Impact of the Immunomodulatory Factor Soluble B7-H4 in the Progress of Preeclampsia by Inhibiting Essential Functions of Extravillous Trophoblast Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. MTT Proliferation Assay
2.3. Wound Healing and Transwell Assay
2.4. Immunofluorescence Staining
2.5. Patient Cohort
2.6. Therapeutic Plasma Exchange
2.7. Immunoblotting
2.8. Immunohistochemical Staining
2.9. Statistical Analysis
3. Results
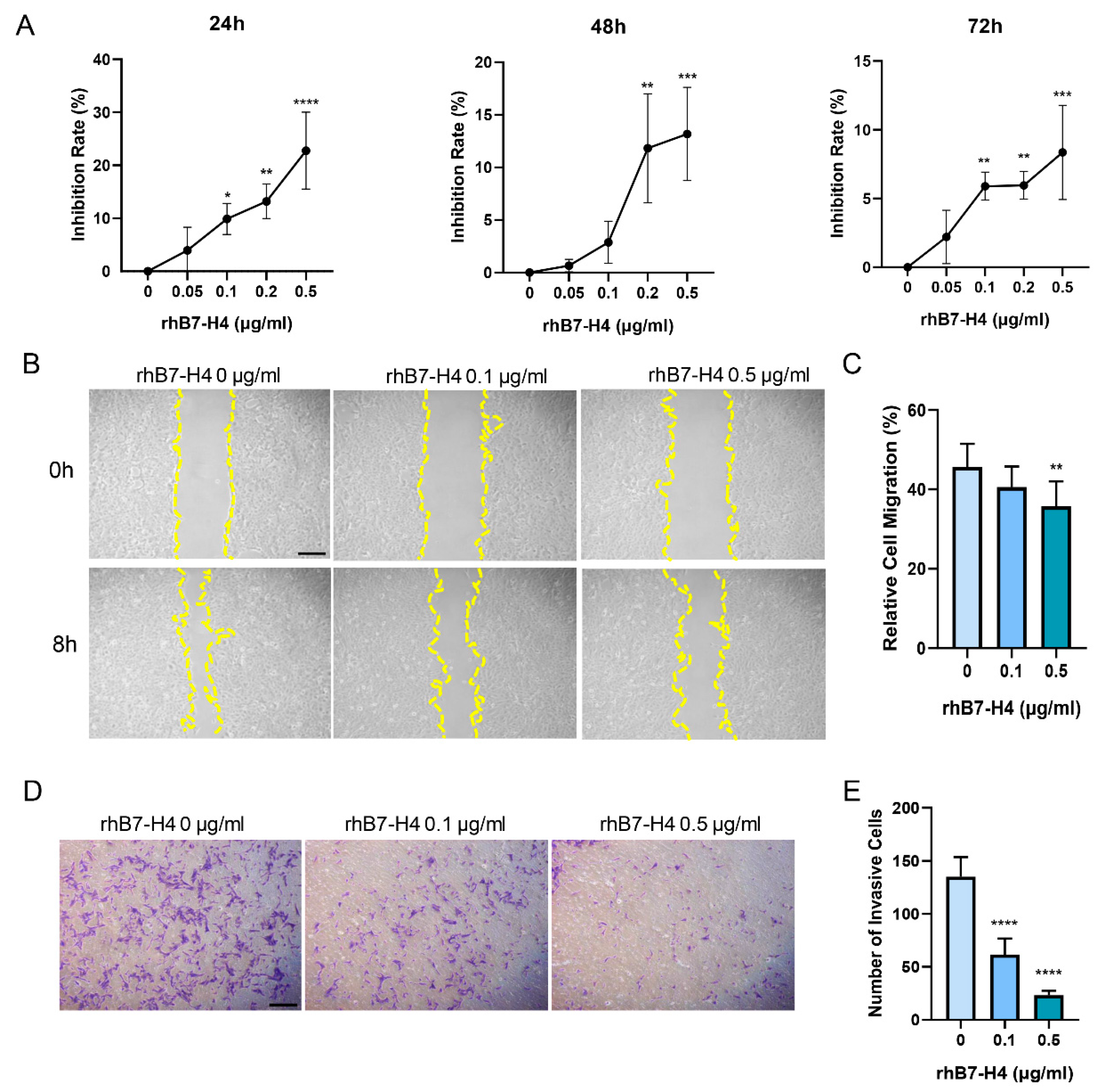
3.1. B7-H4 Reduces the Proliferative, Migratory, and Invasion Capacity of SGHPL-5 Cells
