Temporal Transcriptomic Profiling of the Developing Xenopus laevis Eye
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Surgeries
2.2. Sample Collection and RNA Extraction
2.3. Library Preparation and Sequencing
2.4. Data Preprocessing
2.5. Reference Genome and Annotation
2.6. Alignment, Quantification, and Quality Control Assessment
2.7. RNA-seq Data Analysis
2.8. Enrichment Analysis
3. Results and Discussion
3.1. Transcriptional Analysis of Developing Optic Tissues
3.2. Eye Development Gene Expression across Retinogenesis
3.3. Gene Ontology (GO) Analysis of Developing Optic Tissues
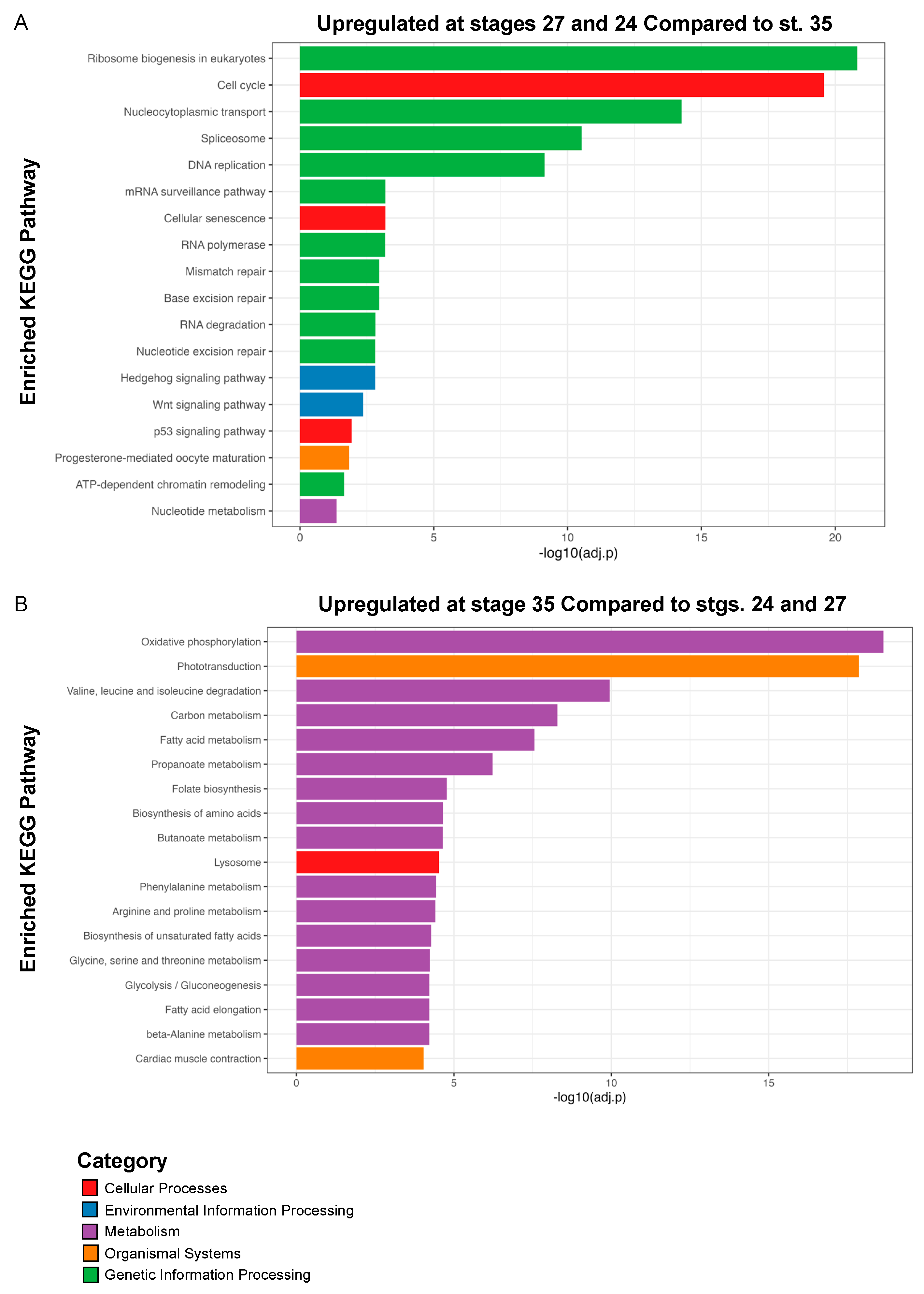
3.4. KEGG Pathway Analyses Identify Hedgehog, PPAR, and Wnt Signaling as Potential Regulators of Eye Development
3.5. Conserved Eye Regeneration Gene Expression Analysis across Retinal Development
3.6. Long versus Short Gene Homeologs Are Differentially Expressed during Eye Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wetts, R.; Fraser, S.E. Multipotent precursors can give rise to all major cell types of the frog retina. Science 1988, 239, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.L.; Cepko, C.L. A common progenitor for neurons and glia persists in rat retina late in development. Nature 1987, 328, 131–136. [Google Scholar] [CrossRef]
- Holt, C.E.; Bertsch, T.W.; Ellis, H.M.; Harris, W.A. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron 1988, 1, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Viet, J.; Reboutier, D.; Hardy, S.; Lachke, S.A.; Paillard, L.; Gautier-Courteille, C. Modeling ocular lens disease in Xenopus. Dev. Dyn. 2020, 249, 610–621. [Google Scholar] [CrossRef]
- Pratt, K.G.; Khakhalin, A.S. Modeling human neurodevelopmental disorders in the Xenopus tadpole: From mechanisms to therapeutic targets. Dis. Model. Mech. 2013, 6, 1057–1065. [Google Scholar] [CrossRef]
- Nakayama, T.; Fisher, M.; Nakajima, K.; Odeleye, A.O.; Zimmerman, K.B.; Fish, M.B.; Yaoita, Y.; Chojnowski, J.L.; Lauderdale, J.D.; Netland, P.A.; et al. Xenopus pax6 mutants affect eye development and other organ systems, and have phenotypic similarities to human aniridia patients. Dev. Biol. 2015, 408, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Lee-Liu, D.; Méndez-Olivos, E.E.; Muñoz, R.; Larraín, J. The African clawed frog Xenopus laevis: A model organism to study regeneration of the central nervous system. Neurosci. Lett. 2017, 652, 82–93. [Google Scholar] [CrossRef]
- Kha, C.X.; Son, P.H.; Lauper, J.; Tseng, K.A. A model for investigating developmental eye repair in Xenopus laevis. Exp. Eye Res. 2018, 169, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kha, C.X.; Guerin, D.J.; Tseng, K.A. Using the Xenopus Developmental Eye Regrowth System to Distinguish the Role of Developmental Versus Regenerative Mechanisms. Front. Physiol. 2019, 10, 502. [Google Scholar] [CrossRef]
- Zuber, M.E. Eye field specification in Xenopus laevis. Curr. Top. Dev. Biol. 2010, 93, 29–60. [Google Scholar] [CrossRef]
