A Pilot Study on Proteomic Predictors of Mortality in Stable COPD
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Study Population
2.3. Biological Sample Obtention
2.4. Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS)
2.5. Immune-Based Multiplexing
2.6. Data Analysis
2.6.1. Calculation of the Sample Size
2.6.2. Descriptive Statistics and Comparisons between Groups
2.7. Functional Classification of Proteins and Network Analysis
2.8. Generation of Predictive Models
3. Results
3.1. General Characteristics of the Patients
3.2. Proteomic Profile
3.3. Prediction of Death and Days of Survival (Table 4 and Table 5)
3.3.1. Conventional Approach
| Fitting | Prediction (Internal Validation) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model Name | Prot | Se/Sp/Acc/MCC | Cov | Se | Sp | MCC | Cov | PPV (Rep|Our) | NPV (Rep|Our) | Acc (Rep|Our) |
| 31 | 1.00 | 1.00 | 0.78 | 1.00 | 0.79 | 0.77 | 1.00|1.00 | 0.84|0.91 | 0.90|0.93 |
| 10 | 1.00 | 1.00 | 0.89 | 1.00 | 0.89 | 0.82 | 1.00|1.00 | 0.91|0.95 | 0.95|0.96 |
| 10 | 1.00 | 1.00 | 1.00 | 0.90 | 0.88 | 0.73 | 0.90|0.82 | 1.00|1.00 | 0.95|0.93 |
| 10 | 1.00 | 0.68 | 0.80 | 1.00 | 0.80 | 0.53 | 0.82|0.70 | 1.00|1.00 | 0.89|0.86 |
| Fitting | Prediction | ||||
|---|---|---|---|---|---|
| Model Name | Proteins | R2 | Conformal Accuracy | Q2 | Conformal Accuracy |
| 31 | 0.64 | 1.00 | 0.18 | 0.95 |
| 10 | 0.81 | 1.00 | 0.52 | 0.95 |
| 10 | 0.64 | 1.00 | 0.25 | 0.91 |
| 10 | 0.71 | 1.00 | 0.36 | 0.95 |
3.3.2. AI Free Choice of Proteins
4. Discussion
4.1. Previous Studies
4.2. Interpretation of Novel Findings
4.2.1. Differentially Abundant Proteins
4.2.2. Prediction of Death and Days of Survival
4.3. Strengths and Potential Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- WHO. COPD Factsheet. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 19 July 2024).
- Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). 2023. Available online: www.goldcopd.org (accessed on 19 July 2024).
- Celli, B.R.; Fabbri, L.M.; Aaron, S.D.; Agusti, A.; Brook, R.; Criner, G.J.; Franssen, F.M.E.; Humbert, M.; Hurst, J.R.; O’donnell, D.; et al. An Updated Definition and Severity Classification of Chronic Obstructive Pulmonary Disease Exacerbations: The Rome Proposal. Am. J. Respir. Crit. Care Med. 2021, 204, 1251–1258. [Google Scholar] [CrossRef]
- Esteban, C.; Quintana, J.M.; Aburto, M.; Moraza, J.; Egurrola, M.; España, P.P.; Pérez-Izquierdo, J.; Capelastegui, A. Predictors of mortality in patients with stable COPD. J. Gen. Intern. Med. 2008, 23, 1829–1834. [Google Scholar] [CrossRef]
- Nishimura, K.; Izumi, T.; Tsukino, M.; Oga, T. Dyspnea Is a Better Predictor of 5-Year Survival Than Airway Obstruction in Patients with COPD. Chest 2002, 121, 1434–1440. [Google Scholar] [CrossRef]
- Oga, T.; Nishimura, K.; Tsukino, M.; Sato, S.; Hajiro, T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: Role of exercise capacity and health status. Am. J. Respir. Crit. Care Med. 2003, 167, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Salvany, A.; Lamarca, R.; Ferrer, M.; Garcia-Aymerich, J.; Alonso, J.; Félez, M.; Khalaf, A.; Marrades, R.M.; Monsó, E.; Serra-Batlles, J.; et al. Health-related Quality of Life and Mortality in Male Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2002, 166, 680–685. [Google Scholar] [CrossRef]
- Almagro, P.; Calbo, E.; de Echaguïen, A.O.; Barreiro, B.; Quintana, S.; Heredia, J.L.; Garau, J. Mortality After Hospitalization for COPD. Chest 2002, 121, 1441–1448. [Google Scholar] [CrossRef]
