Anticancer Activity of Benzo[a]phenoxazine Compounds Promoting Lysosomal Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds, Cell Lines, and Culture Conditions
2.2. Sulforhodamine B Assay
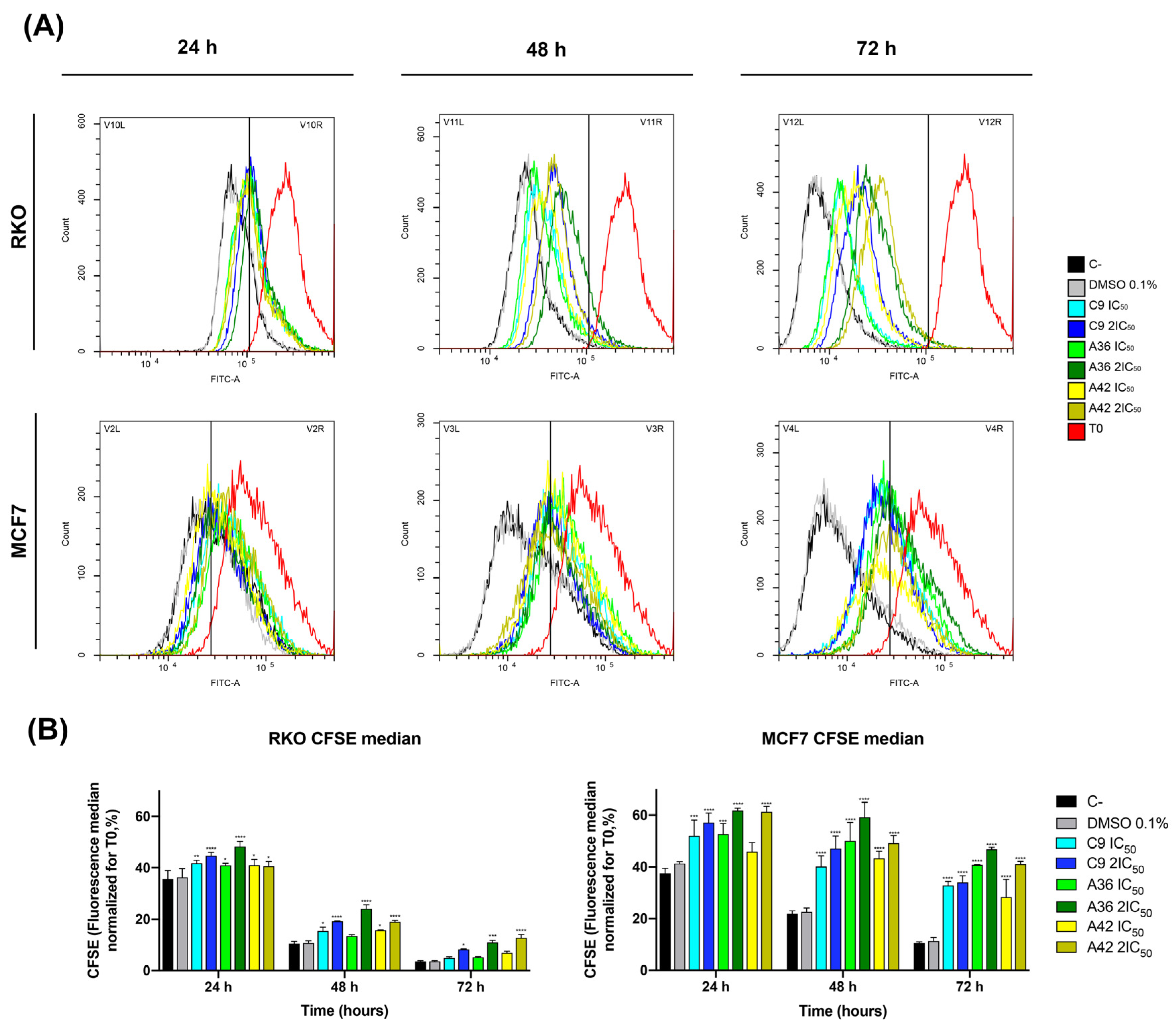
2.3. Cell Proliferation by CFSE
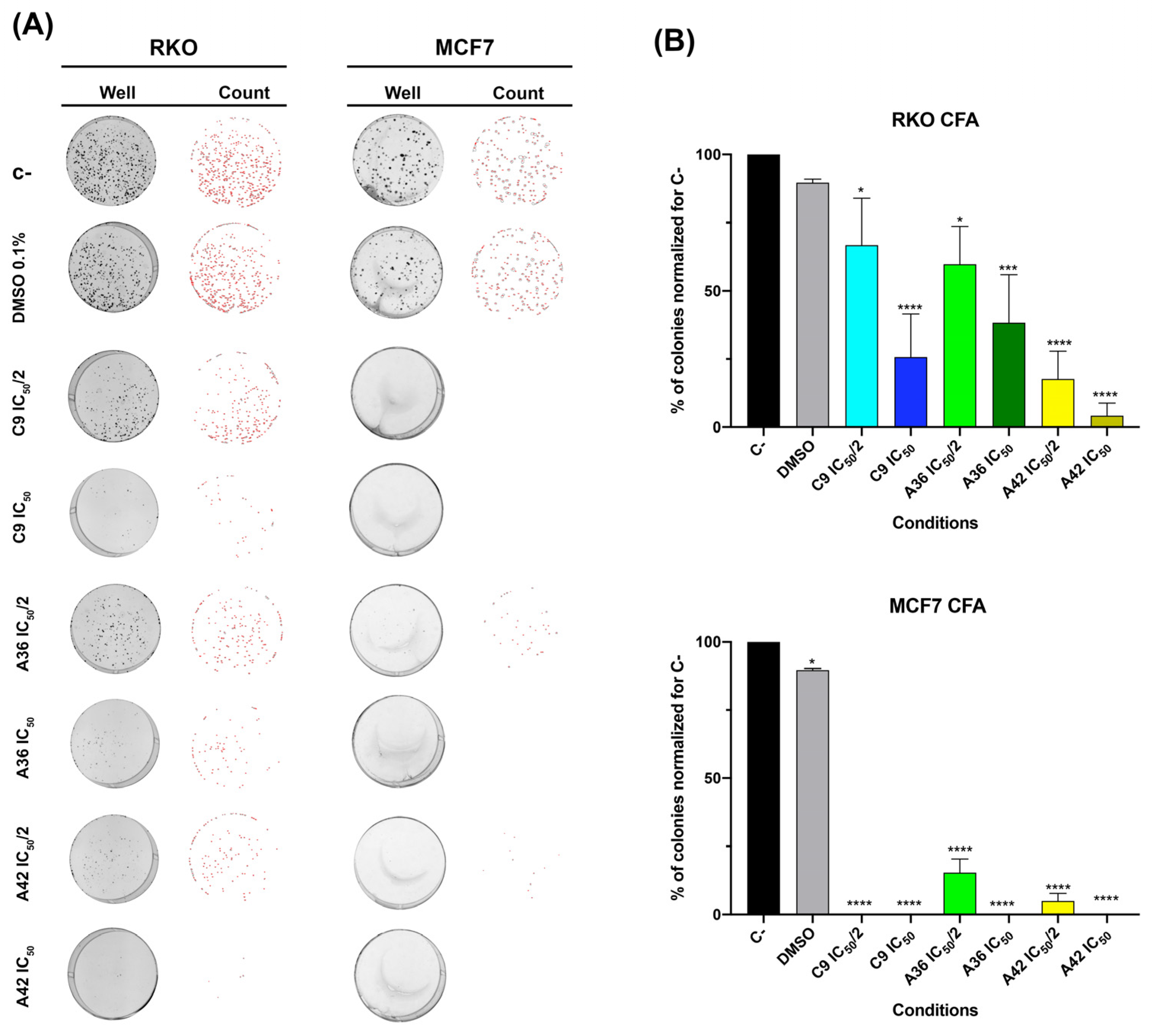
2.4. Colony Formation Assay
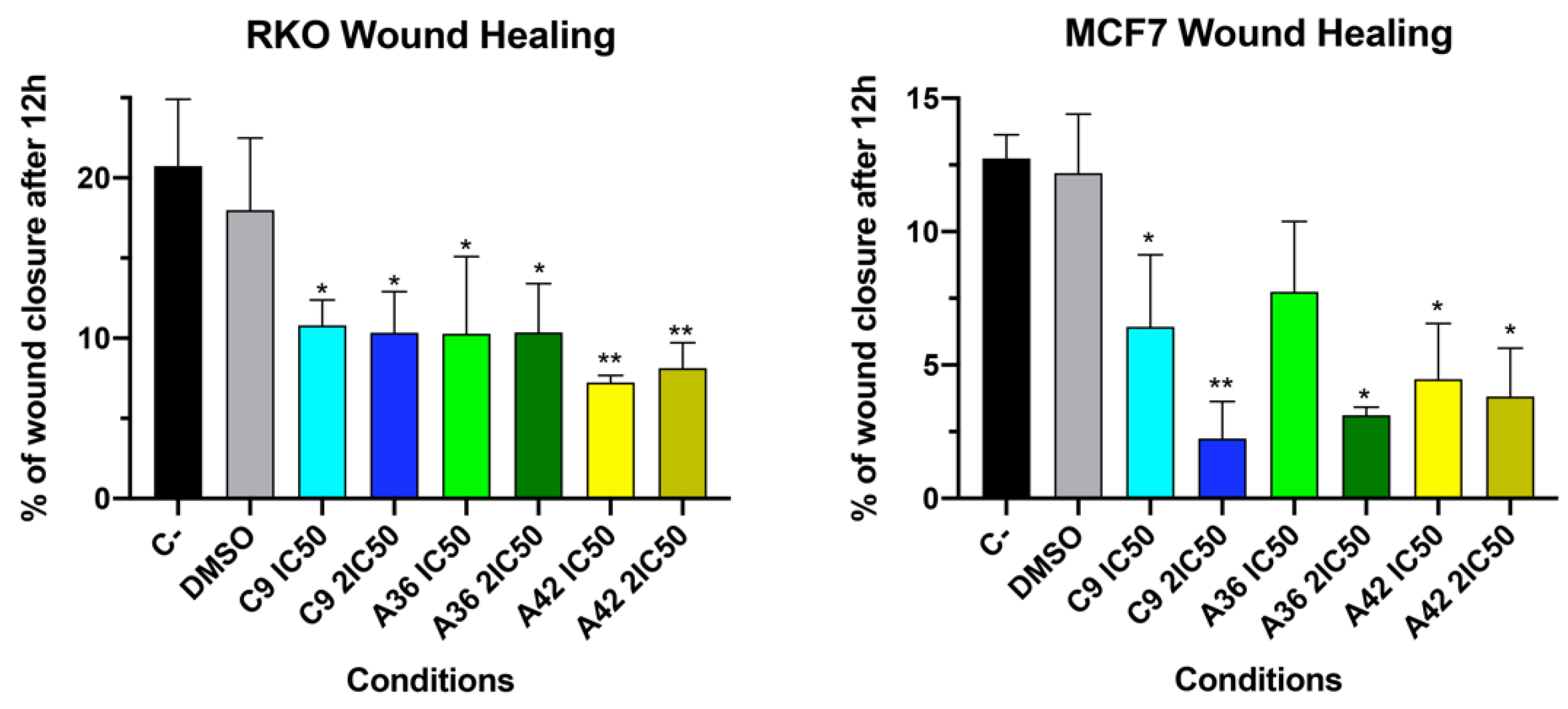
2.5. Wound Healing Assessment Assay
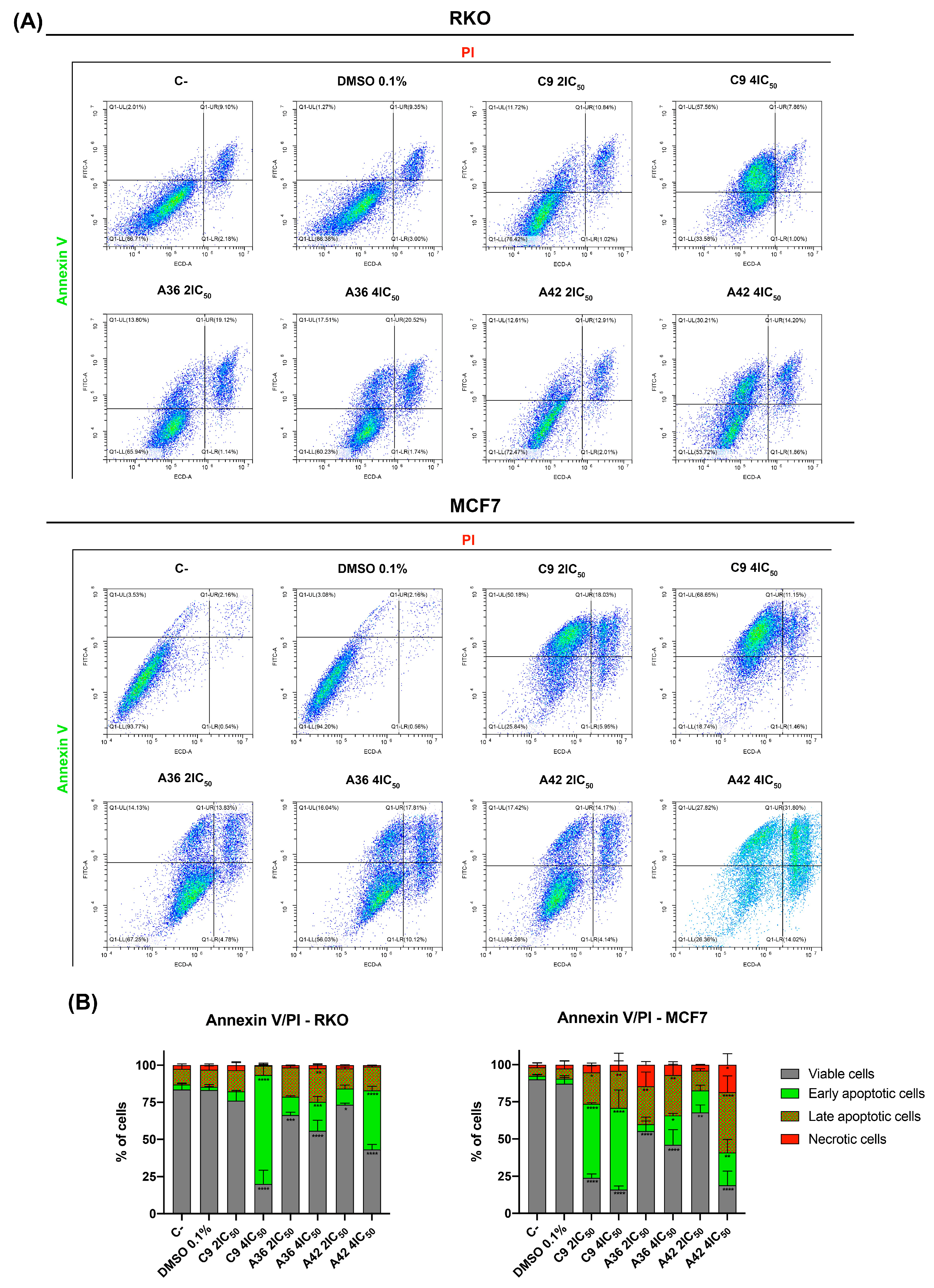
2.6. Annexin V/PI Assay
2.7. Evaluation of ROS Levels
2.8. Fluorescence Staining Evaluation
2.9. Lysosomal Membrane Permeabilization Assessment
2.10. Intracellular pH Evaluation
2.11. Fluorescence Microscopy and Flow Cytometry General Considerations
2.12. Data Analysis
3. Results and Discussion
3.1. Biological Activity of the Benzo[a]phenoxazine Compounds C9, A36, and A42
3.1.1. Effect of C9, A36, and A42 on Cell Viability
3.1.2. C9, A36, and A42 Decrease RKO and MCF7 Cell Proliferation
3.1.3. C9, A36, and A42 Reduce Cell Migration of RKO and MCF7 Cells
3.1.4. C9, A36, and A42 Reduce RKO and MCF7 Cell Survival
3.1.5. C9, A36, and A42 Induce RKO and MCF7 Cell Death
3.2. Intracellular Target Evaluation of the Benzo[a]phenoxazine Compounds C9, A36, and A42
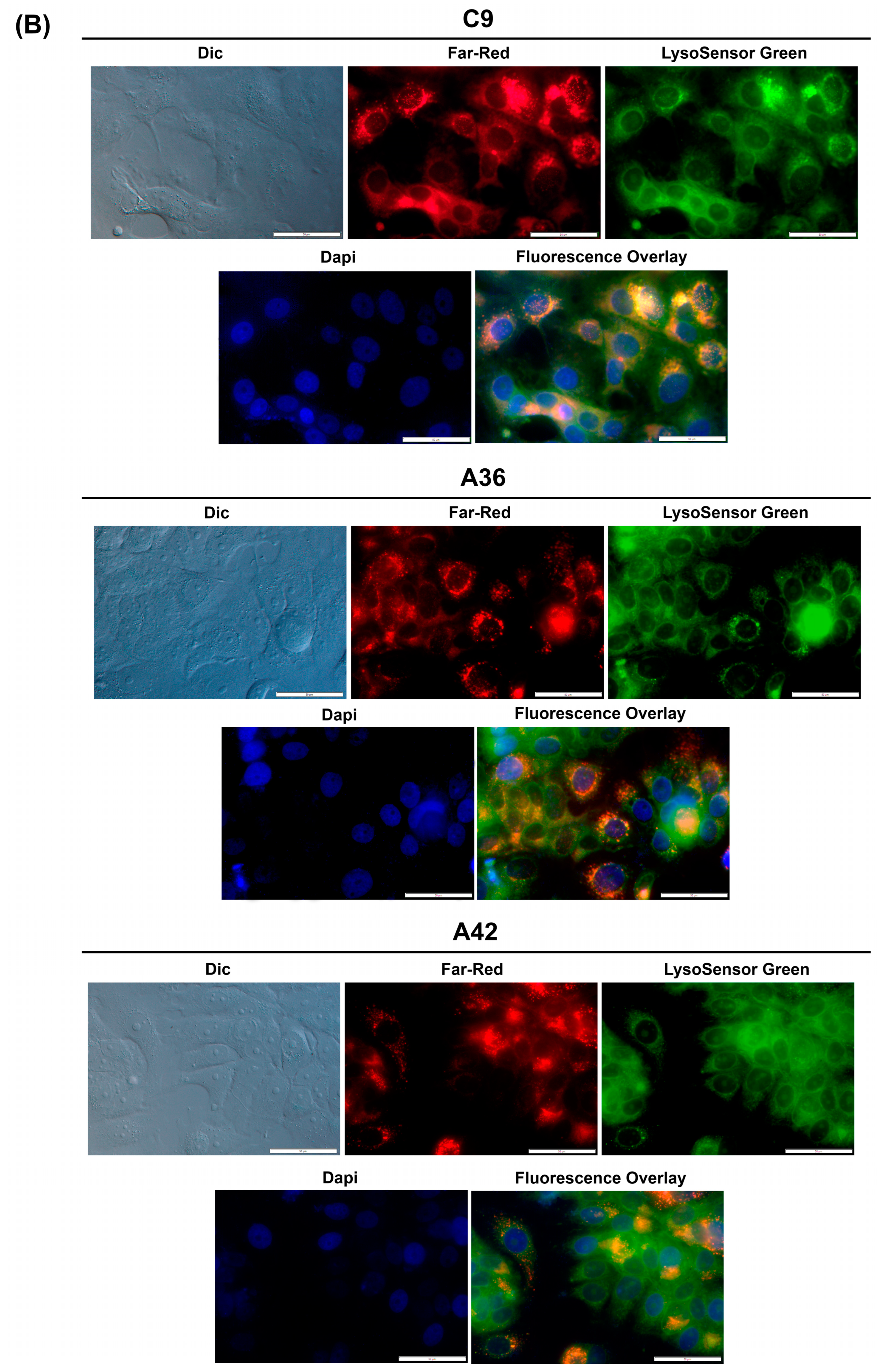
3.2.1. C9, A36, and A42 Accumulate and Emit Fluorescence on RKO and MCF7 Cell Lysosomes
3.2.2. C9, A36, and A42 Lysosomal Membrane Permeabilization, Cytosolic Acidification, and ROS Generation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Linnekamp, J.F.; Wang, X.; Medema, J.P.; Vermeulen, L. Colorectal cancer heterogeneity and targeted therapy: A case for molecular disease subtypes. Cancer Res. 2015, 75, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Sanz-Pamplona, R.; Nadal, E.; Grasselli, J.; Pernas, S.; Dienstmann, R.; Moreno, V.; Tabernero, J.; Salazar, R. Intrinsic cancer subtypes-next steps into personalized medicine. Cells Oncol. 2015, 38, 3–16. [Google Scholar] [CrossRef]
- Turashvili, G.; Brogi, E. Tumor heterogeneity in breast cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic resistance in colorectal cancer: A review. Ther. Adv. Med. Oncol. 2016, 8, 57–84. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Fakih, M.; Tabernero, J. Management of BRAF-mutant metastatic colorectal cancer: A review of treatment options and evidence-based guidelines. Ann. Oncol. 2021, 32, 959–967. [Google Scholar] [CrossRef]
- Peart, O. Breast intervention and breast cancer treatment options. Radiol. Technol. 2015, 86, 535M–558M. [Google Scholar]