- Zuber, M.E.; Gestri, G.; Viczian, A.S.; Barsacchi, G.; Harris, W.A. Specification of the vertebrate eye by a network of eye field transcription factors. Development 2003, 130, 5155–5167. [Google Scholar] [CrossRef] [PubMed]
- Diacou, R.; Nandigrami, P.; Fiser, A.; Liu, W.; Ashery-Padan, R.; Cvekl, A. Cell fate decisions, transcription factors and signaling during early retinal development. Prog. Retin. Eye Res. 2022, 91, 101093. [Google Scholar] [CrossRef] [PubMed]
- Holt, C. Cell movements in Xenopus eye development. Nature 1980, 287, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Harris, W.A. Sequential genesis and determination of cone and rod photoreceptors in Xenopus. J. Neurobiol. 1998, 35, 227–244. [Google Scholar] [PubMed]
- Le Blay, K.; Préau, L.; Morvan-Dubois, G.; Demeneix, B. Expression of the inactivating deiodinase, Deiodinase 3, in the pre-metamorphic tadpole retina. PLoS ONE 2018, 13, e0195374. [Google Scholar] [CrossRef]
- Straznicky, K.; Gaze, R.M. The growth of the retina in Xenopus laevis: An autoradiographic study. Development 1971, 26, 67–79. [Google Scholar] [CrossRef]
- Man, L.L.H.; Storey, S.S.; Bertolesi, G.E.; McFarlane, S. Cell-type expression and activation by light of neuropsins in the developing and mature Xenopus retina. Front. Cell Neurosci. 2023, 17, 1266945. [Google Scholar] [CrossRef]
- Liao, Y.; Ma, L.; Guo, Q.; E, W.; Fang, X.; Yang, L.; Ruan, F.; Wang, J.; Zhang, P.; Sun, Z.; et al. Publisher Correction: Cell landscape of larval and adult Xenopus laevis at single-cell resolution. Nat. Commun. 2022, 13, 5142. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.; Weinreb, C.; Wagner, D.E.; Megason, S.; Peshkin, L.; Kirschner, M.W.; Klein, A.M. The dynamics of gene expression in vertebrate embryogenesis at single-cell resolution. Science 2018, 360, eaar5780. [Google Scholar] [CrossRef]
- Ding, Y.; Colozza, G.; Zhang, K.; Moriyama, Y.; Ploper, D.; Sosa, E.A.; Benitez, M.D.J.; De Robertis, E.M. Genome-wide analysis of dorsal and ventral transcriptomes of the Xenopus laevis gastrula. Dev. Biol. 2017, 426, 176–187. [Google Scholar] [CrossRef]
- Giudetti, G.; Giannaccini, M.; Biasci, D.; Mariotti, S.; Degl’innocenti, A.; Perrotta, M.; Barsacchi, G.; Andreazzoli, M. Characterization of the Rx1-dependent transcriptome during early retinal development. Dev. Dyn. 2014, 243, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Viczian, A.S.; Solessio, E.C.; Lyou, Y.; Zuber, M.E. Generation of functional eyes from pluripotent cells. PLoS Biol. 2009, 7, e1000174. [Google Scholar] [CrossRef]
- Casarosa, S.; Amato, M.A.; Andreazzoli, M.; Gestri, G.; Barsacchi, G.; Cremisi, F. Xrx1 controls proliferation and multipotency of retinal progenitors. Mol. Cell Neurosci. 2003, 22, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Louie, S.H.; Fisher, M.; Grainger, R.M. Elucidating the framework for specification and determination of the embryonic retina. Exp. Cell Res. 2020, 397, 112316. [Google Scholar] [CrossRef] [PubMed]
