Breath and Sputum Analyses in Asthmatic Patients: An Overview
Abstract
1. Introduction
2. Sputum
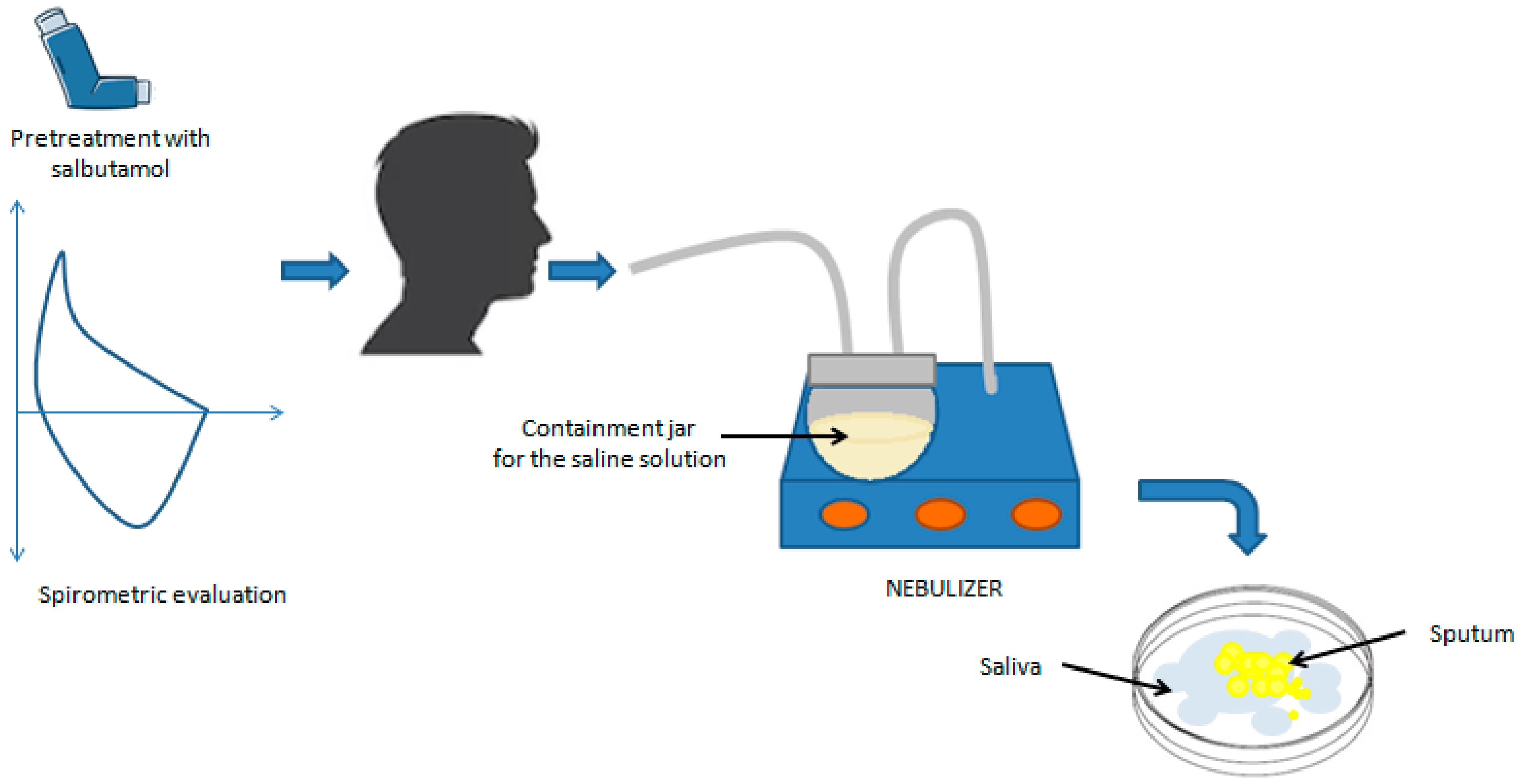
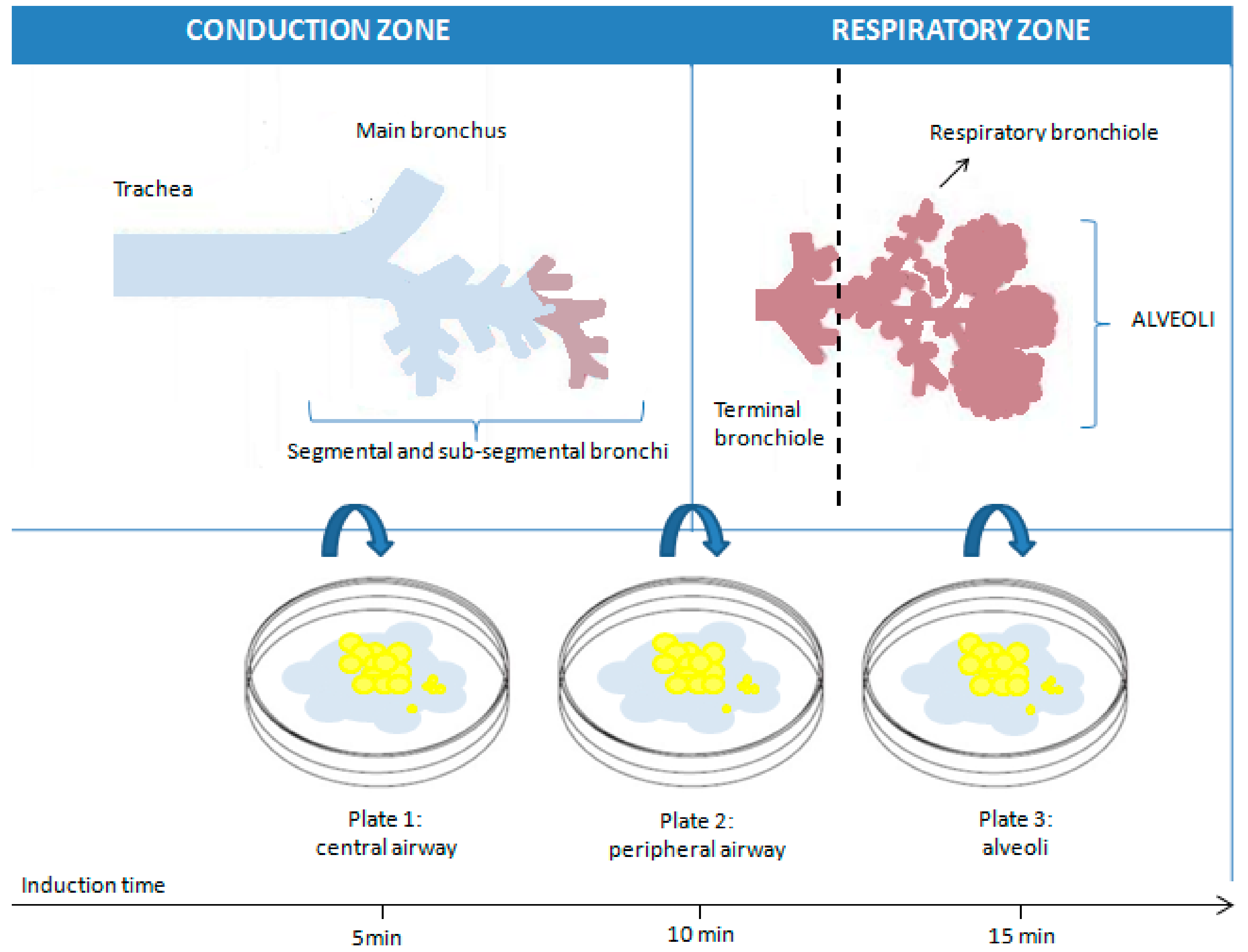
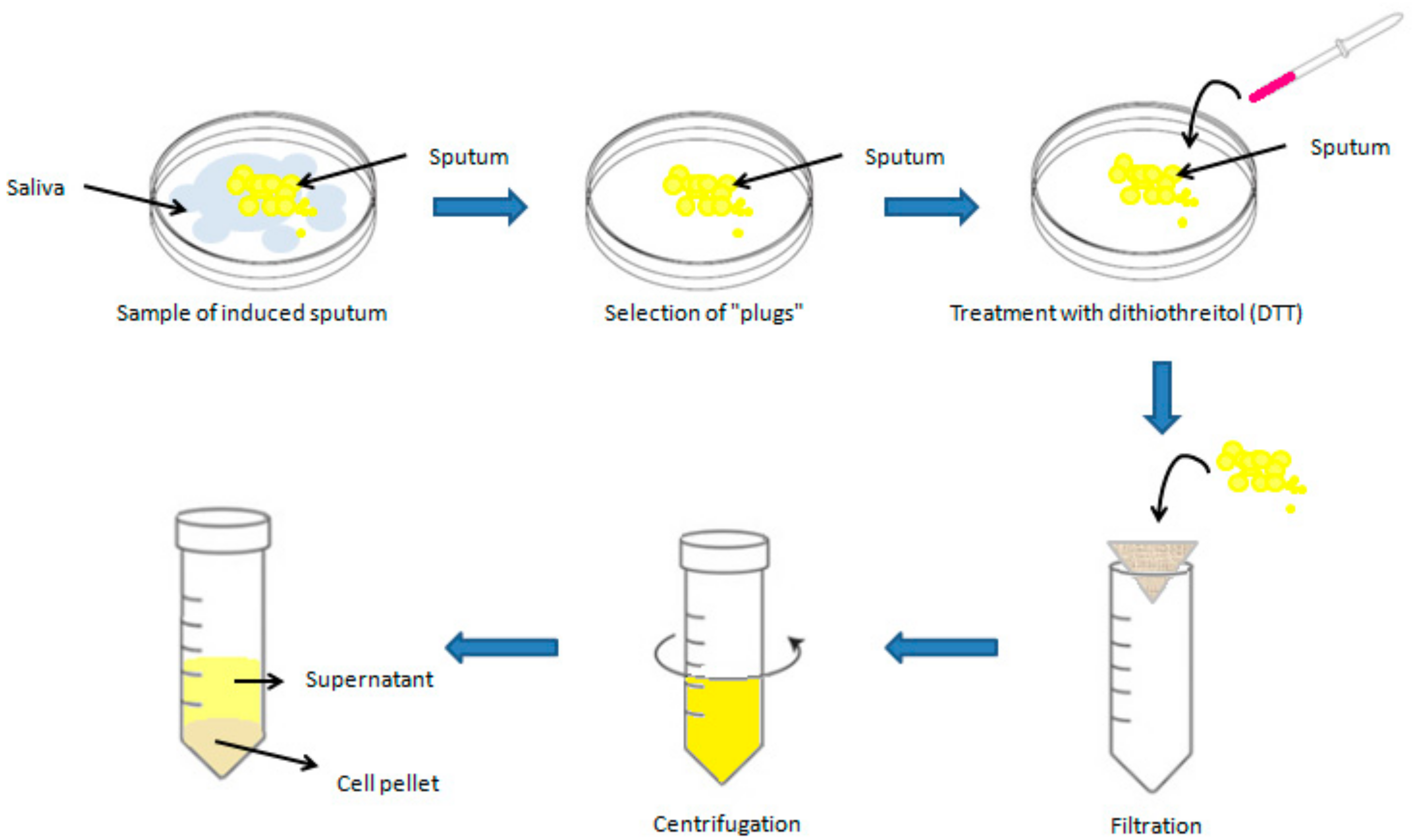
2.1. Sputum Induction and Processing Procedure
2.2. Sputum Analysis: Unveiling Insights into Asthma
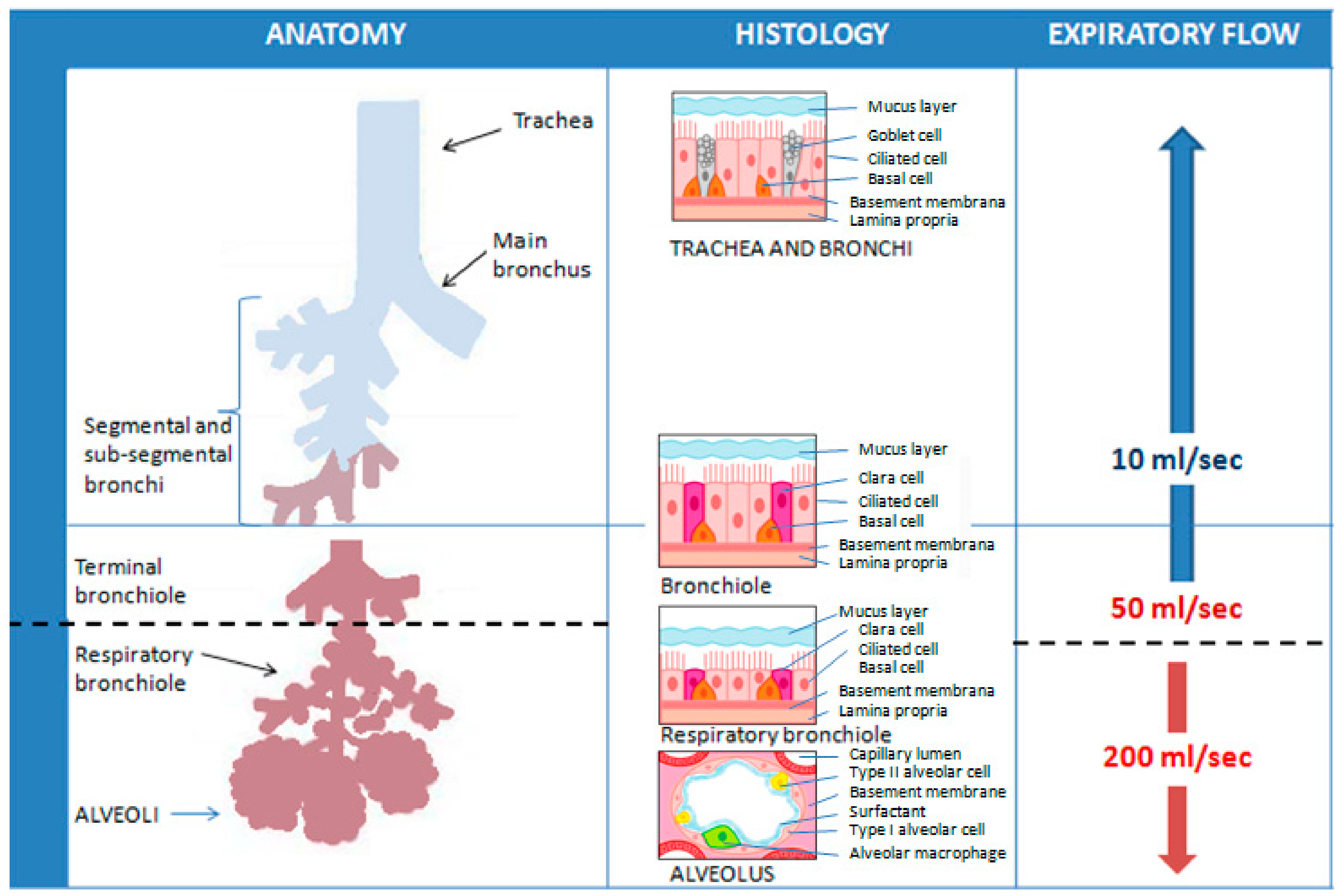
3. Exhaled Breath Condensate (EBC)
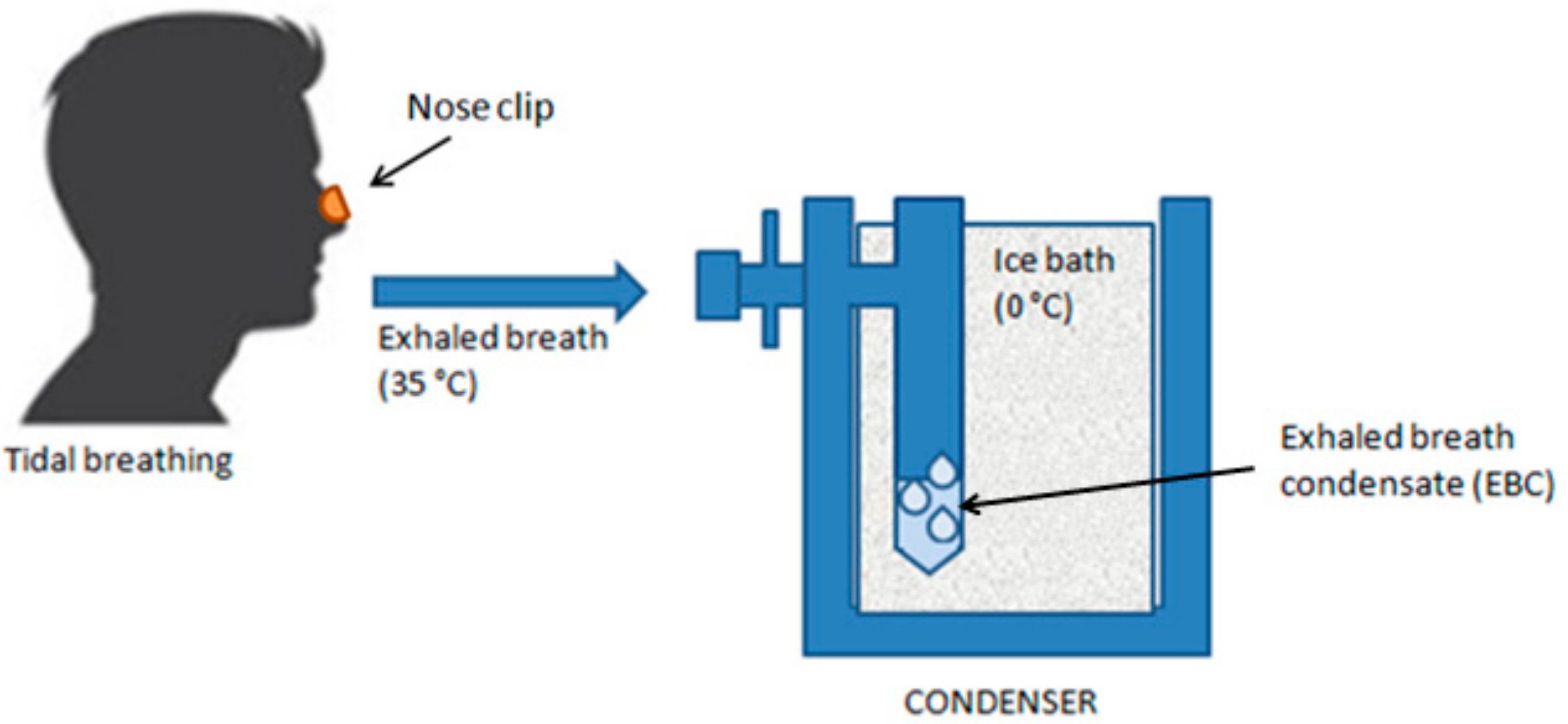
3.1. Collection and Analysis of EBC
3.2. Exhaled Breath Condensate (EBC) in Asthma: Challenges and Future Directions
4. Fractional Exhaled Nitric Oxide (FeNO)
4.1. Measurement of FeNO Levels
4.2. FeNO Measurement: Confounding Factors
4.3. Interpreting FeNO Levels in Asthma
4.4. FeNO in Asthma: Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. 2024. Available online: https://ginasthma.org/2024-report/ (accessed on 21 June 2024).
