Adenosine Metabolism Pathway Alterations in Frontal Cortical Neurons in Schizophrenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Laser Microdissection (LMD)
2.3. RNA Isolation, Reverse Transcription, and Complementary DNA (cDNA) Pre-Amplification
2.4. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.5. Data Analysis
3. Results
3.1. mRNA Expression of Adenosine Pathway Components
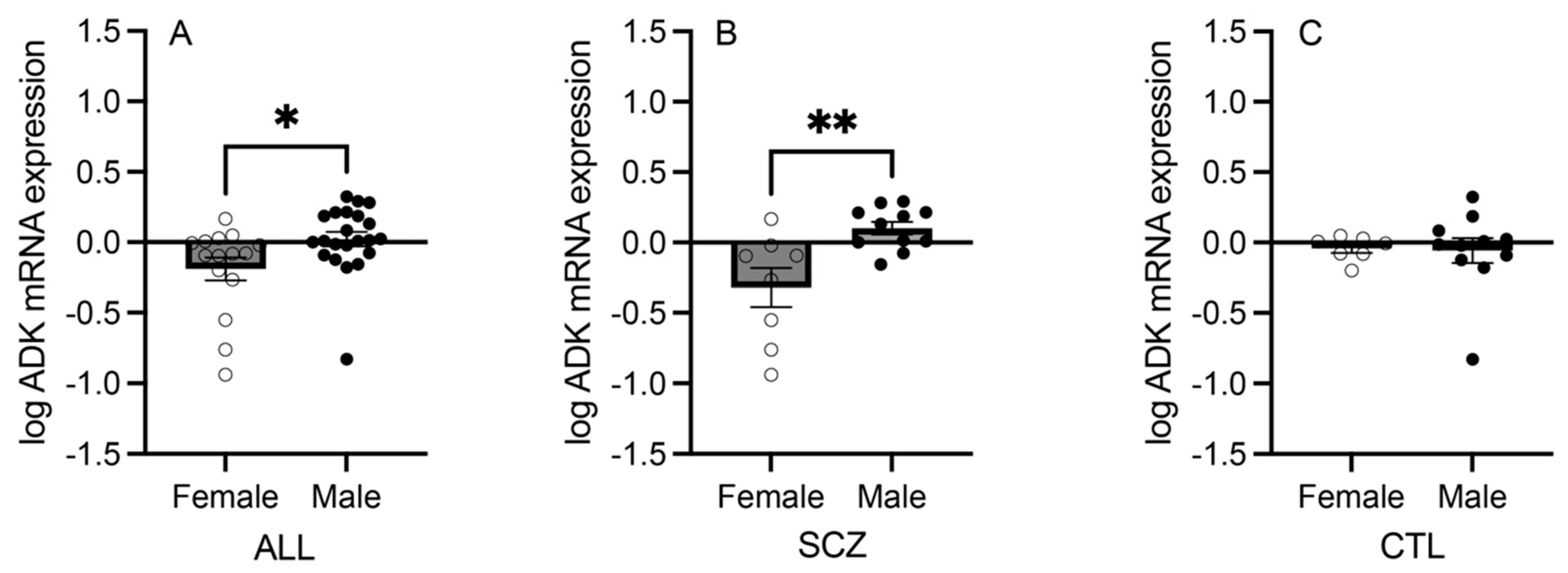
3.2. mRNA Expression of Adenosine Pathway Components in Females versus Males
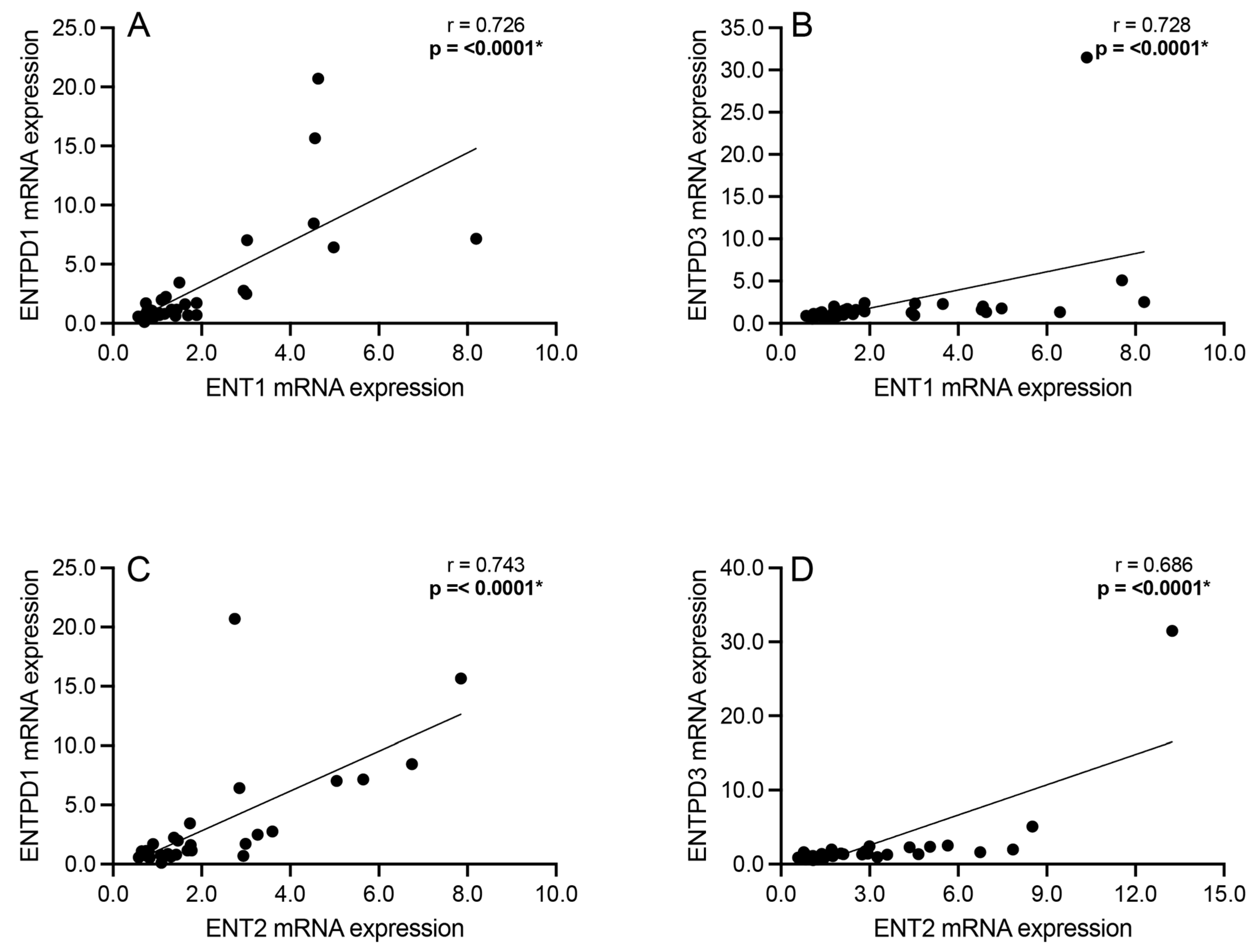
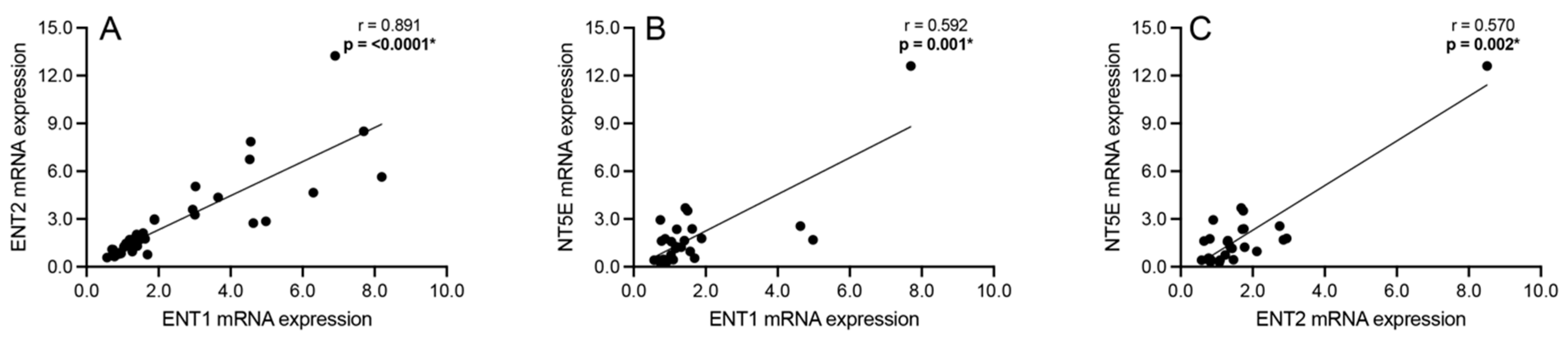
3.3. Correlation Analyses: Intracellular Adenosine Metabolism and Adenosine Transport
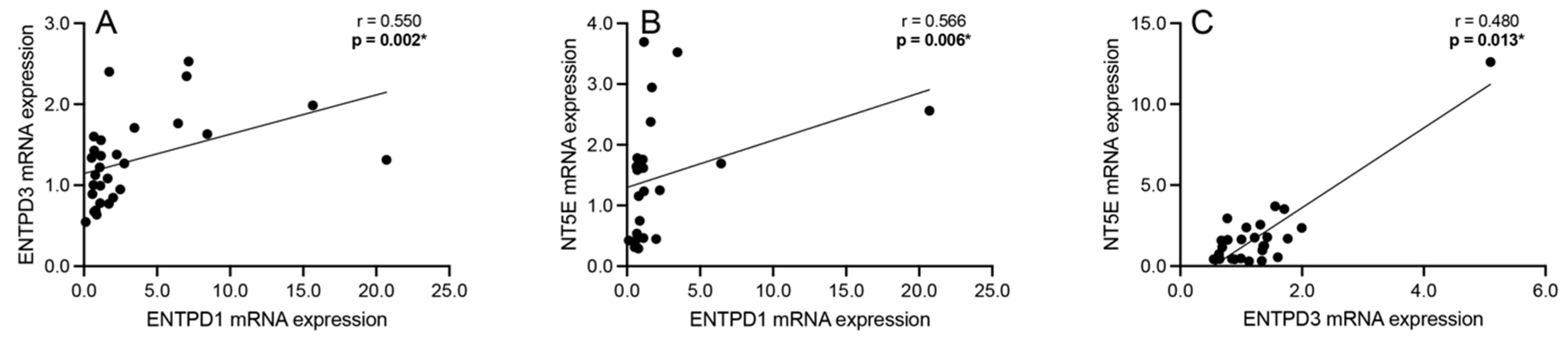
3.4. Correlation Analyses: Extracellular Adenosine Catabolism Enzymes
3.5. Correlation Analyses: Adenosine Transport and Primary Extracellular Catabolism Enzyme
3.6. Correlation Analyses: Adenosine Transport and Rate-Limiting Extracellular Catabolism Enzyme
3.7. mRNA Expression of Adenosine Pathway Components across CDR Bins
3.8. Antipsychotic Medication’s Effect on Adenosine Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sullivan, C.R.; O’Donovan, S.M.; McCullumsmith, R.E.; Ramsey, A. Defects in Bioenergetic Coupling in Schizophrenia. Biol. Psychiatry 2018, 83, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R. Schizophrenia. N. Engl. J. Med. 2003, 349, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- O’donovan, S.M.; Sullivan, C.; Koene, R.; Devine, E.; Hasselfeld, K.; Moody, C.L.; McCullumsmith, R.E. Cell-subtype-specific changes in adenosine pathways in schizophrenia. Neuropsychopharmacology 2018, 43, 1667–1674. [Google Scholar] [CrossRef]
- Sahay, S.; Devine, E.A.; McCullumsmith, R.E.; O’Donovan, S.M. Adenosine Receptor mRNA Expression in Frontal Cortical Neurons in Schizophrenia. Cells 2023, 13, 32. [Google Scholar] [CrossRef]
- Mackay, A.V.P.; Iversen, L.L.; Rossor, M.; Spokes, E.; Bird, E.; Arregui, A.; Creese, I.; Snyder, S.H. Increased brain dopamine and dopamine receptors in schizophrenia. Arch. Gen. Psychiatry 1982, 39, 991–997. [Google Scholar] [CrossRef]
