A Dual Role of the Senescence Marker P16Ink4a in Liver Endothelial Cell Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Liver Endothelial Cell Isolation and Culture
2.2. Lentivirus Production
2.3. Migration Assays
2.4. In Vitro Angiogenesis Assays
2.5. SDS–Polyacrylamide Gel Electrophoresis and Western Blot
2.6. RNA Isolation, Reverse Transcription, and Quantitative Polymerase Chain Reaction (PCR)
2.7. Apoptosis Assays
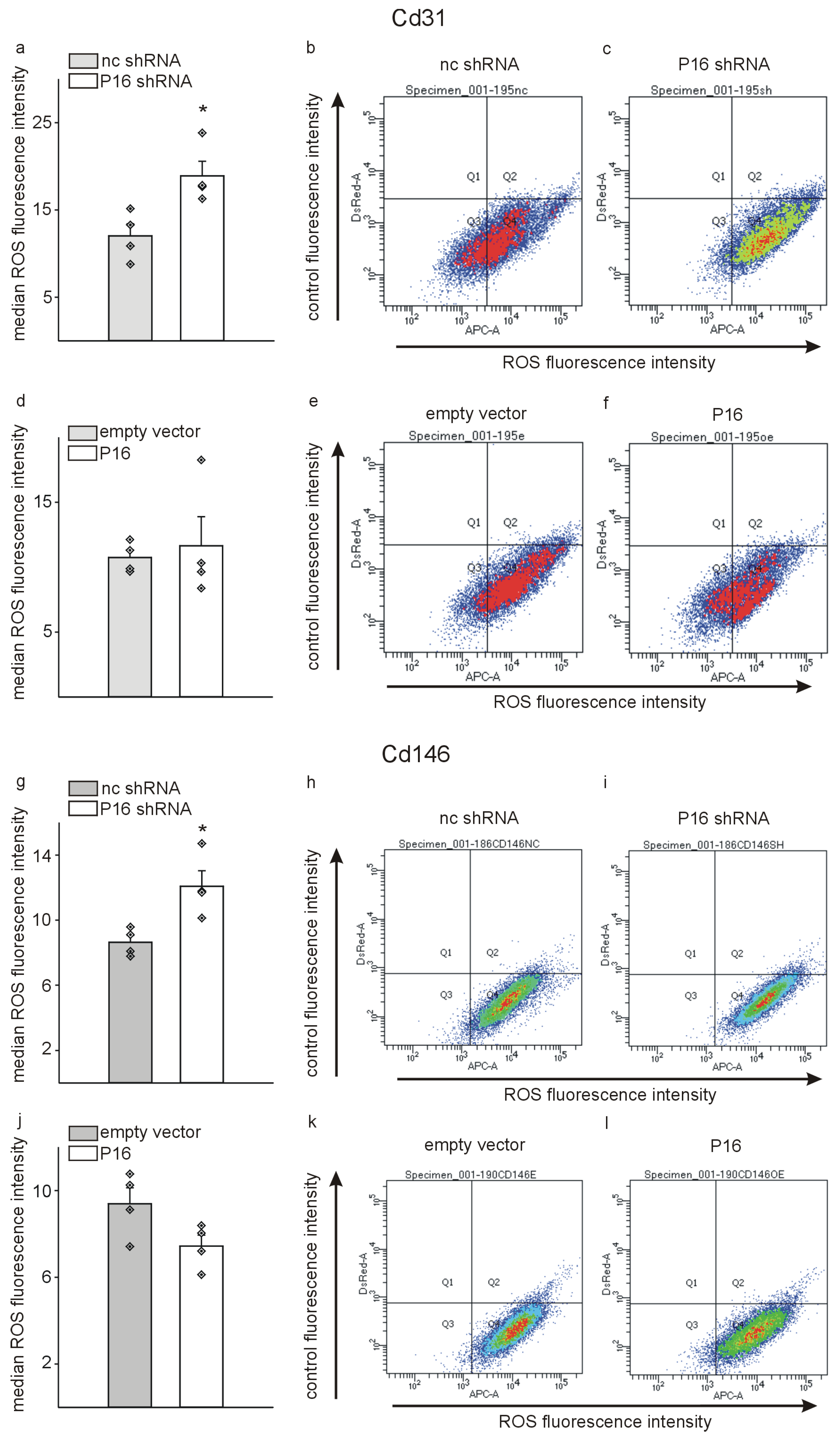
2.8. Reactive Oxygen Species Measurement
2.9. Detection of Cell Proliferation
2.10. Cellular Senescence Assays
2.11. Endothelial Cell Permeability Assays
2.12. mRNA Sequencing
2.13. Statistical Analysis
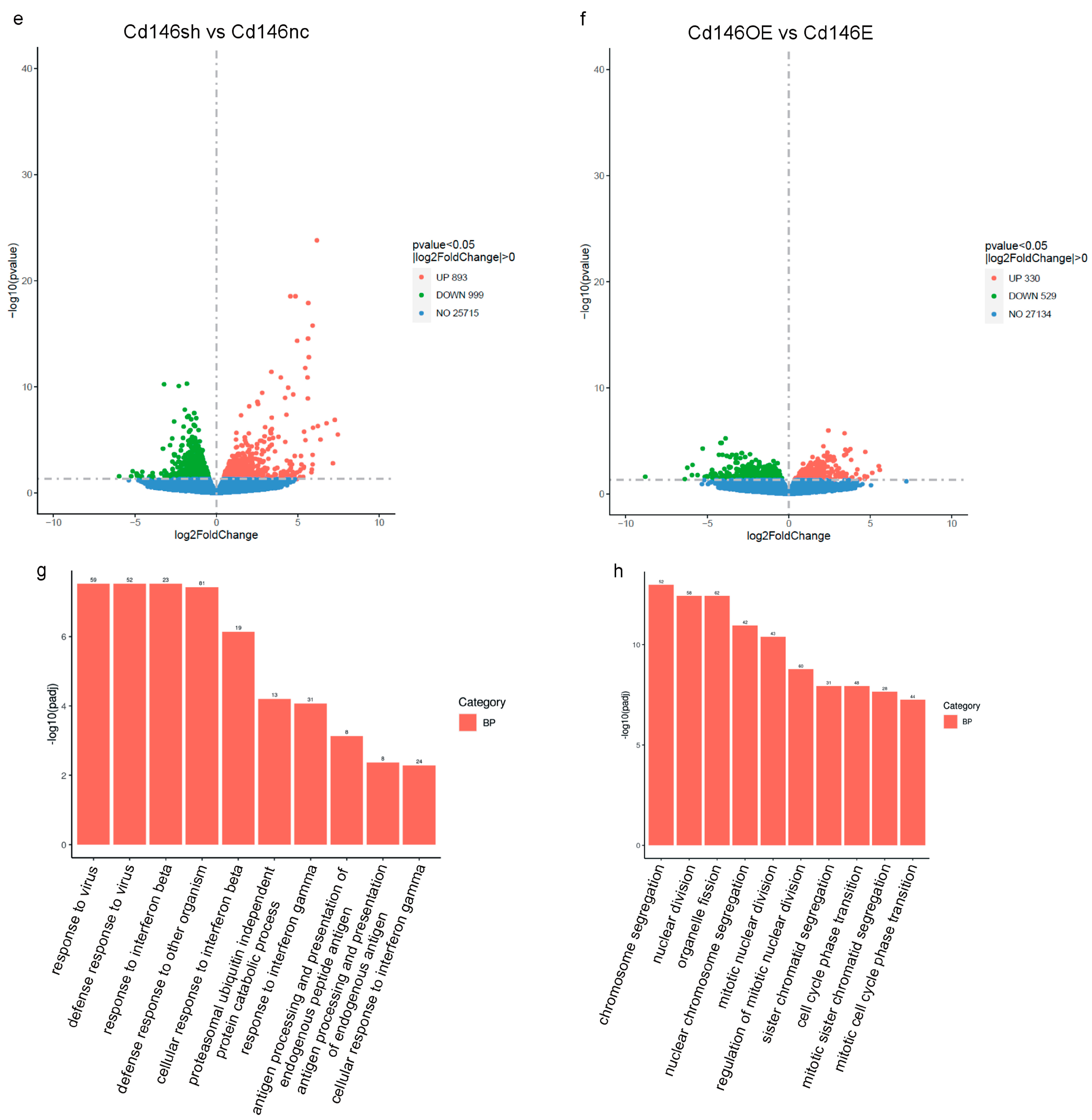
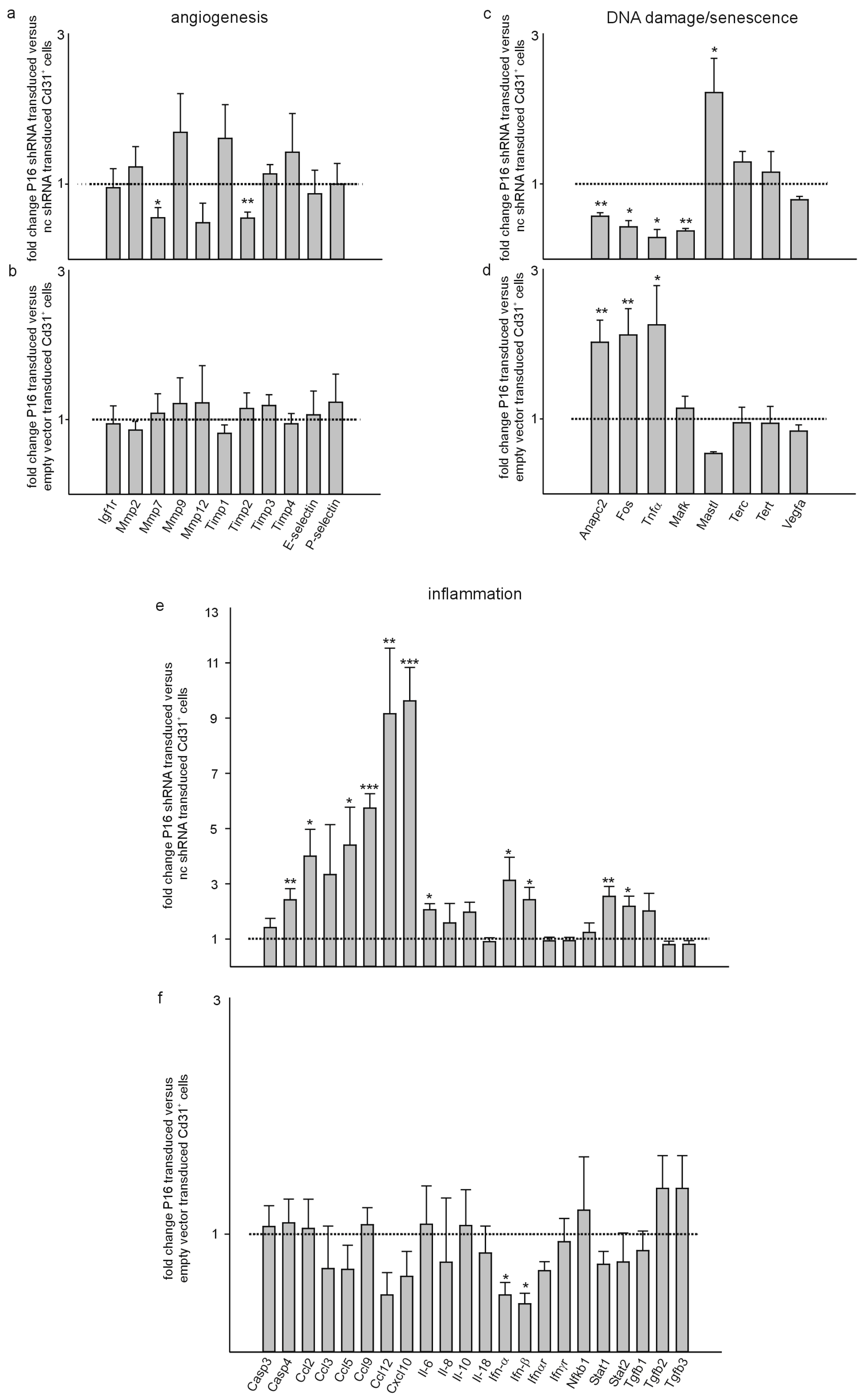
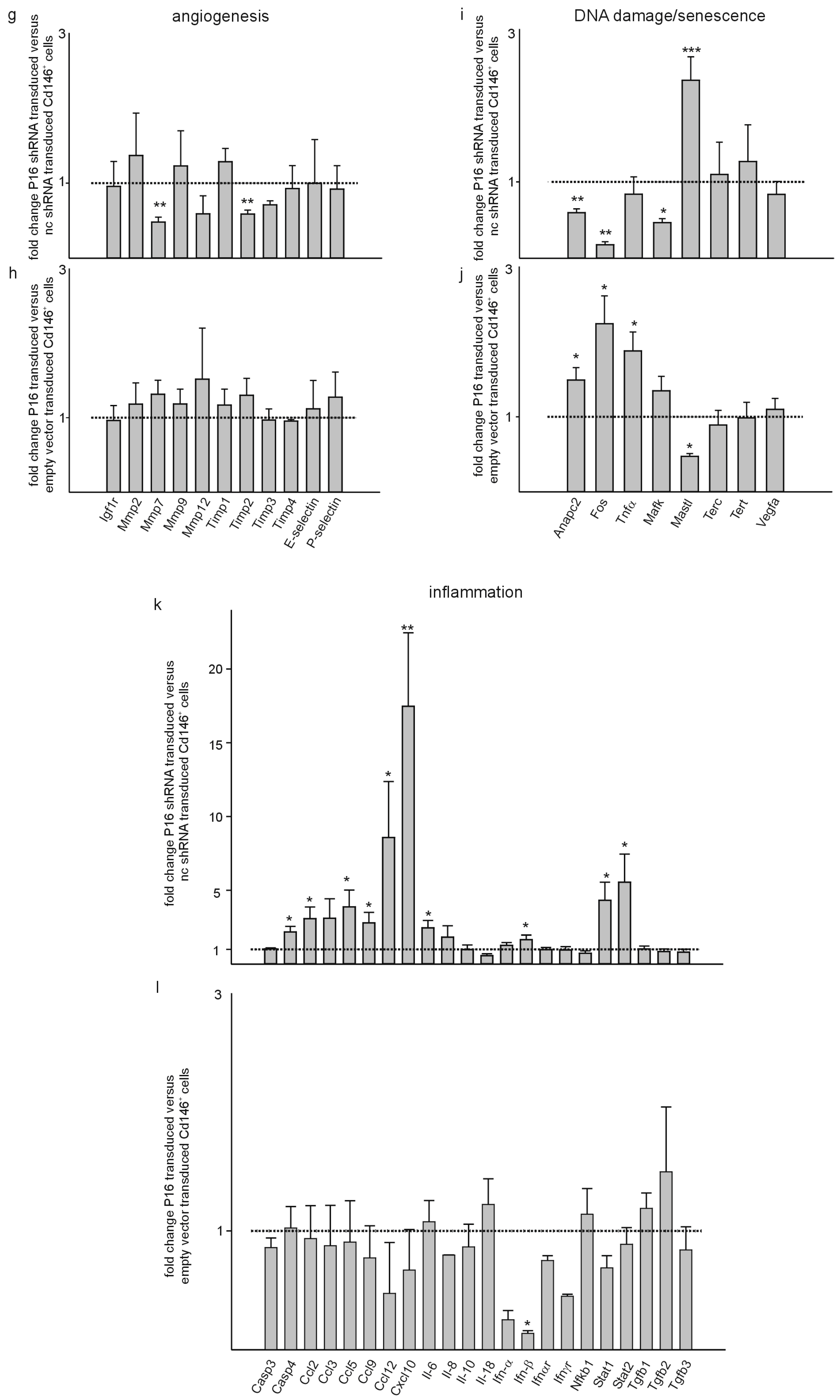
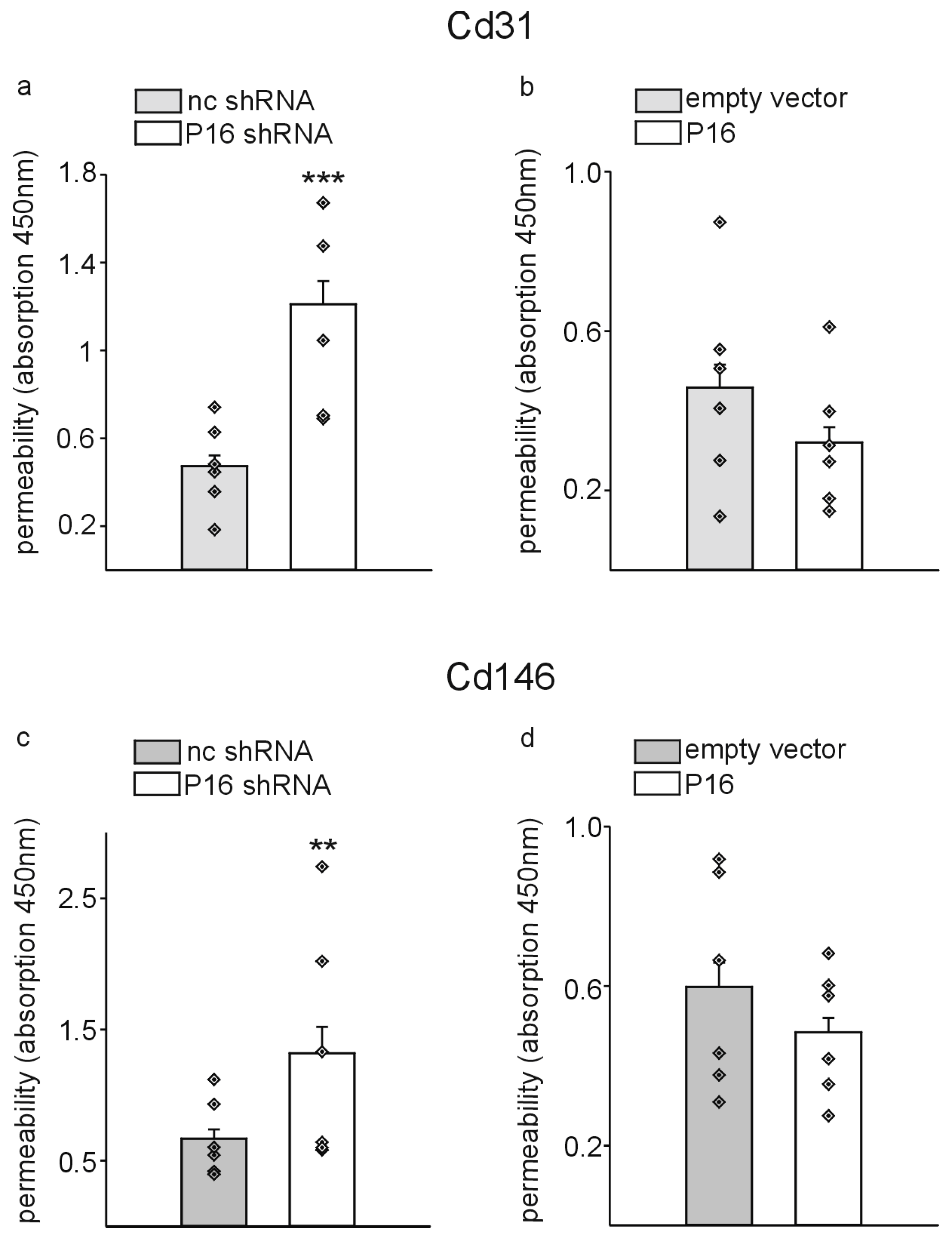
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Kirkwood, T.B.; Rose, M.R. Evolution of senescence: Late survival sacrificed for reproduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1991, 332, 15–24. [Google Scholar] [CrossRef]
- Rose, M.; Charlesworth, B. A test of evolutionary theories of senescence. Nature 1980, 287, 141–142. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Tripathi, U.; Misra, A.; Tchkonia, T.; Kirkland, J.L. Impact of Senescent Cell Subtypes on Tissue Dysfunction and Repair: Importance and Research Questions. Mech. Ageing Dev. 2021, 198, 111548. [Google Scholar] [CrossRef]
- Hara, E.; Smith, R.; Parry, D.; Tahara, H.; Stone, S.; Peters, G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol. Cell. Biol. 1996, 16, 859–867. [Google Scholar] [CrossRef]
- Safwan-Zaiter, H.; Wagner, N.; Wagner, K.D. P16INK4A-More Than a Senescence Marker. Life 2022, 12, 1332. [Google Scholar] [CrossRef]
