α-Parvin Expression in Breast Cancer Tissues: Correlation with Clinical Parameters and Prognostic Significance
Abstract
1. Introduction
2. Materials and Methods
2.1. In Silico Analysis
2.2. Cell Culture
2.3. Immunoblotting (IB) Analysis
2.4. Tissue Microarray and Patient Information
2.5. Study Design
2.6. Immunohistochemistry
2.7. Image Analysis
2.8. Statistical Analyses
3. Results
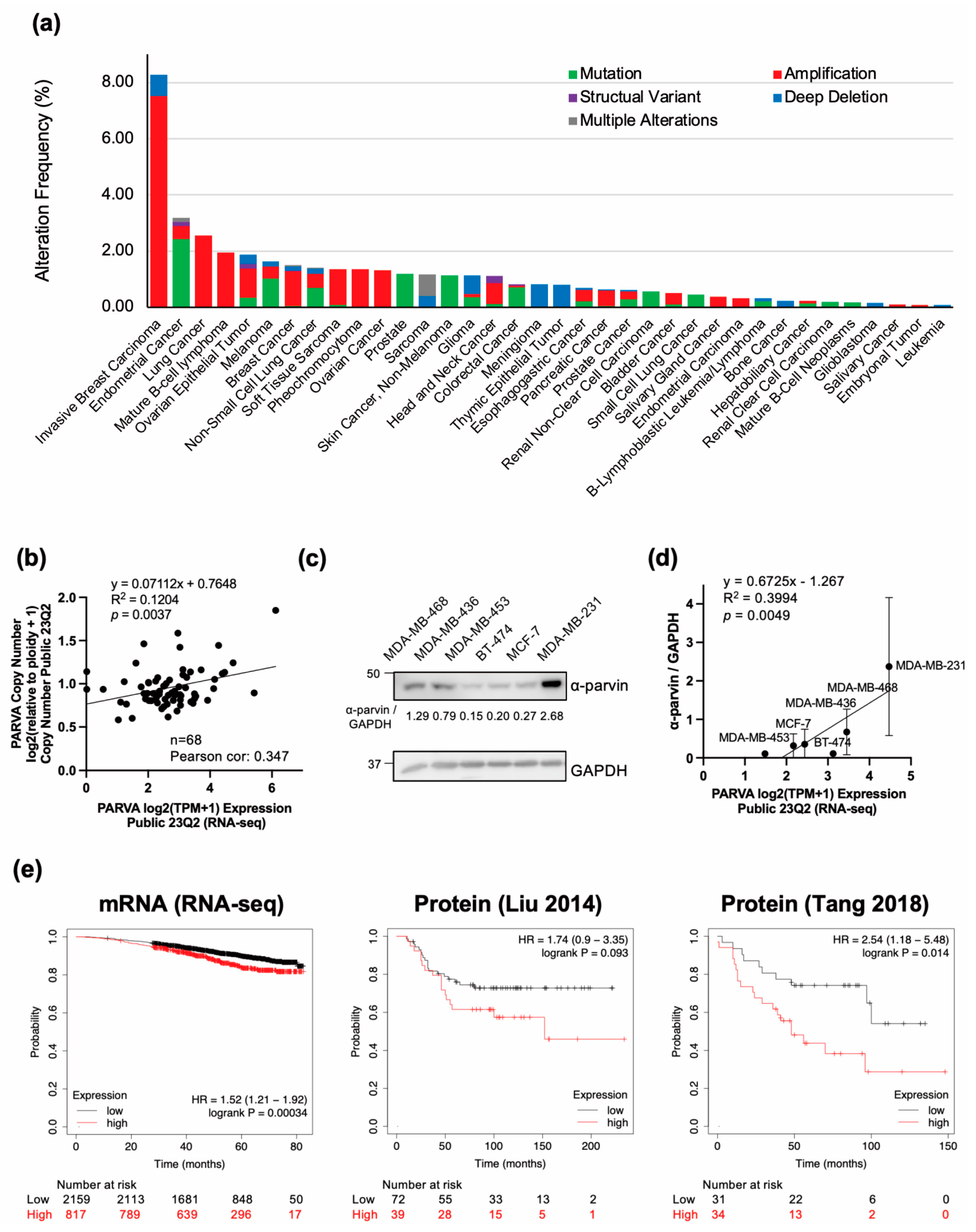
3.1. PARVA Amplification Is Frequently Observed in BC, and Its High Expression Is Associated with Poor Prognosis
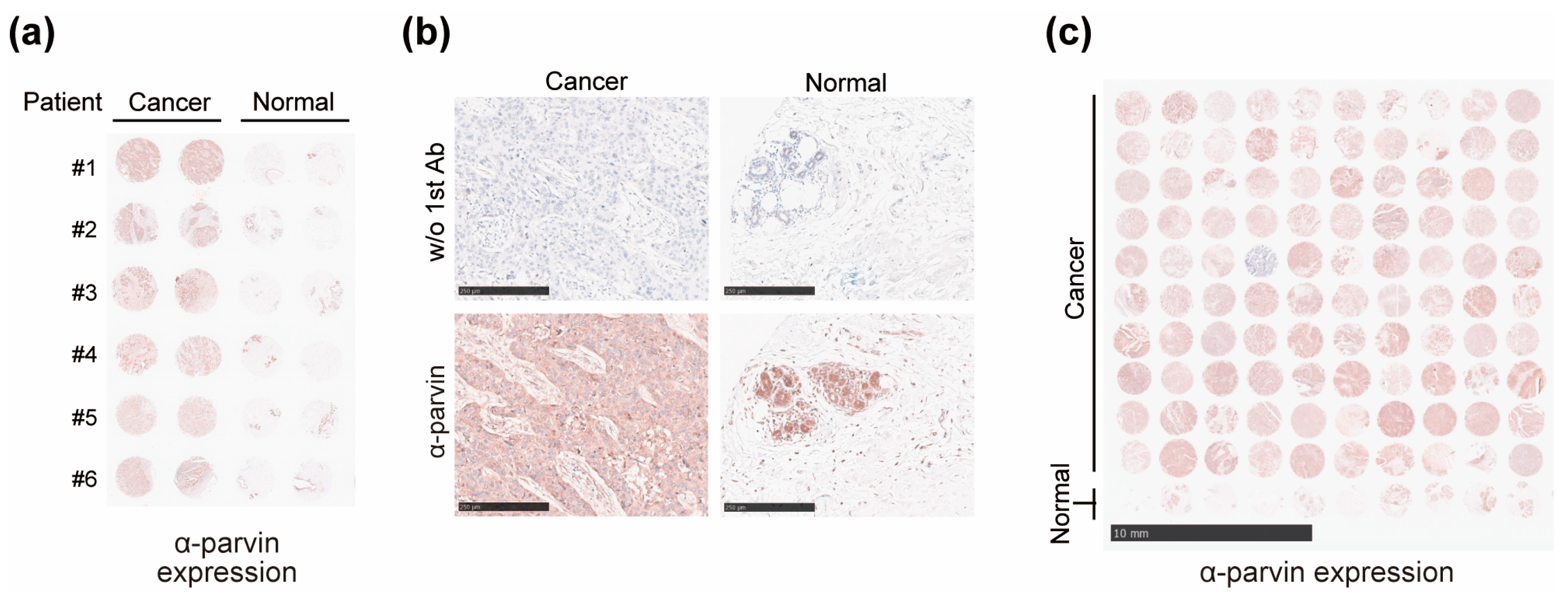
3.2. Expression of α-Parvin Increased in BC Patients
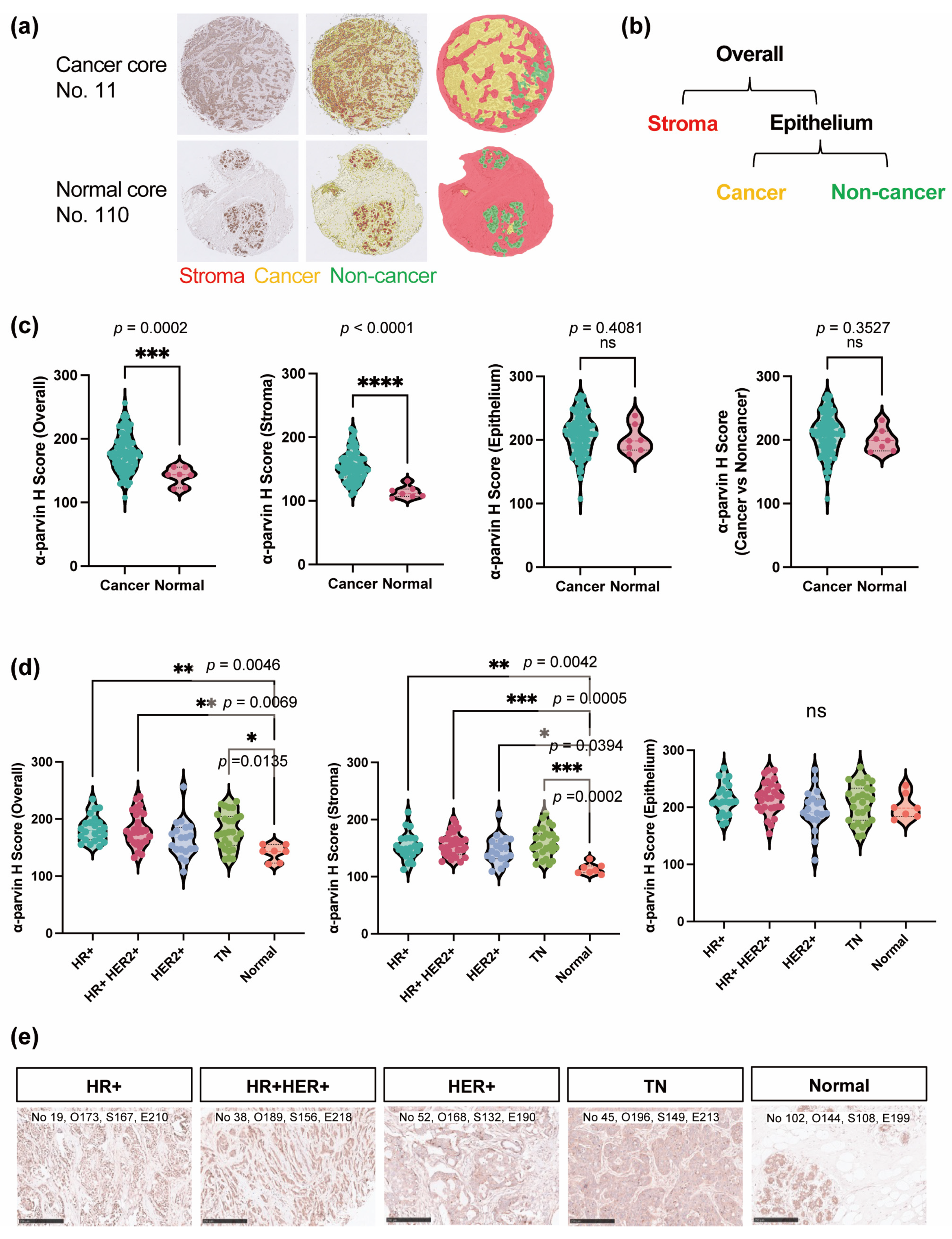
3.3. α-Parvin Level Elevated in the Stromal Region in BC
3.4. All BC Subtypes Had Elevated α-Parvin Expression Levels in Stroma
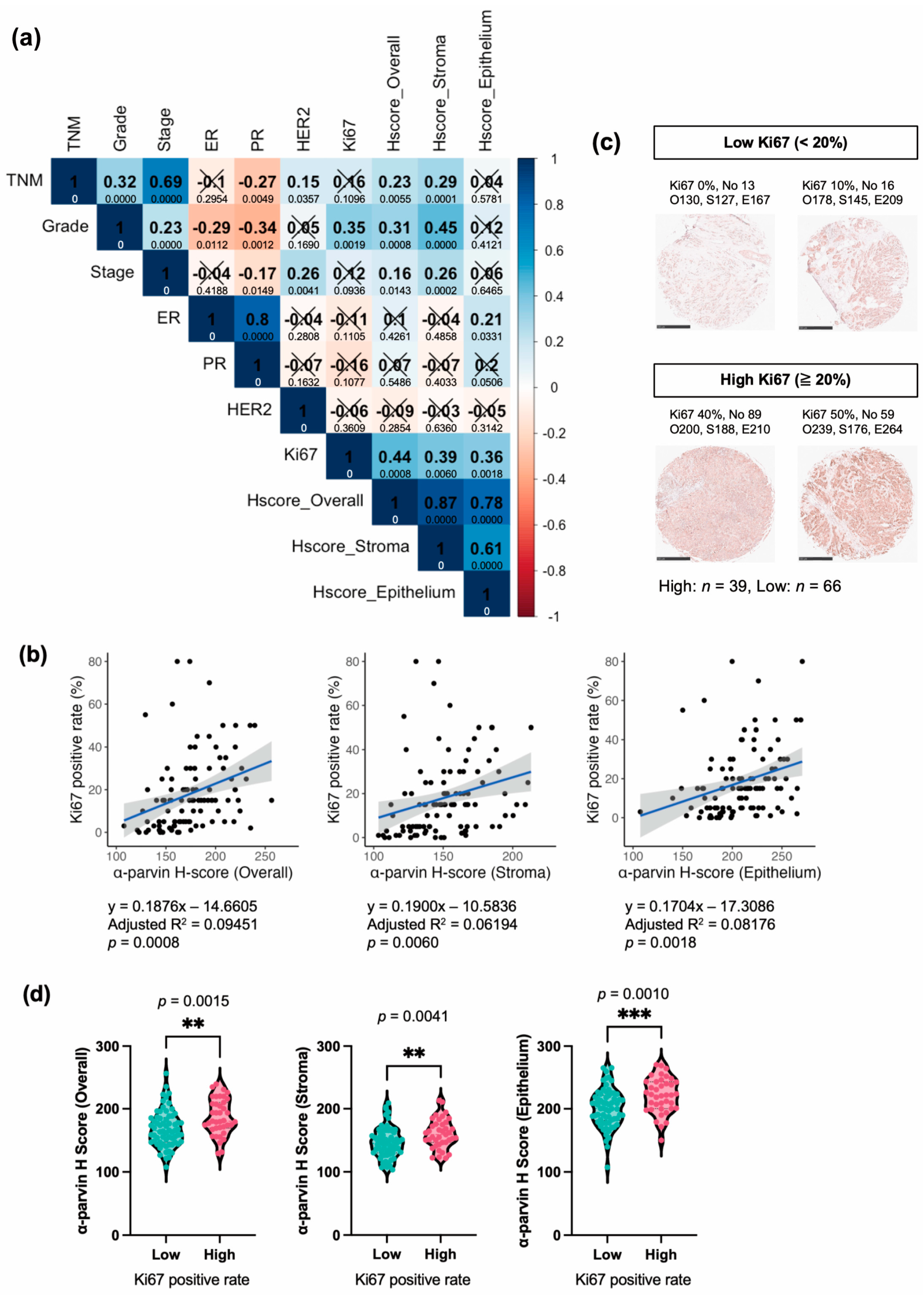
3.5. α-Parvin Expression in BC Correlates Positively with Ki67, While That in the Stroma Correlates with Grade, TNM, and Stage
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Fallahpour, S.; Navaneelan, T.; De, P.; Borgo, A. Breast cancer survival by molecular subtype: A population-based analysis of cancer registry data. CMAJ Open 2017, 5, E734–E739. [Google Scholar] [CrossRef]
- Howard, F.M.; Olopade, O.I. Epidemiology of Triple-Negative Breast Cancer: A Review. Cancer J. 2021, 27, 8–16. [Google Scholar] [CrossRef]