- Yu, J.X.; Hubbard-Lucey, V.M.; Tang, J. The global pipeline of cell therapies for cancer. Nat. Rev. Drug Discov. 2019, 18, 821–822. [Google Scholar]
- Blakemore, D.C.; Castro, L.; Churcher, I.; Rees, D.C.; Thomas, A.W.; Wilson, D.M.; Wood, A. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018, 10, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.; Hashem, A.H.; El-Rashedy, A.A.; Kamel, S. Synthesis of novel heterocyclic compounds based on dialdehyde cellulose: Characterization, antimicrobial, antitumor activity, molecular dynamics simulation and target identification. Cellulose 2021, 28, 8355–8374. [Google Scholar] [CrossRef]
- Grygorenko, O.O.; Volochnyuk, D.M.; Ryabukhin, S.V.; Judd, D.B. The Symbiotic Relationship Between Drug Discovery and Organic Chemistry. Chem. A Eur. J. 2020, 26, 1196–1237. [Google Scholar] [CrossRef]
- Ezeokonkwo, M.A.; Okafor, S.N.; Ogbonna, O.N.; Onoabedje, E.A.; Ibeanu, F.N.; Godwin-Nwakwasi, E.U.; Ezema, B.E. New antimalarial agents derived from nonlinear phenoxazine ring system. Med. Chem. Res. 2020, 29, 63–74. [Google Scholar] [CrossRef]
- Patil, V.S.; Padalkar, V.S.; Phatangare, K.R.; Umape, P.G.; Borase, B.N.; Sekar, N. Synthesis, Characterization, and Antibacterial Activity of Novel (1H-Benzo[d]imidazole-2-yl)-6-(diethylamino)-3H-one-xanthene, Phenoxazine, and Oxazine. J. Heterocycl. Chem. 2015, 52, 124–129. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Jegan, A.; Premnath, D.; Periasamy, V.S.; Vasanthkumar, S. Design, synthesis, molecular docking as histone deacetylase (HDAC8) inhibitors, cytotoxicity and antibacterial evaluation of novel 6-(4-(4-aminophenylsulfonyl)phenylamino)-5H-benzo[a]phenoxazin-5-one derivatives. Med. Chem. Res. 2015, 24, 197–208. [Google Scholar] [CrossRef]
- Kozlovskaya, L.I.; Andrei, G.; Orlov, A.A.; Khvatov, E.V.; Koruchekov, A.A.; Belyaev, E.S.; Nikolaev, E.N.; Korshun, V.A.; Snoeck, R.; Osolodkin, D.I.; et al. Antiviral activity spectrum of phenoxazine nucleoside derivatives. Antiviral Res. 2019, 163, 117–124. [Google Scholar] [CrossRef]
- Sousa, R.P.C.L.; Ferreira, J.C.C.; Sousa, M.J.; Gonçalves, M.S.T. N-(5-Amino-9H-benzo[a]phenoxazin-9-ylidene)propan-1-aminium chlorides as antifungal agents and NIR fluorescent probes. New J. Chem. 2021, 45, 7808–7815. [Google Scholar] [CrossRef]