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373, Erratum in Eur. Respir. J. 2022, 59, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Uribe, V.; Romero-Tapia, S.J.; Castro-Rodriguez, J.A. Asthma Phenotypes in the Era of Personalized Medicine. J. Clin. Med. 2023, 12, 6207. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef] [PubMed]
- Dragonieri, S.; Bikov, A.; Capuano, A.; Scarlata, S.; Carpagnano, G.E. Methodological Aspects of Induced Sputum. Adv. Respir. Med. 2023, 91, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Savito, L.; Scarlata, S.; Bikov, A.; Carratù, P.; Carpagnano, G.E.; Dragonieri, S. Exhaled volatile organic compounds for diagnosis and monitoring of asthma. World J. Clin. Cases 2023, 11, 4996. [Google Scholar] [CrossRef]
- Ragnoli, B.; Radaeli, A.; Pochetti, P.; Kette, S.; Morjaria, J.; Malerba, M. Fractional nitric oxide measurement in exhaled air (FeNO): Perspectives in the management of respiratory diseases. Ther. Adv. Chronic Dis. 2023, 14, 20406223231190480. [Google Scholar] [CrossRef]
- Połomska, J.; Bar, K.; Sozańska, B. Exhaled Breath Condensate—A Non-Invasive Approach for Diagnostic Methods in Asthma. J. Clin. Med. 2021, 10, 2697. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, B.; Eze, U.A. Sputum induction and its diagnostic applications in inflammatory airway disorders: A review. Front. Allergy 2023, 4, 1282782. [Google Scholar] [CrossRef]
- Murugesan, N.; Saxena, D.; Dileep, A.; Adrish, M.; Hanania, N.A. Update on the Role of FeNO in Asthma Management. Diagnostics 2023, 13, 1428. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Scioscia, G.; Lacedonia, D.; Soccio, P.; Quarato, C.M.I.; Cotugno, G.; Palumbo, M.G.; Foschino Barbaro, M.P. Searching for Inflammatory and Oxidative Stress Markers Capable of Clustering Severe Asthma. Arch. Bronconeumol. 2021, 57, 338–344. [Google Scholar] [CrossRef]
- Coop, C.; Hagan, L.L.; Dice, J.P. Exhaled breath condensate pH in the evaluation of asthma. Allergy Asthma Proc. 2008, 29, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Pizzichini, E.; Pizzichini, M.M.M.; Efthimiadis, A.; Evans, S.; Morris, M.M.; Squillace, D.; Gleich, G.J.; Dolovich, J.; Hargreave, F.E. Indices of airway inflammation in induced sputum: Reproducibility and validity of cell and fluid-phase measurements. Am. J. Respir. Crit. Care Med. 1996, 154, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Spanevello, A.; Migliori, G.B.; Sharara, A.; Ballardini, L.; Bridge, P.; Pisati, P.; Neri, M.; Ind, P.W. Induced sputum to assess airway inflammation: A study of reproducibility. Clin. Exp. Allergy 1997, 27, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Pizzichini, M.M.M.; Pizzichini, E.; Hargreave, F.E. The use of induced sputum to investigate airway inflammation. Thorax 1997, 52, 498. [Google Scholar] [CrossRef] [PubMed]
- Pizzichini, E.; Pizzichini, M.M.M.; Leigh, R.; Djukanović, R.; Sterk, P.J. Safety of sputum induction. Eur. Respir. J. 2002, 20, 9s–18s. [Google Scholar] [CrossRef]
- Smith, C.M.; Anderson, S.D. Inhalation provocation tests using nonisotonic aerosols. J. Allergy Clin. Immunol. 1989, 84, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Djukanovic’, R.D.; Sterk, P.J.; Fahy, J.V.; Hargreave, F.E. Standardised methodology of sputum induction and processing. Eur. Respir. J. 2002, 20, 1s–2s. [Google Scholar] [CrossRef] [PubMed]
- Del Giacco, S.R.; Garcia-Larsen, V. Aerobic exercise training reduces bronchial hyper-responsiveness and serum pro-inflammatory cytokines in patients with asthma. BMJ Evid.-Based Med. 2016, 21, 70. [Google Scholar] [CrossRef]
- Leynaert, B.; Bousquet, J.; Neukirch, C.; Liard, R.; Neukirch, F. Perennial rhinitis: An independent risk factor for asthma in nonatopic subjects: Results from the European Community Respiratory Health Survey. J. Allergy Clin. Immunol. 1999, 104, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Bacci, E.; Cianchetti, S.; Paggiaro, P.L.; Carnevali, S.; Bancalari, L.; Dente, F.L.; Di Franco, A.; Giannini, D.; Vagaggini, B.; Giuntini, C. Comparison between hypertonic and isotonic saline-induced sputum in the evaluation of airway inflammation in subjects with moderate asthma. Clin. Exp. Allergy 1996, 26, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, D.; Foidart, J.M.; Laurie, L.; Bartsch, P.; Djukanovic, R.; Louis, R. Induced Sputum: Comparison Between Isotonic and Hypertonic Saline Solution Inhalation in Patients With Asthma. Chest 2001, 120, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Holz, O.; Jörres, R.A.; Koschyk, S.; Speckin, P.; Welker, L.; Magnussen, H. Changes in sputum composition during sputum induction in healthy and asthmatic subjects. Clin. Exp. Allergy 1998, 28, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Gravelyn, T.R.; Pan, P.M.; Eschenbacher, W.L.; Capper, M.; Ciffin, K. Mediator Release in an Isolated Airway Segment in Subjects with Asthma. Am. Rev. Respir. Dis. 2012, 137, 641–646. [Google Scholar] [CrossRef]
- Saetta, M.; Di Stefano, A.; Turato, G.; De Caro, R.; Bordignon, D.; Holgate, S.T.; Fabbri, L.M. Fatal asthma attack during an inhalation challenge with ultrasonically nebulized distilled water. J. Allergy Clin. Immunol. 1995, 95, 1285–1287. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, M.L.; Bacci, E.; Carnevali, S.; Cianchetti, S.; Dente, F.L.; Di Franco, A.; Giannini, D.; Taccola, M.; Vagaggini, B.; Paggiaro, P.L. Quality Evaluation of Samples Obtained by Spontaneous or Induced Sputum: Comparison between Two Methods of Processing and Relationship with Clinical and Functional Findings. J. Asthma 2002, 39, 479–486. [Google Scholar] [CrossRef]
- Belda, J.; Hussack, P.; Dolovich, M.; Efthimiadis, A.; Hargreave, F.E. Sputum induction: Effect of nebulizer output and inhalation time on cell counts and fluid-phase measures. Clin. Exp. Allergy 2001, 31, 1740–1744. [Google Scholar] [CrossRef] [PubMed]
- Toungoussova, O.; Migliori, G.B.; Foschino Barbaro, M.P.; Esposito, L.M.; Dragonieri, S.; Carpagnano, G.E.; Salerno, F.G.; Neri, M.; Spanevello, A. Changes in sputum composition during 15 min of sputum induction in healthy subjects and patients with asthma and chronic obstructive pulmonary disease. Respir. Med. 2007, 101, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Paggiaro, P.L.; Chanez, P.; Holz, O.; Ind, P.W.; Djukanović, R.; Maestrelli, P.; Sterk, P.J. Sputum induction. Eur. Respir. J. 2002, 20, 3s–8s. [Google Scholar] [CrossRef]
- Delvaux, M.; Henket, M.; Lau, L.; Kange, P.; Bartsch, P.; Djukanovic, R.; Louis, R. Nebulised salbutamol administered during sputum induction improves bronchoprotection in patients with asthma. Thorax 2004, 59, 111. [Google Scholar] [CrossRef]
- Pizzichini, E.; Pizzichini, M.M.M.; Efthimiadis, A.; Hargreave, F.E.; Dolovich, J. Measurement of inflammatory indices in induced sputum: Effects of selection of sputum to minimize salivary contamination. Eur. Respir. J. 1996, 9, 1174–1180. [Google Scholar] [CrossRef]