- Connors, A.F., Jr.; Dawson, N.V.; Thomas, C.; Harrell, F.E., Jr.; Desbiens, N.; Fulkerson, W.J.; Kussin, P.; Bellamy, P.; Goldman, L.; Knaus, W.A. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am. J. Respir. Crit. Care Med. 1996, 154, 959–967. [Google Scholar] [CrossRef]
- Solanes-Garcia, I.; Casan, P. Causes of death and prediction of mortality in COPD. Arch Bronconeumol. 2010, 46, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Lüthi-Corridori, G.; Boesing, M.; Roth, A.; Giezendanner, S.; Leuppi-Taegtmeyer, A.B.; Schuetz, P.; Leuppi, J.D. Predictors of Length of Stay, Rehospitalization and Mortality in Community-Acquired Pneumonia Patients: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 5601. [Google Scholar] [CrossRef]
- Hartl, S.; Lopez-Campos, J.L.; Pozo-Rodriguez, F.; Castro-Acosta, A.; Studnicka, M.; Kaiser, B.; Roberts, C.M. Risk of death and re-admission of hospital-admitted COPD exacerbations: European COPD Audit. Eur. Respir. J. 2016, 47, 113–121. [Google Scholar] [CrossRef]
- Han, M.-Z.; Hsiue, T.-R.; Tsai, S.-H.; Huang, T.-H.; Liao, X.-M.; Chen, C.-Z. Validation of the GOLD 2017 and new 16 subgroups (1A–4D) classifications in predicting exacerbation and mortality in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3425–3433. [Google Scholar] [CrossRef] [PubMed]
- de Torres, J.P.; Casanova, C.; Marín, J.M.; Pinto-Plata, V.; Divo, M.; Zulueta, J.J.; Berto, J.; Zagaceta, J.; Sanchez-Salcedo, P.; Cabrera, C.; et al. Prognostic evaluation of COPD patients: GOLD 2011 versus BODE and the COPD comorbidity index COTE. Thorax 2014, 69, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.W.V.; MacDonald, T.M.; Chalmers, J.D.; Schembri, S. The effect of changes to GOLD severity stage on long term morbidity and mortality in COPD. Respir. Res. 2018, 19, 249. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Wrobel, J.P. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and Risk of Mortality in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef]
- Negewo, N.A.; Gibson, P.G.; McDonald, V.M. COPD and its comorbidities: Impact, measurement and mechanisms. Respirology 2015, 20, 1160–1171. [Google Scholar] [CrossRef]
- Sato, S.; Oga, T.; Muro, S.; Tanimura, K.; Tanabe, N.; Nishimura, K.; Hirai, T. Changes in mortality among patients with chronic obstructive pulmonary disease from the 1990s to the 2000s: A pooled analysis of two prospective cohort studies. BMJ Open 2023, 13, e065896. [Google Scholar] [CrossRef]
- Fähndrich, S.; Herr, C.; Teuteberg, S.; Söhler, S.; Soriano, D.; Classen, J.; Adams, J.; Weinhold, V.; Waschki, B.; Zeller, T.; et al. Midregional proatrial naturetic peptide (MRproANP) and copeptin (COPAVP) as predictors of all-cause mortality in recently diagnosed mild to moderate COPD—Results from COSYCONET. Respir. Res. 2024, 25, 56. [Google Scholar] [CrossRef]
- Hu, H.S.; Wang, Z.; Jian, L.Y.; Zhao, L.M.; Liu, X.D. Optimizing inhaled corticosteroid use in patients with chronic obstructive pul-monary disease: Assessing blood eosinophils, neutrophil-to-lymphocyte ratio, and mortality outcomes in US adults. Front Immunol. 2023, 14, 1230766. [Google Scholar] [CrossRef]
- Echevarria, C.; Steer, J.; Prasad, A.; Quint, J.K.; Bourke, S.C. Admission blood eosinophil count, inpatient death and death at 1 year in exacerbating patients with COPD. Thorax 2023, 78, 1090–1096. [Google Scholar] [CrossRef]
- Husebø, G.R.; Gabazza, E.C.; D’Alessandro Gabazza, C.; Yasuma, T.; Toda, M.; Aanerud, M.; Nielsen, R.; Bakke, P.S.; Eagan, T.M.L. Coag-ulation markers as predictors for clinical events in COPD. Respirology 2021, 26, 342–351. [Google Scholar] [CrossRef]