- Koshibu-Koizumi, J.; Akazawa, M.; Iwamoto, T.; Takasaki, M.; Mizuno, F.; Kobayashi, R.; Abe, A.; Tomoda, A.; Hamatake, M.; Ishida, R. Antitumor activity of a phenoxazine compound, 2-amino-4, 4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one against human B cell and T cell lymphoblastoid cell lines: Induction of mixed types of cell death, apoptosis, and necrosis. J. Cancer Res. Clin. Oncol. 2002, 128, 363–368. [Google Scholar]
- Kozlovskaya, L.I.; Volok, V.P.; Shtro, A.A.; Nikolaeva, Y.V.; Chistov, A.A.; Matyugina, E.S.; Belyaev, E.S.; Jegorov, A.V.; Snoeck, R.; Korshun, V.A.; et al. Phenoxazine nucleoside derivatives with a multiple activity against RNA and DNA viruses. Eur. J. Med. Chem. 2021, 220, 113467. [Google Scholar] [CrossRef]
- Wesolowska, O.; Molnar, J.; Westman, G.; Samuelsson, K.; Kawase, M.; Ocsovszki, I.; Motohashi, N.; Michalak, K. Benzo[a]phenoxazines: A new group of potent P-glycoprotein inhibitors. In Vivo 2006, 20, 109–114. [Google Scholar] [PubMed]
- Pal, S.; Konkimalla, V.B.; Kathawate, L.; Rao, S.S.; Gejji, S.P.; Puranik, V.G.; Weyhermüller, T.; Salunke-Gawali, S. Targeting a chemorefractory COLO205 (BRAF V600E) cell line using substituted benzo[a]phenoxazines. RSC Adv. 2015, 5, 82549–82563. [Google Scholar] [CrossRef]
- Sen, K.; Sen, K.; Shirley, D.A. Potential Carcinostatic Derivatives of Benzo[a]- and Benzo[b]phenoxazine. J. Org. Chem. 1961, 26, 3861–3863. [Google Scholar] [CrossRef]
- Thorne, S.H.; Barak, Y.; Liang, W.; Bachmann, M.H.; Rao, J.; Contag, C.H.; Matin, A. CNOB/ChrR6, a new prodrug enzyme cancer chemotherapy. Mol. Cancer Ther. 2009, 8, 333–341. [Google Scholar] [CrossRef]
- Lopes, M.; Alves, C.T.; Rama Raju, B.; Gonçalves, M.S.T.; Coutinho, P.J.G.; Henriques, M.; Belo, I. Application of benzo[a]phenoxazinium chlorides in antimicrobial photodynamic therapy of Candida albicans biofilms. J. Photochem. Photobiol. B Biol. 2014, 141, 93–99. [Google Scholar] [CrossRef]
- Raju, B.R.; Garcia, A.M.F.; Costa, A.L.S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Synthesis of new benzo[a]phenoxazinium probes possessing carboxylic ester, hydroxyl and amino functional groups: Photophysical studies in dry ethanol and conjugation with CdTe quantum dots. Dye. Pigment. 2014, 110, 203–213. [Google Scholar] [CrossRef]
- Raju, B.R.; Carvalho, M.M.T.; Leitão, M.I.P.S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Synthesis, photophysical characterisation and photostability studies of NIR probes with aliphatic, aromatic and chlorinated terminals in 5- and 9-amino positions of benzo[a]phenoxazines. Dye. Pigment. 2016, 132, 204–212. [Google Scholar] [CrossRef][Green Version]