- Efthimiadis, A.; Pizzichini, M.M.M.; Pizzichini, E.; Kolendowicz, R.; Weston, S.; Dolovich, J. The influence of cell viability and squamous epithelial cell contamination on the reliability of sputum differential cell counts. J. Respir. Crit. Care Med. 1995, 151, A384. [Google Scholar]
- Efthimiadis, A.; Spanevello, A.; Hamid, Q.; Kelly, M.M.; Linden, M.; Louis, R.; Pizzichini, M.M.M.; Pizzichini, E.; Ronchi, C.; Van Overveld, F.; et al. Methods of sputum processing for cell counts, immunocytochemistry and in situ hybridisation. Eur. Respir. J. 2002, 20, 19s–23s. [Google Scholar] [CrossRef]
- Efthimiadis, A.; Pizzichini, M.M.M.; Pizzichini, E.; Dolovich, J.; Hargreave, F.E. Induced sputum cell and fluid-phase indices of inflammation: Comparison of treatment with dithiothreitol vs phosphate-buffered saline. Eur. Respir. J. 1997, 10, 1336–1340. [Google Scholar] [CrossRef]
- Louis, R.; Shute, J.; Goldring, K.; Perks, B.; Lau, L.C.K.; Radermecker, M.; Djukanovic, R. The effect of processing on inflammatory markers in induced sputum. Eur. Respir. J. 1999, 13, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, I.S.; Bayley, D.L.; Stockley, R.A. Effect of sputum processing with dithiothreitol on the detection of inflammatory mediators in chronic bronchitis and bronchiectasis. Thorax 2002, 57, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Louhelainen, N.; Stark, H.; Mazur, W.; Rytilä, P.; Djukanovic, R.; Kinnula, V.L. Elevation of sputum matrix metalloproteinase-9 persists up to 6 months after smoking cessation: A research study. BMC Pulm. Med. 2010, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Spanevello, A.; Confalonieri, M.; Sulotto, F.; Romano, F.; Balzano, G.; Migliori, G.B.; Bianchi, A.; Michetti, G. Induced Sputum Cellularity. Am. J. Respir. Crit. Care Med. 2012, 162, 1172–1174. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Sterk, P.J.; Hargreave, F.E.; Kips, J.C.; Inman, M.D.; Louis, R.; Pizzichini, M.M.M.; Bel, E.H.; Pin, I.; Grootendorst, D.C.; et al. Clinical applications of assessment of airway inflammation using induced sputum. Eur. Respir. J. 2002, 20, 40s–43s. [Google Scholar] [CrossRef]
- Jayaram, L.; Parameswaran, K.; Sears, M.R.; Hargreave, F.E. Induced sputum cell counts: Their usefulness in clinical practice. Eur. Respir. J. 2000, 16, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Demarche, S.; Henket, M.; Paulus, V.; Graff, S.; Schleich, F.; Corhay, J.L.; Louis, R.; Moermans, C. Methodology for Sputum Induction and Laboratory Processing. J. Vis. Exp. 2017, 2017, 56612. [Google Scholar] [CrossRef]
- Mukherjee, M.; Kjarsgaard, M.; Radford, K.; Huang, C.; Leigh, R.; Dorscheid, D.R.; Lemiere, C.; Boulet, L.P.; Waserman, S.; Martin, J.; et al. Omalizumab in patients with severe asthma and persistent sputum eosinophilia. Allergy Asthma. Clin. Immunol. 2019, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Buhl, R.; Kraft, M.; Prazma, C.M.; Price, R.G.; Howarth, P.H.; Yancey, S.W. Evaluation of sputum eosinophil count as a predictor of treatment response to mepolizumab. ERJ Open Res. 2022, 8, 00560-2021. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Paramo, F.A.; Kjarsgaard, M.; Salter, B.; Nair, G.; LaVigne, N.; Radford, K.; Sehmi, R.; Nair, P. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am. J. Respir. Crit. Care Med. 2018, 197, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Schleich, F.; Moermans, C.; Seidel, L.; Kempeneers, C.; Louis, G.; Rogister, F.; Tombu, S.; Pottier, L.; Poirrier, A.L.; Ziant, S.; et al. Benralizumab in severe eosinophilic asthma in real life: Confirmed effectiveness and contrasted effect on sputum eosinophilia versus exhaled nitric oxide fraction—PROMISE. ERJ Open Res. 2023, 9, 00383-2023. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, S.; Kjarsgaard, M.; Haider, E.; Venegas, C.; Konyer, N.; Friedlander, Y.; Nasir, N.; Boylan, C.; Kirby, M.; Nair, P. Effects of Dupilumab on Mucus Plugging and Ventilation Defects in Patients with Moderate-to-Severe Asthma: A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Respir. Crit. Care Med. 2023, 208, 995–997. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; O’Byrne, P.M.; Boulet, L.-P.; Wang, Y.; Cockcroft, D.; Bigler, J.; FitzGerald, J.M.; Boedigheimer, M.; Davis, B.E.; Dias, C.; et al. Effects of an Anti-TSLP Antibody on Allergen-Induced Asthmatic Responses. N. Engl. J. Med. 2014, 370, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Medrek, S.K.; Parulekar, A.D.; Hanania, N.A. Predictive Biomarkers for Asthma Therapy. Curr. Allergy Asthma Rep. 2017, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Girgis-Gabardo, A.; Kanai, N.; Denburg, J.A.; Hargreave, F.E.; Jordana, M.; Dolovich, J. Immunocytochemical detection of granulocyte-macrophage colony-stimulating factor and eosinophil cationic protein in sputum cells. J. Allergy Clin. Immunol. 1994, 93, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Parkash, V.; Kant, S.; Verma, A.K.; Sankhwar, S.N.; Agrawal, A.; Parmar, D.; Verma, S.; Ahmad, M.K. An update on the diagnostic biomarkers for asthma. J. Fam. Med. Prim. Care 2021, 10, 1139. [Google Scholar] [CrossRef]
- Grebski, E.; Peterson, C.; Medici, T.C. Effect of physical and chemical methods of homogenization on inflammatory mediators in sputum of asthma patients. Chest 2001, 119, 1521–1525. [Google Scholar] [CrossRef]
- Lee, T.H.; Jang, A.S.; Park, J.S.; Kim, T.H.; Choi, Y.S.; Shin, H.R.; Park, S.W.; Uh, S.T.; Choi, J.S.; Kim, Y.H.; et al. Elevation of S100 calcium binding protein A9 in sputum of neutrophilic inflammation in severe uncontrolled asthma. Ann. Allergy Asthma Immunol. 2013, 111, 268–275.e1. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Pavlidis, S.; Ng Kee Kwong, F.; Hoda, U.; Rossios, C.; Sun, K.; Loza, M.; Baribaud, F.; Chanez, P.; Fowler, S.J.; et al. Sputum proteomics and airway cell transcripts of current and ex-smokers with severe asthma in U-BIOPRED: An exploratory analysis. Eur. Respir. J. 2018, 51, 1702173. [Google Scholar] [CrossRef] [PubMed]
- Tariq, K.; Schofield, J.P.R.; Nicholas, B.L.; Burg, D.; Brandsma, J.; Bansal, A.T.; Wilson, S.J.; Lutter, R.; Fowler, S.J.; Caruso, B.M.; et al. Sputum proteomic signature of gastro-oesophageal reflux in patients with severe asthma. Respir. Med. 2019, 150, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.L.; Chen, A.; Diaz, M.P.; Zirn, N.; Gupta, A.; Britto, C.; Sauler, M.; Yan, X.; Stewart, E.; Santerian, K.; et al. A Network of Sputum MicroRNAs Is Associated with Neutrophilic Airway Inflammation in Asthma. Am. J. Respir. Crit. Care Med. 2020, 202, 51. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.; Fang, X.; Qin, L.; Fan, Y.; Ding, D.; Liu, X.; Xie, M. Plasma miR-199a-5p is increased in neutrophilic phenotype asthma patients and negatively correlated with pulmonary function. PLoS ONE 2018, 13, e0193502. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Tay, H.L.; Maltby, S.; Xiang, Y.; Eyers, F.; Hatchwell, L.; Zhou, H.; Toop, H.D.; Morris, J.C.; Nair, P.; et al. MicroRNA-9 regulates steroid-resistant airway hyperresponsiveness by reducing protein phosphatase 2A activity. J. Allergy Clin. Immunol. 2015, 136, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liang, Y.; Feng, Y.; Wu, W.; Zhang, H.; He, J.; Hu, Q.; Zhao, J.; Xu, Y.; Liu, Z.; et al. Decreased epithelial and sputum miR-221-3p associates with airway eosinophilic inflammation and CXCL17 expression in asthma. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2018, 315, L253–L264. [Google Scholar] [CrossRef] [PubMed]