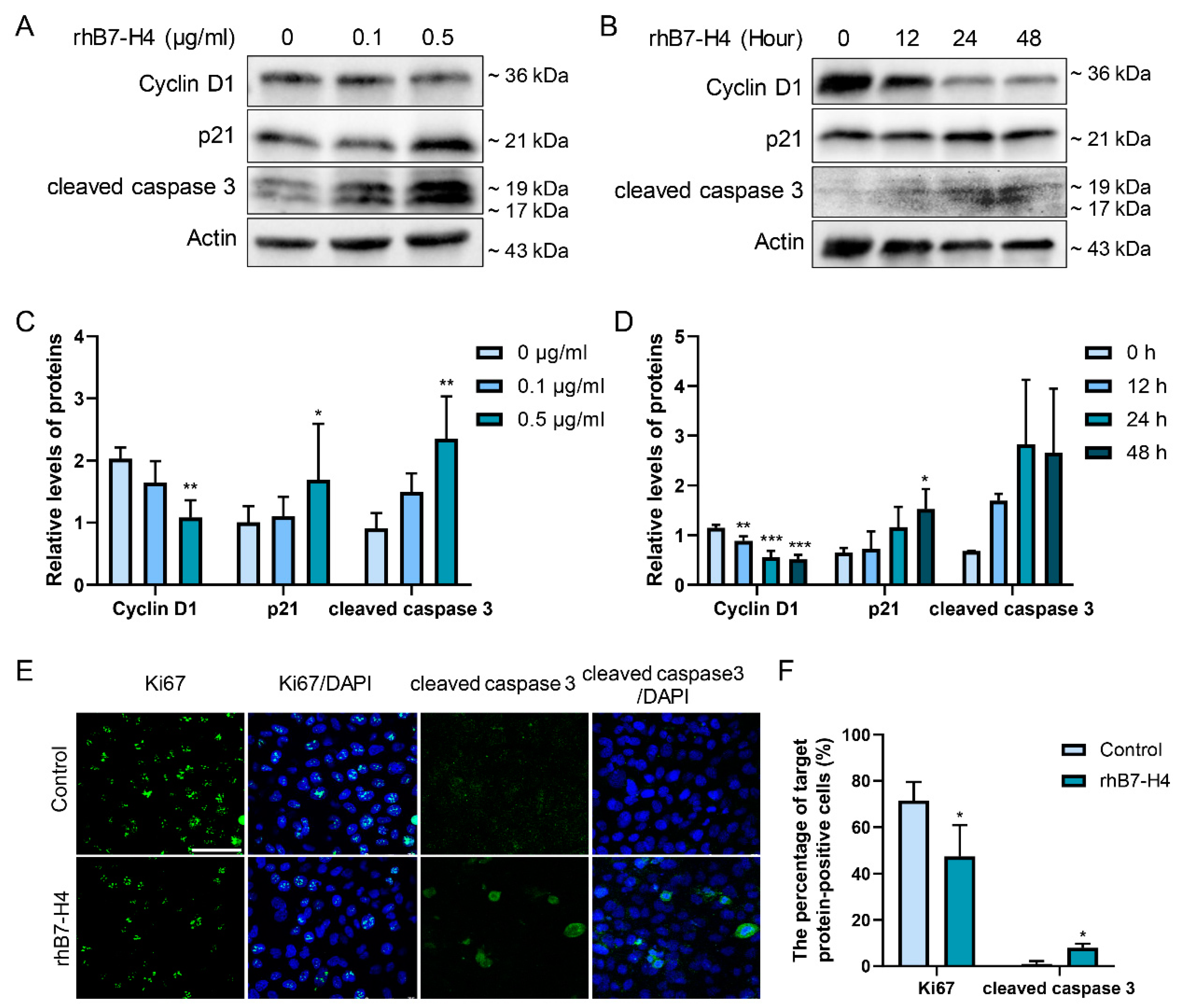
3.2. B7-H4 Reduces Cyclin D1, Induces p21 Protein Expression, and Promotes Apoptosis in SGHPL-5 Cells
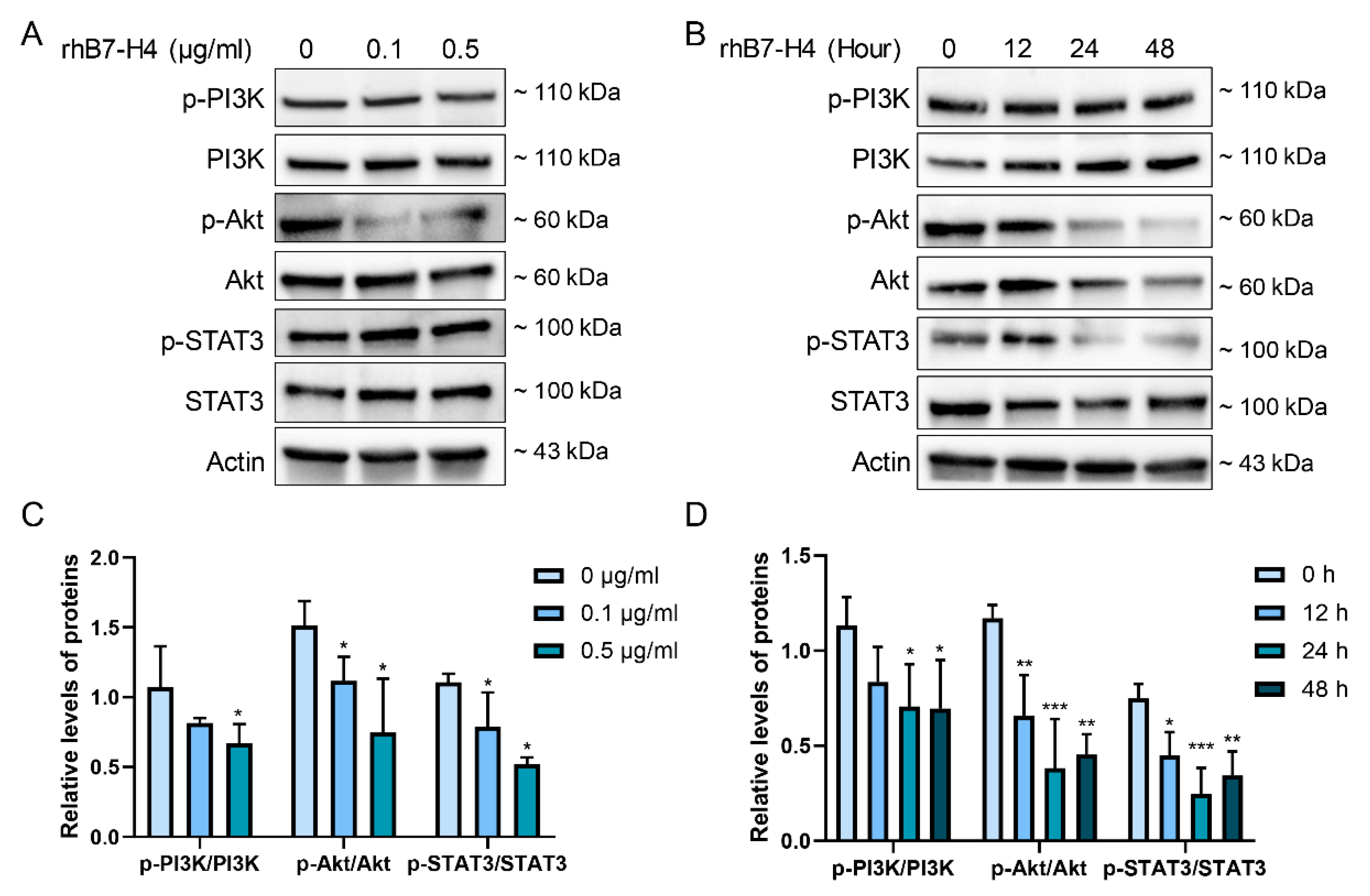
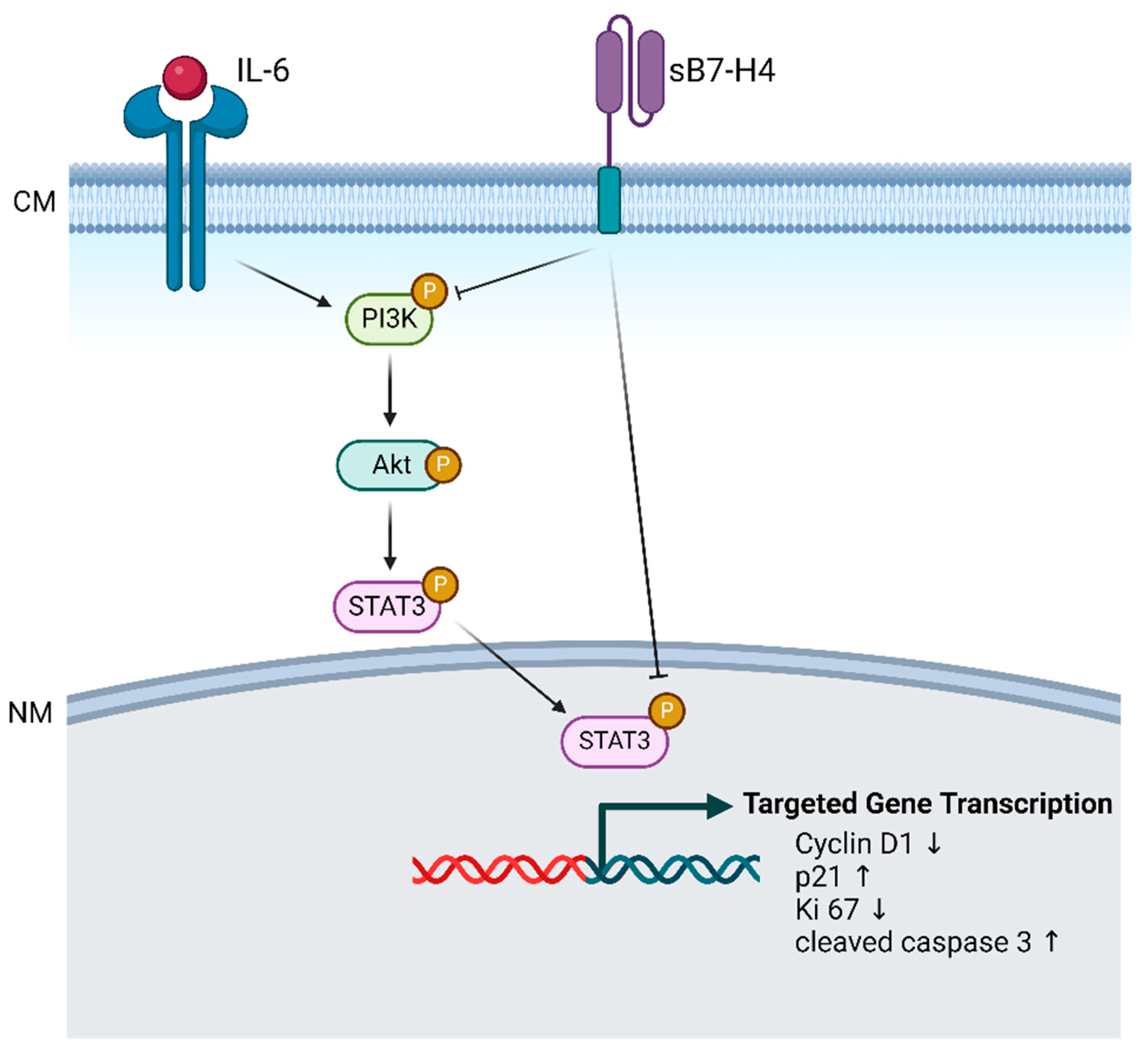
3.3. B7-H4 Downregulates the PI3K/Akt/STAT3 Signalling Pathway in SGHPL-5 Cells
3.4. IL-6, a PI3K/Akt/STAT3 Activator, Attenuated B7-H4-Induced Inhibitory Effects in SGHPL-5 Cells
3.5. Serum sB7-H4 Levels Vary among Control and PE Patients Receiving Standard of Care or TPE Treatment
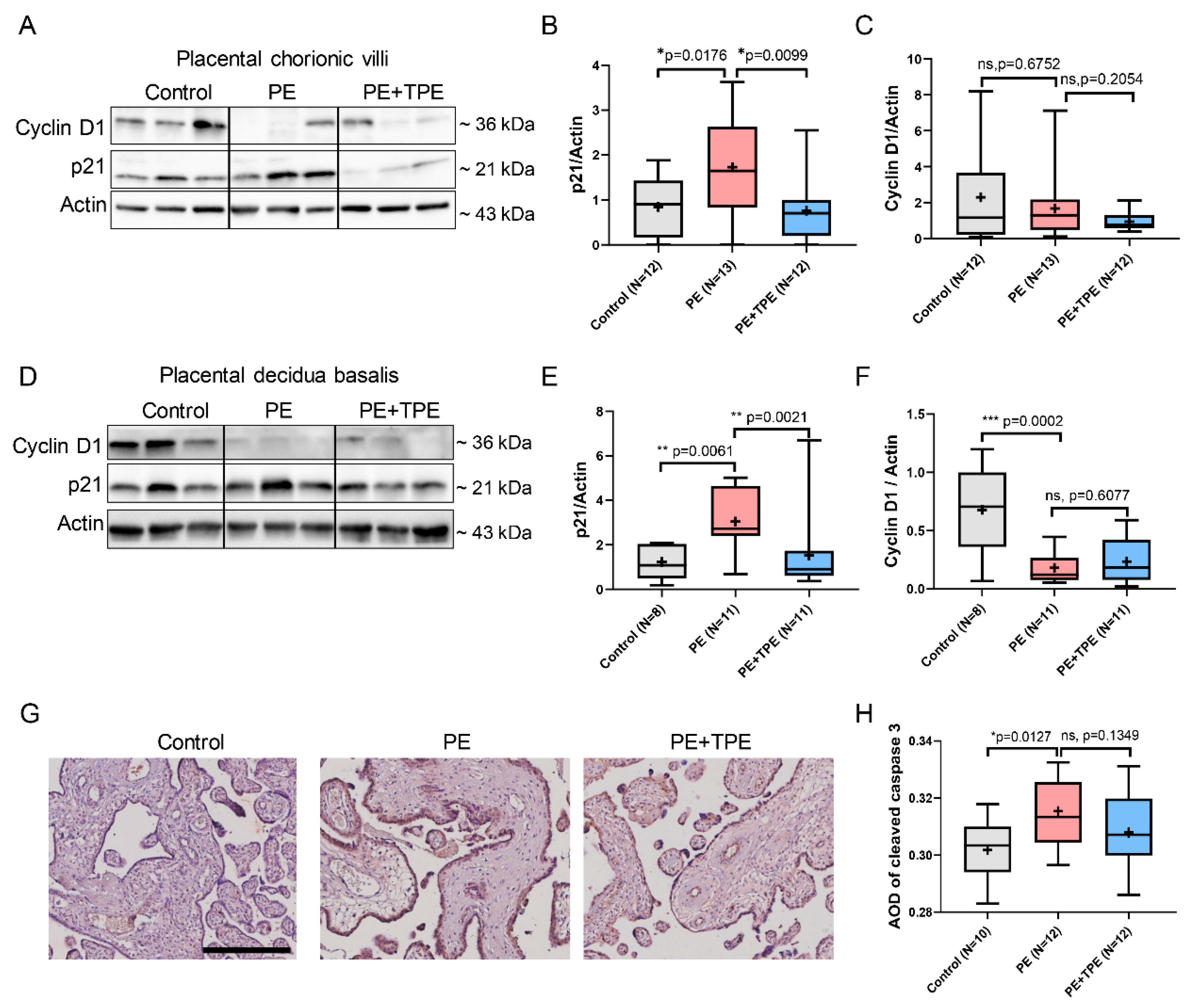
