Evaluation of Anti-Inflammatory Activity of the New Cardiotonic Steroid γ-Benzylidene Digoxin 8 (BD-8) in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of γ-Benzylidene Digoxin 8 (BD-8)
2.2. In Silico Analysis
2.3. Animals
2.4. Macrophage Isolation
2.5. Macrophage Viability
2.6. Nitric Oxide Production
2.7. Quantification of Cytokine Production
2.8. Zymosan Phagocytosis Assay
2.9. Effect of BD-8 on Macrophages Functions and Its Mechanism of Action
2.10. Model of Paw Edema
2.11. Histological Analysis
2.12. Statistical Analysis
3. Results
3.1. In Silico Analyses
3.2. Results In Vitro
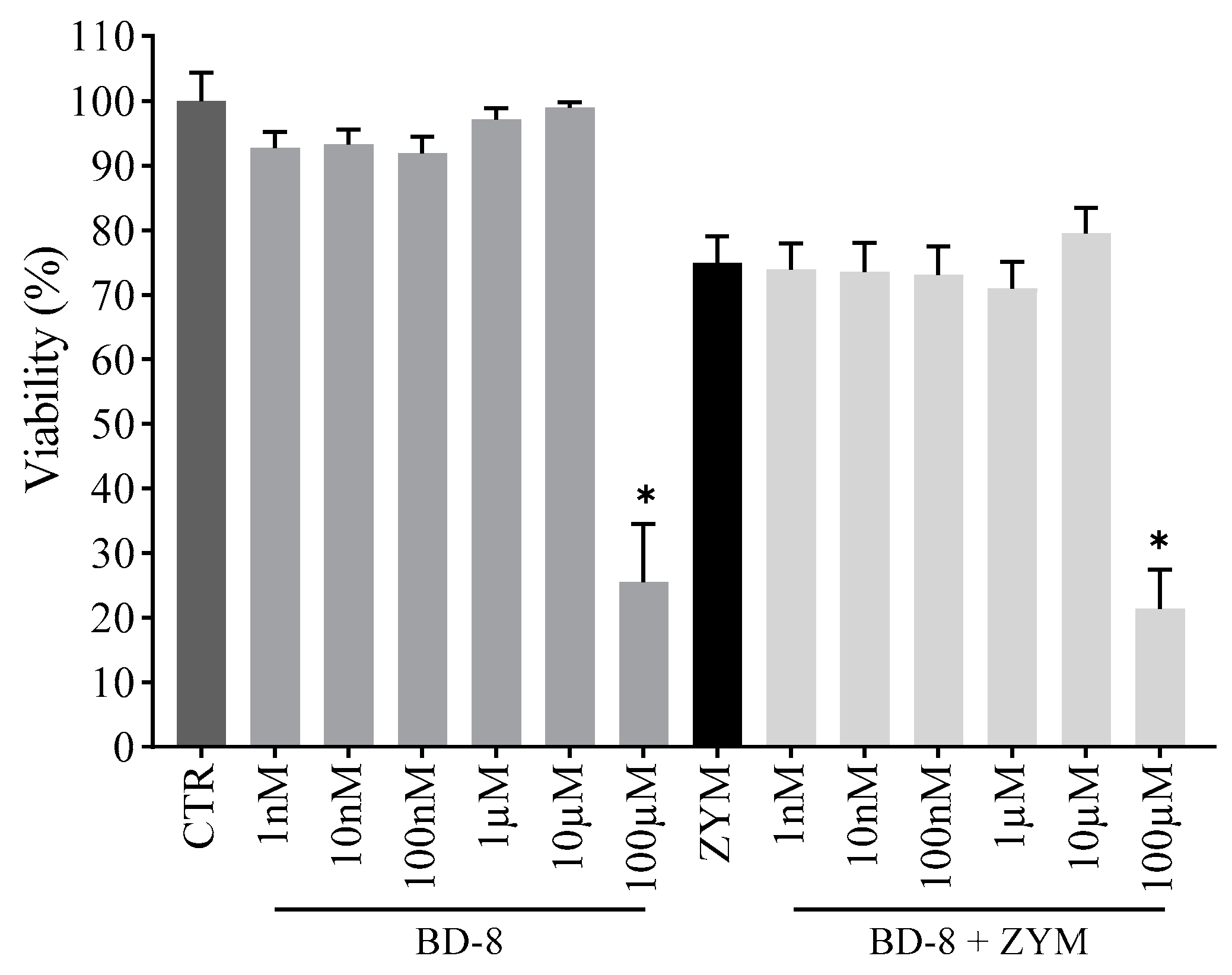
3.2.1. Effect of BD-8 on Macrophage Viability
3.2.2. Effect of BD-8 on Nitric Oxide Production
3.2.3. Effect of BD-8 on Cytokine Production
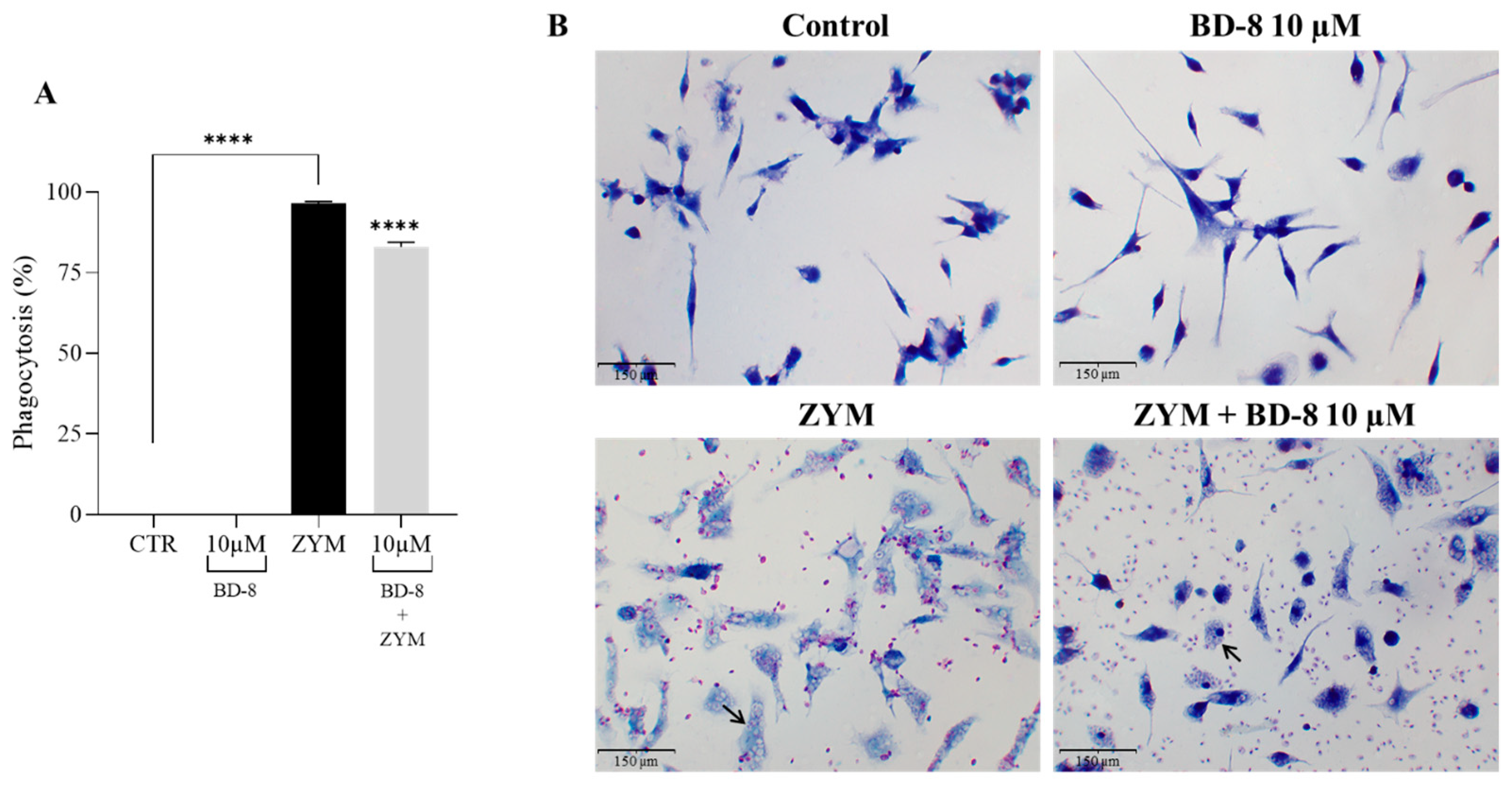
3.2.4. Effect of BD-8 on Phagocytosis of ZYM Particles
3.2.5. Effect of BD-8 on Phagocytosis of Fluorescent Red-ZYM Particles
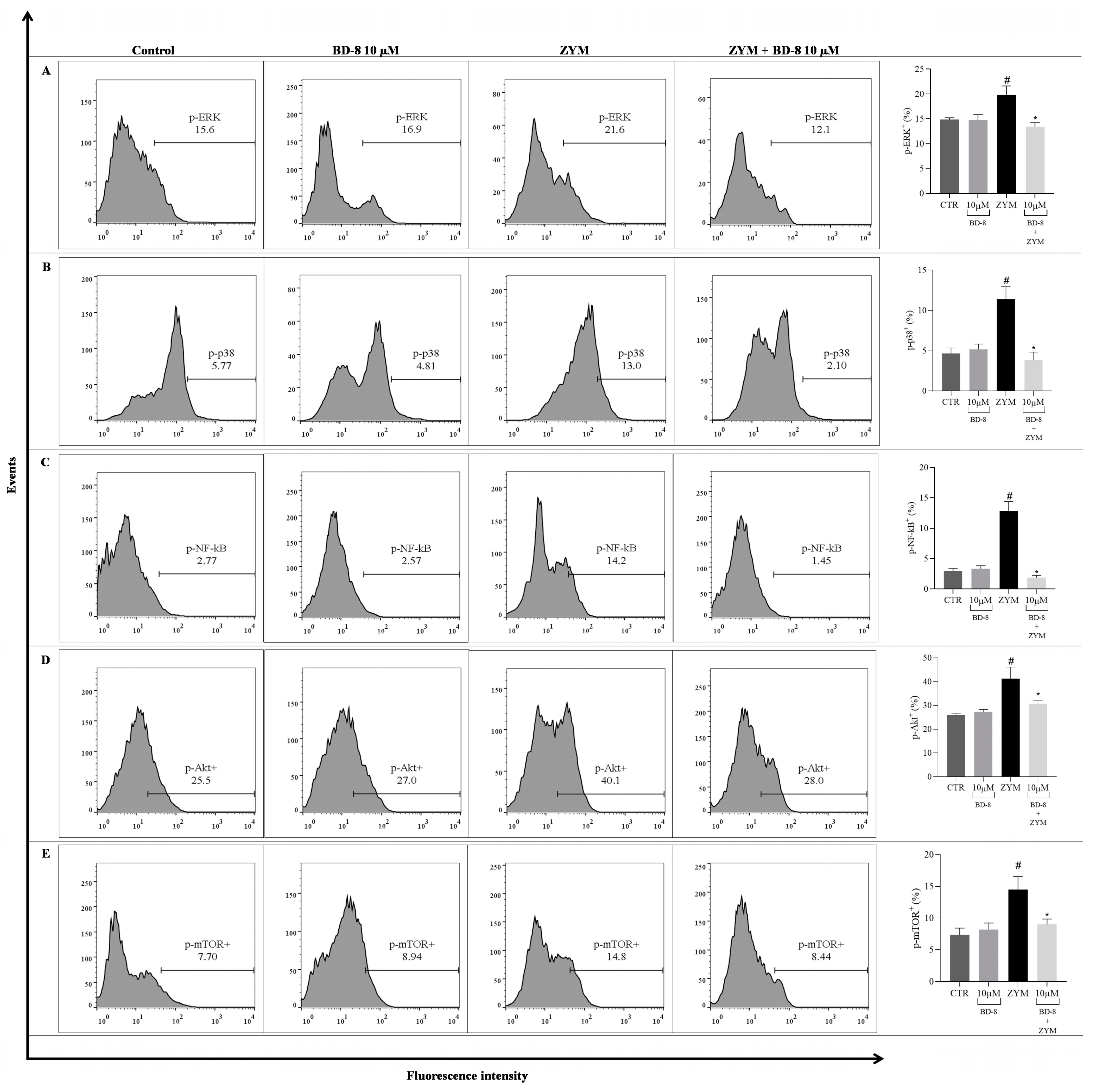
3.2.6. Effect of BD-8 on ROS Production, iNOS and COX-2 Expression, and NF-κB p65, MAPK–ERK/p38, Akt/mTOR, and Src Phosphorylation
3.3. Results from In Vivo Model
Effect of BD-8 in Paw Edema
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mia, M.M.; Singh, M.K. Emerging roles of the Hippo signaling pathway in modulating immune response and inflammation-driven tissue repair and remodeling. FEBS J. 2022, 289, 4061–4081. [Google Scholar] [CrossRef]
- Télez-Rizo, S.A.; Filep, J.G. Beyond host defense and tissue injury: The emerging role of neutrophils in tissue repair. Am. J. Physiol. Cell Physiol. 2024, 326, C661–C683. [Google Scholar] [CrossRef] [PubMed]
- Vago, J.P.; Amaral, F.A.; Loo, F.A.J.V. Resolving inflammation by TAM receptor activation. Pharmacol. Ther. 2021, 227, 107893. [Google Scholar] [CrossRef]
- Bell, R.M.B.; Conway, B.R. Macrophages in the kidney in health, injury and repair. Int. Rev. Cell Mol. Biol. 2022, 367, 101–147. [Google Scholar] [CrossRef] [PubMed]
- Arya, P.; Nabi, S.; Bhandari, U. Modulatory role of atorvastatin against high-fat diet and zymosan-induced activation of TLR2/NF-ƙB signaling pathway in C57BL/6 mice. Iran. J. Basic Med. Sci. 2021, 24, 1023–1032. [Google Scholar] [CrossRef]
- Dvela, M.; Rosen, H.; Feldmann, T.; Nesher, M.; Lichtstein, D. Diverse biological responses to different cardiotonic steroids. Pathophysiology 2007, 14, 159–166. [Google Scholar] [CrossRef]
