Investigation of Biological Activity of Fucoidan and Laminarin as Bioactive Polysaccharides from Irish Brown Macroalgae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction of Seaweed Samples
2.3. Antioxidant Activity
2.3.1. DPPH Radical Scavenging Assay
2.3.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.4. Immune Stimulation and Inflammatory Activity
2.5. In Vitro Gastrointestinal Digestion
2.6. Anticancer Activity
2.6.1. Cell Culture
2.6.2. Two-Dimensional Cell Culture Method
2.6.3. Three-Dimensional Cell Culture Method
2.6.4. Alamar Blue™ Cell Viability Assay
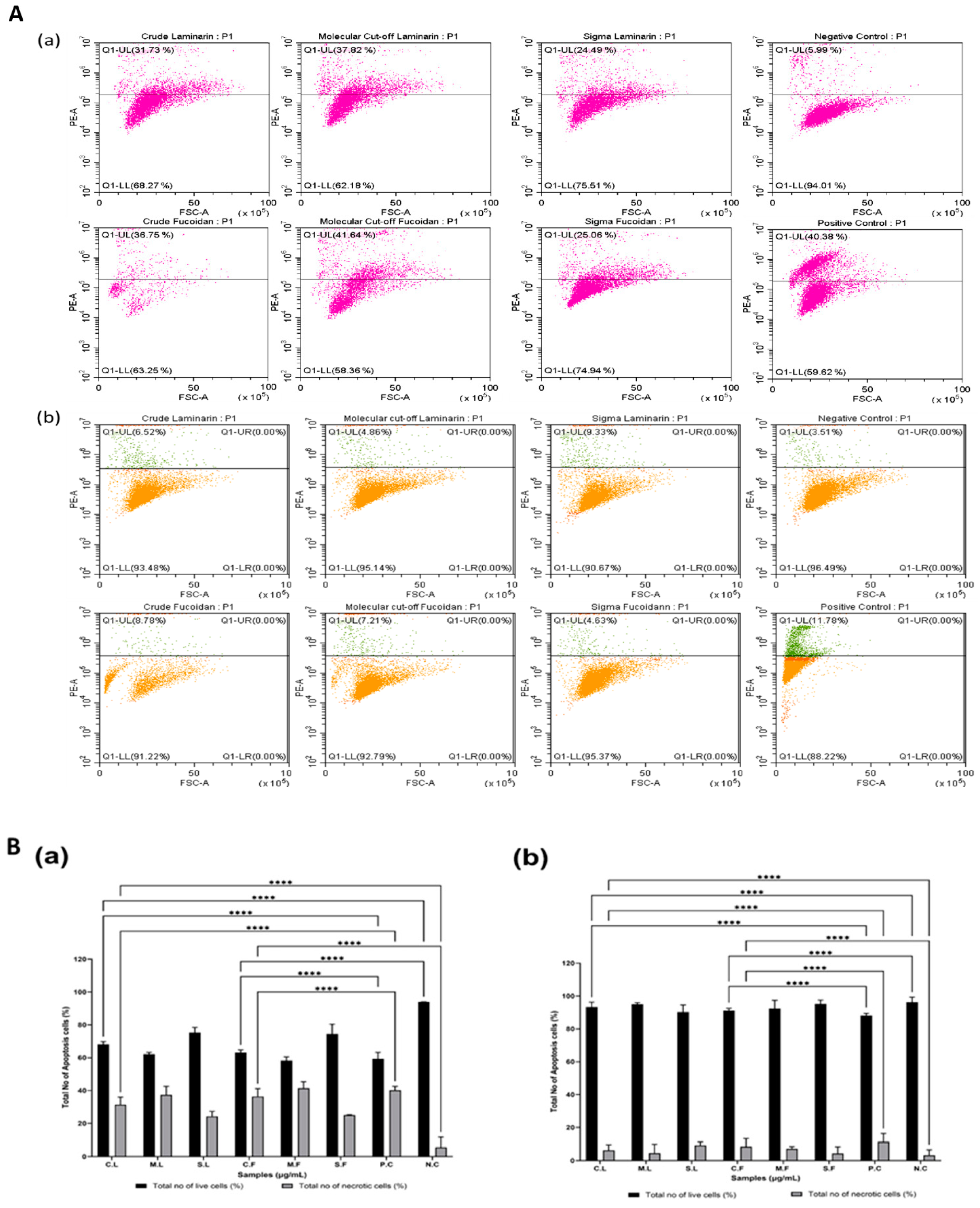
2.6.5. Live/Dead Cell Analysis Using Propidium Iodide (PI) for Flow Cytometry
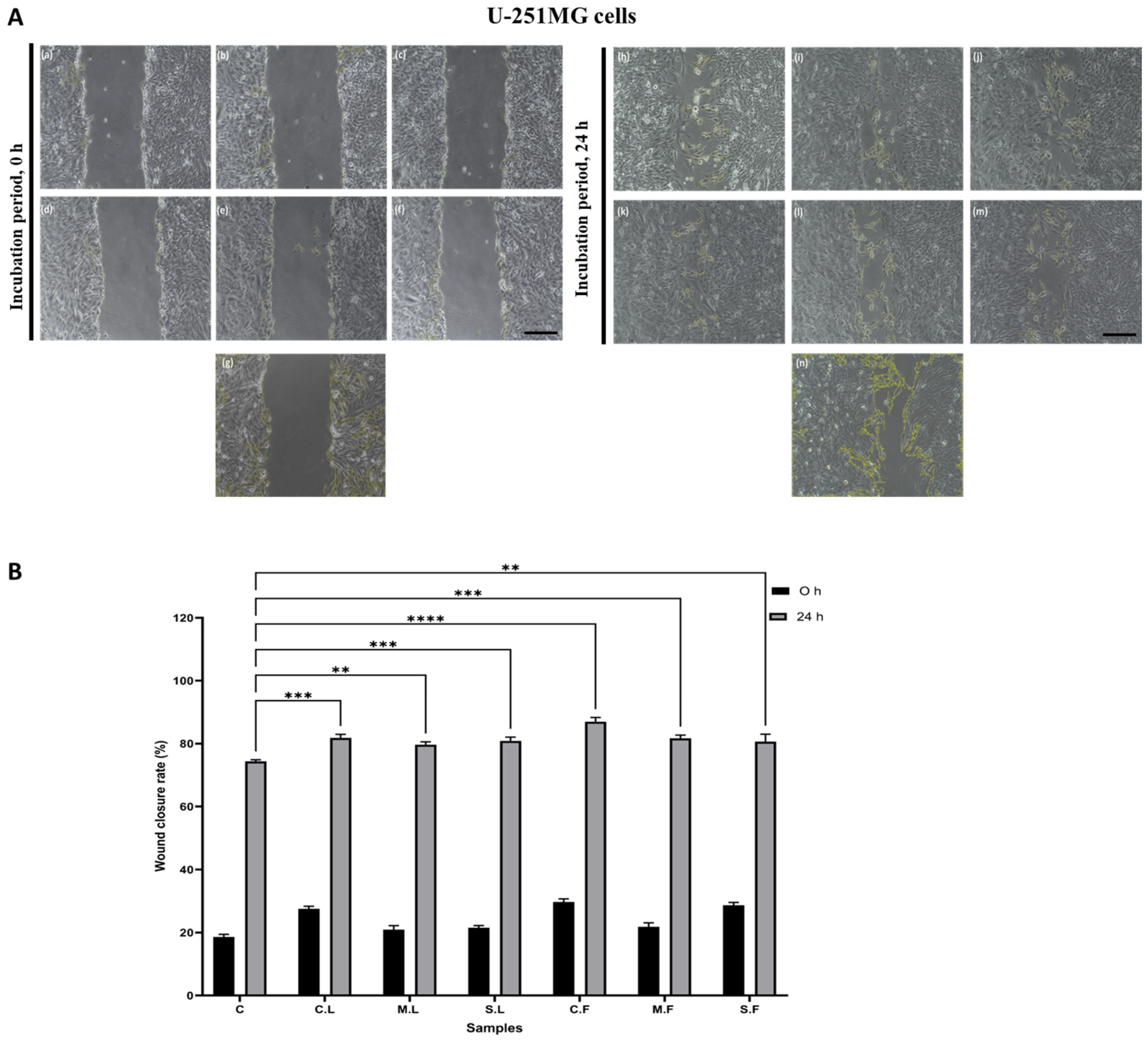
2.7. Wound-Healing Assay for Cell Migration
2.8. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Activity
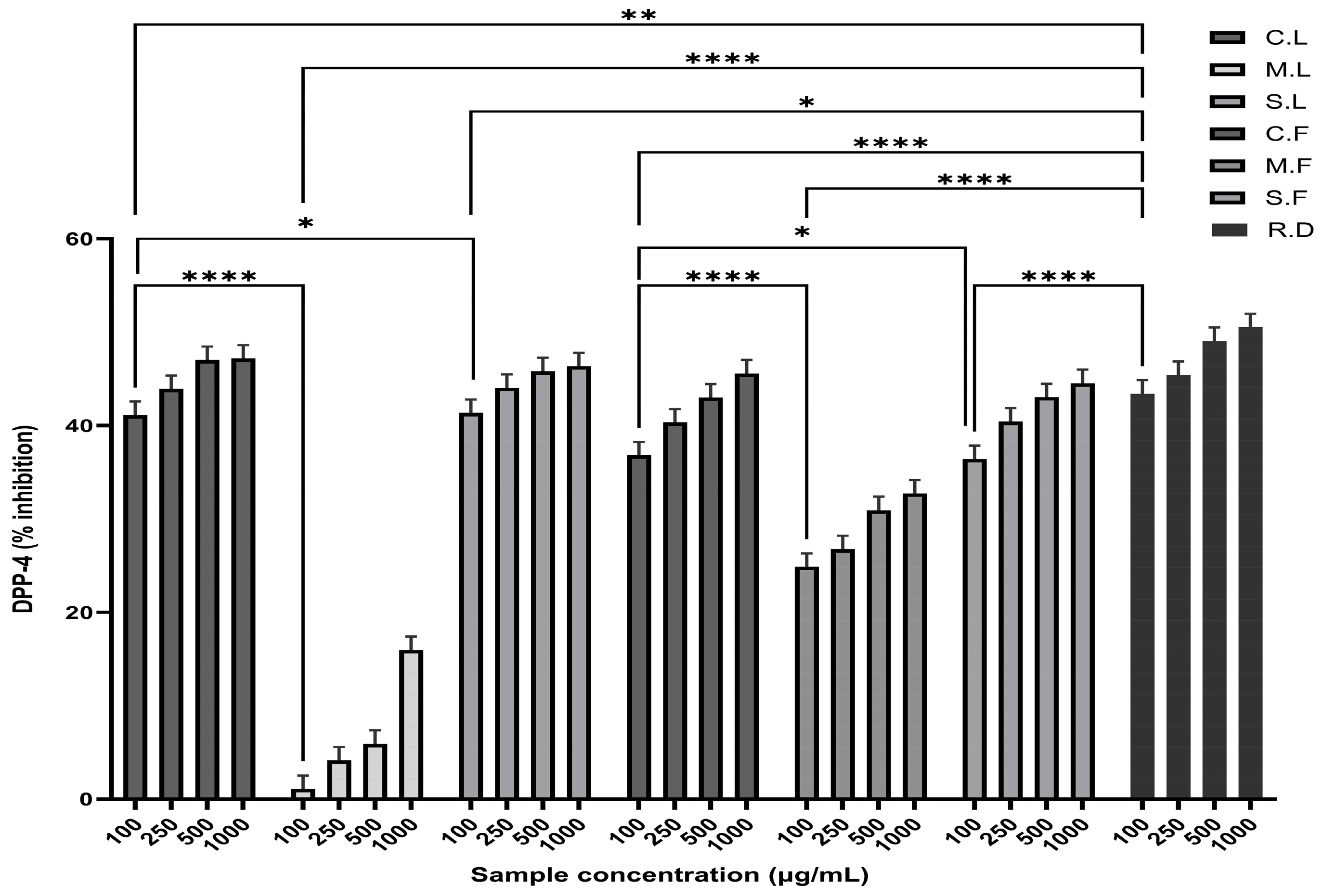
3.3. Antidiabetic Activity
3.4. Cytotoxicity of Laminarin and Fucoidan in Cancer Cells in 2D and 3D Cell Culture Models
3.5. Wound-Healing Assay for Cell Migration
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gultekin, M.; Ramirez, P.T.; Broutet, N.; Hutubessy, R. World Health Organization call for action to eliminate cervical cancer globally. Int. J. Gynecol. Cancer 2020, 30, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Wanigasekara, J.; Barcia, C.; Cullen, P.J.; Tiwari, B.; Curtin, J.F. Plasma induced reactive oxygen species-dependent cytotoxicity in glioblastoma 3D tumourspheres. Plasma Process. Polym. 2022, 19, 2100157. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Pacheco, D.; Gonçalves, A.M.; Silva, P.; Carvalho, L.G.; Pereira, L. Seaweeds’ nutraceutical and biomedical potential in cancer therapy: A concise review. J. Cancer Metastasis Treat. 2021, 7, 13. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. Epidermal growth factor receptor conjugated fucoidan/alginates loaded hydrogel for activating EGFR/AKT signaling pathways in colon cancer cells during targeted photodynamic therapy. Int. J. Biol. Macromol. 2020, 158, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. Fucoidan-loaded hydrogels facilitates wound healing using photodynamic therapy by in vitro and in vivo evaluation. Carbohydr. Polym. 2020, 247, 116624. [Google Scholar] [CrossRef]