- Liu, J.; Chen, K.; Jiang, W.; Mao, K.; Li, S.; Kim, M.J.; Liu, Q.; Jacobs, L.K. Chemotherapy response and survival of inflammatory breast cancer by hormone receptor- and HER2-defined molecular subtypes approximation: An analysis from the National Cancer Database. J. Cancer Res. Clin. Oncol. 2017, 143, 161–168. [Google Scholar] [CrossRef]
- Morris, G.J.; Naidu, S.; Topham, A.K.; Guiles, F.; Xu, Y.; McCue, P.; Schwartz, G.F.; Park, P.K.; Rosenberg, A.L.; Brill, K. Differences in breast carcinoma characteristics in newly diagnosed African–American and Caucasian patients: A single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and end results database. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 110, 876–884. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Jin, X.; Mu, P. Targeting breast cancer metastasis. Breast Cancer Basic Clin. Res. 2015, 9s1. [Google Scholar] [CrossRef]
- Hanley, C.J.; Henriet, E.; Sirka, O.K.; Thomas, G.J.; Ewald, A.J. Tumor-Resident Stromal Cells Promote Breast Cancer Invasion through Regulation of the Basal Phenotype. Mol. Cancer Res. 2020, 18, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.S.; Sarnella, A.; D’Avino, G.; Zannetti, A. Recruitment of stromal cells into tumour microenvironment promote the metastatic spread of breast cancer. Semin. Cancer Biol. 2020, 60, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Legate, K.R.; Montañez, E.; Kudlacek, O.; Füssler, R. ILK, PINCH and parvin: The tIPP of integrin signalling. Nat. Rev. Mol. Cell Biol. 2006, 7, 20–31. [Google Scholar] [CrossRef]
- Nikolopoulos, S.N.; Turner, C.E. Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J. Cell Biol. 2000, 151, 1435–1448. [Google Scholar] [CrossRef]