- Stone, S.; Jiang, P.; Dayananth, P.; Tavtigian, S.V.; Katcher, H.; Parry, D.; Peters, G.; Kamb, A. Complex structure and regulation of the P16 (MTS1) locus. Cancer Res. 1995, 55, 2988–2994. [Google Scholar] [PubMed]
- Kamb, A.; Gruis, N.A.; Weaver-Feldhaus, J.; Liu, Q.; Harshman, K.; Tavtigian, S.V.; Stockert, E.; Day, R.S.; Johnson, B.E.; Skolnick, M.H. A cell cycle regulator potentially involved in genesis of many tumor types. Science 1994, 264, 436–440. [Google Scholar] [CrossRef]
- Serrano, M.; Hannon, G.J.; Beach, D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 1993, 366, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Liggett, W.H.; Sidransky, D. Role of the p16 tumor suppressor gene in cancer. J. Clin. Oncol. 1998, 16, 1197–1206. [Google Scholar] [CrossRef]
- Sharpless, N.E.; Bardeesy, N.; Lee, K.H.; Carrasco, D.; Castrillon, D.H.; Aguirre, A.J.; Wu, E.A.; Horner, J.W.; DePinho, R.A. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 2001, 413, 86–91. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.-M.; Vijg, J.; Van Steeg, H.; Dollé, M.E.T.; et al. An Essential Role for Senescent Cells in Optimal Wound Healing through Secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef]
- Grosse, L.; Wagner, N.; Emelyanov, A.; Molina, C.; Lacas-Gervais, S.; Wagner, K.-D.; Bulavin, D.V. Defined p16High Senescent Cell Types Are Indispensable for Mouse Healthspan. Cell Metab. 2020, 32, 87–99.e86. [Google Scholar] [CrossRef]
- Reyes, N.S.; Krasilnikov, M.; Allen, N.C.; Lee, J.Y.; Hyams, B.; Zhou, M.; Ravishankar, S.; Cassandras, M.; Wang, C.; Khan, I.; et al. Sentinel. Science 2022, 378, 192–201. [Google Scholar] [CrossRef]
- Wagner, K.D.; Wagner, N. The Senescence Markers p16INK4A, p14ARF/p19ARF, and p21 in Organ Development and Homeostasis. Cells 2022, 11, 1966. [Google Scholar] [CrossRef] [PubMed]
