Bromodomain-Containing 4 Is a Positive Regulator of Interleukin-34 Production in the Gut
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Lamina Propria Mononuclear Cell Isolation and Culture
2.3. In Vivo Mouse Studies
2.4. Real-Time PCR
2.5. Total Protein Extraction and Western Blotting
2.6. Immunofluorescence
2.7. Flow Cytometry Analysis
2.8. Statistical Analysis
3. Results
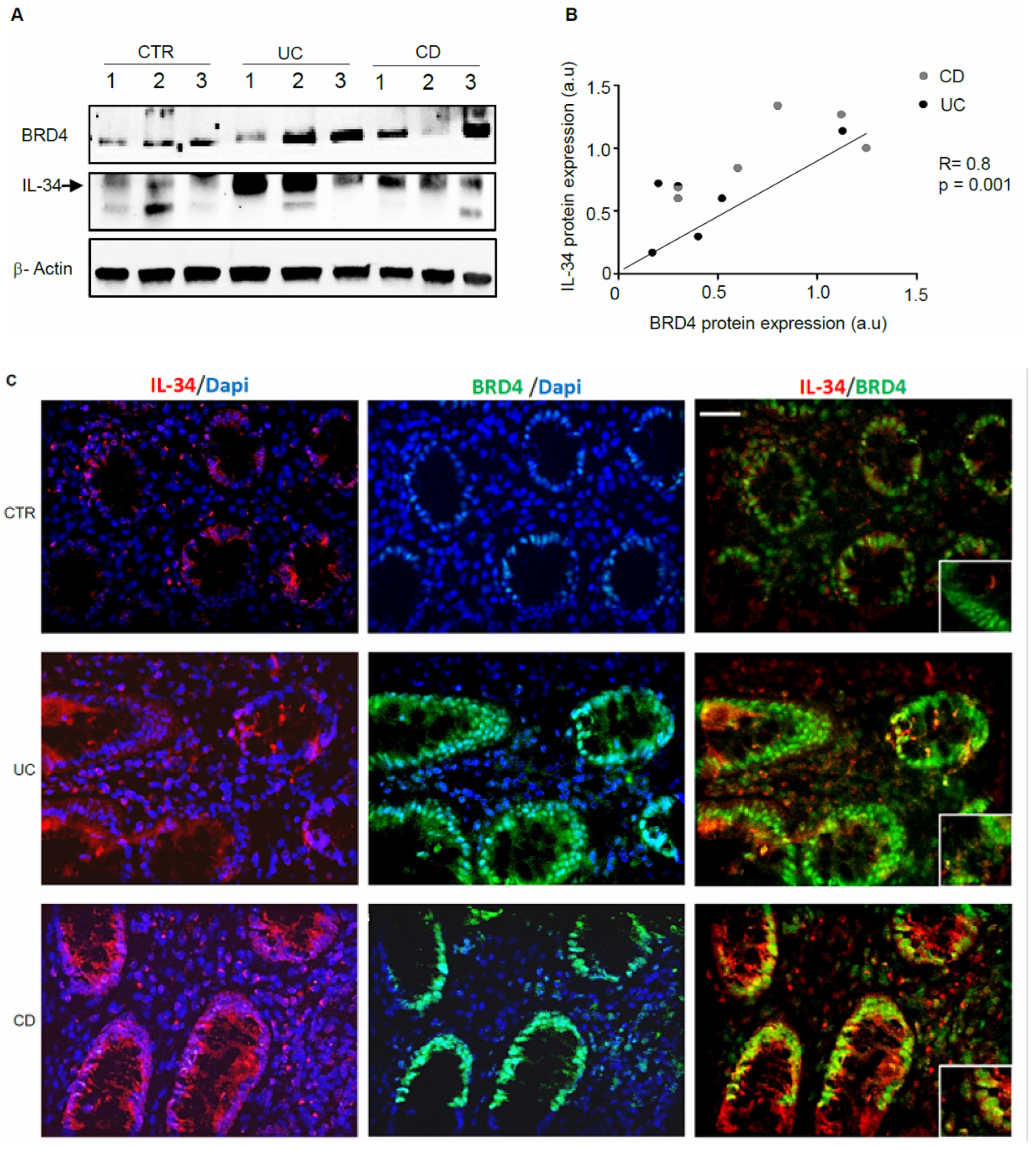
3.1. IL-34 and BRD4 Are Over-Expressed in IBD
3.2. The Fractions of IL-34 and/or BRD4-Expressing LPMCs Are Increased in IBD
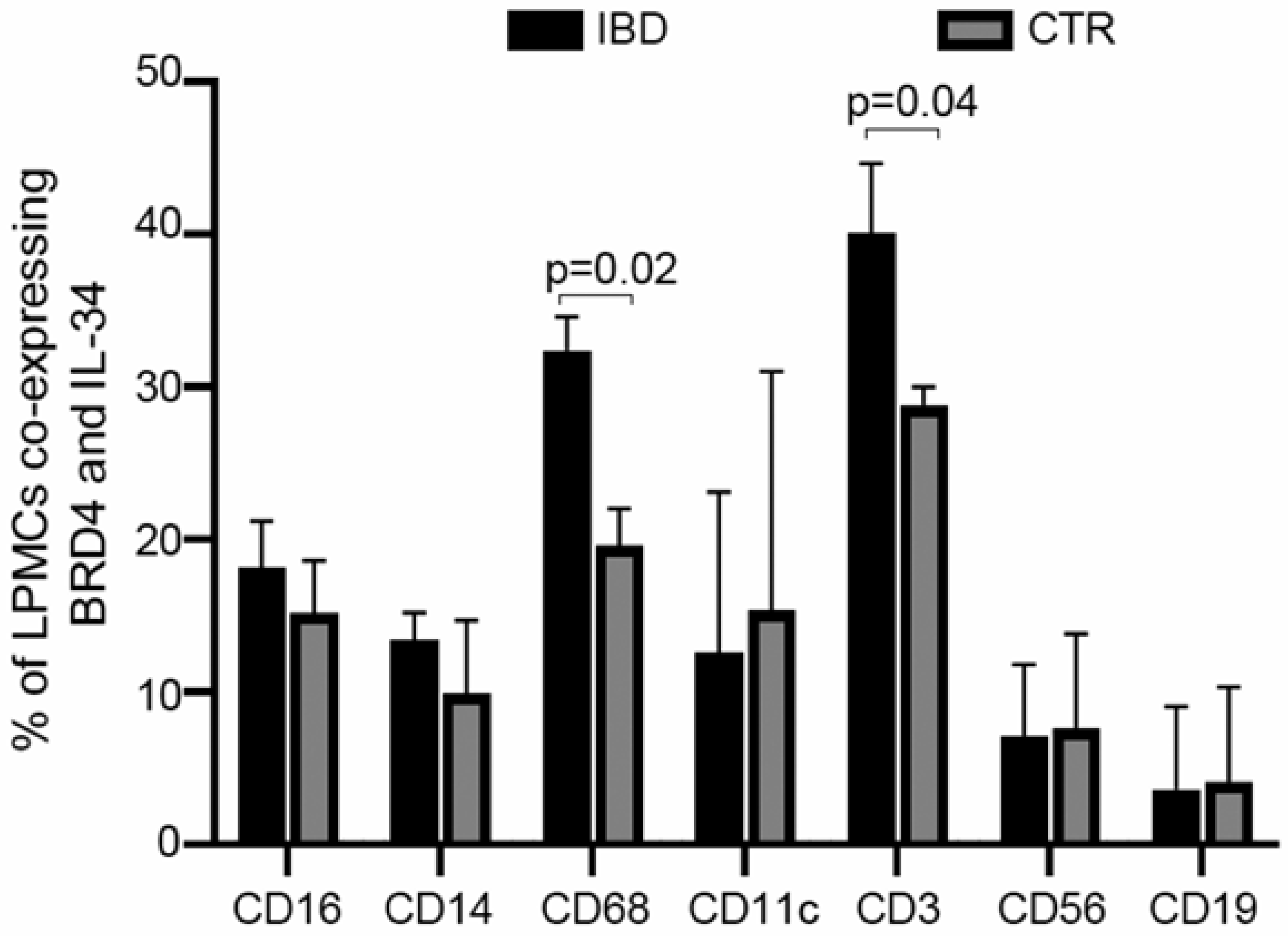
3.3. IL-34 and BRD4 Are Co-Expressed by Multiple Immune Cells in IBD
3.4. BRD4 Inhibition Reduces the Intestinal Expression of IL-34
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Jans, D.; Cleynen, I. The genetics of non-monogenic IBD. Hum. Genet. 2023, 142, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Mack, D.R.; Guttman, A.; Nguyen, G.C.; To, T.; Mojaverian, N.; Quach, P.; Manuel, D.G. Inflammatory bowel disease in immigrants to Canada and their children: A population-based cohort. Am. J. Gastro. 2015, 110, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Suo, Z.; Lin, J.; Cong, Y.; Liu, Z. Monocyte-macrophages modulate intestinal homeostasis in inflammatory bowel disease. Biomark. Res. 2024, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yu, L.; Fang, L.; Yang, W.; Yu, T.; Miao, Y.; Chen, M.; Wu, K.; Chen, F.; Cong, Y.; et al. CD177+ neutrophils as functionally activated neutrophils negatively regulate IBD. Gut 2018, 67, 1052–1063. [Google Scholar] [CrossRef]
- Kosinsky, R.L.; Gonzalez, M.M.; Saul, D.; Barros, L.L.; Sagstetter, M.R.; Fedyshyn, Y.; Nair, A.; Sun, Z.; Hamdan, F.H.; Gibbons, H.R.; et al. The FOXP3+ Pro-Inflammatory T Cell: A Potential Therapeutic Target in Crohn’s Disease. Gastroenterology 2024, 166, 631–644. [Google Scholar] [CrossRef]
- Vebr, M.; Pomahačová, R.; Sýkora, J.; Schwarz, J. A Narrative Review of Cytokine Networks: Pathophysiological and Therapeutic Implications for Inflammatory Bowel Disease Pathogenesis. Biomedicines 2023, 11, 3229. [Google Scholar] [CrossRef]