- Agalakova, N.I.; Kolodkin, N.I.; Adair, D.C.; Trashkov, A.P.; Bagrov, A.Y. Preeclampsia: Cardiotonic Steroids, Fibrosis, Fli1 and Hint to Carcinogenesis. Int. J. Mol. Sci. 2021, 22, 1941. [Google Scholar] [CrossRef]
- Manna, S.K.; Sreenivasan, Y.; Sarkar, A. Cardiac glycoside inhibits IL-8-induced biological responses by downregulating IL-8 receptors through altering membrane fluidity. J. Cell. Physiol. 2006, 207, 195–207. [Google Scholar] [CrossRef]
- Tverskoi, A.M.; Poluektov, Y.M.; Klimanova, E.A.; Mitkevich, V.A.; Makarov, A.A.; Orlov, S.N.; Petrushanko, I.Y.; Lopina, O.D. Depth of the Steroid Core Location Determines the Mode of Na,K-ATPase Inhibition by Cardiotonic Steroids. Int. J. Mol. Sci. 2021, 22, 13268. [Google Scholar] [CrossRef]
- Ihenetu, K.; Espinosa, R.; Leon, R.; Planas, G.; Perez-Pinero, A.; Waldbeser, L. Digoxin and digoxin-like immunoreactive factors (DLIF) modulate the release of pro-inflammatory cytokines. Inflamm. Res. 2008, 57, 519–523. [Google Scholar] [CrossRef]
- Orlov, S.N.; Tverskoi, A.M.; Sidorenko, S.V.; Smolyaninova, L.V.; Lopina, O.D.; Dulin, N.O.; Klimanova, E.A. Na,K-ATPase as a target for endogenous cardiotonic steroids: What’s the evidence? Genes Dis. 2021, 8, 259–271. [Google Scholar] [CrossRef]
- Klimanova, E.A.; Fedorov, D.A.; Sidorenko, S.V.; Abramicheva, P.A.; Lopina, O.D.; Orlov, S.N. Ouabain and Marinobufagenin: Physiological Effects on Human Epithelial and Endothelial Cells. Biochemistry 2020, 85, 507–515. [Google Scholar] [CrossRef]
- Pasatetskaya, N.A.; Klimshin, S.I.; Lopatina, E.V. Role of Na+,K+-ATPase in Bone Remodeling. Bull. Exp. Biol. Med. 2023, 174, 678–680. [Google Scholar] [CrossRef]
- Cavalcante-Silva, L.H.A.; Carvalho, D.C.M.; Lima, É.A.; Rodrigues-Mascarenhas, S. Ouabain inhibits p38 activation in mice neutrophils. Inflammopharmacology 2021, 29, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Galvão, J.G.F.M.; Cavalcante-Silva, L.H.A.; de Almeida Lima, É.; Carvalho, D.C.M.; Alves, A.F.; Mascarenhas, S.R. Ouabain modulates airway remodeling caused by Th2-high asthma in mice. Int. Immunopharmacol. 2022, 109, 108808. [Google Scholar] [CrossRef]
- Carvalho, D.C.M.; Cavalcante-Silva, L.H.A.; Lima, É.A.; Galvão, J.G.F.M.; Alves, A.K.A.; Feijó, P.R.O.; Quintas, L.E.M.; Rodrigues-Mascarenhas, S. Marinobufagenin inhibits neutrophil migration and proinflammatory cytokines. J. Immunol. Res. 2019, 2019, 1094520. [Google Scholar] [CrossRef]