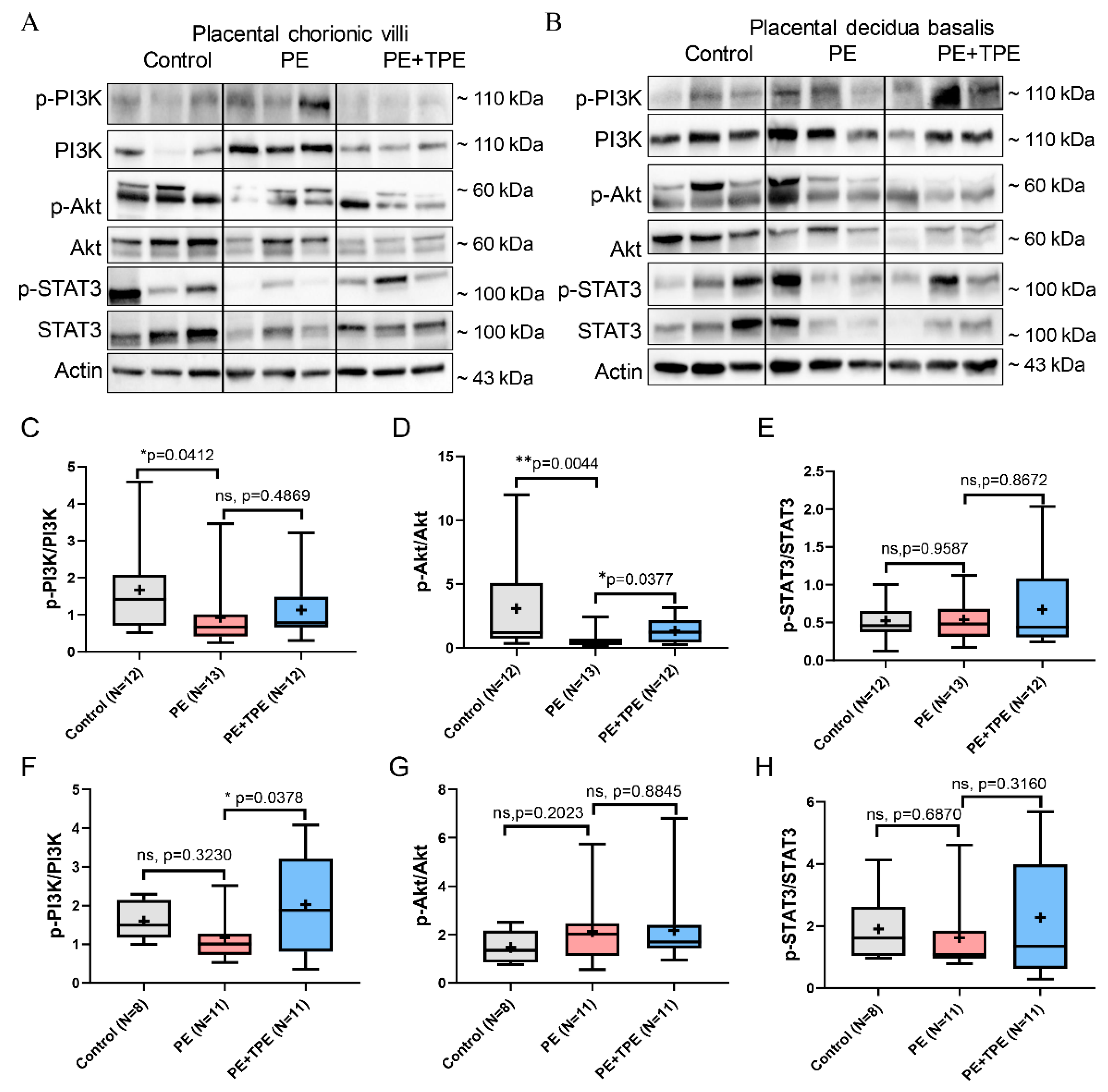
3.6. B7-H4 May Suppress Trophoblast Cell Proliferation and Induce Apoptosis in PE Patients by Downregulating PI3K/Akt/STAT3 Signalling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karrar, S.A.; Martingano, D.J.; Hong, P.L. Preeclampsia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Bisson, C.; Dautel, S.; Patel, E.; Suresh, S.; Dauer, P.; Rana, S. Preeclampsia pathophysiology and adverse outcomes during pregnancy and postpartum. Front. Med. 2023, 10, 1144170. [Google Scholar] [CrossRef]