- Bessodes, N.; Parain, K.; Bronchain, O.; Bellefroid, E.J.; Perron, M. Prdm13 forms a feedback loop with Ptf1a and is required for glycinergic amacrine cell genesis in the Xenopus Retina. Neural Dev. 2017, 12, 16. [Google Scholar] [CrossRef]
- Parain, K.; Mazurier, N.; Bronchain, O.; Borday, C.; Cabochette, P.; Chesneau, A.; Colozza, G.; El Yakoubi, W.; Hamdache, J.; Locker, M.; et al. A large scale screen for neural stem cell markers in Xenopus retina. Dev. Neurobiol. 2012, 72, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Digregorio, M.; Voto, I.A.P.; Morabito, G.; Degl’Innocenti, A.; Giudetti, G.; Giannaccini, M.; Andreazzoli, M. Kdm7a expression is spatiotemporally regulated in developing Xenopus laevis embryos, and its overexpression influences late retinal development. Dev. Dyn. 2024, 253, 508–518. [Google Scholar] [CrossRef]
- Ledford, K.L.; Martinez-De Luna, R.I.; Theisen, M.A.; Rawlins, K.D.; Viczian, A.S.; Zuber, M.E. Distinct cis-acting regions control six6 expression during eye field and optic cup stages of eye formation. Dev. Biol. 2017, 426, 418–428. [Google Scholar] [CrossRef]
- Zaghloul, N.A.; Moody, S.A. Alterations of rx1 and pax6 expression levels at neural plate stages differentially affect the production of retinal cell types and maintenance of retinal stem cell qualities. Dev. Biol. 2007, 306, 222–240. [Google Scholar] [CrossRef]
- Zaghloul, N.A.; Moody, S.A. Changes in Rx1 and Pax6 activity at eye field stages differentially alter the production of amacrine neurotransmitter subtypes in Xenopus. Mol. Vis. 2007, 13, 86–95. [Google Scholar]
- Wong, L.L.; Rapaport, D.H. Defining retinal progenitor cell competence in Xenopus laevis by clonal analysis. Development 2009, 136, 1707–1715. [Google Scholar] [CrossRef]
- Sive, H.L.; Grainger, R.M.; Harland, R.M. Early Development of Xenopus laevis: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2000. [Google Scholar]
- Nieuwkoop, P.D.; Faber, J. Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Matamorphosis; North-Holland Publishing Company: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Kha, C.X.; Guerin, D.J.; Tseng, K.A. Studying In Vivo Retinal Progenitor Cell Proliferation in Xenopus laevis. Methods Mol. Biol. 2020, 2092, 19–33. [Google Scholar] [CrossRef]
- McDonough, M.J.; Allen, C.E.; Ng-Sui-Hing, N.K.; Rabe, B.A.; Lewis, B.B.; Saha, M.S. Dissection, culture, and analysis of Xenopus laevis embryonic retinal tissue. J. Vis. Exp. 2012, 70, e4377. [Google Scholar] [CrossRef]
- Bowes, J.B.; Snyder, K.A.; Segerdell, E.; Jarabek, C.J.; Azam, K.; Zorn, A.M.; Vize, P.D. Xenbase: Gene expression and improved integration. Nucleic Acids Res. 2010, 38, D607–D612. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; James-Zorn, C.; Ponferrada, V.; Bell, A.J.; Sundararaj, N.; Segerdell, E.; Chaturvedi, P.; Bayyari, N.; Chu, S.; Pells, T.; et al. Xenbase: Key features and resources of the Xenopus model organism knowledgebase. Genetics 2023, 224, iyad018. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.E.; Segerdell, E.; Matentzoglu, N.; Nenni, M.J.; Fortriede, J.D.; Chu, S.; Pells, T.J.; Osumi-Sutherland, D.; Chaturvedi, P.; James-Zorn, C.; et al. The Xenopus phenotype ontology: Bridging model organism phenotype data to human health and development. BMC Bioinform. 2022, 23, 99. [Google Scholar] [CrossRef]