- Raju, B.R.; Sampaio, D.M.F.; Silva, M.M.; Coutinho, P.J.G.; Gonçalves, M.S.T. Ultrasound promoted synthesis of Nile Blue derivatives. Ultrason. Sonochem. 2014, 21, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Frade, V.H.J.; Coutinho, P.J.G.; Moura, J.C.V.P.; Gonçalves, M.S.T. Functionalised benzo[a]phenoxazine dyes as long-wavelength fluorescent probes for amino acids. Tetrahedron 2007, 63, 1654–1663. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Barros, S.A.; Moura, J.C.V.P.; Coutinho, P.J.G.; Gonçalves, M.S.T. Synthesis of short and long-wavelength functionalised probes: Amino acids’ labelling and photophysical studies. Tetrahedron 2007, 63, 12405–12418. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Sousa, M.J.; Moura, J.C.V.P.; Gonçalves, M.S.T. Synthesis, characterisation and antimicrobial activity of new benzo[a]phenoxazine based fluorophores. Tetrahedron Lett. 2007, 48, 8347–8352. [Google Scholar] [CrossRef]
- Leitão, M.I.P.S.; Rama Raju, B.; Cerqueira, N.M.F.S.A.; Sousa, M.J.; Gonçalves, M.S.T. Benzo[a]phenoxazinium chlorides: Synthesis, antifungal activity, in silico studies and evaluation as fluorescent probes. Bioorg. Chem. 2020, 98, 103730. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.C.; Lopes, C.; Preto, A.; Gonçalves, M.S.T.; Sousa, M.J. Novel Nile Blue Analogue Stains Yeast Vacuolar Membrane, Endoplasmic Reticulum, and Lipid Droplets, Inducing Cell Death through Vacuole Membrane Permeabilization. J. Fungi 2021, 7, 971. [Google Scholar] [CrossRef]
- Rama Raju, B.; Naik, S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Novel Nile Blue derivatives as fluorescent probes for DNA. Dye. Pigment. 2013, 99, 220–227. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Gonçalves, M.S.T.; Moura, J.C.V.P. Synthesis of fluorescent water-soluble functionalised benzo[a]phenoxazinium salts. Tetrahedron Lett. 2006, 47, 8567–8570. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Barros, S.A.; Moura, J.C.V.P.; Gonçalves, M.S.T. Fluorescence derivatisation of amino acids in short and long-wavelengths. Tetrahedron Lett. 2007, 48, 3403–3407. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Gonçalves, M.S.T.; Moura, J.C.V.P. Synthesis and fluorescence properties of side-chain carboxylated 5,9-diaminobenzo[a]phenoxazinium salts. Tetrahedron Lett. 2005, 46, 4949–4952. [Google Scholar] [CrossRef]
- Firmino, A.D.G.; Gonçalves, M.S.T. Bifunctionalised long-wavelength fluorescent probes for biological applications. Tetrahedron Lett. 2012, 53, 4946–4950. [Google Scholar] [CrossRef]
