Screening a Compound Library to Identify Additives That Boost Cytochrome P450 Enzyme Function in Vascularised Liver Spheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Differentiation
2.1.1. Hepatic Differentiation
2.1.2. Endothelial Cell Differentiation
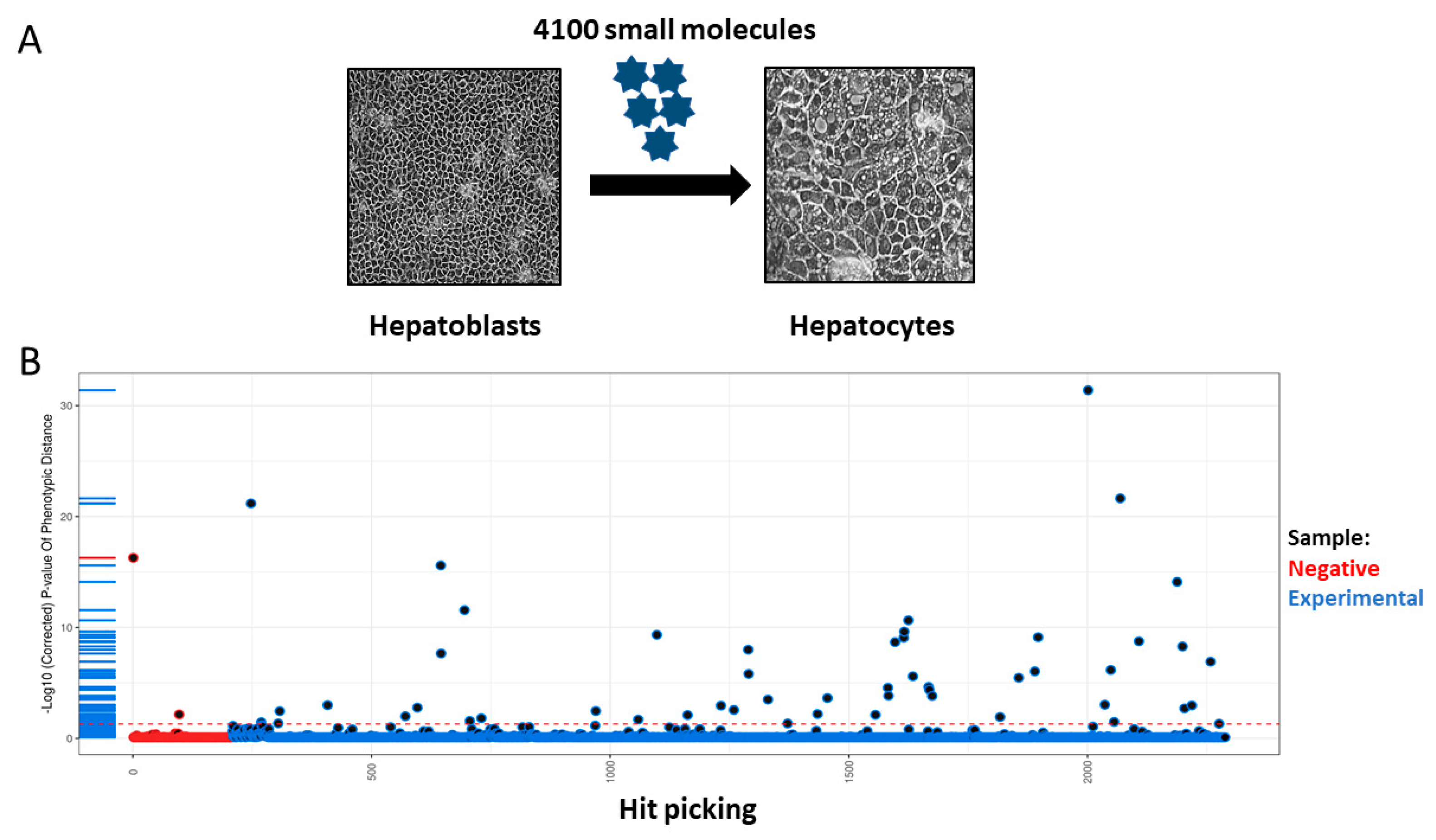
2.2. Automated Production of Liver Spheres
2.3. Compound Exposure
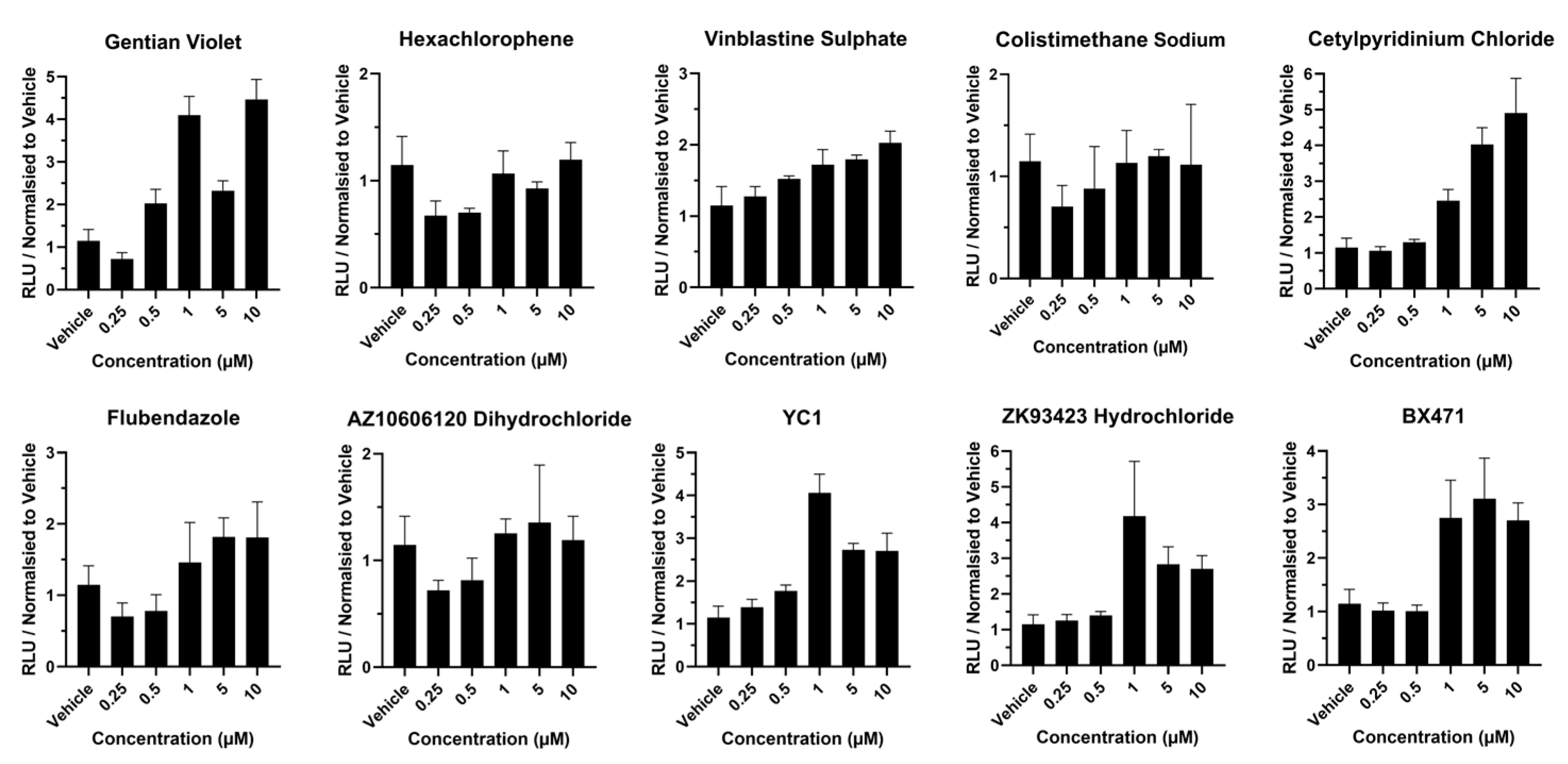
2.4. Cell Health in Response to Hit Compounds
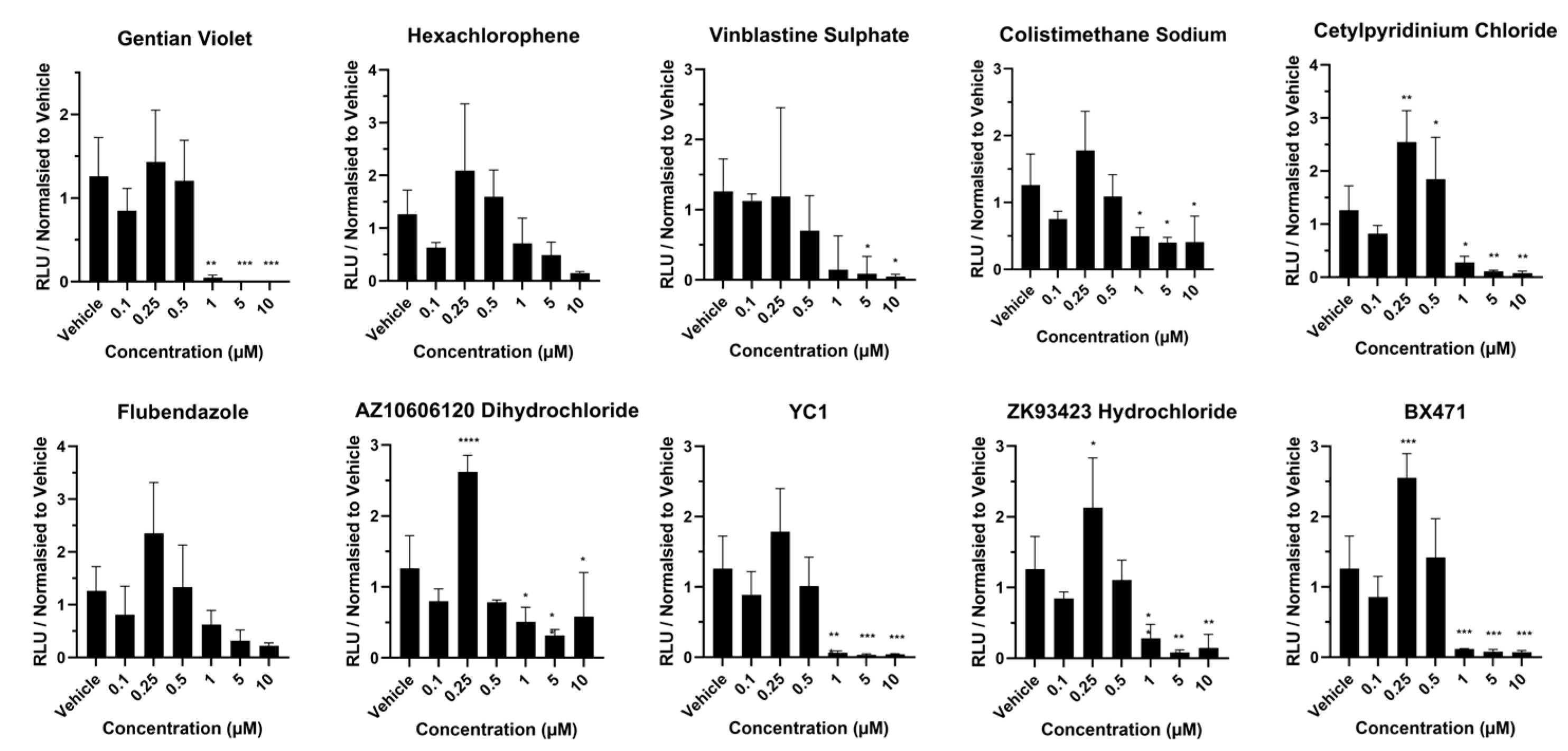
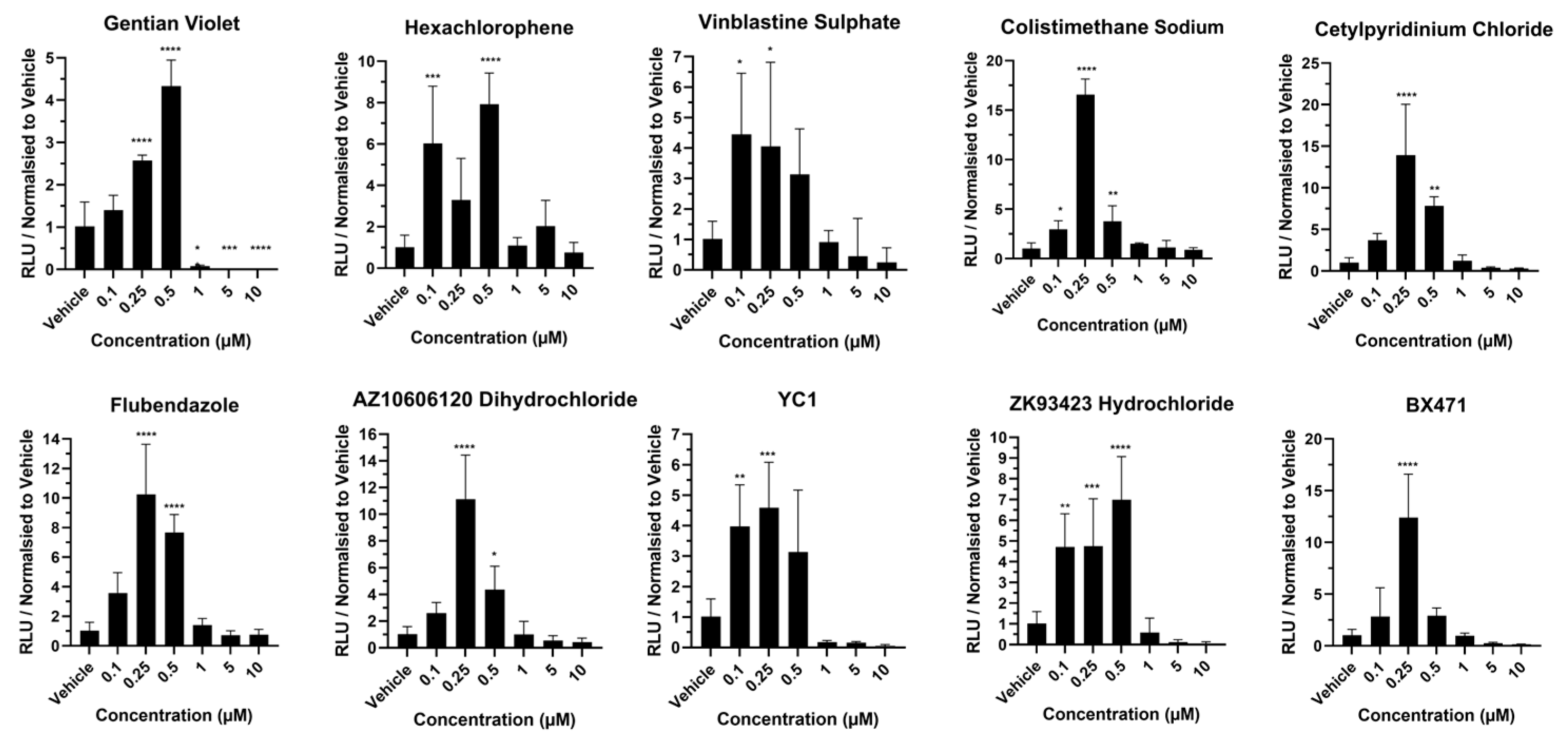
2.5. Cytochrome P450 Activity in Response to Hit Compounds
2.6. Statistics
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasarinaite, A.; Sinton, M.; Saunders, P.T.K.; Hay, D.C. The Influence of Sex Hormones in Liver Function and Disease. Cells 2023, 12, 1604. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Chalasani, N.; Björnsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, M.I.; Kaplowitz, N.; et al. Drug-induced liver injury. Nat. Rev. Dis. Prim. 2019, 5, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kidambi, S.; Ortega-Ribera, M.; Thuy, L.T.T.; Nieto, N.; Cogger, V.C.; Xie, W.-F.; Tacke, F.; Gracia-Sancho, J. In Vitro Models for the Study of Liver Biology and Diseases: Advances and Limitations. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Szkolnicka, D.; Hay, D.C. Concise Review: Advances in Generating Hepatocytes from Pluripotent Stem Cells for Translational Medicine. Stem Cells 2016, 34, 1421–1426. [Google Scholar] [CrossRef]