- Safwan-Zaiter, H.; Wagner, N.; Michiels, J.F.; Wagner, K.D. Dynamic Spatiotemporal Expression Pattern of the Senescence-Associated Factor p16Ink4a in Development and Aging. Cells 2022, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.D.; Du, S.; Martin, L.; Leccia, N.; Michiels, J.F.; Wagner, N. Vascular PPARβ/δ Promotes Tumor Angiogenesis and Progression. Cells 2019, 8, 1623. [Google Scholar] [CrossRef] [PubMed]
- Schrage, A.; Loddenkemper, C.; Erben, U.; Lauer, U.; Hausdorf, G.; Jungblut, P.R.; Johnson, J.; Knolle, P.A.; Zeitz, M.; Hamann, A.; et al. Murine CD146 is widely expressed on endothelial cells and is recognized by the monoclonal antibody ME-9F1. Histochem. Cell Biol. 2008, 129, 441–451. [Google Scholar] [CrossRef]
- El Maï, M.; Wagner, K.D.; Michiels, J.F.; Ambrosetti, D.; Borderie, A.; Destree, S.; Renault, V.; Djerbi, N.; Giraud-Panis, M.J.; Gilson, E.; et al. The Telomeric Protein TRF2 Regulates Angiogenesis by Binding and Activating the PDGFRβ Promoter. Cell Rep. 2014, 9, 1047–1060. [Google Scholar] [CrossRef]
- Wagner, K.D.; Cherfils-Vicini, J.; Hosen, N.; Hohenstein, P.; Gilson, E.; Hastie, N.D.; Michiels, J.F.; Wagner, N. The Wilms’ tumour suppressor Wt1 is a major regulator of tumour angiogenesis and progression. Nat. Commun. 2014, 5, 5852. [Google Scholar] [CrossRef]
- Wagner, K.D.; Benchetrit, M.; Bianchini, L.; Michiels, J.F.; Wagner, N. Peroxisome proliferator-activated receptor β/δ (PPARβ/δ) is highly expressed in liposarcoma and promotes migration and proliferation. J. Pathol. 2011, 224, 575–588. [Google Scholar] [CrossRef]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Keber, R.; Motaln, H.; Wagner, K.D.; Debeljak, N.; Rassoulzadegan, M.; Ačimovič, J.; Rozman, D.; Horvat, S. Mouse knockout of the cholesterogenic cytochrome P450 lanosterol 14alpha-demethylase (Cyp51) resembles Antley-Bixler syndrome. J. Biol. Chem. 2011, 286, 29086–29097. [Google Scholar] [CrossRef]