- Uno, Y.; Coyle, J.T. Glutamate hypothesis in schizophrenia. Psychiatry Clin. Neurosci. 2019, 73, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Lara, D.R.; Dall’Igna, O.P.; Ghisolfi, E.S.; Brunstein, M.G. Involvement of adenosine in the neurobiology of schizophrenia and its therapeutic implications. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 617–629. [Google Scholar] [CrossRef]
- Singer, P.; Yee, B.K. The adenosine hypothesis of schizophrenia into its third decade: From neurochemical imbalance to early life etiological risks. Front. Cell Neurosci. 2023, 17, 1120532. [Google Scholar] [CrossRef]
- Lara, D.R.; Souza, D.O. Schizophrenia: A purinergic hypothesis. Med. Hypotheses 2000, 54, 157–166. [Google Scholar] [CrossRef]
- Malewska-Kasprzak, M.K.; Permoda-Osip, A.; Rybakowski, J. Disturbances of purinergic system in affective disorders and schizophrenia. Psychiatr. Pol. 2019, 53, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Boison, D.; Singer, P.; Shen, H.Y.; Feldon, J.; Yee, B.K. Adenosine hypothesis of schizophrenia--opportunities for pharmacotherapy. Neuropharmacology 2012, 62, 1527–1543. [Google Scholar] [CrossRef] [PubMed]
- Yee, B.K.; Singer, P.; Chen, J.F.; Feldon, J.; Boison, D. Transgenic overexpression of adenosine kinase in brain leads to multiple learning impairments and altered sensitivity to psychomimetic drugs. Eur. J. Neurosci. 2007, 26, 3237–3252. [Google Scholar] [CrossRef] [PubMed]
- Boison, D.; Chen, J.F.; Fredholm, B.B. Adenosine signaling and function in glial cells. Cell Death Differ. 2010, 17, 1071–1082. [Google Scholar] [CrossRef]
- Boison, D. Adenosine kinase: Exploitation for therapeutic gain. Pharmacol. Rev. 2013, 65, 906–943. [Google Scholar] [CrossRef]
- Moody, C.L.; Funk, A.J.; Devine, E.; Homan, R.C.D.; Boison, D.; E McCullumsmith, R.; O’donovan, S.M. Adenosine Kinase Expression in the Frontal Cortex in Schizophrenia. Schizophr. Bull. 2020, 46, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Pascual, O.; Casper, K.B.; Kubera, C.; Zhang, J.; Revilla-Sanchez, R.; Sul, J.