- Rodríguez-Antona, C.; Donato, M.T.; Boobis, A.; Edwards, R.J.; Watts, P.S.; Castell, J.V.; Gómez-Lechón, M.-J. Cytochrome P450 expression in human hepatocytes and hepatoma cell lines: Molecular mechanisms that determine lower expression in cultured cells. Xenobiotica 2002, 32, 505–520. [Google Scholar] [CrossRef]
- Yang, S.; Ooka, M.; Margolis, R.J.; Xia, M. Liver three-dimensional cellular models for high-throughput chemical testing. Cell Rep. Methods 2023, 3, 100432. [Google Scholar] [CrossRef] [PubMed]
- Lucendo-Villarin, B.; Meseguer-Ripolles, J.; Drew, J.; Fischer, L.; Ma, E.; Flint, O.; Simpson, K.J.; Machesky, L.M.; Mountford, J.C.; Hay, D.C. Development of a cost-effective automated platform to produce human liver spheroids for basic and applied research. Biofabrication 2020, 13, 015009. [Google Scholar] [CrossRef]
- Meseguer-Ripolles, J.; Lucendo-Villarin, B.; Tucker, C.; Ferreira-Gonzalez, S.; Homer, N.; Wang, Y.; Lewis, P.J.S.; Toledo, E.M.; Mellado-Gomez, E.; Simpson, J.; et al. Dimethyl fumarate reduces hepatocyte senescence following paracetamol exposure. iScience 2021, 24, 102552. [Google Scholar] [CrossRef]