- Chen, H.R.; Yeh, T.M. Assays for Measuring Endothelial Permeability byTranswells and Electrical Impedance Systems. Bio Protoc. 2017, 7, e2273. [Google Scholar] [CrossRef] [PubMed]
- Born, E.; Maisonnasse, P.; Fouillade, C.; Pascal, Q.; Marcos, E.; Londono-Vallejo, A.; Le Grand, R.; Adnot, S.; Lipskaia, L. SARS-COV-2 infection causes massive lung-cell senescence. Rev. Des Mal. Respir. 2022, 39, 121. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Miwa, S.; Tchkonia, T.; Tiniakos, D.; Wilson, C.L.; Lahat, A.; Day, C.P.; Burt, A.; Palmer, A.; Anstee, Q.M.; et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 2017, 8, 15691. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.M.; Balan, V.; Gleiberman, A.S.; Strom, E.; Krasnov, P.; Virtuoso, L.P.; Rydkina, E.; Vujcic, S.; Balan, K.; Gitlin, I.I.; et al. p16(Ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging 2017, 9, 1867–1884. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.M.; Balan, V.; Gleiberman, A.S.; Strom, E.; Krasnov, P.; Virtuoso, L.P.; Rydkina, E.; Vujcic, S.; Balan, K.; Gitlin, I.; et al. Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging 2016, 8, 1294–1315. [Google Scholar] [CrossRef]
- Sharpless, N.E.; Sherr, C.J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 2015, 15, 397–408. [Google Scholar] [CrossRef]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef]
- Collins, C.J.; Sedivy, J.M. Involvement of the INK4a/Arf gene locus in senescence. Aging Cell 2003, 2, 145–150. [Google Scholar] [CrossRef]
- Michaelis, U.R. Mechanisms of endothelial cell migration. Cell Mol. Life Sci. 2014, 71, 4131–4148. [Google Scholar] [CrossRef]
- Jonkman, J.E.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adh. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef]