- Yu, S.; Tripod, M.; Atasoy, U.; Chen, J. HuR Plays a Positive Role to Strengthen the Signaling Pathways of CD4+ T Cell Activation and Th17 Cell Differentiation. J. Immunol. Res. 2021, 2021, 9937243. [Google Scholar] [CrossRef]
- Saeed, H.; Somaiya Mateen, S.; Moin, S.; Khan, A.Q.; Owais, M. Cardiac glycoside digoxin ameliorates pro-inflammatory cytokines in PBMCs of rheumatoid arthritis patients in vitro. Int. Immunopharmacol. 2020, 82, 106331. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, K.; Liu, Z.; Liu, J.; Tian, Z.; Qin, S.; Wei, J.; Cheng, L. Digoxin protects against intervertebral disc degeneration via TNF/NF-κB and LRP4 signaling. Front. Immunol. 2023, 14, 1251517. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.; Saldanha, A.A.; Moraes, A.M.; Oliveira, F.M.; Lopes, D.O.; Barbosa, L.A.O.; Ribeiro, R.I.M.A.; Thomé, R.G.; Santos, H.B.D.; Villar, J.A.F.P.; et al. 21 Benzylidene digoxin, a novel digoxin hemi-synthetic derivative, presents an anti-inflammatory activity through inhibition of edema, tumor necrosis factor-alpha production, inducible nitric oxide synthase expression, and leucocyte migration. Int. Immunopharmacol. 2018, 65, 174–181. [Google Scholar] [CrossRef] [PubMed]
- de Souza Gonçalves, B.; de Moura Valadares, J.M.; Alves, S.L.G.; Silva, S.C.; Rangel, L.P.; Cortes, V.F.; Villar, J.A.F.P.; Barbosa, L.A.; de Lima Santos, H. Evaluation of neuroprotective activity of digoxin and semisynthetic derivatives against partial chemical ischemia. J. Cell. Biochem. 2019, 120, 17108–17122. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Yosri, N.; El-Aarag, B.; Mahmoud, S.H.; Zayed, A.; Du, M.; Saeed, A.; Musharraf, S.G.; El-Garawani, I.M.; Habib, M.R.; et al. Chemistry and the Potential Antiviral, Anticancer, and Anti-Inflammatory Activities of Cardiotonic Steroids Derived from Toads. Molecules 2022, 27, 6586. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, G.C.; Rocha, S.C.; Lopes, M.A.S.; Paixão, N.; Alves, S.L.G.; Pessoa, M.T.C.; Noël, F.; Quintas, E.M.; Barbosa, L.A.; Villar, J.A.F.P.; et al. Implications of Synthetic Modifications of the Cardiotonic Steroid Lactone Ring on Cytotoxicity. J. Membr. Biol. 2021, 254, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Pessôa, M.T.C.; Valadares, J.M.M.; Rocha, S.C.; Silva, S.C.; McDermott, J.P.; Sánchez, G.; Varotti, F.P.; Scavone, C.; Ribeiro, R.I.M.A.; Villar, J.A.F.P.; et al. 21-Benzylidene digoxin decreases proliferation by inhibiting the EGFR/ERK signaling pathway and induces apoptosis in HeLa cells. Steroids 2020, 155, 108551. [Google Scholar] [CrossRef]