- MacAskill, M.G.; Saif, J.; Condie, A.; Jansen, M.A.; MacGillivray, T.J.; Tavares, A.A.; Fleisinger, L.; Spencer, H.L.; Besnier, M.; Martin, E.; et al. Robust Revascularization in Models of Limb Ischemia Using a Clinically Translatable Human Stem Cell-Derived Endothelial Cell Product. Mol. Ther. 2018, 26, 1669–1684. [Google Scholar] [CrossRef]
- Meseguer-Ripolles, J.; Lucendo-Villarin, B.; Wang, Y.; Hay, D.C. Semi-automated Production of Hepatocyte Like Cells from Pluripotent Stem Cells. J. Vis. Exp. 2018, e57995. [Google Scholar] [CrossRef]
- Bell, C.C.; A Dankers, A.C.; Lauschke, V.M.; Sison-Young, R.; Jenkins, R.; Rowe, C.; E Goldring, C.; Park, K.; Regan, S.L.; Walker, T.; et al. Comparison of Hepatic 2D Sandwich Cultures and 3D Spheroids for Long-term Toxicity Applications: A Multicenter Study. Toxicol. Sci. 2018, 162, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Pona, A.; Quan, E.Y.; Cline, A.; Feldman, S.R. Review of the use of gentian violet in dermatology practice. Dermatol. Online J. 2020, 26. [Google Scholar] [CrossRef]
- Gibson, J.W. Comparative antibacterial activity of hexachlorophane in different formulations used for skin disinfection. J. Clin. Pathol. 1969, 22, 90–98. [Google Scholar] [CrossRef]