- Neurath, M.F. Strategies for targeting cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2024, 24, 559–576. [Google Scholar] [CrossRef]
- Franze, E.; Monteleone, I.; Cupi, M.L.; Mancia, P.; Caprioli, F.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Laudisi, F.; Sica, G.; et al. Interleukin-34 sustains inflammatory pathways in the gut. Clin. Sci. 2015, 129, 271–280. [Google Scholar] [CrossRef]
- Massimino, L.; Lamparelli, L.A.; Houshyar, Y.; D’Alessio, S.; Peyrin-Biroulet, L.; Vetrano, S.; Danese, S.; Ungaro, F. The Inflammatory Bowel Disease Transcriptome and Metatranscriptome Meta-Analysis (IBD TaMMA) framework. Nat. Comput. Sci. 2021, 1, 511–515. [Google Scholar] [CrossRef]
- Lin, H.; Lee, E.; Hestir, K.; Leo, C.; Huang, M.; Bosch, E.; Halenbeck, R.; Wu, G.; Zhou, A.; Behrens, D.; et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 2008, 320, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Xu, D.; Austin, C.D.; Caplazi, P.; Senger, K.; Sun, Y.; Jeet, S.; Young, J.; Delarosa, D.; Suto, E.; et al. Function of Csf1 and Il34 in Macrophage Homeostasis, Inflammation, and Cancer. Front. Immunol. 2019, 10, 2019. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Franze, E.; Troncone, E.; Maresca, C.; Marafini, I. Interleukin-34 Mediates Cross-Talk Between Stromal Cells and Immune Cells in the Gut. Front. Immunol. 2022, 13, 873332. [Google Scholar] [CrossRef] [PubMed]
- Altendorfer, E.; Mochalova, Y.; Mayer, A. BRD4: A general regulator of transcription elongation. Transcription 2022, 13, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y. The Bromodomain and Extra-Terminal Domain (BET) Family: Functional Anatomy of BET Paralogous Proteins. Int. J. Mol. Sci. 2016, 17, 1849. [Google Scholar] [CrossRef]
- Franze, E.; Laudisi, F.; Maresca, C.; Di Grazia, A.; Iannucci, A.; Pacifico, T.; Ortenzi, A.; Sica, G.; Lolli, E.; Stolfi, C.; et al. Bromodomain-containing 4 is a positive regulator of the inflammatory cytokine response in the gut. J. Crohns Colitis 2024. [Google Scholar] [CrossRef]
- Han, N.; Anwar, D.; Hama, N.; Kobayashi, T.; Suzuki, H.; Takahashi, H.; Wada, H.; Otsuka, R.; Baghdadi, M.; Seino, K.I. Bromodomain-containing protein 4 regulates interleukin-34 expression in mouse ovarian cancer cells. Inflamm. Regen. 2020, 40, 25. [Google Scholar] [CrossRef]