- Pessôa, M.T.C.; Alves, S.L.G.; Taranto, A.G.; Villar, J.A.F.P.; Blanco, G.; Barbosa, L.A. Selectivity analyses of γ-benzylidene digoxin derivatives to different Na, K-ATPase α isoforms: A molecular docking approach. J. Enzym. Inhib. Med. Chem. 2018, 33, 85–97. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- Myung, Y.; de Sá, A.G.C.; Ascher, D.B. Deep-PK: Deep Learning for Small Molecule Pharmacokinetic and Toxicity Prediction. Nucleic Acids Res. 2024, 52, W469–W475. [Google Scholar] [CrossRef]
- Lagunin, A.A.; Dubovskaja, V.I.; Rudik, A.V.; Pogodin, P.V.; Druzhilovskiy, D.S.; Gloriozova, T.A.; Filimonov, D.A.; Sastry, N.G.; Poroikov, V.V. CLC-Pred: A Freely Available Web-Service for in Silico Prediction of Human Cell Line Cytotoxicity for Drug-like Compounds. PLoS ONE 2018, 13, e0191838. [Google Scholar] [CrossRef]
- Lagunin, A.; Ivanov, S.; Rudik, A.; Filimonov, D.; Poroikov, V. DIGEP-Pred: Web Service for in Silico Prediction of Drug-Induced Gene Expression Profiles Based on Structural Formula. Bioinformatics 2013, 29, 2062–2063. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, A.V.; Filimonov, D.A.; Rudik, A.V.; Pogodin, P.V.; Karasev, D.A.; Lagunin, A.A.; Poroikov, V.V. Drug-Drug Interaction Prediction Using PASS. SAR QSAR Environ. Res. 2019, 30, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Pogodin, P.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. PASS Targets: Ligand-Based Multi-Target Computational System Based on a Public Data and Naïve Bayes Approach. SAR QSAR Environ. Res. 2015, 26, 783–793. [Google Scholar] [CrossRef] [PubMed]
- de França, J.S.; de Sales-Neto, J.M.; Carvalho, D.C.M.; de Almeida Lima, É.; Olegário, T.R.; Mendes, R.K.S.; Lima-Junior, C.G.; de Almeida, V.M.L.A.; Rodrigues-Mascarenhas, S. Morita-Baylis-Hillman Adduct 2-(3-Hydroxy-2-oxoindolin-3-yl)acrylonitrile (ISACN) Modulates Inflammatory Process In vitro and In vivo. Inflammation 2021, 44, 899–907. [Google Scholar] [CrossRef]
- Sales-Neto, J.M.; da Silva, J.S.F.; Carvalho, D.C.M.; Lima, É.A.; Cavalcante-Silva, L.H.A.; Lettnin, A.P.; Votto, A.P.S.; Vasconcelos, U.; Rodrigues-Mascarenhas, S. Patulin inhibits LPS-induced nitric oxide production by suppressing MAPKs signaling pathway. Nat. Prod. Res. 2022, 36, 5879–5883. [Google Scholar] [CrossRef]