- Ouyang, Y.; Qiu, Y.; Liu, Y.; Zhu, R.; Chen, Y.; El-Seedi, H.R.; Chen, X.; Zhao, C. Cancer-fighting potentials of algal polysaccharides as nutraceuticals. Food Res. Int. 2021, 147, 110522. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant therapy in cancer: Rationale and progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef]
- Zhu, X.; Healy, L.; Wanigasekara, J.; Zhao, M.; Padamati, R.B.; Karuppusamy, S.; Curtin, J.F.; Sivagnanam, S.P.; Rai, D.K.; Sun, D.-W. Characterisation of laminarin extracted from brown seaweed Laminaria digitata, using optimized ultrasound-and ultrafiltration-assisted extraction method. Algal Res. 2023, 75, 103277. [Google Scholar] [CrossRef]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The antioxidant activity of polysaccharides derived from marine organisms: An overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.C.L.; dos Santos Lisboa, L.; Paiva, W.S.; Batista, L.A.N.C.; Luchiari, A.C.; Rocha, H.A.O.; Camara, R.B.G. Comparison of in vitro and in vivo antioxidant activities of commercial fucoidans from Macrocystis pyrifera, Undaria pinnatifida, and Fucus vesiculosus. Int. J. Biol. Macromol. 2022, 216, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Ummat, V.; Sivagnanam, S.P.; Rai, D.K.; O’Donnell, C.; Conway, G.E.; Heffernan, S.M.; Fitzpatrick, S.; Lyons, H.; Curtin, J.; Tiwari, B.K. Conventional extraction of fucoidan from Irish brown seaweed Fucus vesiculosus followed by ultrasound-assisted depolymerization. Sci. Rep. 2024, 14, 6214. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I.; Ustyuzhanina, N.E.; Nifantiev, N.E. Fucoidans of Brown Algae: Comparison of Sulfated Polysaccharides from Fucus vesiculosus and Ascophyllum nodosum. Mar. Drugs 2022, 20, 638. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The Biochemical composition and antioxidant properties of Fucus vesiculosus from the Arctic region. Mar. Drugs 2022, 20, 193. [Google Scholar] [CrossRef]