- Bleehen, N.M.; Jelliffe, A.M. Vinblastine Sulphate in the Treatment of Malignant Disease. Br. J. Cancer 1965, 19, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Auer, D.L.; Buchalla, W.; Hiller, K.-A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium Chloride: Mechanism of Action, Antimicrobial Efficacy in Biofilms, and Potential Risks of Resistance. Antimicrob. Agents Chemother. 2020, 64, 1110–1128. [Google Scholar] [CrossRef]
- Huang, C.; Yu, W.; Cui, H.; Wang, Y.; Zhang, L.; Han, F.; Huang, T. P2X7 blockade attenuates mouse liver fibrosis. Mol. Med. Rep. 2013, 9, 57–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stasch, J.-P.; Pacher, P.; Evgenov, O.V. Soluble Guanylate Cyclase as an Emerging Therapeutic Target in Cardiopulmonary Disease. Circulation 2011, 123, 2263–2273. [Google Scholar] [CrossRef]
- Löscher, W.; Hönack, D.; Hashem, A. Anticonvulsant efficacy of clonazepam and the β-carboline ZK 93423 during chronic treatment in amygdala-kindled rats. Eur. J. Pharmacol. 1987, 143, 403–414. [Google Scholar] [CrossRef]
- El-Sayed Ahmed, M.A.E.G.; Zhong, L.-L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.-B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhao, R.; Wang, M.; Jiang, X.; Gao, F.; Fu, L.; Zhang, L.; Zhou, X.-L. Flubendazole elicits anti-cancer effects via targeting EVA1A-modulated autophagy and apoptosis in Triple-negative Breast Cancer. Theranostics 2020, 10, 8080–8097. [Google Scholar] [CrossRef] [PubMed]