- Lacedonia, D.; Palladino, G.P.; Foschino-Barbaro, M.P.; Scioscia, G.; Elisiana, G. Carpagnano Expression profiling of miRNA-145 and miRNA-338 in serum and sputum of patients with COPD, asthma, and asthma–COPD overlap syndrome phenotype. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1811. [Google Scholar] [CrossRef]
- Malmhäll, C.; Johansson, K.; Winkler, C.; Alawieh, S.; Ekerljung, L.; Rådinger, M. Altered miR-155 Expression in Allergic Asthmatic Airways. Scand. J. Immunol. 2017, 85, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Jiang, Y.; Zhao, Q.; Li, J.; Zhao, Y. lncRNA-NEAT1 Sponges miR-128 to Promote Inflammatory Reaction and Phenotypic Transformation of Airway Smooth Muscle Cells. Comput. Math. Methods Med. 2022, 2022, 7499911. [Google Scholar] [CrossRef]
- Soccio, P.; Moriondo, G.; Lacedonia, D.; Tondo, P.; Pescatore, D.; Quarato, C.M.I.; Carone, M.; Foschino Barbaro, M.P.; Scioscia, G. MiRNA and Exosomal miRNA as New Biomarkers Useful to Phenotyping Severe Asthma. Biomolecules 2023, 13, 1542. [Google Scholar] [CrossRef]
- Patsiris, S.; Exarchos, T.; Vlamos, P. Exhaled Breath Condensate (EBC): Is It a Viable Source of Biomarkers for Lung Diseases? Adv. Exp. Med. Biol. 2020, 1195, 13–18. [Google Scholar] [CrossRef]
- Kazeminasab, S.; Emamalizadeh, B.; Jouyban, A.; Shoja, M.M.; Khoubnasabjafari, M. Macromolecular Biomarkers of Chronic Obstructive Pulmonary Disease in Exhaled Breath Condensate. Biomark. Med. 2020, 14, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Ghelli, F.; Panizzolo, M.; Garzaro, G.; Squillacioti, G.; Bellisario, V.; Colombi, N.; Bergamaschi, E.; Guseva Canu, I.; Bono, R. Inflammatory Biomarkers in Exhaled Breath Condensate: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9820. [Google Scholar] [CrossRef] [PubMed]
- Effros, R.M.; Hoagland, K.W.; Bosbous, M.; Castillo, D.; Foss, B.; Dunning, M.; Gare, M.; Lin, W.; Feng, S. Dilution of Respiratory Solutes in Exhaled Condensates. Am. J. Respir. Crit. Care Med. 2012, 165, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Effros, R.M.; Biller, J.; Foss, B.; Hoagland, K.; Dunning, M.B.; Castillo, D.; Bosbous, M.; Sun, F.; Shaker, R. A Simple Method for Estimating Respiratory Solute Dilution in Exhaled Breath Condensates. Am. J. Respir. Crit. Care Med. 2012, 168, 1500–1505. [Google Scholar] [CrossRef]
- Zacharasiewicz, A.; Wilson, N.; Lex, C.; Li, A.; Kemp, M.; Donovan, J.; Hooper, J.; Kharitonov, S.A.; Bush, A. Repeatability of sodium and chloride in exhaled breath condensates. Pediatr. Pulmonol. 2004, 37, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, T.M. Sampling airway surface liquid: Non-volatiles in the exhaled breath condensate. Lung 2004, 182, 241–250. [Google Scholar] [CrossRef]
- Haslam, P.; Baughman, R.P. Report of ERS Task Force: Guidelines for measurement of acellular components and standardization of BAL. Eur. Respir. J. 1999, 14, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Carpagnano, G.E.; Lacedonia, D.; Foschino-Barbaro, M.P. Non-invasive study of airways inflammation in sleep apnea patients. Sleep Med. Rev. 2011, 15, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J. Exhaled breath condensate: An evolving tool for noninvasive evaluation of lung disease. J. Allergy Clin. Immunol. 2002, 110, 28–34. [Google Scholar] [CrossRef]
- Ahmadzai, H.; Huang, S.; Hettiarachchi, R.; Lin, J.L.; Thomas, P.S.; Zhang, Q. Exhaled breath condensate: A comprehensive update. Clin. Chem. Lab. Med. 2013, 51, 1343–1361. [Google Scholar] [CrossRef] [PubMed]
- Grob, N.M.; Aytekin, M.; Dweik, R.A. Biomarkers in exhaled breath condensate: A review of collection, processing and analysis. J. Breath Res. 2008, 2, 037004. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.; Ngamtrakulpanit, L.; Pajewski, T.N.; Turner, R.; Nguyen, T.A.; Smith, A.; Urban, P.; Hom, S.; Gaston, B.; Hunt, J. Exhaled breath condensate pH is a robust and reproducible assay of airway acidity. Eur. Respir. J. 2003, 22, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Kubáň, P.; Foret, F. Exhaled breath condensate: Determination of non-volatile compounds and their potential for clinical diagnosis and monitoring. A review. Anal. Chim. Acta 2013, 805, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, N.C.; Lowe, L.A.; Murray, C.S.; Woodcock, A.; Simpson, A.; Custovic, A. Exhaled Breath Condensate pH and Childhood Asthma. Am. J. Respir. Crit. Care Med. 2012, 174, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Kostikas, K.; Koutsokera, A.; Papiris, S.; Gourgoulianis, K.I.; Loukides, S. Exhaled breath condensate in patients with asthma: Implications for application in clinical practice. Clin. Exp. Allergy 2008, 38, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.W.S.; Leung, T.F.; Hui, D.S.C. Are exhaled breath condensates useful in monitoring asthma? Curr. Allergy Asthma Rep. 2007, 7, 65–71. [Google Scholar] [CrossRef]
- Hunt, J.F.; Fang, K.; Malik, R.; Snyder, A.; Malhotra, N.; Platts-Mills, T.A.E.; Gaston, B. Endogenous Airway Acidification. Implications for asthma pathophysiology. Am. J. Respir. Crit. Care Med. 2012, 161, 694–699. [Google Scholar] [CrossRef]
- Gaston, B.; Kelly, R.; Urban, P.; Liu, L.; Henderson, E.M.; Doctor, A.; Teague, W.G.; Fitzpatrick, A.; Erzurum, S.; Hunt, J.F. Buffering airway acid decreases exhaled nitric oxide in asthma. J. Allergy Clin. Immunol. 2006, 118, 817–822. [Google Scholar] [CrossRef]
- Bikov, A.; Galffy, G.; Tamasi, L.; Bartusek, D.; Antus, B.; Losonczy, G.; Horvath, I. Exhaled breath condensate pH decreases during exercise-induced bronchoconstriction. Respirology 2014, 19, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, E.; Carraro, S.; Alinovi, R.; Pesci, A.; Ghiro, L.; Bodini, A.; Piacentini, G.; Zacchello, F.; Zanconato, S. Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbations. Thorax 2003, 58, 505. [Google Scholar] [CrossRef] [PubMed]
- Carraro, S.; Corradi, M.; Zanconato, S.; Alinovi, R.; Pasquale, M.F.; Zacchello, F.; Baraldi, E. Exhaled breath condensate cysteinyl leukotrienes are increased in children with exercise-induced bronchoconstriction. J. Allergy Clin. Immunol. 2005, 115, 764–770. [Google Scholar] [CrossRef]
- Huszár, É.; Vass, G.; Vizi, É.; Csoma, Z.; Barát, E.; Molnár-Világos, G.; Herjavecz, I.; Horváth, I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur. Respir. J. 2002, 20, 1393–1398. [Google Scholar] [CrossRef]