-Y.; Takano, H.; Moss, S.J.; McCarthy, K.; Haydon, P.G. Astrocytic purinergic signaling coordinates synaptic networks. Science 2005, 310, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Aliagas, E.; Villar-Menéndez, I.; Sévigny, J.; Roca, M.; Romeu, M.; Ferrer, I.; Martín-Satué, M.; Barrachina, M. Reduced striatal ecto-nucleotidase activity in schizophrenia patients supports the “adenosine hypothesis”. Purinergic Signal. 2013, 9, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Borycz, J.; Pereira, M.F.; Melani, A.; Rodrigues, R.J.; Köfalvi, A.; Panlilio, L.; Pedata, F.; Goldberg, S.R.; Cunha, R.A.; Ferré, S. Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J. Neurochem. 2007, 101, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.; Lacey, M.G. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J. Neurosci. 1997, 17, 5271–5280. [Google Scholar] [CrossRef] [PubMed]
- Augusto, E.; Matos, M.; Sévigny, J.; El-Tayeb, A.; Bynoe, M.S.; Müller, C.E.; Cunha, R.A.; Chen, J.-F. Ecto-5’-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J. Neurosci. 2013, 33, 11390–11399. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Ferré, S.; Canals, M.; Torvinen, M.; Terasmaa, A.; Marcellino, D.; Goldberg, S.R.; Staines, W.; Jacobsen, K.X.; Lluis, C.; et al. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J. Mol. Neurosci. 2005, 26, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Brundege, J.M.; Dunwiddie, T.V. Metabolic regulation of endogenous adenosine release from single neurons. Neuroreport 1998, 9, 3007–3011. [Google Scholar] [CrossRef] [PubMed]
- Latini, S.; Pedata, F. Adenosine in the central nervous system: Release mechanisms and extracellular concentrations. J. Neurochem. 2001, 79, 463–484. [Google Scholar] [CrossRef]
- Cunha, R.A. Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem. Int. 2008, 52, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Dunwiddie, T.V.; Masino, S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Haroutunian, V.; Meador-Woodruff, J.H.; McCullumsmith, R.E. Expression of equilibrative nucleoside transporter type 1 protein in elderly patients with schizophrenia. Neuroreport 2012, 23, 224–227. [Google Scholar] [CrossRef]