- Horuk, R. BX471: A CCR1 Antagonist with Anti-Inflammatory Activity in Man. Mini-Reviews Med. Chem. 2005, 5, 791–804. [Google Scholar] [CrossRef] [PubMed]

| Compound Name | Vendor | Catalogue Number |
|---|---|---|
| Gentian Violet | Microsource Discovery | 01500315 |
| Hexachlorophene | Microsource Discovery | 01500328 |
| Vinblastine Sulphate | Microsource Discovery | 01500611 |
| Colistimethane Sodium | Microsource Discovery | 01500206 |
| Cetylpyridinium Chloride | Microsource Discovery | 01500169 |
| Flubendazole | Microsource Discovery | 01505043 |
| AZ10606120 Dihydrochloride | Tocris | 3323 |
| YC1 | Tocris | 4307 |
| ZK93423 Hydrochloride | Tocris | 1994 |
| BX471 | Tocris | 3496 |
| Compound | Viability Reduction at 50% | Peak Caspase Activity | Peak CYP1A2 Activity | Peak CYP3A4 Activity |
|---|---|---|---|---|
| Gentian Violet | 0.25 µM | 0.50 µM | 0.25 µM | 0.50 µM |
| Hexachlorophene | 0.25 µM | >10 µM | 0.25 µM | 0.50 µM |
| Vinblastine Sulphate | 0.25 µM | >10 µM | 0.25 µM | 0.10 µM |
| Colistimethane Sodium | 5 µM | >10 µM | 0.25 µM | 0.25 µM |
| Cetylpyridinium Chloride | 0.25 µM | >5 µM | 0.25 µM | 0.25 µM |
| Flubendazole | 5 µM | 5 µM | 0.25 µM | 0.25 µM |
| AZ10606120 Dihydrochloride | 0.25 µM | >10 µM | 0.25 µM | 0.25 µM |