- Khong, T.Y.; De Wolf, F.; Robertson, W.B.; Brosens, I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br. J. Obstet. Gynaecol. 1986, 93, 1049–1059. [Google Scholar] [CrossRef]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef]
- Gubensek, J.; Ponikvar, R.; Premru Srsen, T.; Fabjan Vodusek, V.; Moertl, M.G.; Lucovnik, M. Therapeutic plasma exchange and dextran-sulfate plasma adsorption as extracorporeal treatments of extremely preterm preeclampsia with fetal growth restriction. J. Clin. Apher. 2021, 36, 595–605. [Google Scholar] [CrossRef]
- Iannaccone, A.; Reisch, B.; Kimmig, R.; Schmidt, B.; Mavarani, L.; Darkwah Oppong, M.; Tyczynski, B.; Dzietko, M.; Jahn, M.; Gellhaus, A.; et al. Therapeutic Plasma Exchange in Early-Onset Preeclampsia: A 7-Year Monocentric Experience. J. Clin. Med. 2023, 12, 4289. [Google Scholar] [CrossRef]
- Duan, L.; Ma, Y.; Reisch, B.; Hadrovic, E.; Mach, P.; Kimmig, R.; Jahn, M.; Köninger, A.; Iannaccone, A.; Gellhaus, A. Alteration in sB7-H4 serum levels and placental biomarker expression after therapeutic plasma exchange in early-onset preeclampsia patients. Int. J. Mol. Sci. 2024, submitted.