- Wu, C. The PINCH–ILK–parvin complexes: Assembly, functions and regulation. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2004, 1692, 55–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Tu, Y.; Velyvis, A.; Yang, Y.; Qin, J.; Wu, C. Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J. Cell Sci. 2002, 115, 4777–4786. [Google Scholar] [CrossRef]
- Wickström, S.A.; Lange, A.; Montanez, E.; Fässler, R. The ILK/PINCH/parvin complex: The kinase is dead, long live the pseudokinase! EMBO J. 2010, 29, 281–291. [Google Scholar] [CrossRef]
- Huang, A.H.; Pan, S.H.; Chang, W.H.; Hong, Q.S.; Chen, J.J.; Yu, S.L. PARVA promotes metastasis by modulating ILK signalling pathway in lung adenocarcinoma. PLoS ONE 2015, 10, e0118530. [Google Scholar] [CrossRef]
- Ito, M.; Hagiyama, M.; Mimae, T.; Inoue, T.; Kato, T.; Yoneshige, A.; Nakanishi, J.; Kondo, T.; Okada, M.; Ito, A. α-Parvin, a pseudopodial constituent, promotes cell motility and is associated with lymph node metastasis of lobular breast carcinoma. Breast Cancer Res. Treat. 2014, 144, 59–69. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, Y.; Guo, C.; Liu, C.; Ma, P.; Ma, S.; Wang, Z.; Liu, J.; Qian, T.; Ma, L.; et al. α-Parvin promotes breast cancer progression and metastasis through interaction with G3BP2 and regulation of TWIST1 signaling. Oncogene 2019, 38, 4856–4874. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shen, Q.; Song, G.; He, S.; Zhou, L. Downregulation of PARVA promotes metastasis by modulating integrin-linked kinase activity and regulating MAPK/ERK and MLC2 signaling in prostate cancer. Transl. Androl. Urol. 2021, 10, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Györffy, B.; Lanczky, A.; Eklund, A.C.; Denkert, C.; Budczies, J.; Li, Q.; Szallasi, Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1809 patients. Breast Cancer Res. Treat. 2010, 123, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Ősz, Á.; Lánczky, A.; Győrffy, B. Survival analysis in breast cancer using proteomic data from four independent datasets. Sci. Rep. 2021, 11, 16787. [Google Scholar] [CrossRef]
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef]
- Liu, N.Q.; Stingl, C.; Look, M.P.; Smid, M.; Braakman, R.B.; De Marchi, T.; Sieuwerts, A.M.; Span, P.N.; Sweep, F.C.; Linderholm, B.K.; et al. Comparative proteome analysis revealing an 11-protein signature for aggressive triple-negative breast cancer. J. Natl. Cancer Inst. 2014, 106, djt376. [Google Scholar] [CrossRef]
- Tang, W.; Zhou, M.; Dorsey, T.H.; Prieto, D.A.; Wang, X.W.; Ruppin, E.; Veenstra, T.D.; Ambs, S. Integrated proteotranscriptomics of breast cancer reveals globally increased protein-mRNA concordance associated with subtypes and survival. Genome Med. 2018, 10, 94. [Google Scholar] [CrossRef]
- Conklin, M.W.; Keely, P.J. Why the stroma matters in breast cancer: Insights into breast cancer patient outcomes through the examination of stromal biomarkers. Cell Adh. Migr. 2012, 6, 249–260. [Google Scholar] [CrossRef]
