tanihara, H.; Inoue, T. TGF-β-Induced Activation of Conjunctival Fibroblasts Is Modulated by FGF-2 and Substratum Stiffness. PLoS ONE 2020, 15, e0242626. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cui, R.; Meng, H.; Liu, X.; Liu, X.; Lu, Y.; Liu, K.; Jia, L.; Zheng, Y. Gene Suppression of the Chloride Channel 2 Suppressed TGF-Β1-Induced Proliferation, Collagen Synthesis, and Collagen Gel Contraction Mediated by Conjunctival Fibroblasts. Ophthalmic Res. 2021, 64, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Dong, Y.; Zhao, J.; Yin, Y.; Zheng, Y. The CLC-2 Chloride Channel Modulates ECM Synthesis, Differentiation, and Migration of Human Conjunctival Fibroblasts via the PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2016, 17, 910. [Google Scholar] [CrossRef]
- Watanabe, M.; Tsugeno, Y.; Sato, T.; Umetsu, A.; Nishikiori, N.; Furuhashi, M.; Ohguro, H. TGF-β Isoforms Affect the Planar and Subepithelial Fibrogenesis of Human Conjunctival Fibroblasts in Different Manners. Biomedicines 2023, 11, 2005. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Chen, L.; Yan, D.; Zhou, M.; Shao, C.; Lu, Y.; Yao, Q.; Sun, H.; Fu, Y. Trehalose Attenuates TGF-Β1-Induced Fibrosis of hSCFs by Activating Autophagy. Mol. Cell. Biochem. 2020, 470, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Sandner, P.; Follmann, M.; Becker-Pelster, E.; Hahn, M.G.; Meier, C.; Freitas, C.; Roessig, L.; Stasch, J.-P. Soluble GC Stimulators and Activators: Past, Present and Future. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Li, Q.; Hu, L.; Yu, Z.; Yang, J.; Chang, Q.; Chen, Z.; Hu, G. Soluble Guanylate Cyclase Stimulators and Activators: Where Are We and Where to Go? Mini-Rev. Med. Chem. 2019, 19, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Reich, N.; Schindler, S.C.; Akhmetshina, A.; Dees, C.; Tomcik, M.; Hirth-Dietrich, C.; von Degenfeld, G.; Sandner, P.; Distler, O.; et al. Stimulation of Soluble Guanylate Cyclase Reduces Experimental Dermal Fibrosis. Ann. Rheum. Dis. 2012, 71, 1019–1026. [Google Scholar] [CrossRef]
- Dees, C.; Beyer, C.; Distler, A.; Soare, A.; Zhang, Y.; Palumbo-Zerr, K.; Distler, O.; Schett, G.; Sandner, P.; Distler, J.H.W. Stimulators of Soluble Guanylate Cyclase (sGC) Inhibit Experimental Skin Fibrosis of Different Aetiologies. Ann. Rheum. Dis. 2015, 74, 1621–1625. [Google Scholar] [CrossRef]
- Knorr, A.; Hirth-Dietrich, C.; Alonso-Alija, C.; Härter, M.; Hahn, M.; Keim, Y.; Wunder, F.; Stasch, J.-P. Nitric Oxide-Independent Activation of Soluble Guanylate Cyclase by BAY 60-2770 in Experimental Liver Fibrosis. Arzneimittelforschung 2008, 58, 71–80. [Google Scholar] [CrossRef]
- Masuyama, H.; Tsuruda, T.; Sekita, Y.; Hatakeyama, K.; Imamura, T.; Kato, J.; Asada, Y.; Stasch, J.-P.; Kitamura, K. Pressure-Independent Effects of Pharmacological Stimulation of Soluble Guanylate Cyclase on Fibrosis in Pressure-Overloaded Rat Heart. Hypertens. Res. 2009, 32, 597–603. [Google Scholar] [CrossRef]
- Xie, G.; Wang, X.; Wang, L.; Wang, L.; Atkinson, R.D.; Kanel, G.C.; Gaarde, W.A.; Deleve, L.D. Role of Differentiation of Liver Sinusoidal Endothelial Cells in Progression and Regression of Hepatic Fibrosis in Rats. Gastroenterology 2012, 142, 918–927.e6. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Okano, T.; Yamada, H.; Akashi, K.; Sendo, S.; Ueda, Y.; Morinobu, A.; Saegusa, J. Soluble Guanylate Cyclase Stimulator Reduced the Gastrointestinal Fibrosis in Bleomycin-Induced Mouse Model of Systemic Sclerosis. Arthritis Res. Ther. 2021, 23, 133. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Zenzmaier, C.; Palumbo-Zerr, K.; Mancuso, R.; Distler, A.; Dees, C.; Zerr, P.; Huang, J.; Maier, C.; Pachowsky, M.L.; et al. Stimulation of the Soluble Guanylate Cyclase (sGC) Inhibits Fibrosis by Blocking Non-Canonical TGFβ Signalling. Ann. Rheum. Dis. 2015, 74, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Lambers, C.; Boehm, P.M.; Karabacak, Y.; Samaha, E.; Benazzo, A.; Jaksch, P.; Roth, M. Combined Activation of Guanylate Cyclase and Cyclic AMP in Lung Fibroblasts as a Novel Therapeutic Concept for Lung Fibrosis. Biomed. Res. Int. 2019, 2019, 1345402. [Google Scholar] [CrossRef] [PubMed]
- Zenzmaier, C.; Kern, J.; Heitz, M.; Plas, E.; Zwerschke, W.; Mattesich, M.; Sandner, P.; Berger, P. Activators and Stimulators of Soluble Guanylate Cyclase Counteract Myofibroblast Differentiation of Prostatic and Dermal Stromal Cells. Exp. Cell Res. 2015, 338, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Romano, E.; Rosa, I.; Fioretto, B.S.; Giuggioli, D.; Manetti, M.; Matucci-Cerinic, M. Soluble Guanylate Cyclase Stimulation Fosters Angiogenesis and Blunts Myofibroblast-like Features of Systemic Sclerosis Endothelial Cells. Rheumatology 2023, 62, SI125–SI137. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.; Fioretto, B.S.; Romano, E.; Buzzi, M.; Mencucci, R.; Marini, M.; Manetti, M. The Soluble Guanylate Cyclase Stimulator BAY 41-2272 Attenuates Transforming Growth Factor Β1-Induced Myofibroblast Differentiation of Human Corneal Keratocytes. Int. J. Mol. Sci. 2022, 23, 15325. [Google Scholar] [CrossRef]
- Andreucci, E.; Fioretto, B.S.; Rosa, I.; Matucci-Cerinic, M.; Biagioni, A.; Romano, E.; Calorini, L.; Manetti, M. Extracellular Lactic Acidosis of the Tumor Microenvironment Drives Adipocyte-to-Myofibroblast Transition Fueling the Generation of Cancer-Associated Fibroblasts. Cells 2023, 12, 939. [Google Scholar] [CrossRef]
- Zada, M.; Pattamatta, U.; White, A. Modulation of Fibroblasts in Conjunctival Wound Healing. Ophthalmology 2018, 125, 179–192. [Google Scholar] [CrossRef]
- Das, A.; Kashyap, O.; Singh, A.; Shree, J.; Namdeo, K.P.; Bodakhe, S.H. Oxymatrine Protects TGFβ1-Induced Retinal Fibrosis in an Animal Model of Glaucoma. Front. Med. 2022, 8, 750342. [Google Scholar] [CrossRef]
- Chen, K.; Lai, K.; Zhang, X.; Qin, Z.; Fu, Q.; Luo, C.; Jin, X.; Hu, J.; Liu, S.; Yao, K. Bromfenac Inhibits TGF-Β1-Induced Fibrotic Effects in Human Pterygium and Conjunctival Fibroblasts. Invest. Ophthalmol. Vis. Sci. 2019, 60, 1156–1164. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, X.; Jiang, Y.; Chen, L.; Sheng, M.; Chen, Y. SPARC Knockdown Attenuated TGF-Β1-Induced Fibrotic Effects through Smad2/3 Pathways in Human Pterygium Fibroblasts. Arch. Biochem. Biophys. 2021, 713, 109049. [Google Scholar] [CrossRef]
- Tsutsumi-Kuroda, U.; Inoue, T.; Futakuchi, A.; Shobayashi, K.; Takahashi, E.; Kojima, S.; Inoue-Mochita, M.; Fujimoto, T.; Tanihara, H. Decreased MCP-1/CCR2 Axis-Mediated Chemotactic Effect of Conjunctival Fibroblasts after Transdifferentiation into Myofibroblasts. Exp. Eye Res. 2018, 170, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Stasch, J.-P.; Schlossmann, J.; Hocher, B. Renal Effects of Soluble Guanylate Cyclase Stimulators and Activators: A Review of the Preclinical Evidence. Curr. Opin. Pharmacol. 2015, 21, 95–104. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, M.; Yim, B.; Park, C.Y. Nitric Oxide Attenuated Transforming Growth Factor-β Induced Myofibroblast Differentiation of Human Keratocytes. Sci. Rep. 2021, 11, 8183. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ye, Q.; Lan, C.; Wang, X.; Zhu, Y. AZD6738 Inhibits Fibrotic Response of Conjunctival Fibroblasts by Regulating Checkpoint Kinase 1/P53 and PI3K/AKT Pathways. Front. Pharmacol. 2022, 13, 990401. [Google Scholar] [CrossRef] [PubMed]