- Ajjarapu, S.M.; Tiwari, A.; Kumar, S. Applications and Utility of Three-Dimensional In Vitro Cell Culture for Therapeutics. Future Pharmacol. 2023, 3, 213–228. [Google Scholar] [CrossRef]
- Amaro, H.M.; Pagels, F.; Tavares, T.G.; Costa, I.; Sousa-Pinto, I.; Guedes, A.C. Antioxidant and Anti-Inflammatory Potential of Seaweed Extracts as Functional Ingredients. Hydrobiology 2022, 1, 469–482. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.; Barqawi, A.A.; Mansour, A.T. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef]
- Jayapala, N.; Perumal, M.K.; Baskaran, R.; Vallikannan, B. Pharmacological Importance of Bioactive Molecules of Seaweeds. In Sustainable Global Resources of Seaweeds Volume 2: Food, Pharmaceutical and Health Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 597–613. [Google Scholar]
- Gabbia, D.; De Martin, S. Brown seaweeds for the management of metabolic syndrome and associated diseases. Molecules 2020, 25, 4182. [Google Scholar] [CrossRef]
- Liyanage, N.; Nagahawatta, D.; Jayawardena, T.U.; Jeon, Y.-J. The Role of Seaweed Polysaccharides in Gastrointestinal Health: Protective Effect against Inflammatory Bowel Disease. Life 2023, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.H.; Pinto, D.C.; Silva, A. Macroalgae Specialized Metabolites: Evidence for Their Anti-Inflammatory Health Benefits. Mar. Drugs 2022, 20, 789. [Google Scholar] [CrossRef] [PubMed]
- Gunathilaka, T.L.; Samarakoon, K.; Ranasinghe, P.; Peiris, L.D.C. Antidiabetic potential of marine brown algae—A mini review. J. Diabetes Res. 2020, 2020, 1230218. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, D.; Rafferty, E.; Fitzgerald, C.; Stoilova, V.; Wylie, A.; Gilmore, B.F.; Castaneda, F.; Israel, A.; Maggs, C.A.; Green, B.D. Profiling the activity of edible European macroalgae towards pharmacological targets for type 2 diabetes mellitus. Appl. Phycol. 2021, 2, 10–21. [Google Scholar] [CrossRef]
- Unnikrishnan, P.; Suthindhiran, K.; Jayasri, M. Antidiabetic potential of marine algae by inhibiting key metabolic enzymes. Front. Life Sci. 2015, 8, 148–159. [Google Scholar] [CrossRef]