- Sica, G.L.; Choi, I.H.; Zhu, G.; Tamada, K.; Wang, S.D.; Tamura, H.; Chapoval, A.I.; Flies, D.B.; Bajorath, J.; Chen, L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 2003, 18, 849–861. [Google Scholar] [CrossRef]
- Duan, L.; Reisch, B.; Iannaccone, A.; Hadrovic, E.; Wu, Y.; Vogtmann, R.; Winterhager, E.; Kimmig, R.; Koninger, A.; Mach, P.; et al. Abnormal expression of the costimulatory molecule B7-H4 in placental chorionic villous and decidual basalis tissues of patients with preeclampsia and HELLP syndrome. Am. J. Reprod. Immunol. 2021, 86, e13430. [Google Scholar] [CrossRef]
- Mach, P.; Nolte-Boenigk, L.; Droste, L.; Fox, L.; Frank, M.; Schmidt, B.; Herse, F.; Verlohren, S.; Wicherek, L.; Iannaccone, A.; et al. Soluble B7-H4 blood serum levels are elevated in women at high risk for preeclampsia in the first trimester, as well as in patients with confirmed preeclampsia. Am. J. Reprod. Immunol. 2018, 80, e12988. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Di, Z.M.; Cao, Q.; Xu, W.S.; Bi, S.X.; Yu, J.S.; Shen, Y.J.; Yu, Y.Q.; Shen, Y.X.; Feng, L.J. Xanthatin induces glioma cell apoptosis and inhibits tumor growth via activating endoplasmic reticulum stress-dependent CHOP pathway. Acta Pharmacol. Sin. 2020, 41, 404–414. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef]
- Tchakarska, G.; Sola, B. The double dealing of cyclin D1. Cell Cycle 2020, 19, 163–178. [Google Scholar] [CrossRef]
- Li, J.; Ying, H.; Cai, G.; Guo, Q.; Chen, L. Pre-Eclampsia-Associated Reduction in Placental Growth Factor Impaired Beta Cell Proliferation Through PI3k Signalling. Cell. Physiol. Biochem. 2015, 36, 34–43. [Google Scholar] [CrossRef]
- Besson, A.; Dowdy, S.F.; Roberts, J.M. CDK inhibitors: Cell cycle regulators and beyond. Dev. Cell 2008, 14, 159–169. [Google Scholar] [CrossRef]
- Mohan, C.D.; Rangappa, S.; Preetham, H.D.; Chandra Nayaka, S.; Gupta, V.K.; Basappa, S.; Sethi, G.; Rangappa, K.S. Targeting STAT3 signaling pathway in cancer by agents derived from Mother Nature. Semin. Cancer Biol. 2022, 80, 157–182. [Google Scholar] [CrossRef]
- Neumeister, P.; Pixley, F.J.; Xiong, Y.; Xie, H.; Wu, K.; Ashton, A.; Cammer, M.; Chan, A.; Symons, M.; Stanley, E.R.; et al. Cyclin D1 governs adhesion and motility of macrophages. Mol. Biol. Cell 2003, 14, 2005–2015. [Google Scholar] [CrossRef]
- Allaire, A.D.; Ballenger, K.A.; Wells, S.R.; McMahon, M.J.; Lessey, B.A. Placental apoptosis in preeclampsia. Obstet. Gynecol. 2000, 96, 271–276. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, W.P. B7-H4, a promising target for immunotherapy. Cell. Immunol. 2020, 347, 104008. [Google Scholar] [CrossRef]
- Wang, Y.T.; Tang, F.; Hu, X.; Zheng, C.X.; Gong, T.J.; Zhou, Y.; Luo, Y.; Min, L. Role of crosstalk between STAT3 and mTOR signaling in driving sensitivity to chemotherapy in osteosarcoma cell lines. IUBMB Life 2020, 72, 2146–2153. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Guan, H.; Cai, W.; Bai, X.; Fang, X.; Hu, X.; Wang, Y.; Wang, H.; Zheng, Z.; et al. Anti-fibrotic actions of interleukin-10 against hypertrophic scarring by activation of PI3K/AKT and STAT3 signaling pathways in scar-forming fibroblasts. PLoS ONE 2014, 9, e98228. [Google Scholar] [CrossRef]
- Kang, S.; Kishimoto, T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp. Mol. Med. 2021, 53, 1116–1123. [Google Scholar] [CrossRef]
- Sharifpanah, F.; Ali, E.H.; Wartenberg, M.; Sauer, H. The milk thistle (Silybum marianum) compound Silibinin stimulates leukopoiesis from mouse embryonic stem cells. Phytother. Res. 2019, 33, 452–460. [Google Scholar] [CrossRef]
- Guttmacher, A.E.; Maddox, Y.T.; Spong, C.Y. The Human Placenta Project: Placental structure, development, and function in real time. Placenta 2014, 35, 303–304. [Google Scholar] [CrossRef]
- Rossant, J.; Cross, J.C. Placental development: Lessons from mouse mutants. Nat. Rev. Genet. 2001, 2, 538–548. [Google Scholar] [CrossRef]
- James, J.L.; Whitley, G.S.; Cartwright, J.E. Pre-eclampsia: Fitting together the placental, immune and cardiovascular pieces. J. Pathol. 2010, 221, 363–378. [Google Scholar] [CrossRef]