- Choi, D.-S.; Cascini, M.-G.; Mailliard, W.; Young, H.; Paredes, P.; McMahon, T.; Diamond, I.; Bonci, A.; O Messing, R. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat. Neurosci. 2004, 7, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.L.; Sherali, A.; Mo, Z.P.; Tse, C.M. Kinetic and pharmacological properties of cloned human equilibrative nucleoside transporters, ENT1 and ENT2, stably expressed in nucleoside transporter-deficient PK15 cells. Ent2 exhibits a low affinity for guanosine and cytidine but a high affinity for inosine. J. Biol. Chem. 2000, 275, 8375–8381. [Google Scholar] [CrossRef]
- Zhou, X.; He, Y.; Xu, T.; Wu, Z.; Guo, W.; Xu, X.; Liu, Y.; Zhang, Y.; Shang, H.; Huang, L.; et al. 40 Hz light flickering promotes sleep through cortical adenosine signaling. Cell Res. 2024, 34, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef]
- Hall, J.; Trent, S.; Thomas, K.L.; O’Donovan, M.C.; Owen, M.J. Genetic risk for schizophrenia: Convergence on synaptic pathways involved in plasticity. Biol. Psychiatry 2015, 77, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.R.; Koene, R.H.; Hasselfeld, K.; O’Donovan, S.M.; Ramsey, A.; McCullumsmith, R.E. Neuron-specific deficits of bioenergetic processes in the dorsolateral prefrontal cortex in schizophrenia. Mol. Psychiatry 2019, 24, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- McCullumsmith, R.E.; O’donovan, S.M.; Drummond, J.B.; Benesh, F.S.; Simmons, M.; Roberts, R.; Lauriat, T.; Haroutunian, V.; Meador-Woodruff, J.H. Cell-specific abnormalities of glutamate transporters in schizophrenia: Sick astrocytes and compensating relay neurons? Mol Psychiatry 2016, 21, 823–830. [Google Scholar] [CrossRef]
- Sodhi, M.S.; Simmons, M.; McCullumsmith, R.; Haroutunian, V.; Meador-Woodruff, J.H. Glutamatergic gene expression is specifically reduced in thalamocortical projecting relay neurons in schizophrenia. Biol. Psychiatry 2011, 70, 646–654. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Hasselfeld, K.; Bauer, D.; Simmons, M.; Roussos, P.; Haroutunian, V.; Meador-Woodruff, J.H.; E McCullumsmith, R. Glutamate transporter splice variant expression in an enriched pyramidal cell population in schizophrenia. Transl. Psychiatry 2015, 5, e579. [Google Scholar] [CrossRef]