- Plava, J.; Cihova, M.; Burikova, M.; Matuskova, M.; Kucerova, L.; Miklikova, S. Recent advances in understanding tumor stroma-mediated chemoresistance in breast cancer. Mol. Cancer 2019, 18, 67. [Google Scholar] [CrossRef]
- Zuo, T.; Zeng, H.; Li, H.; Liu, S.; Yang, L.; Xia, C.; Zheng, R.; Ma, F.; Liu, L.; Wang, N. The influence of stage at diagnosis and molecular subtype on breast cancer patient survival: A hospital-based multi-center study. Cancer Commun. 2017, 36, 1–10. [Google Scholar] [CrossRef]
- Deluche, E.; Venat-Bouvet, L.; Leobon, S.; Fermeaux, V.; Mollard, J.; Saidi, N.; Jammet, I.; Aubard, Y.; Tubiana-Mathieu, N. Assessment of Ki67 and uPA/PAI-1 expression in intermediate-risk early stage breast cancers. BMC Cancer 2017, 17, 662. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations From the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Ain, U.; Firdaus, H. Parvin: A hub of intracellular signalling pathways regulating cellular behaviour and disease progression. Acta Histochem. 2022, 124, 151935. [Google Scholar] [CrossRef]
- Qu, M.; Yu, K.; Rehman Aziz, A.U.; Zhang, H.; Zhang, Z.; Li, N.; Liu, B. The role of Actopaxin in tumor metastasis. Prog. Biophys. Mol. Biol. 2022, 175, 90–102. [Google Scholar] [CrossRef]
- Velazquez-Torres, G.; Shoshan, E.; Ivan, C.; Huang, L.; Fuentes-Mattei, E.; Paret, H.; Kim, S.J.; Rodriguez-Aguayo, C.; Xie, V.; Brooks, D.; et al. A-to-I miR-378a-3p editing can prevent melanoma progression via regulation of PARVA expression. Nat. Commun. 2018, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.C.; Dedhar, S. New Perspectives on the Role of Integrin-Linked Kinase (ILK) Signaling in Cancer Metastasis. Cancers 2022, 14, 3209. [Google Scholar] [CrossRef]