- Vizi, É.; Huszár, É.; Csoma, Z.; Böszörményi-Nagy, G.; Barát, E.; Horváth, I.; Herjavecz, I.; Kollai, M. Plasma adenosine concentration increases during exercise: A possible contributing factor in exercise-induced bronchoconstriction in asthma. J. Allergy Clin. Immunol. 2002, 109, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.; Hunt, J.; Barnes, P.J.; Alving, K.; Antczak, A.; Baraldi, E.; Becher, G.; van Beurden, W.J.C.; Corradi, M.; Dekhuijzen, R.; et al. Exhaled breath condensate: Methodological recommendations and unresolved questions. Eur. Respir. J. 2005, 26, 523–548. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.C.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European Respiratory Society technical standard: Exhaled biomarkers in lung disease. Eur. Respir. J. 2017, 49, 1600965. [Google Scholar] [CrossRef] [PubMed]
- Urs, R.; Ni Chin, R.; Hemy, N.; Wilson, A.C.; Pillow, J.J.; Hall, G.L.; Simpson, S.J. Elevated leukotriene B4 and 8-isoprostane in exhaled breath condensate from preterm-born infants. BMC Pediatr. 2023, 23, 386. [Google Scholar] [CrossRef]
- Galiniak, S.; Rohovyk, N.; Rachel, M. Biomarkers of nitrosative stress in exhaled breath condensate and serum among patients with cystic fibrosis. Adv. Med. Sci. 2023, 68, 202–207. [Google Scholar] [CrossRef]
- Berumen-Rodríguez, A.A.; Alcántara-Quintana, L.E.; Pérez-Vázquez, F.J.; Zamora-Mendoza, B.N.; Díaz de León-Martínez, L.; Díaz Barriga, F.; Flores-Ramírez, R. Assessment of inflammatory cytokines in exhaled breath condensate and exposure to mixtures of organic pollutants in brick workers. Environ. Sci. Pollut. Res. 2023, 30, 13270–13282. [Google Scholar] [CrossRef]
- Guzmán-Beltrán, S.; Carreto-Binaghi, L.E.; Carranza, C.; Torres, M.; Gonzalez, Y.; Muñoz-Torrico, M.; Juárez, E. Oxidative Stress and Inflammatory Mediators in Exhaled Breath Condensate of Patients with Pulmonary Tuberculosis. A Pilot Study with a Biomarker Perspective. Antioxidants 2021, 10, 1572. [Google Scholar] [CrossRef] [PubMed]
- Carpagnano, G.E.; Scioscia, G.; Lacedonia, D.; Soccio, P.; Lepore, G.; Saetta, M.; Foschino Barbaro, M.P.; Barnes, P.J. Looking for Airways Periostin in Severe Asthma: Could It Be Useful for Clustering Type 2 Endotype? Chest 2018, 154, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Cherchi, R.; Cusano, R.; Orrù, S.; Ferrari, P.A.; Massidda, M.; Fotia, G.; De Matteis, S.; Cocco, P. Next Generation Sequencing for miRNA Detection on the Exhaled Breath Condensate: A Pilot Study. Epigenetics Insights 2023, 16, 25168657231160985. [Google Scholar] [CrossRef]
- Kierbiedź-Guzik, N.; Sozańska, B. miRNAs as Modern Biomarkers in Asthma Therapy. Int. J. Mol. Sci. 2023, 24, 11499. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, M.; Chinchilli, V.; Banta, E.; Craig, T.; August, A.; Bascom, R.; Cantorna, M.; Harvill, E.; Ishmael, F.T. Differential expression of microRNAs in exhaled breath condensates of patients with asthma, patients with chronic obstructive pulmonary disease, and healthy adults. J. Allergy Clin. Immunol. 2013, 132, 217–219.e2. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.C.; Paciência, I.; Ferreira, A.C.; Martins, C.; Rufo, J.C.; Silva, D.; Cunha, P.; Farraia, M.; Moreira, P.; Delgado, L.; et al. Development and validation of exhaled breath condensate microRNAs to identify and endotype asthma in children. PLoS ONE 2019, 14, e0224983. [Google Scholar] [CrossRef] [PubMed]
- Roff, A.N.; Craig, T.J.; August, A.; Stellato, C.; Ishmael, F.T. MicroRNA-570-3p regulates HuR and cytokine expression in airway epithelial cells. Am. J. Clin. Exp. Immunol. 2014, 3, 68. [Google Scholar]
- Mendes, F.C.; Paciência, I.; Rufo, J.C.; Silva, D.; Delgado, L.; Moreira, A.; Moreira, P. Dietary Acid Load Modulation of Asthma-Related miRNAs in the Exhaled Breath Condensate of Children. Nutrients 2022, 14, 1147. [Google Scholar] [CrossRef]
- Bazan-Socha, S.; Kierbied´zkierbied´z-Guzik, N.; Sozá Nska, B. The Potential Role of Serum and Exhaled Breath Condensate miRNAs in Diagnosis and Predicting Exacerbations in Pediatric Asthma. Biomedicines 2023, 11, 763. [Google Scholar] [CrossRef]
- Griese, M.; Noss, J.; von, B.C. Protein pattern of exhaled breath condensate and saliva. Proteomics 2002, 2, 690–696. [Google Scholar] [CrossRef]
- Maloča Vuljanko, I.; Turkalj, M.; Nogalo, B.; Bulat Lokas, S.; Plavec, D. Diagnostic value of a pattern of exhaled breath condensate biomarkers in asthmatic children. Allergol. Immunopathol. 2016, 45, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.M. Multiple roles of nitric oxide in the airways. Thorax 2003, 58, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.M.; Sterk, P.J.; Gaston, B.; Folkerts, G. Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 2004, 84, 731–765. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.M.; Sorbello, V.; Ciprandi, G. A pathophysiological approach for FeNO: A biomarker for asthma. Allergol. Immunopathol. 2015, 43, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, M.; Vitale, C.; Vatrella, A.; Molino, A.; Bianco, A.; Mazzarella, G. Fractional exhaled nitric oxide-measuring devices: Technology update. Med. Devices Evid. Res. 2016, 9, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Fuschillo, S.; Mosella, M.; Accardo, M.; Guida, P.; Motta, A.; Maniscalco, M. Comparison of three different exhaled nitric oxide analyzers in chronic respiratory disorders. J. Breath Res. 2019, 13, 021002. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar] [CrossRef] [PubMed]
- Silkoff, P.E.; Mcclean, P.A.; Slutsky, A.S.; Furlott, H.G.; Hoffstein, E.; Wakita, S.; Chapman, K.R.; Szalai, J.P.; Zamel, N. Marked flow-dependence of exhaled nitric oxide using a new technique to exclude nasal nitric oxide. Am. J. Respir. Crit. Care Med. 2012, 155, 260–267. [Google Scholar] [CrossRef]
- Tsoukias, N.M.; George, S.C. Impact of volume-dependent alveolar diffusing capacity on exhaled nitric oxide concentration. Ann. Biomed. Eng. 2001, 29, 731–739. [Google Scholar] [CrossRef]
- Högman, M.; Drca, N.; Ehrstedt, C.; Meriläinen, P. Exhaled nitric oxide partitioned into alveolar, lower airways and nasal contributions. Respir. Med. 2000, 94, 985–991. [Google Scholar] [CrossRef]
- Tsoukias, N.M.; Shin, H.W.; Wilson, A.F.; George, S.C. A single-breath technique with variable flow rate to characterize nitric oxide exchange dynamics in the lungs. J. Appl. Physiol. 2001, 91, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, T.; Malinovschi, A.; Janson, C.; Fonseca, J.; Alving, K. Evolution of exhaled nitric oxide levels throughout development and aging of healthy humans. J. Breath Res. 2015, 9, 036005. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Lin, J.; Xie, G.; Lv, C.; Zhang, M. Sex differences of small airway function and fractional exhaled nitric oxide in patients with mild asthma. Ann. Allergy Asthma Immunol. 2023, 130, 187–198.e3. [Google Scholar] [CrossRef] [PubMed]
- Atukorala, K.R.; Fernando, D.M.S.; Silva, W.A.N.Y.; Amarasiri, W.A.D.L. Lung Functions and Airway Inflammatory Markers during Different Phases of Menstrual Cycle in Asthmatic and Healthy Females of Sri Lanka. 2018. Available online: http://repository.kln.ac.lk/handle/123456789/19222 (accessed on 21 June 2024).