- Arnaoutova, I.; Kleinman, H.K. In vitro angiogenesis: Endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 2010, 5, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Minami, R.; Muta, K.; Umemura, T.; Motomura, S.; Abe, Y.; Nishimura, J.; Nawata, H. p16(INK4a) induces differentiation and apoptosis in erythroid lineage cells. Exp. Hematol. 2003, 31, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohanna, M.A.; Manogaran, P.S.; Al-Mukhalafi, Z.; A Al-Hussein, K.; Aboussekhra, A. The tumor suppressor p16(INK4a) gene is a regulator of apoptosis induced by ultraviolet light and cisplatin. Oncogene 2004, 23, 201–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fukuoka, K.; Nishio, K.; Fukumoto, H.; Arioka, H.; Kurokawa, H.; Ishida, T.; Iwamoto, Y.; Tomonari, A.; Suzuki, T.; Usuda, J.; et al. Ectopic p16(ink4) expression enhances CPT-11-induced apoptosis through increased delay in S-phase progression in human non-small-cell-lung-cancer cells. Int. J. Cancer 2000, 86, 197–203. [Google Scholar] [CrossRef]
- Ito, T.K.; Ishii, G.; Saito, S.; Yano, K.; Hoshino, A.; Suzuki, T.; Ochiai, A. Degradation of soluble VEGF receptor-1 by MMP-7 allows VEGF access to endothelial cells. Blood 2009, 113, 2363–2369. [Google Scholar] [CrossRef]
- Stetler-Stevenson, W.G.; Seo, D.W. TIMP-2: An endogenous inhibitor of angiogenesis. Trends Mol. Med. 2005, 11, 97–103. [Google Scholar] [CrossRef]
- Khan, S.Y.; Awad, E.M.; Oszwald, A.; Mayr, M.; Yin, X.; Waltenberger, B.; Stuppner, H.; Lipovac, M.; Uhrin, P.; Breuss, J.M. Premature senescence of endothelial cells upon chronic exposure to TNFα can be prevented by N-acetyl cysteine and plumericin. Sci. Rep. 2017, 7, 39501. [Google Scholar] [CrossRef]
- Voets, E.; Wolthuis, R.M. MASTL is the human orthologue of Greatwall kinase that facilitates mitotic entry, anaphase and cytokinesis. Cell Cycle 2010, 9, 3591–3601. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, H.; Beach, D. Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes. Dev. 1993, 7, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chu, E.S.; Zhang, J.; Li, X.; Liang, Q.; Chen, J.; Chen, M.; Teoh, N.; Farrell, G.; Sung, J.J.; et al. Peroxisome proliferator activated receptor alpha inhibits hepatocarcinogenesis through mediating NF-κB signaling pathway. Oncotarget 2014, 5, 8330–8340. [Google Scholar] [CrossRef] [PubMed]
- Trochon, V.; Mabilat, C.; Bertrand, P.; Legrand, Y.; Smadja-Joffe, F.; Soria, C.; Delpech, B.; Lu, H. Evidence of involvement of CD44 in endothelial cell proliferation, migration and angiogenesis in vitro. Int. J. Cancer 1996, 66, 664–668. [Google Scholar] [CrossRef]
- Reed, M.J.; Edelberg, J.M. Impaired angiogenesis in the aged. Sci. Aging Knowledge Environ. 2004, 2004, pe7. [Google Scholar] [CrossRef]
- Lähteenvuo, J.; Rosenzweig, A. Effects of aging on angiogenesis. Circ. Res. 2012, 110, 1252–1264. [Google Scholar] [CrossRef]
- Suda, M.; Katsuumi, G.; Tchkonia, T.; Kirkland, J.L.; Minamino, T. Potential Clinical Implications of Senotherapies for Cardiovascular Disease. Circ. J. 2024, 88, 277–284. [Google Scholar] [CrossRef]
- Matikainen, S.; Nyman, T.A.; Cypryk, W. Function and Regulation of Noncanonical Caspase-4/5/11 Inflammasome. J. Immunol. 2020, 204, 3063–3069. [Google Scholar] [CrossRef]
- Platnich, J.M.; Chung, H.; Lau, A.; Sandall, C.F.; Bondzi-Simpson, A.; Chen, H.M.; Komada, T.; Trotman-Grant, A.C.; Brandelli, J.R.; Chun, J.; et al. Shiga Toxin/Lipopolysaccharide Activates Caspase-4 and Gasdermin D to Trigger Mitochondrial Reactive Oxygen Species Upstream of the NLRP3 Inflammasome. Cell Rep. 2018, 25, 1525–1536.e1527. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Lum, H.; Roebuck, K.A. Oxidant stress and endothelial cell dysfunction. Am. J. Physiol. Cell Physiol. 2001, 280, C719–C741. [Google Scholar] [CrossRef]
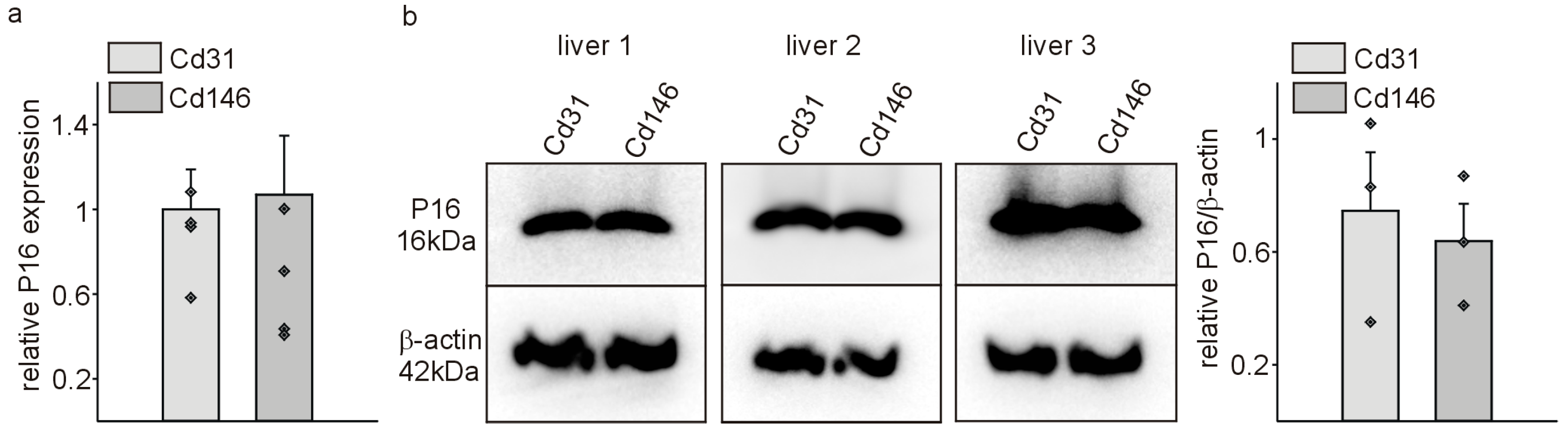


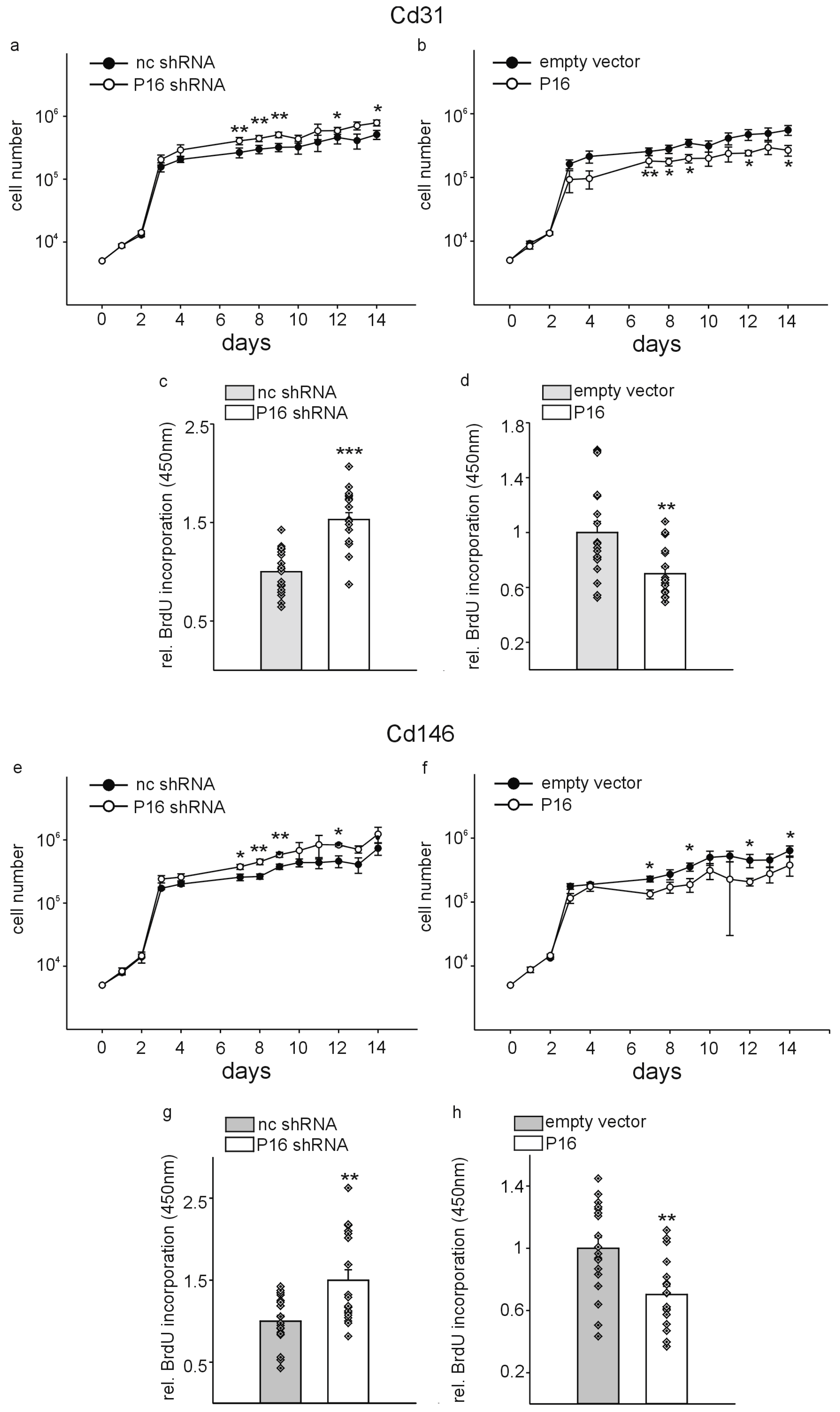
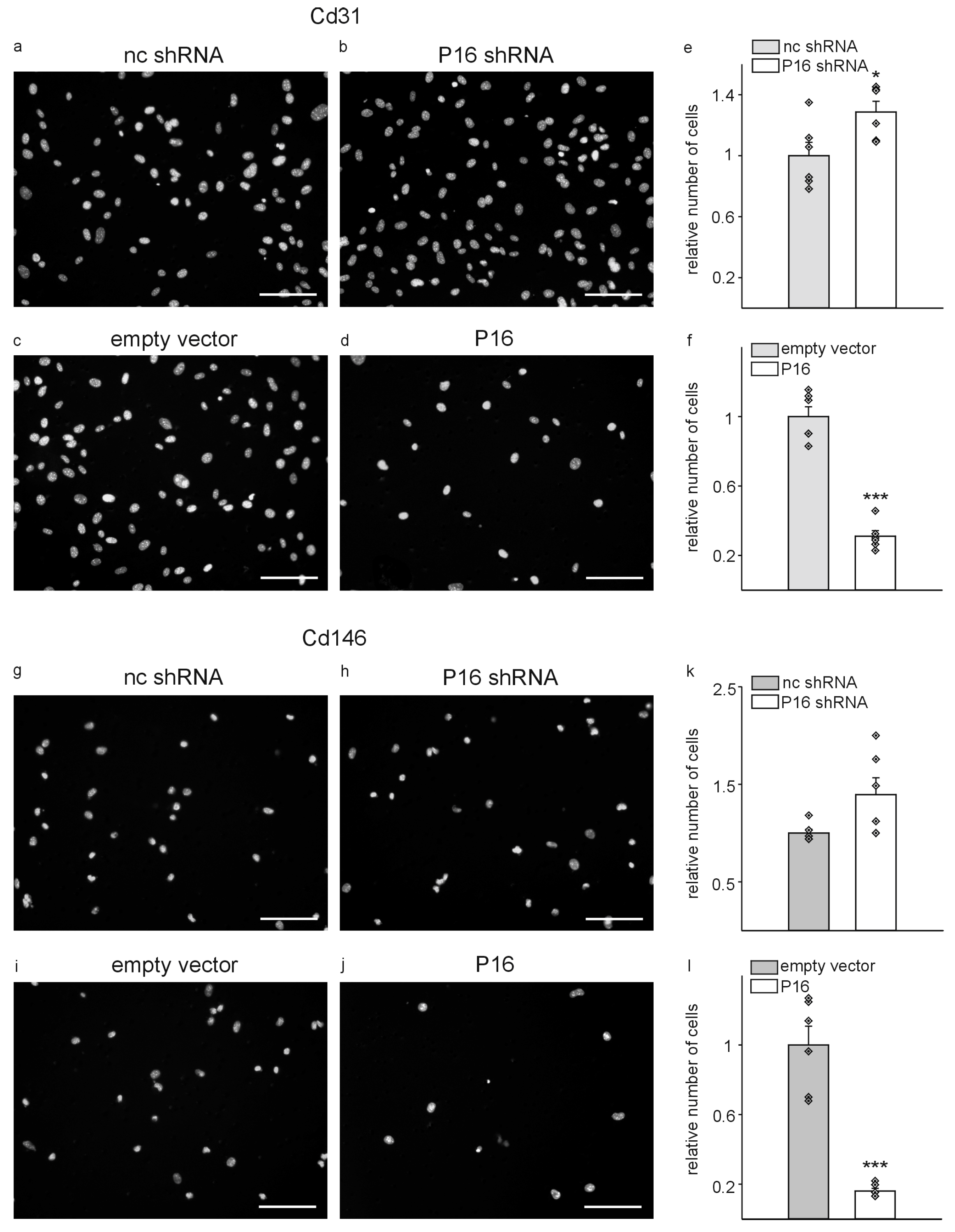