- Rønnow, S.R.; Langholm, L.L.; Karsdal, M.A.; Manon-Jensen, T.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Leeming, D.J.; Sand, J.M.B. Endo-trophin, an extracellular hormone, in combination with neoepitope markers of von Willebrand factor improves prediction of mortality in the ECLIPSE COPD cohort. Respir. Res. 2020, 21, 202. [Google Scholar] [CrossRef]
- Rabe, K.F.; Martinez, F.J.; Ferguson, G.T.; Wang, C.; Singh, D.; Wedzicha, J.A.; Trivedi, R.; Rose, E.S.; Ballal, S.; McLaren, J.; et al. Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD. N. Engl. J. Med. 2020, 383, 35–48. [Google Scholar] [CrossRef]
- Calverley, P.M.; Anzueto, A.R.; Carter, K.; Grönke, L.; Hallmann, C.; Jenkins, C.; Wedzicha, J.; Rabe, K.F. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): A double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir. Med. 2018, 6, 337–344. [Google Scholar] [CrossRef]
- Lipson, D.A.; Crim, C.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Jones, C.E.; Kilbride, S.; Lange, P.; et al. Reduction in All-Cause Mortality with Fluticasone Furoate/Umeclidinium/Vilanterol in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2020, 201, 1508–1516. [Google Scholar] [CrossRef]
- Gea, J.; Pascual, S.; Castro-Acosta, A.; Hernández-Carcereny, C.; Castelo, R.; Márquez-Martín, E.; Montón, C.; Palou, A.; Faner, R.; Furlong, L.I.; et al. The BIOMEPOC Project: Personalized Biomarkers and Clinical Profiles in Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2019, 55, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Enríquez-Rodríguez, C.J.; Casadevall, C.; Faner, R.; Castro-Costa, A.; Pascual-Guàrdia, S.; Seijó, L.; López-Campos, J.L.; Peces-Barba, G.; Monsó, E.; Barreiro, E.; et al. COPD: Systemic proteomic profiles in frequent and infrequent exacerbators. ERJ Open Res. 2024, 10. [Google Scholar] [CrossRef]
- Enríquez-Rodríguez, C.J.; Pascual-Guardia, S.; Casadevall, C.; Caguana-Vélez, O.A.; Rodríguez-Chiaradia, D.; Barreiro, E.; Gea, J. Proteomic Blood Profiles Obtained by Totally Blind Biological Clustering in Stable and Exacerbated COPD Patients. Cells 2024, 13, 866. [Google Scholar] [CrossRef]
- Arostegui, I.; Legarreta, M.J.; Barrio, I.; Esteban, C.; Garcia-Gutierrez, S.; Aguirre, U.; Quintana, J.M. IRYSS-COPD Group A Computer Application to Predict Adverse Events in the Short-Term Evolution of Patients with Exacerbation of Chronic Obstructive Pulmonary Disease. JMIR Med. Inform. 2019, 7, e10773. [Google Scholar] [CrossRef]
- Austin, P.C.; White, I.R.; Lee, D.S.; van Buuren, S. Missing Data in Clinical Research: A Tutorial on Multiple Imputation. Can. J. Cardiol. 2021, 37, 1322–1331. [Google Scholar] [CrossRef]
- Pastor, M.; Gomez-Tamayo, J.C.; Sanz, F. Flame: An open-source framework for model development, hosting, and usage in production envoiironments. J. Cheminform. 2021, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Castillo, E.G.; Pérez, T.A.; Ancochea, J.; Sanz, M.T.P.; Almagro, P.; Martínez-Camblor, P.; Miravitlles, M.; Rodríguez-Carballeira, M.; Navarro, A.; Lamprecht, B.; et al. Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2015 and GOLD 2019 staging: A pooled analysis of individual patient data. ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef]
- Chicco, D.; Warrens, M.J.; Jurman, G. The coefficient of determination R-squared is more informative than SMAPE, MAE, MAPE, MSE and RMSE in regression analysis evaluation. PeerJ Comput. Sci. 2021, 7, e623. [Google Scholar] [CrossRef]
- Kiddle, S.J.; Whittaker, H.R.; Seaman, S.R.; Quint, J.K. Prediction of five-year mortality after COPD diagnosis using primary care records. PLoS ONE 2020, 15, e0236011. [Google Scholar] [CrossRef]