- Lima, É.A.; Cavalcante-Silva, L.H.A.; Carvalho, D.C.M.; Netto, C.D.; Costa, P.R.R.; Rodrigues-Mascarenhas, S. The pterocarpanquinone LQB 118 inhibits inflammation triggered by zymosan in vivo and in vitro. Int. Immunopharmacol. 2020, 83, 106399. [Google Scholar] [CrossRef] [PubMed]
- Benov, L. Improved Formazan Dissolution for Bacterial MTT Assay. Microbiol. Spectr. 2021, 9, e0163721. [Google Scholar] [CrossRef]
- Vargas-Maya, N.I.; Padilla-Vaca, F.; Romero-González, O.E.; Rosales-Castillo, E.A.S.; Rangel-Serrano, Á.; Arias-Negrete, S.; Franco, B. Refinement of the Griess method for measuring nitrite in biological samples. J. Microbiol. Methods 2021, 187, 106260. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, J.P.; Gutiérrez, J.M.; Teixeira, C. Signaling pathways involved in zymosan phagocytosis induced by two secreted phospholipases A2 isolated from Bothrops asper snake venom in macrophages. Int. J. Biol. Macromol. 2018, 113, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.M.; Raslan, M.; Salama, M.M.; Menze, E.T.; El Hawary, S.S. In vivo anti-inflammatory activity and UPLC-MS/MS profiling of the peels and pulps of Cucumis melo var. cantalupensis and Cucumis melo var. reticulatus. J. Ethnopharmacol. 2019, 237, 245–254. [Google Scholar] [CrossRef]
- de Vasconcelos, D.I.; Leite, J.A.; Carneiro, L.T.; Piuvezam, M.R.; de Lima, M.R.; de Morais, L.C.; Rumjanek, V.M.; Rodrigues-Mascarenhas, S. Anti-inflammatory and Antinociceptive Activity of Ouabain in Mice. Mediat. Inflamm. 2011, 2011, 912925. [Google Scholar] [CrossRef]
- Silva, L.R.; Alves, A.F.; Cavalcante-Silva, L.H.A.; Braga, R.M.; Almeida, R.N.; Barbosa-Filho, J.M.; Piuvezam, M.R. Milonine, a Morphinandienone Alkaloid, Has Anti-Inflammatory and Analgesic Effects by Inhibiting TNF-α and IL-1β Production. Inflammation 2017, 40, 2074–2085. [Google Scholar] [CrossRef]
- Park, S.J.; Im, D.-S. 2-Arachidonyl-lysophosphatidylethanolamine Induces Anti-Inflammatory Effects on Macrophages and in Carrageenan-Induced Paw Edema. Int. J. Mol. Sci. 2021, 22, 4865. [Google Scholar] [CrossRef] [PubMed]
- Bassani, D.; Parrott, N.J.; Manevski, N.; Zhang, J.D. Another string to your bow: Machine learning prediction of the pharmacokinetic properties of small molecules. Expert Opin. Drug Discov. 2024, 19, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Orellana, A.M.; Kinoshita, P.F.; Leite, J.A.; Kawamoto, E.; Scavone, C. Cardiotonic Steroids as Modulators of Neuroinflammation. Front. Endocrinol. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Seljelid, R.; Plytycz, B. Role of mast cells in zymosan-induced peritoneal inflammation in Balb/c and mast cell-deficient WBB6F1 mice. J. Leukoc. Biol. 2001, 69, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Koziol, A.; Plytycz, B.; Arnold, B. Inflammatory macrophages, and not only neutrophils, die by apoptosis during acute peritonitis. Immunobiology 2010, 215, 492–504. [Google Scholar] [CrossRef]