- Xue, X.Y.; Harris, W.A. Using myc genes to search for stem cells in the ciliary margin of the Xenopus retina. Dev. Neurobiol. 2012, 72, 475–490. [Google Scholar] [CrossRef]
- Khosrowshahian, F.; Wolanski, M.; Chang, W.Y.; Fujiki, K.; Jacobs, L.; Crawford, M.J. Lens and retina formation require expression of Pitx3 in Xenopus pre-lens ectoderm. Dev. Dyn. 2005, 234, 577–589. [Google Scholar] [CrossRef]
- Furukawa, T.; Morrow, E.M.; Cepko, C.L. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell 1997, 91, 531–541. [Google Scholar] [CrossRef]
- Hoshino, A.; Ratnapriya, R.; Brooks, M.J.; Chaitankar, V.; Wilken, M.S.; Zhang, C.; Starostik, M.R.; Gieser, L.; La Torre, A.; Nishio, M.; et al. Molecular Anatomy of the Developing Human Retina. Dev. Cell 2017, 43, 763–779.e4. [Google Scholar] [CrossRef]
- Clark, B.S.; Stein-O’Brien, G.L.; Shiau, F.; Cannon, G.H.; Davis-Marcisak, E.; Sherman, T.; Santiago, C.P.; Hoang, T.V.; Rajaii, F.; James-Esposito, R.E.; et al. Single-Cell RNA-Seq Analysis of Retinal Development Identifies NFI Factors as Regulating Mitotic Exit and Late-Born Cell Specification. Neuron 2019, 102, 1111–1126.e5. [Google Scholar] [CrossRef] [PubMed]
- Muto, A.; Iida, A.; Satoh, S.; Watanabe, S. The group E Sox genes Sox8 and Sox9 are regulated by Notch signaling and are required for Müller glial cell development in mouse retina. Exp. Eye Res. 2009, 89, 549–558. [Google Scholar] [CrossRef]
- Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; Hill, D.P.; et al. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef]
- Kha, C.X.; Tseng, K.A. Developmental dependence for functional eye regrowth in Xenopus laevis. Neural Regen. Res. 2018, 13, 1735–1737. [Google Scholar] [CrossRef]
- Viczian, A.S.; Zuber, M.E. A simple behavioral assay for testing visual function in Xenopus laevis. J. Vis. Exp. 2014, 88, 51726. [Google Scholar] [CrossRef]
- Locker, M.; Agathocleous, M.; Amato, M.A.; Parain, K.; Harris, W.A.; Perron, M. Hedgehog signaling and the retina: Insights into the mechanisms controlling the proliferative properties of neural precursors. Genes Dev. 2006, 20, 3036–3048. [Google Scholar] [CrossRef]
- Agathocleous, M.; Iordanova, I.; Willardsen, M.I.; Xue, X.Y.; Vetter, M.L.; Harris, W.A.; Moore, K.B. A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development 2009, 136, 3289–3299. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, K.; Gan, L.; Yang, X.J. Distinct effects of Hedgehog signaling on neuronal fate specification and cell cycle progression in the embryonic mouse retina. J. Neurosci. 2009, 29, 6932–6944. [Google Scholar] [CrossRef]