- Gedebjerg, A.; Szépligeti, S.K.; Wackerhausen, L.-M.H.; Horváth-Puhó, E.; Dahl, R.; Hansen, J.G.; Sørensen, H.T.; Nørgaard, M.; Lange, P.; Thomsen, R.W. Prediction of mortality in patients with chronic obstructive pulmonary disease with the new Global Initiative for Chronic Obstructive Lung Disease 2017 classification: A cohort study. Lancet Respir. Med. 2018, 6, 204–212. [Google Scholar] [CrossRef]
- van Hirtum, P.V.; Sprooten, R.T.M.; van Noord, J.A.; van Vliet, M.; de Kruif, M.D. Long term survival after admission for COPD exacerbation: A comparison with the general population. Respir. Med. 2018, 137, 77–82. [Google Scholar] [CrossRef]
- Lorenzana, I.; Galera, R.; Casitas, R.; Martínez-Cerón, E.; Castillo, M.A.; Alfaro, E.; Cubillos-Zapata, C.; García-Río, F. Dynamic hy-perinflation is a risk factor for mortality and severe exacerbations in COPD patients. Respir. Med. 2024, 225, 107597. [Google Scholar] [CrossRef]
- Nishimura, K.; Kusunose, M.; Shibayama, A.; Nakayasu, K. Is Frailty a Mortality Predictor in Subjects with Chronic Obstructive Pulmonary Disease? Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 2955–2960. [Google Scholar] [CrossRef]
- Nishimura, K.; Kusunose, M.; Sanda, R.; Mori, M.; Shibayama, A.; Nakayasu, K. Comparison of Predictive Properties between Tools of Patient-Reported Outcomes: Risk Prediction for Three Future Events in Subjects with COPD. Diagnostics 2023, 13, 2269. [Google Scholar] [CrossRef]
- Medina-Mirapeix, F.; Valera-Novella, E.; Morera-Balaguer, J.; Bernabeu-Mora, R. Prognostic value of the five-repetition sit-to-stand test for mortality in people with chronic obstructive pulmonary disease. Ann. Phys. Rehabil. Med. 2022, 65, 101598. [Google Scholar] [CrossRef]
- Liu, S.-F.; Chin, C.-H.; Tseng, C.-W.; Chen, Y.-C.; Kuo, H.-C. Exertional Desaturation Has Higher Mortality Than Non-Desaturation in COPD. Medicina 2021, 57, 1110. [Google Scholar] [CrossRef]
- Waschki, B.; Kirsten, A.; Holz, O.; Müller, K.C.; Meyer, T.; Watz, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef]
- Deng, M.; Lu, Y.; Zhang, Q.; Bian, Y.; Zhou, X.; Hou, G. Global prevalence of malnutrition in patients with chronic obstructive pulmonary disease: Systemic review and meta-analysis. Clin. Nutr. 2023, 42, 848–858. [Google Scholar] [CrossRef]
- van Gestel, Y.R.; Hoeks, S.E.; Sin, D.D.; Hüzeir, V.; Stam, H.; Mertens, F.W.; van Domburg, R.T.; Bax, J.J.; Poldermans, D. COPD and cancer mortality: The influence of statins. Thorax 2009, 64, 963–967. [Google Scholar] [CrossRef][Green Version]
- Wasswa-Kintu, S.; Gan, W.Q.; Man, S.F.P.; Pare, P.D.; Sin, D.D. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: A systematic review and meta-analysis. Thorax 2005, 60, 570–575. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Zheng, X.; Peng, J.; Chen, Y.; Yu, K.; Yang, Y.; Wang, X.; Yang, X.; Qian, J.; et al. Deaths from COPD in patients with cancer: A population-based study. Aging 2021, 13, 12641–12659. [Google Scholar] [CrossRef]
- Lüthi-Corridori, G.; Boesing, M.; Ottensarendt, N.; Leuppi-Taegtmeyer, A.B.; Schuetz, P.; Leuppi, J.D. Predictors of Length of Stay, Mortality and Rehospitalization in COPD Patients: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 5322. [Google Scholar] [CrossRef]
- Abdullah, A.S.; Eigbire, G.; Ali, M.; Awadalla, M.; Wahab, A.; Ibrahim, H.; Salama, A.; Alweis, R. Relationship of Atrial Fibrillation to Outcomes in Patients Hospitalized for Chronic Obstructive Pulmonary Disease Exacerbation. J. Atr. Fibrillation 2019, 12, 2117. [Google Scholar] [CrossRef]