- Alves, C.M.A.; Naik, S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Novel DNA fluorescence probes based on N-[5-(11-functionalised-undecylamino)-9H-benzo[a]phenoxazin-9-ylidene]propan-1-aminium chlorides: Synthesis and photophysical studies. Tetrahedron Lett. 2011, 52, 112–116. [Google Scholar] [CrossRef]
- Ferreira, J.C.C.; Granja, S.; Almeida, A.F.; Baltazar, F.; Gonçalves, M.S.T.; Preto, A.; Sousa, M.J. Targeting Lysosomes in Colorectal Cancer: Exploring the Anticancer Activity of a New Benzo[a]phenoxazine Derivative. Int. J. Mol. Sci. 2023, 24, 614. [Google Scholar] [CrossRef]
- Alves, C.M.A.; Naik, S.; Coutinho, P.J.G.; Gonçalves, M.S.T. New long alkyl side-chain benzo[a]phenoxazines as micellisation probes. Tetrahedron Lett. 2009, 50, 4470–4474. [Google Scholar] [CrossRef]
- Alves, C.M.A.; Naik, S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Novel long alkyl side chain benzo[a]phenoxazinium chlorides: Synthesis, photophysical behaviour and DNA interaction. Tetrahedron 2009, 65, 10441–10452. [Google Scholar] [CrossRef]
- Leitão, M.I.P.S.; Raju, B.R.; Naik, S.; Coutinho, P.J.G.; Sousa, M.J.; Gonçalves, M.S.T. Synthesis and photophysical studies of new benzo[a]phenoxazinium chlorides as potential antifungal agents. Tetrahedron Lett. 2016, 57, 3936–3941. [Google Scholar] [CrossRef]
- Raju, B.R.; Leitão, M.I.P.S.; Sousa, M.J.; Coutinho, P.J.G.; Gonçalves, M.S.T. New NIR dyes based on quinolizino[1,9-hi]phenoxazin-6-iminium chlorides: Synthesis, photophysics and antifungal activity. Dye. Pigment. 2020, 173, 107870. [Google Scholar] [CrossRef]
- Marques, C.; Oliveira, C.S.F.; Alves, S.; Chaves, S.R.; Coutinho, O.P.; Corte-Real, M.; Preto, A. Acetate-induced apoptosis in colorectal carcinoma cells involves lysosomal membrane permeabilization and cathepsin D release. Cell Death Dis. 2013, 4, e507. [Google Scholar] [CrossRef]
- Samowitz, W.S.; Sweeney, C.; Herrick, J.; Albertsen, H.; Levin, T.R.; Murtaugh, M.A.; Wolff, R.K.; Slattery, M.L. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005, 65, 6063–6069. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- Dienstmann, R.; Tabernero, J. BRAF as a Target for Cancer Therapy. Anticancer Agents Med. Chem. 2012, 11, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; Lapeyre-Prost, A.; Laurent Puig, P.; Zaanan, A. Exploring the best treatment options for BRAF-mutant metastatic colon cancer. Br. J. Cancer 2019, 121, 434–442. [Google Scholar] [CrossRef]