- Almeida, D.; Turecki, G. Profiling cell-type specific gene expression in post-mortem human brain samples through laser capture microdissection. Methods 2022, 207, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Kahn, R.S.; Selten, J.P. Sex differences in the risk of schizophrenia: Evidence from meta-analysis. Arch. Gen. Psychiatry 2003, 60, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, W.; Yi, Z. A glimpse of gender differences in schizophrenia. Gen. Psychiatr. 2022, 35, e100823. [Google Scholar] [CrossRef]
- Boswell-Casteel, R.C.; Hays, F.A. Equilibrative nucleoside transporters-A review. Nucleosides Nucleotides Nucleic Acids 2017, 36, 7–30. [Google Scholar] [CrossRef]
- Naes, S.M.; Ab-Rahim, S.; Mazlan, M.; Abdul Rahman, A. Equilibrative Nucleoside Transporter 2: Properties and Physiological Roles. Biomed. Res. Int. 2020, 2020, 5197626. [Google Scholar] [CrossRef]
- Cunha, R.A. How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Tamura, R.; Ohta, H.; Satoh, Y.; Nonoyama, S.; Nishida, Y.; Nibuya, M. Neuroprotective effects of adenosine deaminase in the striatum. J. Cereb. Blood Flow. Metab. 2016, 36, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Boison, D.; Yegutkin, G.G. Adenosine Metabolism: Emerging Concepts for Cancer Therapy. Cancer Cell 2019, 36, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Wu, K.C.; Lin, C.Y.; Chern, Y. Emerging roles of dysregulated adenosine homeostasis in brain disorders with a specific focus on neurodegenerative diseases. J. Biomed. Sci. 2021, 28, 70. [Google Scholar] [CrossRef] [PubMed]
- Behdad, A.; Sun, X.; Khalpey, Z.; Enjyoji, K.; Wink, M.; Wu, Y.; Usheva, A.; Robson, S.C. Vascular smooth muscle cell expression of ectonucleotidase CD39 (ENTPD1) is required for neointimal formation in mice. Purinergic Signal. 2009, 5, 335–342. [Google Scholar] [CrossRef]