- Beaven, E.; Kumar, R.; Bhatt, H.N.; Esquivel, S.V.; Nurunnabi, M. Myofibroblast Specific Targeting Approaches to Improve Fibrosis Treatment. Chem. Commun. 2022, 58, 13556–13571. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.A.; Lazaropoulos, M.P.; Elrod, J.W. Myofibroblasts and Fibrosis: Mitochondrial and Metabolic Control of Cellular Differentiation. Circ. Res. 2020, 127, 427–447. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, H.; Li, G.; Zhang, Y. Higher TGF-Β1, TGF-Β2, MMP-2, and TIMP-1 Levels in the Aqueous Humor of Patients with Acute Primary Angle Closure. Ophthalmic Res. 2021, 64, 62–67. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.-B.; Liu, M.-Y.; Yao, R.-Y. Correlation between Tissue Characterization and Dynamic Expression of Matrix Metalloproteinase-2 and Its Tissue Inhibitor in Conjunctival Filtering Bleb of Rats. Biomed. Res. Int. 2017, 2017, 1054129. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-M.; Cian, A.-A.; Weng, T.-H.; Liang, C.-M.; Pao, S.-I.; Chen, Y.-J. Beneficial Effects of Hypercapnic Acidosis on the Inhibition of Transforming Growth Factor β-1-Induced Corneal Fibrosis In Vitro. Curr. Eye Res. 2021, 46, 648–656. [Google Scholar] [CrossRef]
- Maruri, D.P.; Iyer, K.S.; Schmidtke, D.W.; Petroll, W.M.; Varner, V.D. Signaling Downstream of Focal Adhesions Regulates Stiffness-Dependent Differences in the TGF-Β1-Mediated Myofibroblast Differentiation of Corneal Keratocytes. Front. Cell Dev. Biol. 2022, 10, 886759. [Google Scholar] [CrossRef]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E.M. Inflammation in Glaucoma: From the Back to the Front of the Eye, and Beyond. Prog. Retin. Eye Res. 2021, 83, 100916. [Google Scholar] [CrossRef]
- Flores-Costa, R.; Duran-Güell, M.; Casulleras, M.; López-Vicario, C.; Alcaraz-Quiles, J.; Diaz, A.; Lozano, J.J.; Titos, E.; Hall, K.; Sarno, R.; et al. Stimulation of Soluble Guanylate Cyclase Exerts Antiinflammatory Actions in the Liver through a VASP/NF-κB/NLRP3 Inflammasome Circuit. Proc. Natl. Acad. Sci. USA 2020, 117, 28263–28274. [Google Scholar] [CrossRef] [PubMed]
- Flores-Costa, R.; Alcaraz-Quiles, J.; Titos, E.; López-Vicario, C.; Casulleras, M.; Duran-Güell, M.; Rius, B.; Diaz, A.; Hall, K.; Shea, C.; et al. The Soluble Guanylate Cyclase Stimulator IW-1973 Prevents Inflammation and Fibrosis in Experimental Non-Alcoholic Steatohepatitis. Br. J. Pharmacol. 2018, 175, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Shea, C.M.; Price, G.M.; Liu, G.; Sarno, R.; Buys, E.S.; Currie, M.G.; Masferrer, J.L. Soluble Guanylate Cyclase Stimulator Praliciguat Attenuates Inflammation, Fibrosis, and End-Organ Damage in the Dahl Model of Cardiorenal Failure. Am. J. Physiol. Renal. Physiol. 2020, 318, F148–F159. [Google Scholar] [CrossRef] [PubMed]
- Collotta, D.; Colletta, S.; Carlucci, V.; Fruttero, C.; Fea, A.M.; Collino, M. Pharmacological Approaches to Modulate the Scarring Process after Glaucoma Surgery. Pharmaceuticals 2023, 16, 898. [Google Scholar] [CrossRef] [PubMed]