- Karuppusamy, S.; Rajauria, G.; Fitzpatrick, S.; Lyons, H.; McMahon, H.; Curtin, J.; Tiwari, B.K.; O’Donnell, C. Biological Properties and Health-Promoting Functions of Laminarin: A Comprehensive Review of Preclinical and Clinical Studies. Mar. Drugs 2022, 20, 772. [Google Scholar] [CrossRef]
- Jayapala, N.; Toragall, V.; Kumar, G.; Chaudhari, S.R.; Baskaran, V. Preparation, characterization, radical scavenging property and antidiabetic potential of laminarioligosaccharides derived from laminarin. Algal Res. 2022, 63, 102642. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Molecular characteristics and antioxidant activity of laminarin extracted from the seaweed species Laminaria hyperborea, using hydrothermal-assisted extraction and a multi-step purification procedure. Food Hydrocoll. 2021, 112, 106332. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Tiwari, B.; Sweeney, T.; O’Doherty, J. Extraction and yield optimisation of fucose, glucans and associated antioxidant activities from Laminaria digitata by applying response surface methodology to high intensity ultrasound-assisted extraction. Mar. Drugs 2018, 16, 257. [Google Scholar] [CrossRef] [PubMed]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar]
- Lu, X.; Dissanayake, A.A.; Xiao, C.; Gao, J.; Zhao, M.; Nair, M.G. The edible seaweed Laminaria japonica contains cholesterol analogues that inhibit lipid peroxidation and cyclooxygenase enzymes. PLoS ONE 2022, 17, e0258980. [Google Scholar] [CrossRef] [PubMed]
- El-Ghoneimy, A.; Ahmed, H. Anti-inflammatory activities of a sulfated polysaccharide isolated from the brown seaweed Padina boergesenii (Phaeophyceae, Dictyotaceae). SVU-Int. J. Vet. Sci. 2022, 5, 83–101. [Google Scholar]
- Khalid, M.F.; Rehman, K.; Irshad, K.; Chohan, T.A.; Akash, M.S.H. Biochemical investigation of inhibitory activities of plant-derived bioactive compounds against carbohydrate and glucagon-like Peptide-1 metabolizing enzymes. Dose-Response 2022, 20, 15593258221093275. [Google Scholar] [CrossRef]
- Wanigasekara, J.; Carroll, L.J.; Cullen, P.J.; Tiwari, B.; Curtin, J.F. Three-Dimensional (3D) in vitro cell culture protocols to enhance glioblastoma research. PLoS ONE 2023, 18, e0276248. [Google Scholar] [CrossRef]