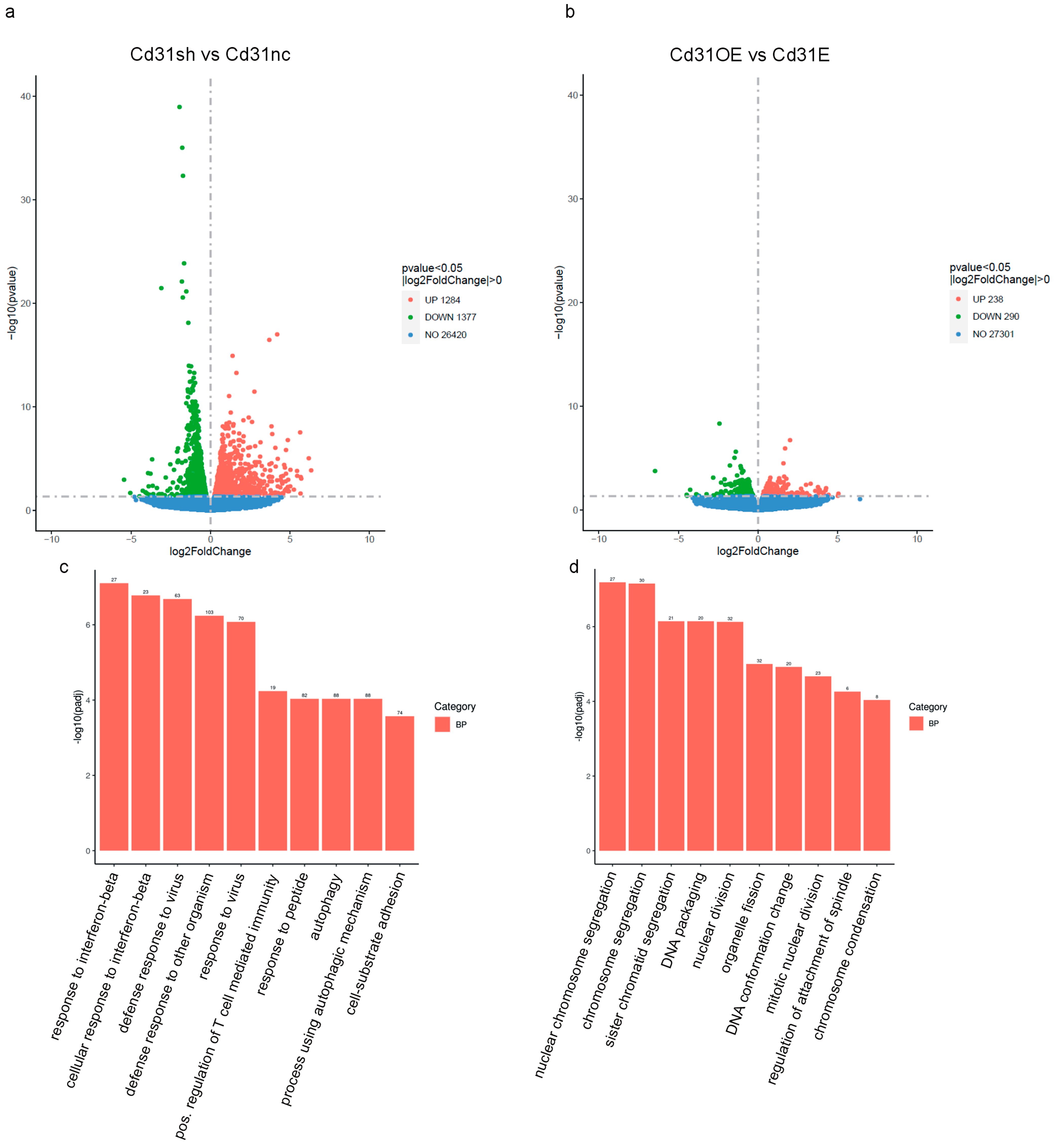
| Gene of Interest | Oligonucleotide Sequences |
|---|---|
| p16ink4 | F: AGGGCCGTGTGCATGACGTG B: GCACCGGGCGGGAGAAGGTA |
| Igf1R | F: GTGGGGGCTCGTGTTTCT B: GATCTCCGTGCAGTTTTCCA |
| Mmp2 | F: CAAGTTCCCCGGCGATGTC B: TTCTGGTCAAGGTCACCTGTC |
| Mmp7 | F: CTGCCACTGTCCCAGGAAG B: GGGAGAGTTTTCCAGTCATGG |
| Mmp9 | F: CCATGCACTGGGCTTAGATCA B: GGCCTTGGGTCAGGCTTAGA |
| Mmp12 | F: GAGTCCAGCCACCAACATTAC B: GCGAAGTGGGTCAAAGACAG |
| Timp1 | F: GCAACTCGGACCTGGTCATAA B: CGGCCCGTGATGAGAAACT |
| Timp2 | F: TCAGAGCCAAAGCAGTGAGC B: GCCGTGTAGATAAACTCGATGTC |
| Timp3 | F: CTTCTGCAACTCCGACATCGT B: GGGGCATCTTACTGAAGCCTC |
| Timp4 | F: TGTGGCTGCCAAATCACCA B: TCATGCAGACATAGTGCTGGG |
| E-selectin | F: ATGCCTCGCGCTTTCTCTC B: GTAGTCCCGCTGACAGTATGC |
| P-selectin | F: CATCTGGTTCAGTGCTTTGATCT B: ACCCGTGAGTTATTCCATGAGT |
| Anapc2 | F: TCCGATGACTGCGACTCTAGG B: CACTTCCACGAACCACTCCT |
| Fos | F: CGGGTTTCAACGCCGACTA B: TTGGCACTAGAGACGGACAGA |
| Tnfα | F: GTAGCCCACGTCGTAGCAAA B: ACAAGGTACAACCCATCGGC |
| Mafk | F: ATGACGACTAATCCCAAGCCC B: CGTAGCCTCTGTTCTTGAGTGT |
| Mastl | F: TCGGCAAGTGAGGAGAATGAA B: CACCACGGCTAATGGGCTT |
| Terc | F: GTGGGTTCTGGTCTTTTGTTCT B: CTGCAGGTCTGGACTTTCCT |
| Tert | F: TCAAGAGCAGTAGTCGCCAG B: TCTCGGGACAGGATAGCATCT |
| Vegfa | F: CTCACCAAAGCCAGCACATA B: AATGCTTTCTCCGCTCTGAA |
| Casp3 | F: ATGGAGAACAACAAAACCTCAGT B: TTGCTCCCATGTATGGTCTTTAC |
| Casp4 | F: ACAAACACCCTGACAAACCAC B: CACTGCGTTCAGCATTGTTAAA |
| Ccl2 | F: AGCTGTAGTTTTTGTCACCAAGC B: GTGCTGAAGACCTTAGGGCA |
| Ccl3 | F: TTCTCTGTACCATGACACTCTGC B: CGTGGAATCTTCCGGCTGTAG |
| Ccl5 | F: GCTGCTTTGCCTACCTCTCC B: TCGAGTGACAAACACGACTGC |
| Ccl9 | F: CCCTCTCCTTCCTCATTCTTACA B: AGTCTTGAAAGCCCATGTGAAA |
| Ccl12 | F: ATTTCCACACTTCTATGCCTCCT B: ATCCAGTATGGTCCTGAAGATCA |
| Cxcl10 | F: CCAAGTGCTGCCGTCATTTTC B: GGCTCGCAGGGATGATTTCAA |
| Il-6 | F: CACTTCACAAGTCGGAGGCT R: TGCCATTGCACAACTCTTTTCT |