- Amato, M.A.; Boy, S.; Perron, M. Hedgehog signaling in vertebrate eye development: A growing puzzle. Cell Mol. Life Sci. 2004, 61, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Cavodeassi, F.; Creuzet, S.; Etchevers, H.C. The hedgehog pathway and ocular developmental anomalies. Hum. Genet. 2019, 138, 917–936. [Google Scholar] [CrossRef]
- Fuhrmann, S. Wnt signaling in eye organogenesis. Organogenesis 2008, 4, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Van Raay, T.J.; Moore, K.B.; Iordanova, I.; Steele, M.; Jamrich, M.; Harris, W.A.; Vetter, M.L. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron 2005, 46, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.J.; Zhang, J.; Brown, E.C.; Van Bibber, A.M.; Van Es, J.; Clevers, H.; Ishikawa, T.O.; Taketo, M.M.; Vetter, M.L.; Fuhrmann, S. Investigation of Frizzled-5 during embryonic neural development in mouse. Dev. Dyn. 2008, 237, 1614–1626. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef] [PubMed]
- Wong-Riley, M.T. Energy metabolism of the visual system. Eye Brain 2010, 2, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Oginuma, M.; Moncuquet, P.; Xiong, F.; Karoly, E.; Chal, J.; Guevorkian, K.; Pourquié, O. A Gradient of Glycolytic Activity Coordinates FGF and Wnt Signaling during Elongation of the Body Axis in Amniote Embryos. Dev. Cell 2017, 40, 342–353.e10. [Google Scholar] [CrossRef]
- Miyazawa, H.; Aulehla, A. Revisiting the role of metabolism during development. Development 2018, 145, dev131110. [Google Scholar] [CrossRef]
- Reddien, P.W. Principles of regeneration revealed by the planarian eye. Curr. Opin. Cell Biol. 2021, 73, 19–25. [Google Scholar] [CrossRef]
- Silva, N.J.; Nagashima, M.; Li, J.; Kakuk-Atkins, L.; Ashrafzadeh, M.; Hyde, D.R.; Hitchcock, P.F. Inflammation and matrix metalloproteinase 9 (Mmp-9) regulate photoreceptor regeneration in adult zebrafish. Glia 2020, 68, 1445–1465. [Google Scholar] [CrossRef]
- Gozlan, O.; Sprinzak, D. Notch signaling in development and homeostasis. Development 2023, 150, dev201138. [Google Scholar] [CrossRef]
- Hori, K.; Sen, A.; Artavanis-Tsakonas, S. Notch signaling at a glance. J. Cell Sci. 2013, 126, 2135–2140. [Google Scholar] [CrossRef]
- Henrique, D.; Schweisguth, F. Mechanisms of Notch signaling: A simple logic deployed in time and space. Development 2019, 146, dev172148. [Google Scholar] [CrossRef] [PubMed]
- Coffman, C.R.; Skoglund, P.; Harris, W.A.; Kintner, C.R. Expression of an extracellular deletion of Xotch diverts cell fate in Xenopus embryos. Cell 1993, 73, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.L.; Turner, D.L.; Vetter, M.L. Notch signaling can inhibit Xath5 function in the neural plate and developing retina. Mol. Cell Neurosci. 2001, 18, 458–472. [Google Scholar] [CrossRef]
- Ohnuma, S.; Philpott, A.; Wang, K.; Holt, C.E.; Harris, W.A. p27Xic1, a Cdk inhibitor, promotes the determination of glial cells in Xenopus retina. Cell 1999, 99, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Marsh-Armstrong, N.; Huang, H.; Remo, B.F.; Liu, T.T.; Brown, D.D. Asymmetric growth and development of the Xenopus laevis retina during metamorphosis is controlled by type III deiodinase. Neuron 1999, 24, 871–878. [Google Scholar] [CrossRef][Green Version]