- Munzone, E.; Colleoni, M. Optimal management of luminal breast cancer: How much endocrine therapy is long enough? Ther. Adv. Med. Oncol. 2018, 10, 175883591877743. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, E.A.; Sutherland, R.L. Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer 2009, 9, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.D.; Dowsett, M. Aromatase inhibitors for breast cancer: Lessons from the laboratory. Nat. Rev. Cancer 2003, 3, 821–831. [Google Scholar] [CrossRef]
- Telang, N.T.; Li, G.; Katdare, M.; Sepkovic, D.W.; Bradlow, H.L.; Wong, G.Y.C. The nutritional herb epimedium grandiflorum inhibits the growth in a model for the luminal a molecular subtype of breast cancer. Oncol. Lett. 2017, 13, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 6024. [Google Scholar] [CrossRef]
- Palmer, T.D.; Ashby, W.J.; Lewis, J.D.; Zijlstra, A. Targeting tumor cell motility to prevent metastasis. Adv. Drug Deliv. Rev. 2011, 63, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Nooter, K.; Stoter, G. Molecular mechanisms of multidrug resistance in cancer chemotherapy. Pathol. Res. Pract. 1996, 192, 768–780. [Google Scholar] [CrossRef]
- Simstein, R.; Burow, M.; Parker, A.; Weldon, C.; Beckman, B. Apoptosis, chemoresistance, and breast cancer: Insights from the MCF-7 cell model system. Exp. Biol. Med. 2003, 228, 995–1003. [Google Scholar] [CrossRef]
- Kawakami, H.; Huang, S.; Pal, K.; Dutta, S.K.; Mukhopadhyay, D.; Sinicrope, F.A. Mutant BRAF Upregulates MCL-1 to Confer Apoptosis Resistance that Is Reversed by MCL-1 Antagonism and Cobimetinib in Colorectal Cancer. Mol. Cancer Ther. 2016, 15, 3015–3027. [Google Scholar] [CrossRef]
- Shirato, K.; Imaizumi, K.; Abe, A.; Tomoda, A. Phenoxazine derivatives 2-amino-4,4alpha-dihydro-4alpha-phenoxazine-3-one and 2-aminophenoxazine-3-one-induced apoptosis through a caspase-independent mechanism in human neuroblastoma cell line NB-1 cells. Biol. Pharm. Bull. 2007, 30, 331–336. [Google Scholar] [CrossRef][Green Version]
- Shirato, K.; Imaizumi, K.; Abe, A.; Tomoda, A. Phenoxazine derivatives induce caspase-independent cell death in human glioblastoma cell lines, A-172 and U-251 MG. Oncol. Rep. 2007, 17, 201–208. [Google Scholar] [CrossRef][Green Version]
- Kato, S.; Shirato, K.; Imaizumi, K.; Toyota, H.; Mizuguchi, J.; Odawara, M.; Che, X.F.; Akiyama, S.; Abe, A.; Tomoda, A. Anticancer effects of phenoxazine derivatives combined with tumor necrosis factor-related apoptosis-inducing ligand on pancreatic cancer cell lines, KLM-1 and MIA-PaCa-2. Oncol. Rep. 2006, 15, 843–848. [Google Scholar] [CrossRef]