- Arya, P.; Bhandari, U. Involvement of the toll-like receptors-2/nuclear factor-kappa B signaling pathway in atherosclerosis induced by high-fat diet and zymosan A in C57BL/6 mice. Indian J. Pharmacol. 2020, 52, 203–209. [Google Scholar] [CrossRef]
- Rahat, M.A.; Brod, V.; Amit-Cohen, B.C.; Henig, O.; Younis, S.; Bitterman, H. Oxygen mitigates the inflammatory response in a model of hemorrhage and zymosan-induced inflammation. Shock 2016, 45, 198–208. [Google Scholar] [CrossRef]
- Fan, X.; Zhu, J.Y.; Sun, Y.; Luo, L.; Yan, J.; Yang, X.; Yu, J.; Tang, W.Q.; Ma, W.; Liang, H.P. Evodiamine inhibits zymosan-induced inflammation in vitro and in vivo: Inactivation of NF-κB by inhibiting IκBα phosphorylation. Inflammation 2017, 40, 1012–1027. [Google Scholar] [CrossRef]
- Kleinert, H.; Schwarz, P.M.; Forstermann, U. Regulation of the expression. of inducible nitric oxide synthase. Biol. Chem. 2003, 384, 1343–1364. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Fan, K.; Zhang, D.; Hu, A.; Zeng, X.; Liu, Y.L.; Tan, G.; Wang, H. Frugoside delays osteoarthritis progression via inhibiting miR-155-modulated synovial macrophage M1 polarization. Rheumatology 2021, 60, 4899–4909. [Google Scholar] [CrossRef]
- Ma, W.T.; Gao, F.; Gu, K.; Chen, D.K. The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 2019, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, M.; Jia, S. Macrophage: Key player in the pathogenesis of autoimmune diseases. Front. Immunol. 2023, 14, 1080310. [Google Scholar] [CrossRef] [PubMed]
- Lendeckel, U.; Venz, S.; Wolke, C. Macrophages: Shapes and functions. ChemTexts 2022, 8, 12. [Google Scholar] [CrossRef]
- Hirayama, D.; Tomoya, I.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2018, 19, 92. [Google Scholar] [CrossRef]