- Wanigasekara, J.; Cullen, P.J.; Bourke, P.; Tiwari, B.; Curtin, J.F. Advances in 3D culture systems for therapeutic discovery and development in brain cancer. Drug Discov. Today 2022, 28, 103426. [Google Scholar] [CrossRef]
- Bonfim-Mendonca, P.d.S.; Capoci, I.R.G.; Tobaldini-Valerio, F.K.; Negri, M.; Svidzinski, T.I.E. Overview of β-glucans from laminaria spp.: Immunomodulation properties and applications on biologic models. Int. J. Mol. Sci. 2017, 18, 1629. [Google Scholar] [CrossRef]
- Michalak, I.; Tiwari, R.; Dhawan, M.; Alagawany, M.; Farag, M.R.; Sharun, K.; Emran, T.B.; Dhama, K. Antioxidant effects of seaweeds and their active compounds on animal health and production–a review. Vet. Q. 2022, 42, 48–67. [Google Scholar] [CrossRef]
- Choi, J.-i.; Kim, H.-J.; Lee, J.-W. Structural feature and antioxidant activity of low molecular weight laminarin degraded by gamma irradiation. Food Chem. 2011, 129, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Zargarzadeh, M.; Amaral, A.J.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef] [PubMed]
- Dhara, S.; Chakraborty, K. Novel Furanyl-Substituted Isochromanyl Class of Anti-Inflammatory Turbinochromanone from Brown Seaweed Turbinaria conoides. Chem. Biodivers. 2022, 19, e202100723. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, C.-M.; Li, Y.-F.; Huang, T.-M.; Chao, N.-X.; Luo, G.-R.; Mo, F.-R. Laminarin from seaweed (Laminaria japonica) inhibits hepatocellular carcinoma through upregulating senescence marker protein-30. Cancer Biother. Radiopharm. 2020, 35, 277–283. [Google Scholar] [CrossRef]
- Bae, H.; Song, G.; Lee, J.-Y.; Hong, T.; Chang, M.-J.; Lim, W. Laminarin-derived from brown algae suppresses the growth of ovarian cancer cells via mitochondrial dysfunction and ER stress. Mar. Drugs 2020, 18, 152. [Google Scholar] [CrossRef] [PubMed]
- Cabral, E.M.; Mondala, J.R.M.; Oliveira, M.; Przyborska, J.; Fitzpatrick, S.; Rai, D.K.; Sivagnanam, S.P.; Garcia-Vaquero, M.; O’Shea, D.; Devereux, M. Influence of molecular weight fractionation on the antimicrobial and anticancer properties of a fucoidan rich-extract from the macroalgae Fucus vesiculosus. Int. J. Biol. Macromol. 2021, 186, 994–1002. [Google Scholar] [CrossRef]