- Hamoud, A.-R.; Bach, K.; Kakrecha, O.; Henkel, N.; Wu, X.; McCullumsmith, R.E.; O’donovan, S.M. Adenosine, Schizophrenia and Cancer: Does the Purinergic System Offer a Pathway to Treatment? Int. J. Mol. Sci. 2022, 23, 11835. [Google Scholar] [CrossRef]
- Friedman, D.J.; Künzli, B.M.; A-Rahim, Y.I.; Sevigny, J.; Berberat, P.O.; Enjyoji, K.; Csizmadia, E.; Friess, H.; Robson, S.C. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2009, 106, 16788–16793. [Google Scholar] [CrossRef]
- Kiss, D.S.; Zsarnovszky, A.; Horvath, K.; Gyorffy, A.; Bartha, T.; Hazai, D.; Sotonyi, P.; Somogyi, V.; Frenyo, L.V.; Diano, S. Ecto-nucleoside triphosphate diphosphohydrolase 3 in the ventral and lateral hypothalamic area of female rats: Morphological characterization and functional implications. Reprod. Biol. Endocrinol. 2009, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.K.; Kauffman, A.L.; Beavers, L.S.; Alston, J.T.; Farb, T.B.; Ficorilli, J.; Marcelo, M.C.; Brenner, M.B.; Bokvist, K.; Barrett, D.G.; et al. Ectonucleotidase NTPDase3 is abundant in pancreatic β-cells and regulates glucose-induced insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1319–E1326. [Google Scholar] [CrossRef] [PubMed]
- Boison, D. Regulation of Extracellular Adenosine. In The Adenosine Receptors; Borea, P.A., Varani, K., Gessi, S., Merighi, S., Vincenzi, F., Eds.; Springer International Publishing: Cham, Swizserland, 2018; pp. 13–32. [Google Scholar]
- Louis, N.A.; Robinson, A.M.; MacManus, C.F.; Karhausen, J.; Scully, M.; Colgan, S.P. Control of IFN-alphaA by CD73: Implications for mucosal inflammation. J. Immunol. 2008, 180, 4246–4255. [Google Scholar] [CrossRef] [PubMed]
- Buckley, P.F. Neuroinflammation and Schizophrenia. Curr. Psychiatry Rep. 2019, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G. Adenosine metabolism in the vascular system. Biochem. Pharmacol. 2021, 187, 114373. [Google Scholar] [CrossRef] [PubMed]
- Lovatt, D.; Xu, Q.; Liu, W.; Takano, T.; Smith, N.A.; Schnermann, J.; Tieu, K.; Nedergaard, M. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc. Natl. Acad. Sci. USA 2012, 109, 6265–6270. [Google Scholar] [CrossRef]