| YC1 | 0.25 µM | >1 µM | 0.25 µM | 0.25 µM |
| ZK93423 Hydrochloride | 0.50 µM | >1 µM | 0.25 µM | 0.50 µM |
| BX471 | 1 µM | 5 µM | 0.25 µM | 0.25 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucendo-Villarin, B.; Wang, Y.; Mallanna, S.K.; Kimbrel, E.A.; Hay, D.C. Screening a Compound Library to Identify Additives That Boost Cytochrome P450 Enzyme Function in Vascularised Liver Spheres. Cells 2024, 13, 1594. https://doi.org/10.3390/cells13181594
Lucendo-Villarin B, Wang Y, Mallanna SK, Kimbrel EA, Hay DC. Screening a Compound Library to Identify Additives That Boost Cytochrome P450 Enzyme Function in Vascularised Liver Spheres. Cells. 2024; 13(18):1594. https://doi.org/10.3390/cells13181594
Chicago/Turabian StyleLucendo-Villarin, Baltasar, Yu Wang, Sunil K. Mallanna, Erin A. Kimbrel, and David C. Hay. 2024. "Screening a Compound Library to Identify Additives That Boost Cytochrome P450 Enzyme Function in Vascularised Liver Spheres" Cells 13, no. 18: 1594. https://doi.org/10.3390/cells13181594
APA StyleLucendo-Villarin, B., Wang, Y., Mallanna, S. K., Kimbrel, E. A., & Hay, D. C. (2024). Screening a Compound Library to Identify Additives That Boost Cytochrome P450 Enzyme Function in Vascularised Liver Spheres. Cells, 13(18), 1594. https://doi.org/10.3390/cells13181594