- Holden, J.M.; Wareham, L.K. cGMP Signaling: A Potential Therapeutic Target for Neurodegeneration in Glaucoma? Neural Regen. Res. 2023, 18, 1267. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, G.; Ferrara, L.; Adams, C.; Ehara, T.; Li, B.; Yang, L.; Xiang, C.; Ng, C.T.H.; Kim, S.; Towler, C.; et al. A Novel Selective Soluble Guanylate Cyclase Activator, MGV354, Lowers Intraocular Pressure in Preclinical Models, Following Topical Ocular Dosing. Invest. Ophthalmol. Vis. Sci. 2018, 59, 1704–1716. [Google Scholar] [CrossRef] [PubMed]
- Stacy, R.; Huttner, K.; Watts, J.; Peace, J.; Wirta, D.; Walters, T.; Sall, K.; Seaman, J.; Ni, X.; Prasanna, G.; et al. A Randomized, Controlled Phase I/II Study to Evaluate the Safety and Efficacy of MGV354 for Ocular Hypertension or Glaucoma. Am. J. Ophthalmol. 2018, 192, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Dumouchel, J.L.; Argikar, U.A.; Adams, C.M.; Prasanna, G.; Ehara, T.; Kim, S.; Breen, C.; Mogi, M. Understanding Metabolism Related Differences in Ocular Efficacy of MGV354. Xenobiotica 2021, 51, 5–14. [Google Scholar] [CrossRef]


| Gene | Assay ID | Catalog Number |
|---|---|---|
| FAP | Hs_FAP_1_SG | QT00074963 |
| ACTA2 | Hs_ACTA2_1_SG | QT00088102 |
| COL1A1 | Hs_COL1A1_1_SG | QT00037793 |
| COL1A2 | Hs_COL1A2_1_SG | QT00072058 |
| FN1 | Hs_FN1_1_SG | QT00038024 |
| MMP2 | Hs_MMP2_1_SG | QT00088396 |
| TIMP1 | Hs_TIMP1_1_SG | QT00084168 |
| TIMP2 | Hs_TIMP2_1_SG | QT00017759 |
| IL1B | Hs_IL1B_1_SG | QT00021385 |
| IL6 | Hs_IL6_1_SG | QT00083720 |
| Primary Antibody | Host Species | Catalog Number | Producer | Dilution |
|---|---|---|---|---|
| anti-α-SMA | Mouse | ab7817 | Abcam, Cambridge, UK | 1:300 |
| anti-N-cadherin | Rabbit | #13116S | Cell Signaling Technology, Danvers, MA, USA | 1:1000 |
| anti-COL1A1 | Rabbit | #39952 | Cell Signaling Technology, Danvers, MA, USA | 1:1000 |
| anti-fibronectin | Mouse | SAB4200880 | Sigma-Aldrich, St. Louis, MO, USA | 1:1000 |
| anti-Smad3 | Rabbit | #9513S | Cell Signaling Technology, Danvers, MA, USA | 1:1000 |
| anti-p-Smad3 | Rabbit | #9520S | Cell Signaling Technology, Danvers, MA, USA | 1:1000 |
| anti-ERK1/2 | Rabbit | ab17942 | Abcam, Cambridge, UK | 1:1000 |
| anti-p-ERK1/2 | Goat | sc-16982 | Santa Cruz Biotechnology, Dallas, TX, USA | 1:1000 |
| anti-α-actinin | Rabbit | #3134 | Cell Signaling Technology, Danvers, MA, USA | 1:1000 |
| anti-GAPDH | Mouse | ab8245 | Abcam, Cambridge, UK | 1:5000 |
| anti-α-tubulin | Rabbit | #2144 | Cell Signaling Technology, Danvers, MA, USA | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fioretto, B.S.; Rosa, I.; Andreucci, E.; Mencucci, R.; Marini, M.; Romano, E.; Manetti, M. Pharmacological Stimulation of Soluble Guanylate Cyclase Counteracts the Profibrotic Activation of Human Conjunctival Fibroblasts. Cells 2024, 13, 360. https://doi.org/10.3390/cells13040360
Fioretto BS, Rosa I, Andreucci E, Mencucci R, Marini M, Romano E, Manetti M. Pharmacological Stimulation of Soluble Guanylate Cyclase Counteracts the Profibrotic Activation of Human Conjunctival Fibroblasts. Cells. 2024; 13(4):360. https://doi.org/10.3390/cells13040360
Chicago/Turabian StyleFioretto, Bianca Saveria, Irene Rosa, Elena Andreucci, Rita Mencucci, Mirca Marini, Eloisa Romano, and Mirko Manetti. 2024. "Pharmacological Stimulation of Soluble Guanylate Cyclase Counteracts the Profibrotic Activation of Human Conjunctival Fibroblasts" Cells 13, no. 4: 360. https://doi.org/10.3390/cells13040360
APA StyleFioretto, B. S., Rosa, I., Andreucci, E., Mencucci, R., Marini, M., Romano, E., & Manetti, M. (2024). Pharmacological Stimulation of Soluble Guanylate Cyclase Counteracts the Profibrotic Activation of Human Conjunctival Fibroblasts. Cells, 13(4), 360. https://doi.org/10.3390/cells13040360