- Perron, M.; Kanekar, S.; Vetter, M.L.; Harris, W.A. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev. Biol. 1998, 199, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, J.; Li, X.; Li, B.; Tao, K.; Yue, S. Notch signaling pathway regulates cell cycle in proliferating hepatocytes involved in liver regeneration. J. Gastroenterol. Hepatol. 2018, 33, 1538–1547. [Google Scholar] [CrossRef]
- Zhao, L.; Borikova, A.L.; Ben-Yair, R.; Guner-Ataman, B.; MacRae, C.A.; Lee, R.T.; Burns, C.G.; Burns, C.E. Notch signaling regulates cardiomyocyte proliferation during zebrafish heart regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 1403–1408. [Google Scholar] [CrossRef]
- Campbell, L.J.; Levendusky, J.L.; Steines, S.A.; Hyde, D.R. Retinal regeneration requires dynamic Notch signaling. Neural Regen. Res. 2022, 17, 1199–1209. [Google Scholar] [CrossRef]
- Kraus, J.M.; Giovannone, D.; Rydzik, R.; Balsbaugh, J.L.; Moss, I.L.; Schwedler, J.L.; Bertrand, J.Y.; Traver, D.; Hankenson, K.D.; Crump, J.G.; et al. Notch signaling enhances bone regeneration in the zebrafish mandible. Development 2022, 149, dev199995. [Google Scholar] [CrossRef]
- Guerin, D.J.; Gutierrez, B.; Zhang, B.; Tseng, K.A.-S. Notch is Required for Neural Progenitor Proliferation during Embryonic Eye Regrowth. bioRxiv 2024. [Google Scholar] [CrossRef]
- Raj, B.; Wagner, D.E.; McKenna, A.; Pandey, S.; Klein, A.M.; Shendure, J.; Gagnon, J.A.; Schier, A.F. Simultaneous single-cell profiling of lineages and cell types in the vertebrate brain. Nat. Biotechnol. 2018, 36, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, X.; Hu, B.; Mao, Y.; Chen, Y.; Yan, L.; Yong, J.; Dong, J.; Wei, Y.; Wang, W.; et al. Dissecting the transcriptome landscape of the human fetal neural retina and retinal pigment epithelium by single-cell RNA-seq analysis. PLoS Biol. 2019, 17, e3000365. [Google Scholar] [CrossRef]
- Lu, Y.; Shiau, F.; Yi, W.; Lu, S.; Wu, Q.; Pearson, J.D.; Kallman, A.; Zhong, S.; Hoang, T.; Zuo, Z.; et al. Single-Cell Analysis of Human Retina Identifies Evolutionarily Conserved and Species-Specific Mechanisms Controlling Development. Dev. Cell 2020, 53, 473–491.e9. [Google Scholar] [CrossRef] [PubMed]
- Session, A.M.; Uno, Y.; Kwon, T.; Chapman, J.A.; Toyoda, A.; Takahashi, S.; Fukui, A.; Hikosaka, A.; Suzuki, A.; Kondo, M.; et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 2016, 538, 336–343. [Google Scholar] [CrossRef]
- Elurbe, D.M.; Paranjpe, S.S.; Georgiou, G.; van Kruijsbergen, I.; Bogdanovic, O.; Gibeaux, R.; Heald, R.; Lister, R.; Huynen, M.A.; van Heeringen, S.J.; et al. Regulatory remodeling in the allo-tetraploid frog Xenopus laevis. Genome Biol. 2017, 18, 198. [Google Scholar] [CrossRef]