- Plaks, V.; Rinkenberger, J.; Dai, J.; Flannery, M.; Sund, M.; Kanasaki, K.; Ni, W.; Kalluri, R.; Werb, Z. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc. Natl. Acad. Sci. USA 2013, 110, 11109–11114. [Google Scholar] [CrossRef]
- de Oliveira, L.G.; Karumanchi, A.; Sass, N. [Preeclampsia: Oxidative stress, inflammation and endothelial dysfunction]. Rev. Bras. Ginecol. Obstet. 2010, 32, 609–616. [Google Scholar] [CrossRef]
- Hutabarat, M.; Wibowo, N.; Huppertz, B. The trophoblast survival capacity in preeclampsia. PLoS ONE 2017, 12, e0186909. [Google Scholar] [CrossRef]
- Abrahams, V.M.; Straszewski-Chavez, S.L.; Guller, S.; Mor, G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol. Hum. Reprod. 2004, 10, 55–63. [Google Scholar] [CrossRef]
- Ashton, S.V.; Whitley, G.S.; Dash, P.R.; Wareing, M.; Crocker, I.P.; Baker, P.N.; Cartwright, J.E. Uterine spiral artery remodeling involves endothelial apoptosis induced by extravillous trophoblasts through Fas/FasL interactions. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 102–108. [Google Scholar] [CrossRef]
- Sharp, A.N.; Heazell, A.E.; Crocker, I.P.; Mor, G. Placental apoptosis in health and disease. Am. J. Reprod. Immunol. 2010, 64, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Wescott, E.C.; Sun, X.; Gonzalez-Ericsson, P.; Hanna, A.; Taylor, B.C.; Sanchez, V.; Bronzini, J.; Opalenik, S.R.; Sanders, M.E.; Wulfkuhle, J.; et al. Epithelial Expressed B7-H4 Drives Differential Immunotherapy Response in Murine and Human Breast Cancer. Cancer Res. Commun. 2024, 4, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Inoue, N.; Iwatani, Y.; Watanabe, A.; Yamamoto, M.; Kitahara, N.; Tanikawa, S.; Noguchi, Y.; Hidaka, Y.; Watanabe, M. Association of B7H3 and B7H4 gene polymorphisms and protein expression with the development and prognosis of autoimmune thyroid diseases. Clin. Endocrinol. 2023, 99, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Albers, H.M.; Reinards, T.H.; Brinkman, D.M.; Kamphuis, S.S.; van Rossum, M.A.; Hoppenreijs, E.P.; Girschick, H.J.; Wouters, C.; Saurenmann, R.K.; Bakker, E.; et al. Genetic variation in VTCN1 (B7-H4) is associated with course of disease in juvenile idiopathic arthritis. Ann. Rheum. Dis. 2014, 73, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Karvas, R.M.; McInturf, S.; Zhou, J.; Ezashi, T.; Schust, D.J.; Roberts, R.M.; Schulz, L.C. Use of a human embryonic stem cell model to discover GABRP, WFDC2, VTCN1 and ACTC1 as markers of early first trimester human trophoblast. Mol. Hum. Reprod. 2020, 26, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tian, Y.; Qu, Y.; Williams, M.; Yuan, Y.; Karvas, R.M.; Sheridan, M.A.; Schulz, L.C.; Ezashi, T.; Roberts, M.R.; et al. The immune checkpoint molecule, VTCN1/B7-H4, guides differentiation and suppresses proinflammatory responses and MHC class I expression in an embryonic stem cell-derived model of human trophoblast. Front. Endocrinol. 2023, 14, 1069395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, H.; Lu, D.; Li, G.; Sun, C.; Song, H.; Li, J.; Zhai, T.; Huang, L.; Hou, C.; et al. The costimulatory molecule B7-H4 promote tumor progression and cell proliferation through translocating into nucleus. Oncogene 2013, 32, 5347–5358. [Google Scholar] [CrossRef]
- Dong, L.; Xie, L.; Li, M.; Dai, H.; Wang, X.; Wang, P.; Zhang, Q.; Liu, W.; Hu, X.; Zhao, M. Downregulation of B7-H4 suppresses tumor progression of hepatocellular carcinoma. Sci. Rep. 2019, 9, 14854. [Google Scholar] [CrossRef] [PubMed]
- Park, G.B.; Song, H.; Kim, Y.S.; Sung, M.; Ryu, J.W.; Lee, H.K.; Cho, D.H.; Kim, D.; Lee, W.J.; Hur, D.Y. Cell cycle arrest induced by engagement of B7-H4 on Epstein-Barr virus-positive B-cell lymphoma cell lines. Immunology 2009, 128, 360–368. [Google Scholar] [CrossRef]
- Sun, X.; Xie, H.; Zhang, H.; Li, Z.; Qi, H.; Yang, C.; Liu, X.; Ren, L.; Jiang, Y.; Hu, X. B7-H4 reduction induced by Toxoplasma gondii infection results in dysfunction of decidual dendritic cells by regulating the JAK2/STAT3 pathway. Parasites Vectors 2022, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Wang, J.; Zhang, L. The decreased expression of Stat3 and p-Stat3 in preeclampsia-like rat placenta. J. Mol. Histol. 2018, 49, 175–183. [Google Scholar] [CrossRef]