- Blake, T.L.; Chang, A.B.; Chatfield, M.D.; Petsky, H.L.; Rodwell, L.T.; Brown, M.G.; Hill, D.C.; McElrea, M.S. Does Ethnicity Influence Fractional Exhaled Nitric Oxide in Healthy Individuals?: A Systematic Review. Chest 2017, 152, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, S.A.; Robbins, R.A.; Yates, D.; Keatings, V.; Barnes, P.J. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am. J. Respir. Crit. Care Med. 2012, 152, 609–612. [Google Scholar] [CrossRef]
- Yurach, M.T.; Davis, B.E.; Cockcroft, D.W. The effect of caffeinated coffee on airway response to methacholine and exhaled nitric oxide. Respir. Med. 2011, 105, 1606–1610. [Google Scholar] [CrossRef][Green Version]
- Imai, T.; Watanabe, K. Effects of acute resistance exercise on exhaled nitric oxide levels in non-asthmatic male. Respir. Physiol. Neurobiol. 2023, 317, 104143. [Google Scholar] [CrossRef] [PubMed]
- Olin, A.-C.; Aldenbratt, A.; Ekman, A.; Ljungkvist, G.; Jungersten, L.; Alving, K.; Toré, K. Increased nitric oxide in exhaled air after intake of a nitrate-rich meal. Respir. Med. 2001, 95, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.C.; Taylor, D.R.; Peterson, L.E.; Cowan, J.O.; Palmay, R.; Williamson, A.; Hammel, J.; Erzurum, S.C.; Hazen, S.L.; Comhair, S.A.A. Biomarker-based Asthma Phenotypes of Corticosteroid Response. J. Allergy Clin. Immunol. 2015, 135, 877. [Google Scholar] [CrossRef] [PubMed]
- Rolla, G.; Brussino, L.; Bertero, M.T.; Colagrande, P.; Converso, M.; Bucca, C.; Polizzi, S.; Caligaris-Cappio, F. Increased nitric oxide in exhaled air of patients with systemic lupus erythematosus—PubMed. J. Rheumatol. 1997, 24, 1066–1071. [Google Scholar] [PubMed]
- Söderman, C.; Leone, A.; Furst, V.; Persson, M.G. Endogenous Nitric Oxide in Exhaled Air from Patients with Liver Cirrhosis. Scand. J. Gastroenterol. 1997, 32, 591–597. [Google Scholar] [CrossRef]
- Ansarin, K.; Chatkin, J.M.; Ferreira, I.M.; Gutierrez, C.A.; Zamel, N.; Chapman, K.R. Exhaled nitric oxide in chronic obstructive pulmonary disease: Relationship to pulmonary function. Eur. Respir. J. 2001, 17, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, A.M.; Sue-Chu, M.; Lingaas Holmen, T.; Langhammer, A.; Bjermer, L. Exhaled and nasal NO levels in allergic rhinitis: Relation to sensitization, pollen season and bronchial hyperresponsiveness. Eur. Respir. J. 1999, 13, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Van Asch, C.J.J.; Balemans, W.A.F.; Rovers, M.M.; Schilder, A.G.M.; Van Der Ent, C.K. Atopic disease and exhaled nitric oxide in an unselected population of young adults. Ann. Allergy Asthma Immunol. 2008, 100, 59–65. [Google Scholar] [CrossRef]
- Maniscalco, M.; Calabrese, C.; D’Amato, M.; Guida, P.; Molino, A.; Aliani, M.; De Tullio, R.; Foschino Barbaro, M.; Ricciardolo, F.L.M.; Carpagnano, G.E. Association between exhaled nitric oxide and nasal polyposis in severe asthma. Respir. Med. 2019, 152, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Ricketts, H.C.; Steffensen, F.; Goodfellow, A.; Cowan, D.C. Obesity affects type 2 biomarker levels in asthma. J. Asthma 2023, 60, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Kiaer, E.; Ravn, A.; Jennum, P.; Prætorius, C.; Welinder, R.; Ørntoft, S.; von Buchwald, C.; Backer, V. Fractional exhaled nitric oxide—A possible biomarker for risk of obstructive sleep apnea in snorers. J. Clin. Sleep Med. 2024, 20, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, P.; Visca, D.; Loukides, S.; Märtson, A.G.; Alffenaar, J.W.C.; Migliori, G.B.; Spanevello, A. A snapshot of exhaled nitric oxide and asthma characteristics: Experience from high to low income countries. Pulmonology 2022, 28, 44–58. [Google Scholar] [CrossRef]
- Alving, K.; Malinovschi, A. Basic aspects of exhaled nitric oxide. Exhaled Biomark. 2010, 49, 1–31. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Busse, W.W.; Wenzel, S.E.; Casale, T.B.; FitzGerald, J.M.; Rice, M.S.; Daizadeh, N.; Deniz, Y.; Patel, N.; Harel, S.; Rowe, P.J.; et al. Baseline FeNO as a prognostic biomarker for subsequent severe asthma exacerbations in patients with uncontrolled, moderate-to-severe asthma receiving placebo in the LIBERTY ASTHMA QUEST study: A post-hoc analysis. Lancet Respir. Med. 2021, 9, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Loewenthal, L.; Menzies-Gow, A. FeNO in Asthma. Semin. Respir. Crit. Care Med. 2022, 43, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Price, D.B.; Buhl, R.; Chan, A.; Freeman, D.; Gardener, E.; Godley, C.; Gruffydd-Jones, K.; McGarvey, L.; Ohta, K.; Ryan, D.; et al. Fractional exhaled nitric oxide as a predictor of response to inhaled corticosteroids in patients with non-specific respiratory symptoms and insignificant bronchodilator reversibility: A randomised controlled trial. Lancet Respir. Med. 2018, 6, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Neelamegan, R.; Saka, V.; Tamilarasu, K.; Rajaram, M.; Selvarajan, S.; Chandrasekaran, A. Clinical Utility of Fractional exhaled Nitric Oxide (FeNO) as a Biomarker to Predict Severity of Disease and Response to Inhaled Corticosteroid (ICS) in Asthma Patients. J. Clin. Diagn. Res. 2016, 10, FC01. [Google Scholar] [CrossRef]