- Irie, T.; Yoshii, D.; Komohara, Y.; Fujiwara, Y.; Kadohisa, M.; Honda, M.; Suzu, S.; Matsuura, T.; Kohashi, K.; Oda, Y.; et al. IL-34 in hepatoblastoma cells potentially promote tumor progression via autocrine and paracrine mechanisms. Cancer Med. 2022, 11, 1441–1453. [Google Scholar] [CrossRef]
- Endo, H.; Hama, N.; Baghdadi, M.; Ishikawa, K.; Otsuka, R.; Wada, H.; Asano, H.; Endo, D.; Konno, Y.; Kato, T.; et al. Interleukin-34 expression in ovarian cancer: A possible correlation with disease progression. Int. Immunol. 2020, 32, 175–186. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Chen, T.; Cui, J.; Li, X.; Liu, A.; Liu, R.; Fang, L.; Jiang, J.; Yang, L.; et al. SAMHD1 dysfunction induces IL-34 expression via NF-kappaB p65 in neuronal SH-SY5Y cells. Mol. Immunol. 2024, 168, 1–9. [Google Scholar] [CrossRef]
- Lin, M.; Liu, X.; Zhang, X.; Wang, H.; Fang, Y.; Wu, X.; Yin, A.; Yang, W.; Zhang, D.; Li, M.; et al. Sp1 Controls the Basal Level of Interleukin-34 Transcription. Immunol. Investig. 2023, 52, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Franze, E.; Dinallo, V.; Laudisi, F.; Di Grazia, A.; Di Fusco, D.; Colantoni, A.; Ortenzi, A.; Giuffrida, P.; Di Carlo, S.; Sica, G.S.; et al. Interleukin-34 Stimulates Gut Fibroblasts to Produce Collagen Synthesis. J. Crohns Colitis 2020, 14, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Lu, G.; Sharma, R.; Vincek, A.; Zhang, R.; Plotnikov, A.N.; Zhang, F.; Zhang, Q.; Ju, Y.; Hu, Y.; et al. BET N-terminal bromodomain inhibition selectively blocks Th17 cell differentiation and ameliorates colitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 2952–2957. [Google Scholar] [CrossRef]
- Freuchet, A.; Salama, A.; Bezie, S.; Tesson, L.; Remy, S.; Humeau, R.; Regue, H.; Serazin, C.; Flippe, L.; Peterson, P.; et al. IL-34 deficiency impairs FOXP3(+) Treg function in a model of autoimmune colitis and decreases immune tolerance homeostasis. Clin. Transl. Med. 2022, 12, e988. [Google Scholar] [CrossRef]