| Il-8 | F: CAAGGCTGGTCCATGCTCC B: TGCTATCACTTCCTTTCTGTTGC |
| Il-10 | F: GCTCTTACTGACTGGCATGAG B: CGCAGCTCTAGGAGCATGTG |
| Il-18 | F: CAAAGTGCCAGTGAACCCCA B: TTCACAGAGAGGGTCACAGC |
| NF-κB | F: ATGGCAGACGATGATCCCTAC B: TGTTGACAGTGGTATTTCTGGTG |
| Stat1 | F: TCACAGTGGTTCGAGCTTCAG B: GCAAACGAGACATCATAGGCA |
| Stat2 | F: TCCTGCCAATGGACGTTCG B: GTCCCACTGGTTCAGTTGGT |
| Tgfb1 | F: AGCTGGTGAAACGGAAGC G B: GCGAGCCTTAGTTTGGACAGG |
| Tgfb2 | F: CCATCCCGCCCACTTTCTAC B: CATCAAAGCGGACGATTCTGA |
| Tgfb3 | F: GGACTTCGGCCACATCAAGAA B: TAGGGGACGTGGGTCATCAC |
| Ifna | F: GACCTGCAAGGCTGTCTGAT B: AGACAGGGCTCTCCAGACTT |
| Ifnar1 | F: AGCCACGGAGAGTCAATGG B: GCTCTGACACGAAACTGTGTTTT |
| Ifnb | F: CAGCTCCAAGAAAGGACGAAC B: GGCAGTGTAACTCTTCTGCAT |
| Ifngr1 | F: CTGGCAGGATGATTCTGCTGG B: GCATACGACAGGGTTCAAGTTAT |
| Gapdh | F: AGGTCGGTGTGAACGGATTTG B: TGTAGACCATGTAGTTGAGGTCA |
| β-actin | F: CTTCCTCCCTGGAGAAGAGC B: ATGCCACAGGATTCCATACC |
| Rplp0 | F: CACTGGTCTAGGACCCGAGAAG B: GGTGCCTCTGGAGATTTTCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, K.-D.; Safwan-Zaiter, H.; Wagner, N. A Dual Role of the Senescence Marker P16Ink4a in Liver Endothelial Cell Function. Cells 2024, 13, 1929. https://doi.org/10.3390/cells13231929
Wagner K-D, Safwan-Zaiter H, Wagner N. A Dual Role of the Senescence Marker P16Ink4a in Liver Endothelial Cell Function. Cells. 2024; 13(23):1929. https://doi.org/10.3390/cells13231929
Chicago/Turabian StyleWagner, Kay-Dietrich, Hasan Safwan-Zaiter, and Nicole Wagner. 2024. "A Dual Role of the Senescence Marker P16Ink4a in Liver Endothelial Cell Function" Cells 13, no. 23: 1929. https://doi.org/10.3390/cells13231929
APA StyleWagner, K.-D., Safwan-Zaiter, H., & Wagner, N. (2024). A Dual Role of the Senescence Marker P16Ink4a in Liver Endothelial Cell Function. Cells, 13(23), 1929. https://doi.org/10.3390/cells13231929