- Watanabe, M.; Yasuoka, Y.; Mawaribuchi, S.; Kuretani, A.; Ito, M.; Kondo, M.; Ochi, H.; Ogino, H.; Fukui, A.; Taira, M.; et al. Conservatism and variability of gene expression profiles among homeologous transcription factors in Xenopus laevis. Dev. Biol. 2017, 426, 301–324. [Google Scholar] [CrossRef]
- Michiue, T.; Yamamoto, T.; Yasuoka, Y.; Goto, T.; Ikeda, T.; Nagura, K.; Nakayama, T.; Taira, M.; Kinoshita, T. High variability of expression profiles of homeologous genes for Wnt, Hh, Notch, and Hippo signaling pathways in Xenopus laevis. Dev. Biol. 2017, 426, 270–290. [Google Scholar] [CrossRef]
- Gout, J.F.; Kahn, D.; Duret, L. The relationship among gene expression, the evolution of gene dosage, and the rate of protein evolution. PLoS Genet. 2010, 6, e1000944. [Google Scholar] [CrossRef]
- Aury, J.M.; Jaillon, O.; Duret, L.; Noel, B.; Jubin, C.; Porcel, B.M.; Ségurens, B.; Daubin, V.; Anthouard, V.; Aiach, N.; et al. Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature 2006, 444, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Mears, A.J.; Kondo, M.; Swain, P.K.; Takada, Y.; Bush, R.A.; Saunders, T.L.; Sieving, P.A.; Swaroop, A. Nrl is required for rod photoreceptor development. Nat. Genet. 2001, 29, 447–452. [Google Scholar] [CrossRef]
- Showell, C.; Christine, K.S.; Mandel, E.M.; Conlon, F.L. Developmental expression patterns of Tbx1, Tbx2, Tbx5, and Tbx20 in Xenopus tropicalis. Dev. Dyn. 2006, 235, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Yoon, J.; Lee, M.H.; Jung, S.K.; Kim, D.J.; Bode, A.M.; Kim, J.; Dong, Z. The role of heterodimeric AP-1 protein comprised of JunD and c-Fos proteins in hematopoiesis. J. Biol. Chem. 2012, 287, 31342–31348. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.D.; Harafuji, N.; Archer, T.; Cunningham, D.D.; Casey, E.S. Xenopus Sox3 activates sox2 and geminin and indirectly represses Xvent2 expression to induce neural progenitor formation at the expense of non-neural ectodermal derivatives. Mech. Dev. 2009, 126, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, Y.; Gu, F.; He, X.; Cao, Z.; Li, X.; Tong, Y.; Ma, X. A novel nonsense mutation in CRYBB1 associated with autosomal dominant congenital cataract. Mol. Vis. 2008, 14, 727–731. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hack, S.J.; Petereit, J.; Tseng, K.A.-S. Temporal Transcriptomic Profiling of the Developing Xenopus laevis Eye. Cells 2024, 13, 1390. https://doi.org/10.3390/cells13161390
Hack SJ, Petereit J, Tseng KA-S. Temporal Transcriptomic Profiling of the Developing Xenopus laevis Eye. Cells. 2024; 13(16):1390. https://doi.org/10.3390/cells13161390
Chicago/Turabian StyleHack, Samantha J., Juli Petereit, and Kelly Ai-Sun Tseng. 2024. "Temporal Transcriptomic Profiling of the Developing Xenopus laevis Eye" Cells 13, no. 16: 1390. https://doi.org/10.3390/cells13161390
APA StyleHack, S. J., Petereit, J., & Tseng, K. A.-S. (2024). Temporal Transcriptomic Profiling of the Developing Xenopus laevis Eye. Cells, 13(16), 1390. https://doi.org/10.3390/cells13161390