- Gkretsi, V.; Kalli, M.; Efstathiades, C.; Papageorgis, P.; Papanikolaou, V.; Zacharia, L.C.; Tsezou, A.; Athanassiou, E.; Stylianopoulos, T. Depletion of Ras Suppressor-1 (RSU-1) promotes cell invasion of breast cancer cells through a compensatory upregulation of a truncated isoform. Sci. Rep. 2019, 9, 10050. [Google Scholar] [CrossRef]
- Penault-Llorca, F.; Radosevic-Robin, N. Ki67 assessment in breast cancer: An update. Pathology 2017, 49, 166–171. [Google Scholar] [CrossRef]
| Characteristic | N = 105 1 |
|---|---|
| Age | 47 (10) |
| Sex | |
| Female | 105/105 (100%) |
| Type | |
| AT | 7/105 (6.7%) |
| Malignant | 98/105 (93%) |
| TNM | |
| − | 7/105 (6.7%) |
| T1NxMx | 2/105 (1.9%) |
| T2NxMx | 75/105 (71%) |
| T3NxMx | 14/105 (13%) |
| T4NxMx | 7/105 (6.7%) |
| Grade | |
| − | 8/105 (7.6%) |
| 1 | 3/105 (2.9%) |
| 2 | 62/105 (59%) |
| 3 | 32/105 (30%) |
| Stage | |
| − | 7/105 (6.7%) |
| IA | 1/105 (1.0%) |
| IIA | 49/105 (47%) |
| IIB | 22/105 (21%) |
| IIIA | 18/105 (17%) |
| IIIB | 7/105 (6.7%) |
| IIIC | 1/105 (1.0%) |
| ER | |
| − | 46/105 (44%) |
| + | 15/105 (14%) |
| ++ | 9/105 (8.6%) |
| +++ | 35/105 (33%) |
| PR | |
| − | 57/105 (54%) |
| + | 14/105 (13%) |
| ++ | 11/105 (10%) |
| +++ | 23/105 (22%) |
| HER2 | |
| 0 | 57/105 (54%) |
| 1+ | 13/105 (12%) |
| 2+ | 12/105 (11%) |
| 3+ | 23/105 (22%) |
| Ki67 | 18 (18) |
| Subtype | |
| HR+ | 23/105 (22%) |
| HER+ | 19/105 (18%) |