- Santhanam, R.C.; Yacoob, S.A.M.; Venkatraman, A. In vitro cytotoxicity assay of Fucoidan extracted from Turbinaria conoides against cancer cell lines MCF7, A549, and normal cell line L929. Braz. J. Pharm. Sci. 2022, 58, e19542. [Google Scholar] [CrossRef]
- Malhão, F.; Ramos, A.A.; Macedo, A.C.; Rocha, E. Cytotoxicity of seaweed compounds, alone or combined to reference drugs, against breast cell lines cultured in 2D and 3D. Toxics 2021, 9, 24. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Vaamonde-García, C.; Capelo-Mera, E.; Flórez-Fernández, N.; Torres, M.D.; Rivas-Murias, B.; Mejide-Faílde, R.; Blanco, F.J.; Domínguez, H. In Vitro Study of the Therapeutic Potential of Brown Crude Fucoidans in Osteoarthritis Treatment. Int. J. Mol. Sci. 2022, 23, 14236. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [PubMed]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D cell culture systems: Tumor application, advantages, and disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Salinas-Vera, Y.M.; Valdés, J.; Pérez-Navarro, Y.; Mandujano-Lazaro, G.; Marchat, L.A.; Ramos-Payán, R.; Nuñez-Olvera, S.I.; Pérez-Plascencia, C.; López-Camarillo, C. Three-dimensional 3D culture models in gynecological and breast cancer research. Front. Oncol. 2022, 12, 826113. [Google Scholar] [CrossRef]
- Ji, C.-F.; Ji, Y.-B.; Meng, D.-Y. Sulfated modification and anti-tumor activity of laminarin. Exp. Ther. Med. 2013, 6, 1259–1264. [Google Scholar] [CrossRef]
- Song, K.; Xu, L.; Zhang, W.; Cai, Y.; Jang, B.; Oh, J.; Jin, J.-O. Laminarin promotes anti-cancer immunity by the maturation of dendritic cells. Oncotarget 2017, 8, 38554. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Thabitha, A.; Vignesh, N.; Manigandan, V.; Saravanan, R.; Daradkeh, G.; Qoronfleh, M.W. Antiskin cancer and antioxidant activities of formulated agar from Brown seaweed Laminaria digitata (hudson) in dimethyl benzanthracene-induced Swiss albino mice. Int. J. Polym. Sci. 2021, 2021, 9930777. [Google Scholar] [CrossRef]