- Christensen, M.; Petersen, J.L.; Sivanandam, P.; Kronborg, C.S.; Knudsen, U.B.; Martensen, P.M. Reduction of serum-induced endothelial STAT3(Y705) activation is associated with preeclampsia. Pregnancy Hypertens. 2021, 25, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.Q.; Tian, F.J.; Li, X.; Zhang, J.; Ma, X.L.; Zeng, W.H.; Lin, Y.; Zhang, Y. ANXA7 regulates trophoblast proliferation and apoptosis in preeclampsia. Am. J. Reprod. Immunol. 2019, 82, e13183. [Google Scholar] [CrossRef] [PubMed]
- Suman, P.; Gupta, S.K. STAT3 and ERK1/2 cross-talk in leukaemia inhibitory factor mediated trophoblastic JEG-3 cell invasion and expression of mucin 1 and Fos. Am. J. Reprod. Immunol. 2014, 72, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sui, L.; Qiu, B.; Yin, X.; Liu, J.; Zhang, X. ANXA4 promotes trophoblast invasion via the PI3K/Akt/eNOS pathway in preeclampsia. Am. J. Physiol.-Cell Physiol. 2019, 316, C481–C491. [Google Scholar] [CrossRef] [PubMed]
- Rduch, T.; Arn, N.; Kinkel, J.; Fischer, T.; Binet, I.; Hornung, R.; Herrmann, I.K. Magnetic blood purification-based soluble fms-like tyrosine kinase-1 removal in comparison with dextran sulfate apheresis and therapeutic plasma exchange. Artif. Organs 2023, 47, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Chowdhry, M.; Agrawal, S.; Gajulapalli, S.P.; Thakur, U.K. Therapeutic plasma exchange in HELLP syndrome: A life savior. Asian J. Transfus. Sci. 2022, 16, 106–110. [Google Scholar] [CrossRef]
- Radulescu, C.; Bacarea, A.; Hutanu, A.; Gabor, R.; Dobreanu, M. Placental Growth Factor, Soluble fms-Like Tyrosine Kinase 1, Soluble Endoglin, IL-6, and IL-16 as Biomarkers in Preeclampsia. Mediat. Inflamm. 2016, 2016, 3027363. [Google Scholar] [CrossRef]

| Antigen | Source | Supplier (Catalogue Number) | Dilution |
|---|---|---|---|
| Primary antibody | |||
| Cyclin D1 | Rabbit | Abcam (ab13417; Cambridge, UK) | 1:150,000 |
| p21 | Rabbit | Cell Signaling Technology (2947s; Danvers, MA, USA) | 1:1000 |
| Cleaved caspase 3 | Rabbit | Cell Signaling Technology (9664s; Danvers, MA, USA) | 1:1000 |
| p-PI3K (p110α) | Rabbit | Cell Signaling Technology (4249; Danvers, MA, USA) | 1:1000 |
| PI3K | Rabbit | Abcam (ab191606; Cambridge, UK) | 1:1000 |
| p-Akt (Ser473) | Rabbit | Cell Signaling Technology (4060s; Danvers, MA, USA) | 1:1000 |
| Akt | Rabbit | Cell Signaling Technology (9272s; Danvers, MA, USA) | 1:1000 |
| p-STAT3 (Tyr705) | Rabbit | Cell Signaling Technology (9145s; Danvers, MA, USA) | 1:1000 |
| STAT3 | Mouse | BD Bioscience (610190; San Jose, CA, USA) | 1:750 |
| β-Actin | Mouse | Sigma-Aldrich (A3854; St. Louis, MO, USA) | 1:100,000 |
| Secondary antibody | |||
| Rabbit, HRP | Goat | Invitrogen (G21234; Carlsbad, CA, USA) | 1:5000 |
| Mouse, HRP | Goat | Pierce (EJ66453; Rockford, IL, USA) | 1:5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Duan, L.; Reisch, B.; Kimmig, R.; Iannaccone, A.; Gellhaus, A. Impact of the Immunomodulatory Factor Soluble B7-H4 in the Progress of Preeclampsia by Inhibiting Essential Functions of Extravillous Trophoblast Cells. Cells 2024, 13, 1372. https://doi.org/10.3390/cells13161372
Ma Y, Duan L, Reisch B, Kimmig R, Iannaccone A, Gellhaus A. Impact of the Immunomodulatory Factor Soluble B7-H4 in the Progress of Preeclampsia by Inhibiting Essential Functions of Extravillous Trophoblast Cells. Cells. 2024; 13(16):1372. https://doi.org/10.3390/cells13161372
Chicago/Turabian StyleMa, Yuyang, Liyan Duan, Beatrix Reisch, Rainer Kimmig, Antonella Iannaccone, and Alexandra Gellhaus. 2024. "Impact of the Immunomodulatory Factor Soluble B7-H4 in the Progress of Preeclampsia by Inhibiting Essential Functions of Extravillous Trophoblast Cells" Cells 13, no. 16: 1372. https://doi.org/10.3390/cells13161372
APA StyleMa, Y., Duan, L., Reisch, B., Kimmig, R., Iannaccone, A., & Gellhaus, A. (2024). Impact of the Immunomodulatory Factor Soluble B7-H4 in the Progress of Preeclampsia by Inhibiting Essential Functions of Extravillous Trophoblast Cells. Cells, 13(16), 1372. https://doi.org/10.3390/cells13161372