- Petsky, H.L.; Kew, K.M.; Turner, C.; Chang, A.B. Exhaled nitric oxide levels to guide treatment for adults with asthma. Cochrane Database Syst. Rev. 2016, 9, CD011440. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Cotton, S.; Wood, J.; Bell, V.; Raja, E.A.; Scott, N.W.; Morgan, H.; Lawrie, L.; Emele, D.; Kennedy, C.; et al. Reducing asthma attacks in children using exhaled nitric oxide (RAACENO) as a biomarker to inform treatment strategy: A multicentre, parallel, randomised, controlled, phase 3 trial. Lancet Respir. Med. 2022, 10, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Wenzel, S.; Roseń, K.; Hsieh, H.J.; Mosesova, S.; Choy, D.F.; Lal, P.; Arron, J.R.; Harris, J.M.; Busse, W. Exploring the effects of omalizumab in allergic asthma: An analysis of biomarkers in the EXTRA study. Am. J. Respir. Crit. Care Med. 2013, 187, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Deniz, Y.; Corren, J.; Casale, T.B.; FitzGerald, J.M.; Izuhara, K.; Daizadeh, N.; Ortiz, B.; Johnson, R.R.; Harel, S.; et al. Baseline FeNO Independently Predicts the Dupilumab Response in Patients With Moderate-to-Severe Asthma. J. Allergy Clin. Immunol. Pract. 2023, 11, 1213–1220.e2. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Hargadon, B.; Morgan, A.; Shelley, M.; Richter, J.; Shaw, D.; Green, R.H.; Brightling, C.; Wardlaw, A.J.; Pavord, I.D. Alveolar nitric oxide in adults with asthma: Evidence of distal lung inflammation in refractory asthma. Eur. Respir. J. 2005, 25, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Dahlan, A.F.; Islam, M.A.; Shukri, N.M.; Abdullah, B. Nasal nitric oxide measurement in allergic rhinitis and non-allergic rhinitis: A meta-analysis. ACTA Otorhinolaryngol. Ital. 2024, 44, 100–112. [Google Scholar] [CrossRef]
- Scadding, G.; Scadding, G.K. Update on the use of nitric oxide as a noninvasive measure of airways inflammation. Rhinology 2009, 47, 115–120. [Google Scholar] [PubMed]

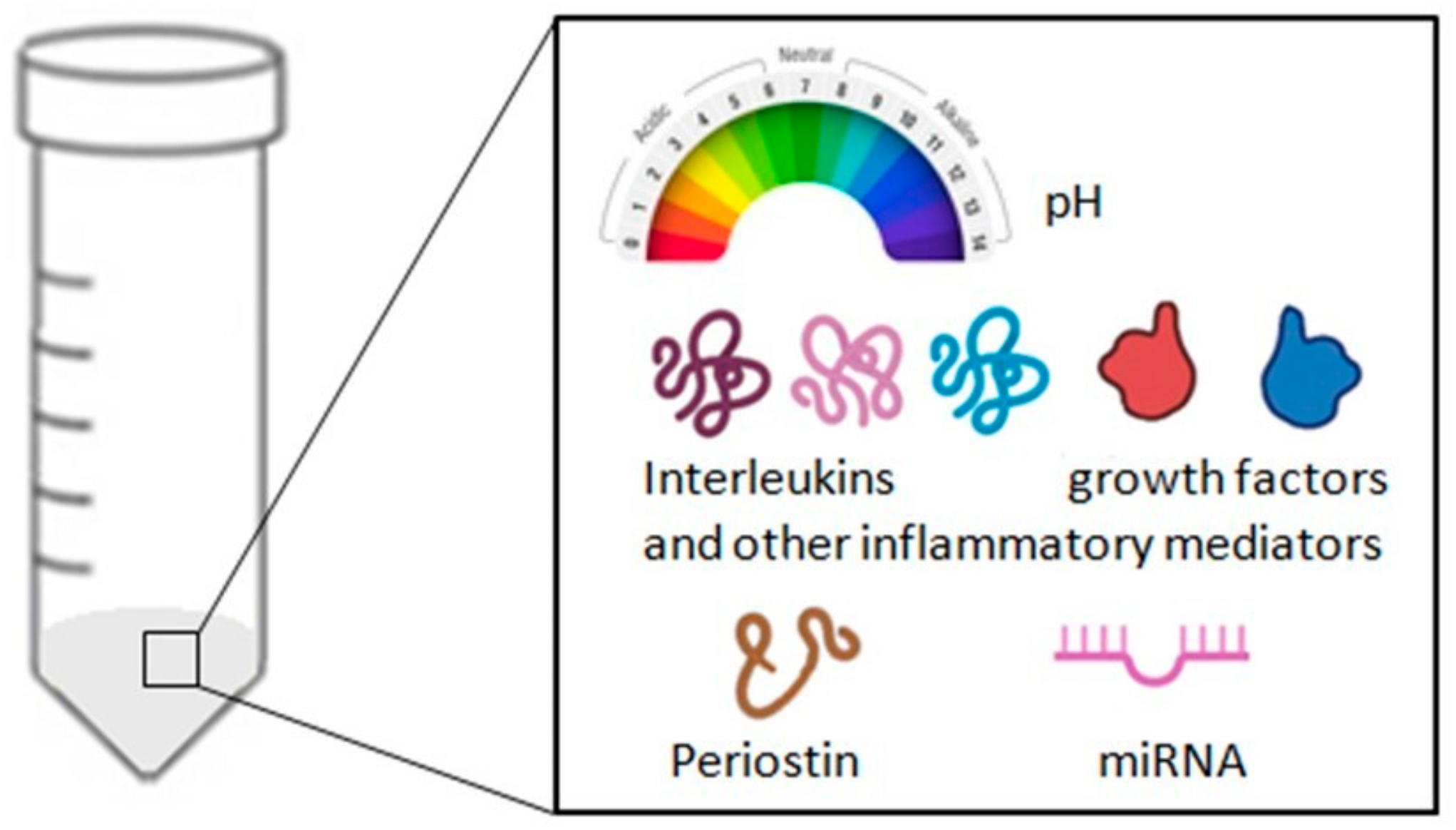

| Cell Type | Normal Range | Pathological Condition |
|---|---|---|
| Squamous epithelial cells (from oropharynx) | <20% | An increased percentage of squamous epithelial cells suggests salivary contamination |
| Epithelial cells | 50–80% | An increased percentage of epithelial cells may reflect inflammation with airway damage |
| Macrophages | 50–80% | Elevated macrophage counts may result from an ongoing immune response |
| Neutrophils | 20–50% | Elevated neutrophil counts (>60–65%) may indicate acute inflammatory processes (e.g., bacterial infections or COPD exacerbations) |
| Lymphocytes | 10–25% | Elevated lymphocyte counts may suggest viral infections or autoimmune disorders |
| Eosinophils | 0–5% | Elevated eosinophil counts (>2%) may suggest eosinophilic airway inflammation |
| ↑ FeNO50 | ↓ FeNO50 |
|---|---|
| Male sex | Tobacco smoke (active and passive) |
| Exposure to allergens and polluted air | Obesity |
| Menstrual cycle (proliferative or follicular phase) | Menstrual cycle (secretory or luteal phase) |
| Diet (rich in nitrates) | Caffeine and alcohol |
| Ethnicity (black and Hispanic) | Ethnicity (Caucasian) |
| Technical factors (environmental NO, incorrect expiratory flow, and mixed oral and nasal NO sampling) | Spirometry and physical exercise (transitory factors) |
| Eosinophilic/allergic asthma | Inhaled or oral corticosteroids |
| Allergic rhinitis | Cystic fibrosis |
| Chronic rhinosinusitis with nasal polyposis (CRNwNP) | Primary ciliary dyskinesia |
| Atopic dermatitis | Bronchiectasis |
| Eosinophilic bronchitis and COPD | Gastroesophageal reflux |
| OSAS | |
| Viral Infections | |
| Systemic lupus erythematosus | |
| Liver cirrhosis |
| FeNO50 < 25 ppb (<20 ppb in Children) | FeNO50 25–50 ppb (20–35 ppb in Children) | FeNO50 > 50 ppb (>35 ppb in Children) |
|---|---|---|
| Eosinophilic airway inflammation unlikely | Be cautious and monitor changes in FeNO50 over time | Eosinophilic airway inflammation present |
| Presence of Respiratory Symptoms | No Respiratory Symptoms | |
|---|---|---|
| FeNO50 < 25 ppb | Evaluate alternative diagnoses | Optimal adherence to inhaled steroid Evaluate step-down |
| FeNO50 25–50 ppb | Suboptimal adherence Inadequate therapeutic dosage Possible steroid resistance Allergen exposure | Optimal adherence and adequate therapeutic dosage Continue FeNO50 monitoring |
| FeNO50 > 50 ppb | Suboptimal adherence Inadequate therapeutic dosage Review inhalation technique Possible steroid resistance Allergen exposure Increased risk of exacerbation | Suboptimal adherence or inadequate therapeutic dosage Review inhalation technique |
| Advantages | Disadvantages | |
|---|---|---|
| Induced sputum | Validated tool for assessment of respiratory inflammation Validated tool for identification of type-2 asthma Validated tool for monitoring of anti-inflammatory drug effectiveness Possibility to analyze multiple biomarkers | Relatively invasive Not repeatable over short time periods Contraindicated in severe obstruction Rescue medication needed Experienced personnel and specialized lab needed Time-consuming |
| EBC | Non-invasive Allows repeated measurements Potential tool for assessment of respiratory inflammation Possibility to analyze multiple biomarkers | Procedure awaits further validation Assays not fully reproducible Soluble markers subject to dilution Experienced personnel and specialized lab needed Expensive equipment |
| FeNO | Non-invasive Allows repeated measurements Validated tool for assessment of respiratory inflammation Validated tool for identification of type-2 asthma Validated tool for monitoring of anti-inflammatory drug effectiveness | Many perturbing factors Expensive equipment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soccio, P.; Quarato, C.M.I.; Tondo, P.; Lacedonia, D.; Hoxhallari, A.; Foschino Barbaro, M.P.; Scioscia, G. Breath and Sputum Analyses in Asthmatic Patients: An Overview. Cells 2024, 13, 1355. https://doi.org/10.3390/cells13161355
Soccio P, Quarato CMI, Tondo P, Lacedonia D, Hoxhallari A, Foschino Barbaro MP, Scioscia G. Breath and Sputum Analyses in Asthmatic Patients: An Overview. Cells. 2024; 13(16):1355. https://doi.org/10.3390/cells13161355
Chicago/Turabian StyleSoccio, Piera, Carla Maria Irene Quarato, Pasquale Tondo, Donato Lacedonia, Anela Hoxhallari, Maria Pia Foschino Barbaro, and Giulia Scioscia. 2024. "Breath and Sputum Analyses in Asthmatic Patients: An Overview" Cells 13, no. 16: 1355. https://doi.org/10.3390/cells13161355
APA StyleSoccio, P., Quarato, C. M. I., Tondo, P., Lacedonia, D., Hoxhallari, A., Foschino Barbaro, M. P., & Scioscia, G. (2024). Breath and Sputum Analyses in Asthmatic Patients: An Overview. Cells, 13(16), 1355. https://doi.org/10.3390/cells13161355