- Haggag, Y.A.; Abd Elrahman, A.A.; Ulber, R.; Zayed, A. Fucoidan in pharmaceutical formulations: A comprehensive review for smart drug delivery systems. Mar. Drugs 2023, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Jesenak, M. β-Glucans: Multi-functional modulator of wound healing. Molecules 2018, 23, 806. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef]

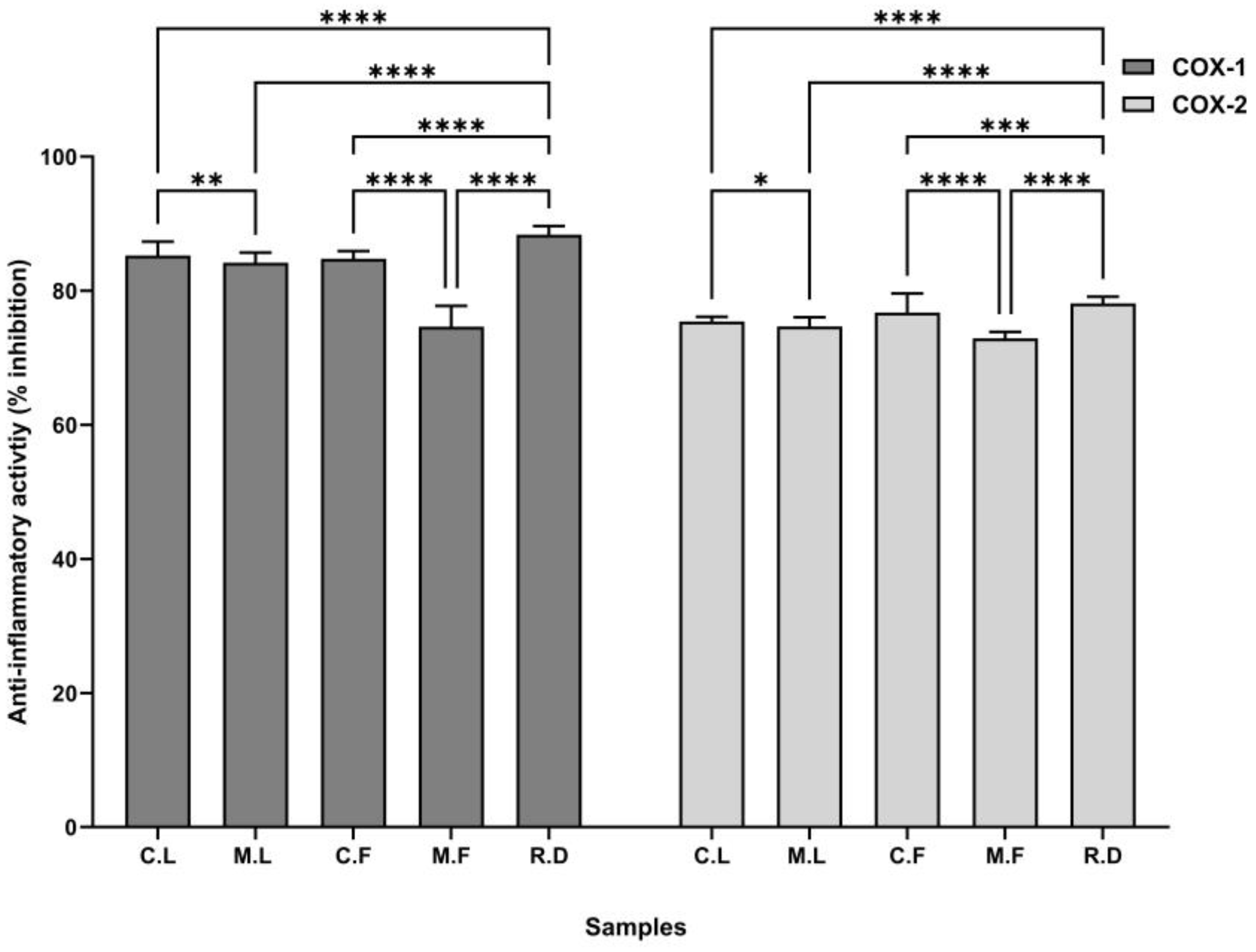



| Sample | IC50 Value (µg/mL) | Selectivity Index, SI | |
|---|---|---|---|
| COX-1 | COX-2 | ||
| C.L. | 48.96 | 42.74 | 1.06 |
| M.L. | 46.35 | 40.06 | 0.88 |
| C.F. | 47.41 | 42.27 | 1.58 |
| M.F. | 44.59 | 40.01 | 0.83 |
| R.D. | 49.64 | 45.19 | 1.07 |
| Sample | Cell Line | 2 Days | 6 Days | ||
|---|---|---|---|---|---|
| IC50 (µg/mL) | IC50 Range | IC50 (µg/mL) | IC50 Range | ||
| C.L. | U-251MG | 0.729 | 0.689 to 0.772 | 0.185 | 0.170 to 0.202 |
| A431 | 0.717 | 0.242 to 2.351 | 0.433 | 0.392 to 0.479 | |
| HepG2 | 1.219 | 1.097 to 1.354 | 0.315 | 0.283 to 0.351 | |
| Caco-2 | 0.390 | 0.373 to 0.408 | 0.404 | 0.365 to 0.448 | |
| HEK293 | 2.841 | 2.323 to 3.530 | 0.542 | 0.490 to 0.601 | |
| M.L. | U-251MG | 1.124 | 1.040 to 1.214 | 0.185 | 0.296 to 0.334 |
| A431 | 3.635 | 2.333 to 6.050 | 0.818 | 0.781 to 0.857 | |
| HepG2 | 1.318 | 1.152 to 1.508 | 0.749 | 0.638 to 0.882 | |
| Caco-2 | 1.283 | 1.186 to 1.389 | 0.840 | 0.767 to 0.920 | |
| HEK293 | 5.267 | 4.833 to 5.770 | 0.703 | 0.649 to 0.763 | |
| S.L. | U-251MG | 4.591 | 4.287 to 4.933 | 0.185 | 1.061 to 1.159 |
| A431 | 15.26 | 6.865 to 15.39 | 1.955 | 1.772 to 2.159 | |
| HepG2 | 8.619 | 7.396 to 10.32 | 7.755 | 6.062 to 10.45 | |
| Caco-2 | 14.47 | 10.67 to 21.64 | 4.515 | 4.094 to 5.006 | |
| HEK293 | 18.29 | 13.57 to 26.81 | 2.936 | 2.611 to 3.310 | |
| C.F. | U-251MG | 0.391 | 0.377 to 0.406 | 0.185 | 0.149 to 0.168 |
| A431 | 0.498 | 0.458 to 0.542 | 0.216 | 0.192 to 0.244 | |
| HepG2 | 1.123 | 1.073 to 1.176 | 0.190 | 0.177 to 0.204 | |
| Caco-2 | 0.330 | 0.303 to 0.362 | 0.237 | 0.230 to 0.244 | |
| HEK293 | 2.092 | 1.875 to 2.342 | 0.465 | 0.438 to 0.494 | |
| M.F. | U-251MG | 1.052 | 0.933 to 1.184 | 0.185 | 0.440 to 0.511 |