- Juva, K.; Sulkava, R.; Erkinjuntti, T.; Ylikoski, R.; Valvanne, J.; Tilvis, R. Usefulness of the Clinical Dementia Rating scale in screening for dementia. Int. Psychogeriatr. 1995, 7, 17–24. [Google Scholar] [CrossRef]
- Ansoleaga, B.; Jové, M.; Schlüter, A.; Garcia-Esparcia, P.; Moreno, J.; Pujol, A.; Pamplona, R.; Portero-Otín, M.; Ferrer, I. Deregulation of purine metabolism in Alzheimer’s disease. Neurobiol. Aging 2015, 36, 68–80. [Google Scholar] [CrossRef]
- Wang, L.; Yang, C.; Mafimoghaddam, S.; Timsina, J.; Gorijala, P.; Wang, F.; Alvarez, I.; Aguilar, M.; Ruiz, A.; Pastor, P.; et al. CSF proteo-genomic studies identify an interaction between LRRK2 genetic variants and, G.R.N.; GPNMB, CTSB, and ENTPD1. Alzheimer’s Dement. 2023, 19, e063181. [Google Scholar] [CrossRef]



| N | Sex | Age | PMI (Hours) | pH | CDR | Medication | |
|---|---|---|---|---|---|---|---|
| Schizophrenia | 20 | 9F/11M | 75 ± 8 (61–90) | 13.1 ± 5.8 (5.8–24) | 6.3 ± 0.2 (5.85–6.74) | 2.3 ± 2 (0–3) | F: 4 on/3 off/2 Unk M: 8 on/3 off/0 Unk |
| Control | 20 | 7F/13M | 78 ± 7 (64–86) | 12.4 ± 7.5 (3.3–24) | 6.6 ± 0.4 (6.04–7.27) | 0.4 ± 0.5 (0–3) | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahay, S.; Devine, E.A.; Vargas, C.F.-A.; McCullumsmith, R.E.; O’Donovan, S.M. Adenosine Metabolism Pathway Alterations in Frontal Cortical Neurons in Schizophrenia. Cells 2024, 13, 1657. https://doi.org/10.3390/cells13191657
Sahay S, Devine EA, Vargas CF-A, McCullumsmith RE, O’Donovan SM. Adenosine Metabolism Pathway Alterations in Frontal Cortical Neurons in Schizophrenia. Cells. 2024; 13(19):1657. https://doi.org/10.3390/cells13191657
Chicago/Turabian StyleSahay, Smita, Emily A. Devine, Christina F.-A. Vargas, Robert E. McCullumsmith, and Sinead M. O’Donovan. 2024. "Adenosine Metabolism Pathway Alterations in Frontal Cortical Neurons in Schizophrenia" Cells 13, no. 19: 1657. https://doi.org/10.3390/cells13191657
APA StyleSahay, S., Devine, E. A., Vargas, C. F.-A., McCullumsmith, R. E., & O’Donovan, S. M. (2024). Adenosine Metabolism Pathway Alterations in Frontal Cortical Neurons in Schizophrenia. Cells, 13(19), 1657. https://doi.org/10.3390/cells13191657