| HR+ HER+ | 29/105 (28%) |
| TN | 27/105 (26%) |
| Normal (AT) | 7/105 (6.7%) |
| Pathology diagnosis | |
| Invasive carcinoma of no special type | 97/105 (92%) |
| Breast carcinoma with apocrine differentiation | 1/105 (1.0%) |
| Cancer adjacent breast tissue | 7/105 (6.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, M.; Ito, H.; Kitahata, K.; Sato, S.; Nishide, A.; Gamo, K.; Managi, S.; Tezuka, T.; Yoshizawa, A.; Kim, M. α-Parvin Expression in Breast Cancer Tissues: Correlation with Clinical Parameters and Prognostic Significance. Cells 2024, 13, 1572. https://doi.org/10.3390/cells13181572
Takeda M, Ito H, Kitahata K, Sato S, Nishide A, Gamo K, Managi S, Tezuka T, Yoshizawa A, Kim M. α-Parvin Expression in Breast Cancer Tissues: Correlation with Clinical Parameters and Prognostic Significance. Cells. 2024; 13(18):1572. https://doi.org/10.3390/cells13181572
Chicago/Turabian StyleTakeda, Midori, Hiroaki Ito, Keisuke Kitahata, Sota Sato, Akira Nishide, Kanae Gamo, Shunsuke Managi, Tohru Tezuka, Akihiko Yoshizawa, and Minsoo Kim. 2024. "α-Parvin Expression in Breast Cancer Tissues: Correlation with Clinical Parameters and Prognostic Significance" Cells 13, no. 18: 1572. https://doi.org/10.3390/cells13181572
APA StyleTakeda, M., Ito, H., Kitahata, K., Sato, S., Nishide, A., Gamo, K., Managi, S., Tezuka, T., Yoshizawa, A., & Kim, M. (2024). α-Parvin Expression in Breast Cancer Tissues: Correlation with Clinical Parameters and Prognostic Significance. Cells, 13(18), 1572. https://doi.org/10.3390/cells13181572