| A431 | 1.707 | 1.548 to 1.883 | 0.329 | 0.304 to 0.357 | |
| HepG2 | 1.622 | 1.492 to 1.763 | 0.559 | 0.461 to 0.679 | |
| Caco-2 | 0.717 | 0.654 to 0.786 | 0.586 | 0.512 to 0.672 | |
| HEK293 | 2.835 | 2.500 to 3.235 | 1.282 | 1.167 to 1.412 | |
| S.F. | U-251MG | 6.972 | 6.585 to 7.404 | 0.185 | 1.621 to 2.145 |
| A431 | 9.520 | 4.088 to 11.37 | 1.601 | 1.517 to 1.731 | |
| HepG2 | 7.896 | 6.924 to 9.164 | 10.80 | 9.095 to 13.30 | |
| Caco-2 | 6.046 | 5.166 to 7.211 | 4.376 | 4.035 to 4.763 | |
| HEK293 | 9.544 | 7.982 to 11.73 | 3.614 | 3.238 to 4.056 | |
| Sample | 6 Days | |
|---|---|---|
| IC50 Value (µg/mL) | IC50 Range | |
| C.L. | 8.583 | 7.639 to 9.644 |
| M.L. | 10.82 | 9.991 to 11.71 |
| S.L. | 30.00 | 24.60 to 36.60 |
| C.F. | 7.237 | 6.625 to 7.906 |
| M.F. | 18.98 | 16.58 to 21.73 |
| S.F. | 33.51 | 27.01 to 41.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karuppusamy, S.; Wanigasekara, J.; Fitzpatrick, S.; Lyons, H.; Curtin, J.; Rajauria, G.; Tiwari, B.K.; O’Donnell, C. Investigation of Biological Activity of Fucoidan and Laminarin as Bioactive Polysaccharides from Irish Brown Macroalgae. Cells 2024, 13, 1938. https://doi.org/10.3390/cells13231938
Karuppusamy S, Wanigasekara J, Fitzpatrick S, Lyons H, Curtin J, Rajauria G, Tiwari BK, O’Donnell C. Investigation of Biological Activity of Fucoidan and Laminarin as Bioactive Polysaccharides from Irish Brown Macroalgae. Cells. 2024; 13(23):1938. https://doi.org/10.3390/cells13231938
Chicago/Turabian StyleKaruppusamy, Shanmugapriya, Janith Wanigasekara, Stephen Fitzpatrick, Henry Lyons, James Curtin, Gaurav Rajauria, Brijesh K. Tiwari, and Colm O’Donnell. 2024. "Investigation of Biological Activity of Fucoidan and Laminarin as Bioactive Polysaccharides from Irish Brown Macroalgae" Cells 13, no. 23: 1938. https://doi.org/10.3390/cells13231938
APA StyleKaruppusamy, S., Wanigasekara, J., Fitzpatrick, S., Lyons, H., Curtin, J., Rajauria, G., Tiwari, B. K., & O’Donnell, C. (2024). Investigation of Biological Activity of Fucoidan and Laminarin as Bioactive Polysaccharides from Irish Brown Macroalgae. Cells, 13(23), 1938. https://doi.org/10.3390/cells13231938