- Galvão, J.G.F.M.; Cavalcante-Silva, L.H.A.; Carvalho, D.C.M.; Ferreira, L.K.D.P.; Monteiro, T.M.; Alves, A.F.; Ferreira, L.A.M.P.; Gadelha, F.A.A.F.; Piuvezam, M.R.; Rodrigues-Mascarenhas, S. Ouabain attenuates ovalbumin-induced airway inflammation. Inflamm. Res. 2017, 66, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Sul, O.J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Diekmann, M.; Hoffmeister, L.; Kühl, F.; Welz, B.; Brand, K. Is an Essential Regulator of Reactive Oxygen Species Production in the Monocytic Cell Type. Antioxidants 2022, 11, 1600. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Jiao, Y.; Chen, Q.; Wu, D.; Yu, P.; Li, Y.; Cai, M.; Zhao, Y. Polystyrene nanoplastic induces ROS production and affects the MAPK-HIF-1/NFkB-mediated antioxidant system in Daphnia pulex. Aquat. Toxicol. 2020, 220, 105420. [Google Scholar] [CrossRef]
- Zhang, J.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Imunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Carey, A.J.; Tan, C.K.; Ulett, G.C. Infection-induced IL-10 and JAK-STAT. JAK-STAT 2012, 1, 159–167. [Google Scholar] [CrossRef]
- Ijomone, O.M.; Iroegbu, J.D.; Aschner, M.; Bornhorst, J. Impact of environmental toxicants on p38- and ERK-MAPK signaling pathways in the central nervous system. Neurotoxicology 2021, 86, 166–171. [Google Scholar] [CrossRef]
- Liu, J.; Du, J.; Chen, X.; Yang, L.; Zhao, W.; Song, M.; Wang, Z.; Wang, Y. The Effects of Mitogen-activated Protein Kinase Signaling Pathways on Lipopolysaccharide-mediated Osteo/Odontogenic Differentiation of Stem Cells from the Apical Papilla. J. Endod. 2019, 45, 161–167. [Google Scholar] [CrossRef]
- Zhakeer, Z.; Hadeer, M.; Tuerxun, Z.; Tuerxun, K. Bufalin Inhibits the Inflammatory Effects in Asthmatic Mice through the Suppression of Nuclear Factor-Kappa B Activity. Pharmacology 2017, 99, 179–187. [Google Scholar] [CrossRef]
- Suarez, R.; Buelvas, N. Inflammasome: Activation mechanisms. Investig. Clin. 2015, 56, 074–099. [Google Scholar] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef]
- Lv, Y.; Fang, L.; Ding, P.; Liu, R. PI3K/Akt-Beclin1 signaling pathway positively regulates phagocytosis and negatively mediates NF-κB-dependent inflammation in Staphylococcus aureus-infected macrophages. Biochem. Biophys. Res. Commun. 2019, 510, 284–289. [Google Scholar] [CrossRef]
- Yang, X.S.; Xu, Z.W.; Yi, T.L.; Xu, R.C.; Li, J.; Zhang, W.B.; Zhang, S.; Sun, H.T.; Yu, Z.Q.; Xu, H.X.; et al. Ouabain suppresses the growth and migration abilities of glioma U-87MG cells through inhibiting the Akt/mTOR signaling pathway and downregulating the expression of HIF-1α. Mol. Med. Rep. 2018, 17, 5595–5600. [Google Scholar] [CrossRef]