- Liao, K.-M.; Chen, C.-Y. Incidence and risk factors of atrial fibrillation in Asian COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2523–2530. [Google Scholar] [CrossRef]
- Warming, P.E.; Garcia, R.; Hansen, C.J.; Simons, S.O.; Torp-Pedersen, C.; Linz, D.; Tfelt-Hansen, J. Atrial fibrillation and chronic ob-structive pulmonary disease: Diagnostic sequence and mortality risk. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 128–134. [Google Scholar] [CrossRef]
- Trudzinski, F.C.; Jörres, R.A.; Alter, P.; Kahnert, K.; Waschki, B.; Herr, C.; Kellerer, C.; Omlor, A.; Vogelmeier, C.F.; Fähndrich, S.; et al. Associations of oxygenated hemoglobin with disease burden and prognosis in stable COPD: Results from COSYCONET. Sci. Rep. 2020, 10, 10544. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, E.C.; Özgül, M.A.; Tutar, N.; Ömür, I.; Uysal, A.; Altın, S. Red Blood Cell Distribution and Survival in Patients with Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 416–424. [Google Scholar] [CrossRef]
- Valvi, D.; Mannino, D.M.; Müllerova, H.; Tal-Singer, R. Fibrinogen, chronic obstructive pulmonary disease (COPD) and outcomes in two United States cohorts. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 173–182. [Google Scholar] [PubMed]
- Oh, Y.-M.; Park, J.H.; Kim, E.-K.; Hwang, S.C.; Kim, H.J.; Kang, D.R.; Yoo, K.H.; Lee, J.-H.; Kim, T.-H.; Lim, S.Y.; et al. Anemia as a clinical marker of stable chronic obstructive pulmonary disease in the Korean obstructive lung disease cohort. J. Thorac. Dis. 2017, 9, 5008–5016. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lim, B.; Kyung, S.Y.; Park, J.-W.; Jeong, S.H. Comorbidity and Inflammatory Markers May Contribute to Predict Mortality of High-Risk Patients with Chronic Obstructive Pulmonary Disease Exacerbation. J. Clin. Med. Res. 2016, 8, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zha, L.; Feng, G.; An, Q.; Shi, F.; Xu, J.; Xu, Q.; Xia, H.; Zhang, M.; Li, L. Prognostic Value of Serum Cholinesterase Levels for In-Hospital Mortality among Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2023, 20, 178–185. [Google Scholar] [CrossRef]
- Urban, M.H.; Stojkovic, S.; Demyanets, S.; Hengstenberg, C.; Valipour, A.; Wojta, J.; Burghuber, O.C. Soluble ST2 and All-Cause Mortality in Patients with Chronic Obstructive Pulmonary Disease—A 10-Year Cohort Study. J. Clin. Med. 2021, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Holmgaard, D.B.; Mygind, L.H.; Titlestad, I.; Madsen, H.; Pedersen, S.S.; Mortensen, O.H.; Pedersen, C. Calprotectin—A Marker of Mortality in COPD? Results from a Prospective Cohort Study. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Tanni, S.E.; Caram, L.M.; Corrêa, C.; Corrêa, C.R.; Godoy, I. Three-year follow-up of Interleukin 6 and C-reactive protein in chronic obstructive pulmonary disease. Respir. Res. 2013, 14, 24. [Google Scholar] [CrossRef]
- Zemans, R.L.; Jacobson, S.; Keene, J.; Kechris, K.; Miller, B.E.; Tal-Singer, R.; Bowler, R.P. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir. Res. 2017, 18, 117. [Google Scholar] [CrossRef]
- Agustí, A.; Edwards, L.D.; Rennard, S.I.; MacNee, W.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Lomas, D.A.; Calverley, P.M.A.; Wouters, E.; et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Persistent Systemic Inflammation is Associated with Poor Clinical Outcomes in COPD: A Novel Phenotype. PLoS ONE 2012, 7, e37483. [Google Scholar] [CrossRef]
- Wu, S.; Huang, K.; Chang, C.; Chu, X.; Zhang, K.; Li, B.; Yang, T. Serum Proteomic Profiling in Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 1623–1635. [Google Scholar] [CrossRef]