- Chadar, D.; Rao, S.S.; Khan, A.; Gejji, S.P.; Bhat, K.S.; Weyhermüller, T.; Salunke-Gawali, S. Benzo[α]phenoxazines and benzo[a]phenothiazine from vitamin K3: Synthesis, molecular structures, DFT studies and cytotoxic activity. RSC Adv. 2015, 5, 57917–57929. [Google Scholar] [CrossRef]
- Sousa, R.P.C.L.; Ferreira, J.C.C.; Sousa, M.J.M.F.; Gonçalves, M.S.T. New Nile Blue derivatives as NIR fluorescent probes and antifungal agents. Proceedings 2019, 9, 65. [Google Scholar]
- Zhan, Y.H.; Li, X.J.; Sun, R.; Xu, Y.J.; Ge, J.F. Distinguishing normal cells from cancer cells via lysosome-targetable pH biomarkers with benzo[a]phenoxazine skeleton. Anal. Chim. Acta 2016, 10, 5796–5803. [Google Scholar] [CrossRef]
- Sun, R.; Liu, W.; Xu, Y.J.; Lu, J.M.; Ge, J.F.; Ihara, M. A cyanobenzo[a]phenoxazine-based near infrared lysosome-tracker for in cellulo imaging. Chem. Commun. 2013, 49, 10709–10711. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.J.; Niu, J.Y.; Sun, R.; Xu, Y.J.; Ge, J.F. The improvement of lysosome targetability with oligoethyleneoxy chains linked benzo[a]phenoxazine. Bioorganic Med. Chem. Lett. 2018, 28, 2953–2956. [Google Scholar] [CrossRef]
- Brunk, U.T.; Svensson, I. Oxidative stress, growth factor starvation and Fas activation may all cause apoptosis through lysosomal leak. Redox Rep. 1999, 4, 3–11. [Google Scholar] [CrossRef]
- Zhao, M.; Eaton, J.W.; Brunk, U.T. Protection against oxidant-mediated lysosomal rupture: A new anti-apoptotic activity of Bcl-2? FEBS Lett. 2000, 485, 104–108. [Google Scholar] [CrossRef]
- Nilsson, C.; Johansson, U.; Johansson, A.C.; Kågedal, K.; Öllinger, K. Cytosolic acidification and lysosomal alkalinization during TNF-α induced apoptosis in U937 cells. Apoptosis 2006, 11, 1149–1159. [Google Scholar] [CrossRef]
- Boya, P.; Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; De Sousa, W.T.; Da Silva, V.C.M.; Rodrigues, M.C.; Morais, J.A.V.; Song, J.L.; Cheng, Z.Q.; Longo, J.P.F.; Azevedo, R.B.; Jiang, C.S.; et al. Synthesis and evaluation of new potential benzo[a]phenoxazinium photosensitizers for anticancer photodynamic therapy. Molecules 2018, 23, 1436. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.-L.; Che, X.-F.; Akiyama, S.-I.; Miyazawa, K.; Tomoda, A. 2-Aminophenoxazine-3-one induces cellular apoptosis by causing rapid intracellular acidification and generating reactive oxygen species in human lung adenocarcinoma cells. Int. J. Oncol. 2010, 36, 641–650. [Google Scholar]