- Cui, S.; Jiang, H.; Chen, L.; Xu, J.; Sun, W.; Sun, H.; Xie, Z.; Xu, Y.; Yang, F.; Liu, W.; et al. Design, synthesis and evaluation of wound healing activity for β-sitosterols derivatives as potent Na+/K+-ATPase inhibitors. Bioorganic Chem. 2020, 98, 103150. [Google Scholar] [CrossRef]
- Chomczynski, P.W.; Vires, K.M.; Rymaszewski, M.; Heiny, J.A. A real-time PCR method to genotype mutant mouse models with altered affinity for cardiotonic steroids on the Na,K-ATPase. PLoS ONE 2022, 17, e0267348. [Google Scholar] [CrossRef]
- Leite, J.A.; Cavalcante-Silva, L.H.A.; Ribeiro, M.R.; de Morais Lima, G.; Scavone, C.; Rodrigues-Mascarenhas, S. Neuroinflammation and Neutrophils: Modulation by Ouabain. Front. Pharmacol. 2022, 13, 824907. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.A.; Isaksen, T.J.; Heuck, A.; Scavone, C.; Lykke-Hartmann, K. The α2 Na+/K+-ATPase isoform mediates LPS-induced neuroinflammation. Sci. Rep. 2020, 10, 14180. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, D.A.; Medeiros, A.B.A.; Soares, M.M.; Lima, É.d.A.; Oliveira, G.C.S.L.d.; Leite, M.B.d.S.; Machado, M.V.; Villar, J.A.F.P.; Barbosa, L.A.; Scavone, C.; et al. Evaluation of Anti-Inflammatory Activity of the New Cardiotonic Steroid γ-Benzylidene Digoxin 8 (BD-8) in Mice. Cells 2024, 13, 1568. https://doi.org/10.3390/cells13181568
Ferreira DA, Medeiros ABA, Soares MM, Lima ÉdA, Oliveira GCSLd, Leite MBdS, Machado MV, Villar JAFP, Barbosa LA, Scavone C, et al. Evaluation of Anti-Inflammatory Activity of the New Cardiotonic Steroid γ-Benzylidene Digoxin 8 (BD-8) in Mice. Cells. 2024; 13(18):1568. https://doi.org/10.3390/cells13181568
Chicago/Turabian StyleFerreira, Davi Azevedo, Anna Beatriz Araujo Medeiros, Mariana Mendonça Soares, Éssia de Almeida Lima, Gabriela Carolina Santos Lima de Oliveira, Mateus Bernardo da Silva Leite, Matheus Vieira Machado, José Augusto Ferreira Perez Villar, Leandro Augusto Barbosa, Cristoforo Scavone, and et al. 2024. "Evaluation of Anti-Inflammatory Activity of the New Cardiotonic Steroid γ-Benzylidene Digoxin 8 (BD-8) in Mice" Cells 13, no. 18: 1568. https://doi.org/10.3390/cells13181568
APA StyleFerreira, D. A., Medeiros, A. B. A., Soares, M. M., Lima, É. d. A., Oliveira, G. C. S. L. d., Leite, M. B. d. S., Machado, M. V., Villar, J. A. F. P., Barbosa, L. A., Scavone, C., Moura, M. T., & Rodrigues-Mascarenhas, S. (2024). Evaluation of Anti-Inflammatory Activity of the New Cardiotonic Steroid γ-Benzylidene Digoxin 8 (BD-8) in Mice. Cells, 13(18), 1568. https://doi.org/10.3390/cells13181568