- Ubhi, B.K.; Cheng, K.K.; Dong, J.; Janowitz, T.; Jodrell, D.; Tal-Singer, R.; MacNee, W.; Lomas, D.A.; Riley, J.H.; Griffin, J.L.; et al. Targeted metabolomics identifies perturbations in amino acid metabolism that sub-classify patients with COPD. Mol. Biosyst. 2012, 8, 3125–3133. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Li, Y.; Liu, F.; Chen, L.; He, S.; Lin, F.; Wei, X.; Fang, Y.; Li, Q.; et al. Proteomics and metabolomics profiling reveal panels of circulating diagnostic biomarkers and molecular subtypes in stable COPD. Respir. Res. 2023, 24, 73. [Google Scholar] [CrossRef]
- Gregory, A.; Xu, Z.; Pratte, K.; Lee, S.; Liu, C.; Chase, R.; Yun, J.; Saferali, A.; Hersh, C.P.; Bowler, R.; et al. Clustering-based COPD subtypes have distinct longitudinal outcomes and multi-omics biomarkers. BMJ Open Respir. Res. 2022, 9, e001182. [Google Scholar] [CrossRef]
- Gandhi, R.; Kalsariya, V.; Katara, R.; Murugan, Y. Sarcopenia, Eosinophil-to-Platelet Ratio, and C-reactive Protein as Predictors of Adverse Events in Patients with Acute Exacerbations of Chronic Obstructive Pulmonary Disease: A Prospective Observational Study. Cureus 2024, 16, e56651. [Google Scholar] [CrossRef]
- Zhou, R.; Pan, D. Association between admission heart rate and in-hospital mortality in patients with acute exacerbation of chronic obstructive pulmonary disease and respiratory failure: A retrospective cohort study. BMC Pulm. Med. 2024, 24, 111. [Google Scholar] [CrossRef]
- Bhat, R.; Kamath, S.; Jain, A.; Acharya, V.; Antony, T.; Holla, R.; Jha, A. RV in COPD—The complicated matters of the heart—Correlation of ECHO and biomarker with COPD severity and outcome. Lung India 2024, 41, 192–199. [Google Scholar] [CrossRef]
- Zhou, W.-Q.; Song, X.; Dong, W.-H.; Chen, Z. Independent effect of the triglyceride-glucose index on all-cause mortality in critically ill patients with chronic obstructive pulmonary disease and asthma: A retrospective cohort study. Chronic Respir. Dis. 2024, 21. [Google Scholar] [CrossRef]
- Holm, A.M.; Andreassen, S.L.; Christensen, V.L.; Kongerud, J.; Almås, Ø.; Auråen, H.; Henriksen, A.H.; Aaberge, I.S.; Klingenberg, O.; Rustøen, T. Hypogammaglobulinemia and Risk of Exacerbation and Mortality in Patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 799–807. [Google Scholar] [CrossRef]
- Fortis, S.; Wan, E.S.; Kunisaki, K.; Eyck, P.T.; Ballas, Z.K.; Bowler, R.P.; Crapo, J.D.; Hokanson, J.E.; Wendt, C.; Silverman, E.K.; et al. Increased mortality associated with frequent exacerbations in COPD patients with mild-to-moderate lung function impairment, and smokers with normal spirometry. Respir. Med. X 2021, 3, 100025. [Google Scholar] [CrossRef]
- Song, W.; Li, D.; Tao, L.; Luo, Q.; Chen, L. Solute carrier transporters: The metabolic gatekeepers of immune cells. Acta Pharm. Sin. B 2020, 10, 61–78. [Google Scholar] [CrossRef]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Mandal, J.; Malla, B.; Steffensen, R.; Costa, L.; Egli, A.; Trendelenburg, M.; Blasi, F.; Kostikas, K.; Welte, T.; Torres, A.; et al. Man-nose-binding lectin protein and its association to clinical outcomes in COPD: A longitudinal study. Respir. Res. 2015, 16, 150. [Google Scholar] [CrossRef]
- Santos, N.C.D.; Miravitlles, M.; Camelier, A.A.; Almeida, V.D.C.; Maciel, R.R.B.T.; Camelier, F.W.R. Prevalence and Impact of Comor-bidities in Individuals with Chronic Obstructive Pulmonary Disease: A Systematic Review. Tuberc. Respir. Dis. 2022, 85, 205–220. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.; Chronis, C.; Papapetrou, E.; Tatsioni, A.; Gartzonika, K.; Tsaousi, C.; Gogali, A.; Katsanos, C.; Vaggeli, A.; Tselepi, C.; et al. Prothrombotic state in patients with stable COPD: An observational study. ERJ Open Res. 2021, 7. [Google Scholar] [CrossRef]