- Cai, X.; Liu, Y.; Hu, Y.; Liu, X.; Jiang, H.; Yang, S.; Shao, Z.; Xia, Y.; Xiong, L. ROS-mediated lysosomal membrane permeabilization is involved in bupivacaine-induced death of rabbit intervertebral disc cells. Redox Biol. 2018, 18, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, N.D.; Zhou, F.; Shen, T.; Duan, T.; Zhou, J.; Shi, Y.; Zhu, X.Q.; Shen, H.M. (-)-Epigallocatechin-3-Gallate Induces Non-Apoptotic Cell Death in Human Cancer Cells via ROS-Mediated Lysosomal Membrane Permeabilization. PLoS ONE 2012, 7, e46749. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Kwon, T.K. Induction of lysosomal membrane permeabilization is a major event of FTY720-mediated non-apoptotic cell death in human glioma cells. Cancers 2020, 12, 3388. [Google Scholar] [CrossRef] [PubMed]

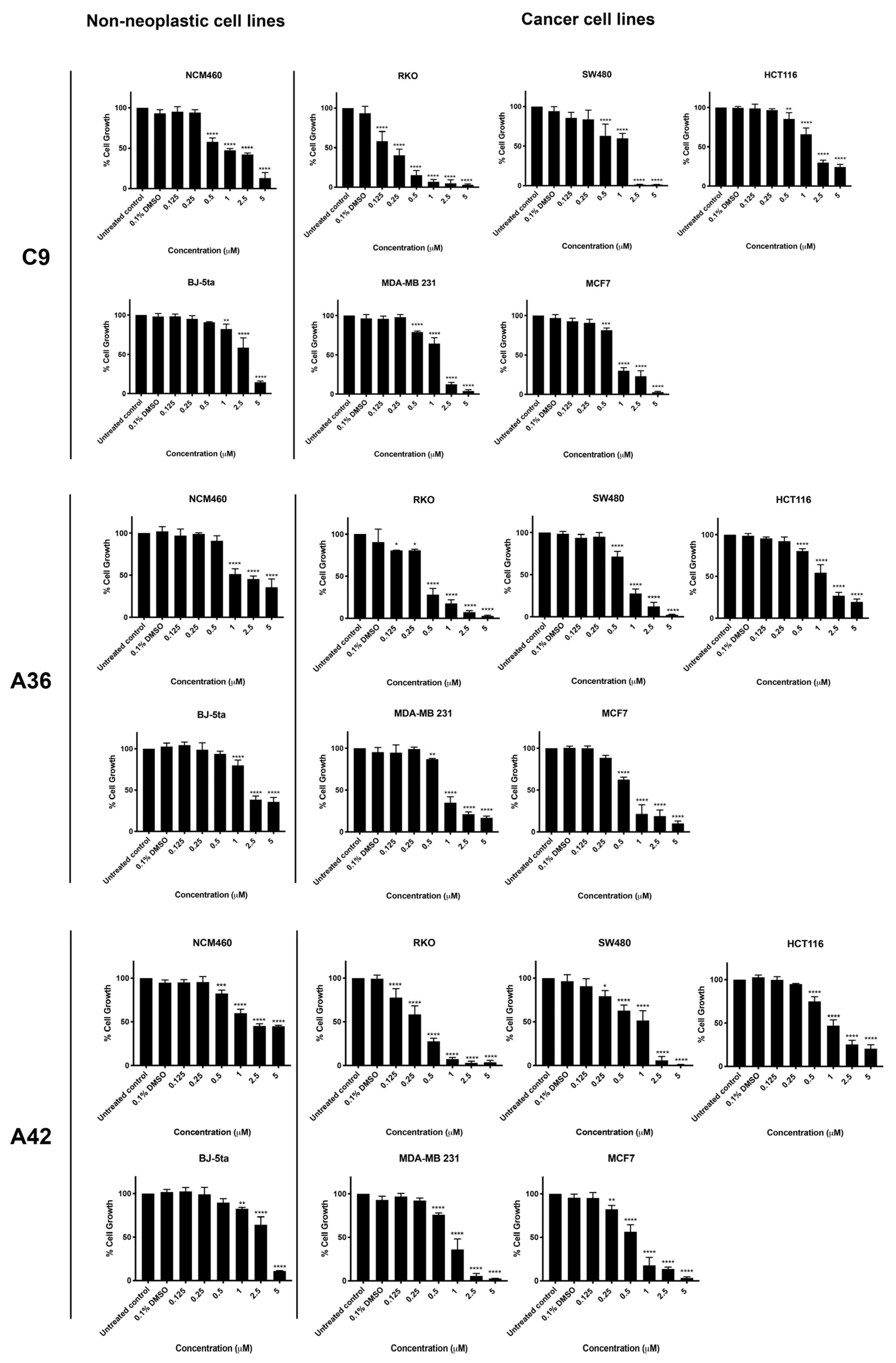



| Cell Lines | IC50 (μM) a ± SD b | Selectivity Index c | ||||
|---|---|---|---|---|---|---|
| BaP1 d | C9 | A36 | A42 | Cell Line IC50/BJ-5ta IC50 | CRC IC50/NCM460 IC50 | |
| BJ-5ta e | 1.83 ± 0.06 | 2.55 ± 0.15 | 2.44 ± 0.18 | 2.58 ± 0.19 | ||
| NCM460 e | 12.80 ± 2.05 | 1.12 ± 0.13 | 2.06 ± 0.25 | 2.59 ± 0.31 | ||
| SW480 f | 5.60 ± 0.19 | 0.78 ± 0.09 | 0.72 ± 0.03 | 0.78 ± 0.07 | 0.33/3.27/3.38/3.31 | 2.28/1.44/2.86/3.32 |
| HCT116 f | 1.90 ± 0.09 | 1.64 ± 0.09 | 1.28 ± 0.06 | 1.13 ± 0.07 | 0.97/1.55/1.91/2.25 | 6.73/0.68/1.61/2.29 |
| RKO f | 1.40 ± 0.08 | 0.17 ± 0.01 | 0.37 ± 0.03 | 0.29 ± 0.01 | 1.30/15/6.59/8.9 | 9.14/6.59/5.56/8.93 |
| MCF7 g | 0.92 ± 0.07 | 0.84 ± 0.06 | 0.63 ± 0.05 | 0.54 ± 0.02 | 1.9/3.04/3.86/4.80 | |
| MDA-MB-231 g | 1.73 ± 0.03 | 1.19 ± 0.05 | 1.01 ± 0.09 | 0.79 ± 0.03 | 1.05/2.14/2.41/3.26 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, J.C.C.; Gonçalves, M.S.T.; Preto, A.; Sousa, M.J. Anticancer Activity of Benzo[a]phenoxazine Compounds Promoting Lysosomal Dysfunction. Cells 2024, 13, 1385. https://doi.org/10.3390/cells13161385
Ferreira JCC, Gonçalves MST, Preto A, Sousa MJ. Anticancer Activity of Benzo[a]phenoxazine Compounds Promoting Lysosomal Dysfunction. Cells. 2024; 13(16):1385. https://doi.org/10.3390/cells13161385
Chicago/Turabian StyleFerreira, João Carlos Canossa, M. Sameiro T. Gonçalves, Ana Preto, and Maria João Sousa. 2024. "Anticancer Activity of Benzo[a]phenoxazine Compounds Promoting Lysosomal Dysfunction" Cells 13, no. 16: 1385. https://doi.org/10.3390/cells13161385
APA StyleFerreira, J. C. C., Gonçalves, M. S. T., Preto, A., & Sousa, M. J. (2024). Anticancer Activity of Benzo[a]phenoxazine Compounds Promoting Lysosomal Dysfunction. Cells, 13(16), 1385. https://doi.org/10.3390/cells13161385