- Park, H.E.; Oh, H.; Baek, J.H. Interleukin-34-regulated T-cell responses in rheumatoid arthritis. Front. Med. 2022, 9, 1078350. [Google Scholar] [CrossRef]
- Van Raemdonck, K.; Umar, S.; Palasiewicz, K.; Volin, M.V.; Elshabrawy, H.A.; Romay, B.; Tetali, C.; Ahmed, A.; Amin, M.A.; Zomorrodi, R.K.; et al. Interleukin-34 Reprograms Glycolytic and Osteoclastic Rheumatoid Arthritis Macrophages via Syndecan 1 and Macrophage Colony-Stimulating Factor Receptor. Arthritis. Rheumatol. 2021, 73, 2003–2014. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, H.M.; Baek, J.H.; Weinmann-Menke, J.; Ajay, A.K.; Charles, J.F.; Noda, M.; Franklin, R.A.; Rodriguez-Morales, P.; Kelley, V.R. IL-34 and protein-tyrosine phosphatase receptor type-zeta-dependent mechanisms limit arthritis in mice. Lab. Investig. 2022, 102, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Sienes, R.; Zanotti, B.; van Raemdonck, K.; Palasiewicz, K.; Mass, D.P.; Volin, M.V.; Shahrara, S. Dysregulation of IL-34 ligation to SDC-1 mitigates collagen-induced arthritis. Cell Mol. Immunol. 2022, 19, 1070–1072. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzè, E.; Laudisi, F.; Frascatani, R.; Tomassini, L.; De Cristofaro, E.; Stolfi, C.; Monteleone, G. Bromodomain-Containing 4 Is a Positive Regulator of Interleukin-34 Production in the Gut. Cells 2024, 13, 1698. https://doi.org/10.3390/cells13201698
Franzè E, Laudisi F, Frascatani R, Tomassini L, De Cristofaro E, Stolfi C, Monteleone G. Bromodomain-Containing 4 Is a Positive Regulator of Interleukin-34 Production in the Gut. Cells. 2024; 13(20):1698. https://doi.org/10.3390/cells13201698
Chicago/Turabian StyleFranzè, Eleonora, Federica Laudisi, Rachele Frascatani, Lorenzo Tomassini, Elena De Cristofaro, Carmine Stolfi, and Giovanni Monteleone. 2024. "Bromodomain-Containing 4 Is a Positive Regulator of Interleukin-34 Production in the Gut" Cells 13, no. 20: 1698. https://doi.org/10.3390/cells13201698
APA StyleFranzè, E., Laudisi, F., Frascatani, R., Tomassini, L., De Cristofaro, E., Stolfi, C., & Monteleone, G. (2024). Bromodomain-Containing 4 Is a Positive Regulator of Interleukin-34 Production in the Gut. Cells, 13(20), 1698. https://doi.org/10.3390/cells13201698