- Manon-Jensen, T.; Langholm, L.L.; Rønnow, S.R.; Karsdal, M.A.; Tal-Singer, R.; Vestbo, J.; Leeming, D.J.; Miller, B.E.; Bülow Sand, J.M. End-product of fibrinogen is elevated in emphysematous chronic obstructive pulmonary disease and is predictive of mortality in the ECLIPSE cohort. Respir. Med. 2019, 160, 105814. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Li, C.; Ma, C.; Ge, W. Proteome Profiling of Lung Tissues in Chronic Obstructive Pulmonary Disease (COPD): Platelet and Macrophage Dysfunction Contribute to the Pathogenesis of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 973–980. [Google Scholar] [CrossRef]
- Jankowski, M.; Undas, A.; Kaczmarek, P.; Butenas, S. Activated factor XI and tissue factor in chronic obstructive pulmonary disease: Links with inflammation and thrombin generation. Thromb. Res. 2011, 127, 242–246. [Google Scholar] [CrossRef]
- Mannino, D.M.; Tal-Singer, R.; Lomas, D.A.; Vestbo, J.; Barr, G.; Tetzlaff, K.; Lowings, M.; Rennard, S.I.; Snyder, J.; Goldman, M.; et al. Plasma Fibrinogen as a Biomarker for Mortality and Hospitalized Exacerbations in People with COPD. Chronic Obstr. Pulm. Dis. 2015, 2, 23–34. [Google Scholar] [CrossRef]
- Sand, J.M.B.; Rønnow, S.R.; Langholm, L.L.; Karsdal, M.A.; Manon-Jensen, T.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Leeming, D.J. Combining biomarkers of clot resolution and alveolar basement membrane destruction predicts mortality in the ECLIPSE COPD cohort. Respir. Med. 2020, 173, 106185. [Google Scholar] [CrossRef]
- Langholm, L.L.; Rønnow, S.R.; Sand, J.M.B.; Leeming, D.J.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Karsdal, M.A.; Manon-Jensen, T. Increased von Willebrand Factor Processing in COPD, Reflecting Lung Epithelium Damage, Is Associated with Emphysema, Exacer-bations and Elevated Mortality Risk. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 543–552. [Google Scholar] [CrossRef]
- Foti, M.; Locati, M. (Eds.) Cytokine Effector Functions in Tissues; Academic Press: Cambridge, MA, USA, 2017; ISBN 978-0-12-804214-4. [Google Scholar]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- Costa, C.; Rufino, R.; Traves, S.L.; e Silva, J.R.L.; Barnes, P.J.; Donnelly, L.E. CXCR3 and CCR5 Chemokines in Induced Sputum from Patients with COPD. Chest 2008, 133, 26–33. [Google Scholar] [CrossRef]
- Peng, J.; Yu, Q.; Fan, S.; Chen, X.; Tang, R.; Wang, D.; Qi, D. High Blood Eosinophil and YKL-40 Levels, as Well as Low CXCL9 Levels, are Associated with Increased Readmission in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 795–806. [Google Scholar] [CrossRef]
- Kubysheva, N.I.; Postnikova, L.B.; Soodaeva, S.K.; Novikov, D.V.; Eliseeva, T.I.; Novikov, V.V.; Karaulov, A.V. Comparative Study of the Levels of IL-1β, IL-4, IL-8, TNFα, and IFNγ in Stable Course and Exacerbation of Chronic Obstructive Pulmonary Disease of Varying Severity. Bull. Exp. Biol. Med. 2022, 173, 745–748. [Google Scholar] [CrossRef]
- Di Francia, M.; Barbier, D.; Mege, J.L.; Orehek, J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pul-monary disease. Am. J. Respir. Crit. Care Med. 1994, 150, 1453–1455. [Google Scholar] [CrossRef]
- Schols, A.M.; Buurman, W.A.; Van den Brekel, A.S.; Dentener, M.A.; Wouters, E.F. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax 1996, 51, 819–824. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Herting, J.R.; Pike, K.C.; Gharib, S.A.; Matute-Bello, G.; Borson, S.; Kohen, R.; Adams, S.G.; Fan, V.S. Symptom profiles and inflammatory markers in moderate to severe COPD. BMC Pulm. Med. 2016, 16, 173. [Google Scholar] [CrossRef]
- Mendy, A.; Forno, E.; Niyonsenga, T.; Gasana, J. Blood biomarkers as predictors of long-term mortality in COPD. Clin. Respir. J. 2018, 12, 1891–1899. [Google Scholar] [CrossRef]
- Dahl, M.; Vestbo, J.; Lange, P.; Bojesen, S.E.; Tybjærg-Hansen, A.; Nordestgaard, B.G. C-reactive Protein as a Predictor of Prognosis in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2007, 175, 250–255. [Google Scholar] [CrossRef]
- Kelly, E.; Owen, C.A.; Pinto-Plata, V.; Celli, B.R. The role of systemic inflammatory biomarkers to predict mortality in chronic obstructive pulmonary disease. Expert. Rev. Respir. Med. 2013, 7, 57–64. [Google Scholar] [CrossRef]
- Gea, J.; Sancho-Muñoz, A.; Chalela, R. Nutritional status and muscle dysfunction in chronic respiratory diseases: Stable phase versus acute exacerbations. J. Thorac. Dis. 2018, 10, S1332–S1354. [Google Scholar] [CrossRef]
- Marquis, K.; Debigaré, R.; Lacasse, Y.; LeBlanc, P.; Jobin, J.; Carrier, G.; Maltais, F. Midthigh Muscle Cross-Sectional Area Is a Better Predictor of Mortality than Body Mass Index in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2002, 166, 809–813. [Google Scholar] [CrossRef]
- Plante, E.; Vance, R. Selection of preschool language tests: A data-based approach. Language, Speech, and Hearing Services in Schools 1994, 25, 15–24. [Google Scholar] [CrossRef]
- Owusuaa, C.; Dijkland, S.A.; Nieboer, D.; van der Rijt, C.C.D.; van der Heide, A. Predictors of mortality in chronic obstructive pulmonary disease: A systematic review and meta-analysis. BMC Pulm. Med. 2022, 22, 125. [Google Scholar] [CrossRef]
- White, N.; Kupeli, N.; Vickerstaff, V.; Stone, P. How accurate is the ‘Surprise Question’ at identifying patients at the end of life? A systematic review and meta-analysis. BMC Med. 2017, 15, 139. [Google Scholar] [CrossRef]
- Aryal, S.; Diaz-Guzman, E.; Mannino, D.M. Influence of sex on chronic obstructive pulmonary disease risk and treatment out-comes. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 1145–1154. [Google Scholar]
- Ntritsos, G.; Franek, J.; Belbasis, L.; Christou, M.A.; Markozannes, G.; Altman, P.; Fogel, R.; Sayre, T.; Ntzani, E.E.; Evangelou, E. Gen-der-specific estimates of COPD prevalence: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1507–1514. [Google Scholar] [CrossRef]
- Drummond, M.B.; Wise, R.A.; Hansel, N.N.; Putcha, N. Comorbidities and Chronic Obstructive Pulmonary Disease: Prevalence, Influence on Outcomes, and Management. Semin. Respir. Crit. Care Med. 2015, 36, 575–591. [Google Scholar] [CrossRef]
- Bai, S.; Ye, R.; Wang, C.; Sun, P.; Wang, D.; Yue, Y.; Wang, H.; Wu, S.; Yu, M.; Xi, S.; et al. Identification of Proteomic Signatures in Chronic Obstructive Pulmonary Disease Emphysematous Phenotype. Front. Mol. Biosci. 2021, 8, 650604. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Enríquez-Rodríguez, C.J.; Pascual-Guardia, S. Metabolomics in COPD. Arch. Bronconeumol. 2023, 59, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Casadevall, C.; Nebot, P.; Enríquez-Rodríguez, C.J.; Faner, R.; Cosio, B.G.; Haro, N.; Pascual-Guardia, S.; Peces-Barba, G.; Monsó, E.; et al. Aging and metabolic changes in COPD patients. Am. J. Respir. Crit. Care Med. 2004, 209, A4314. [Google Scholar]
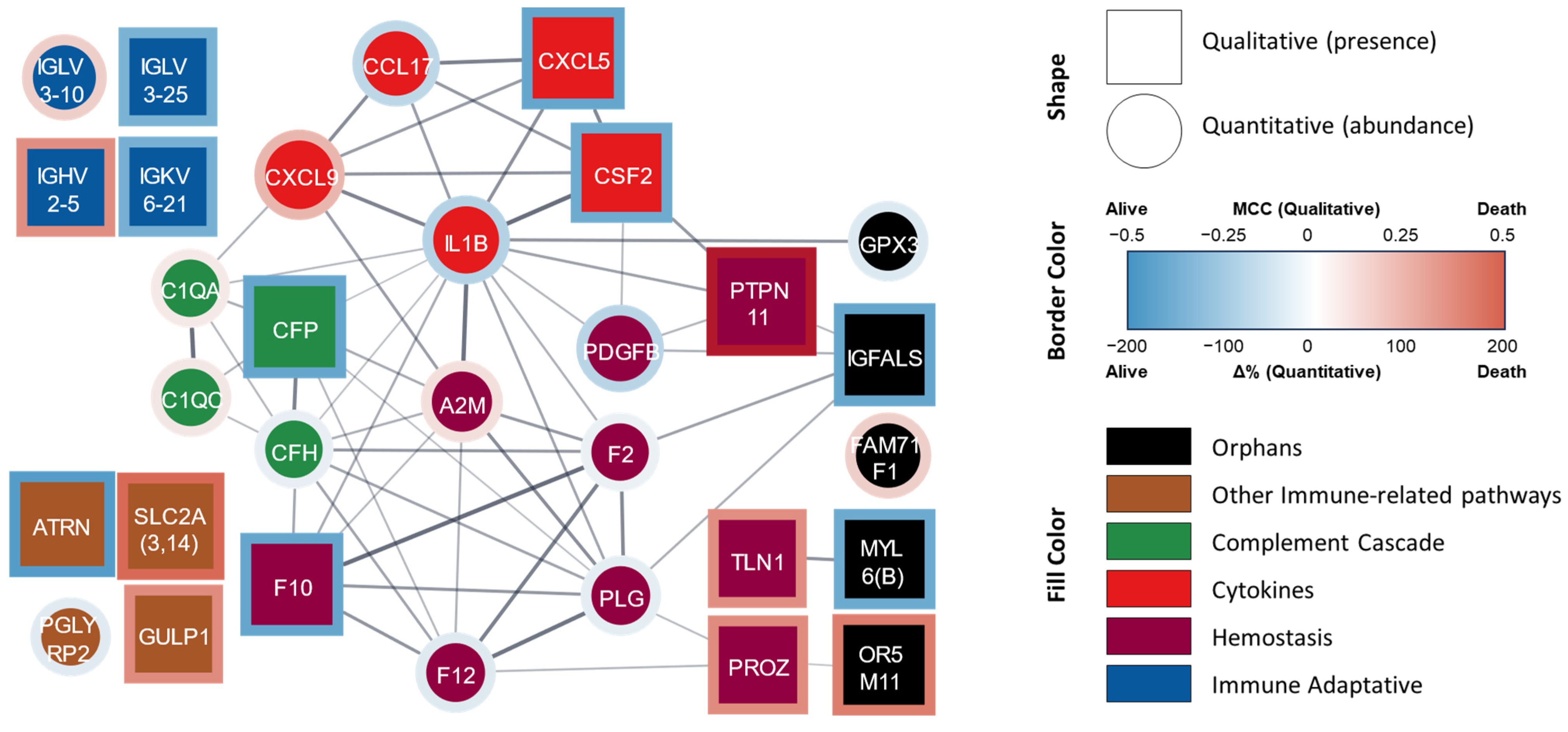
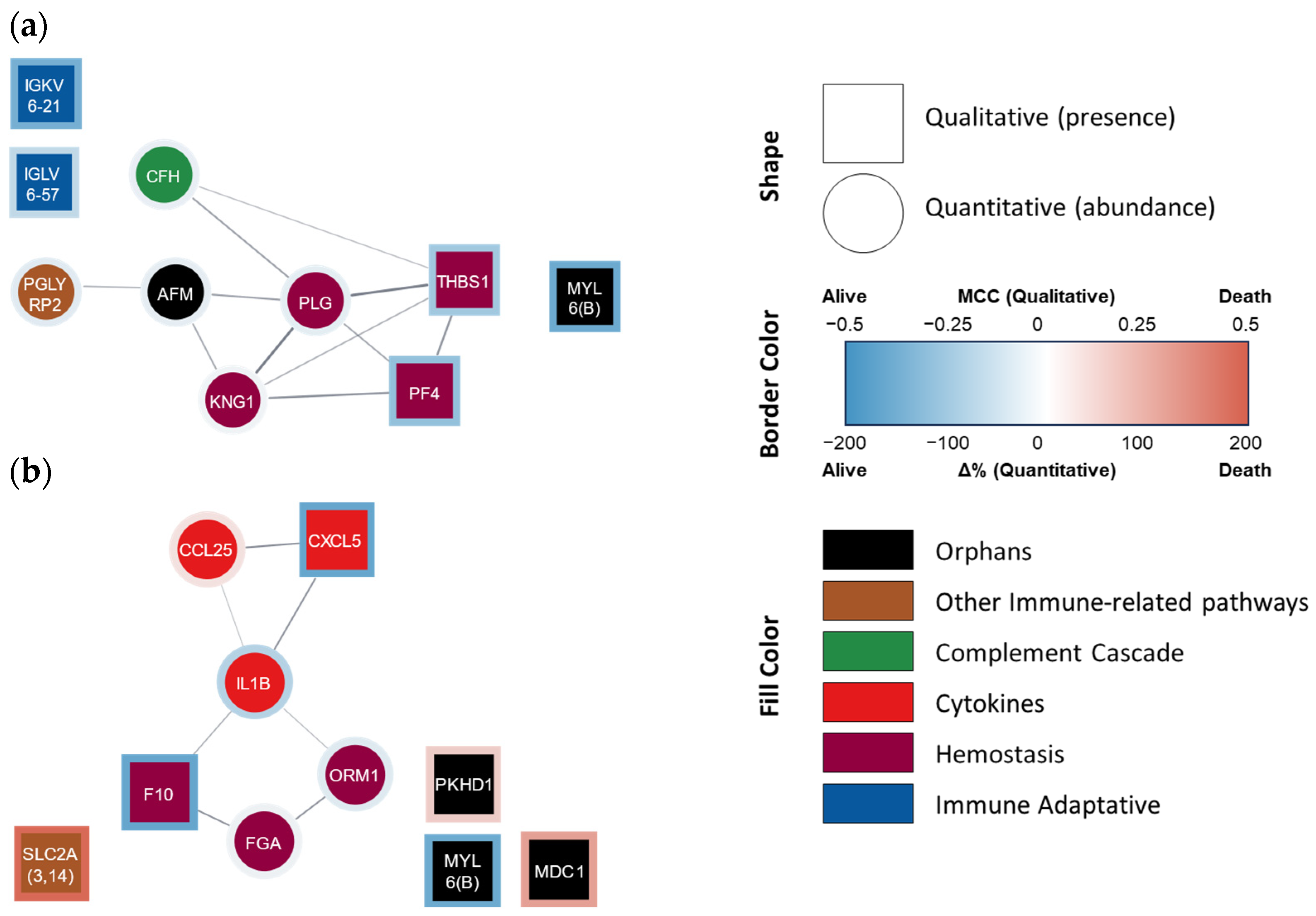
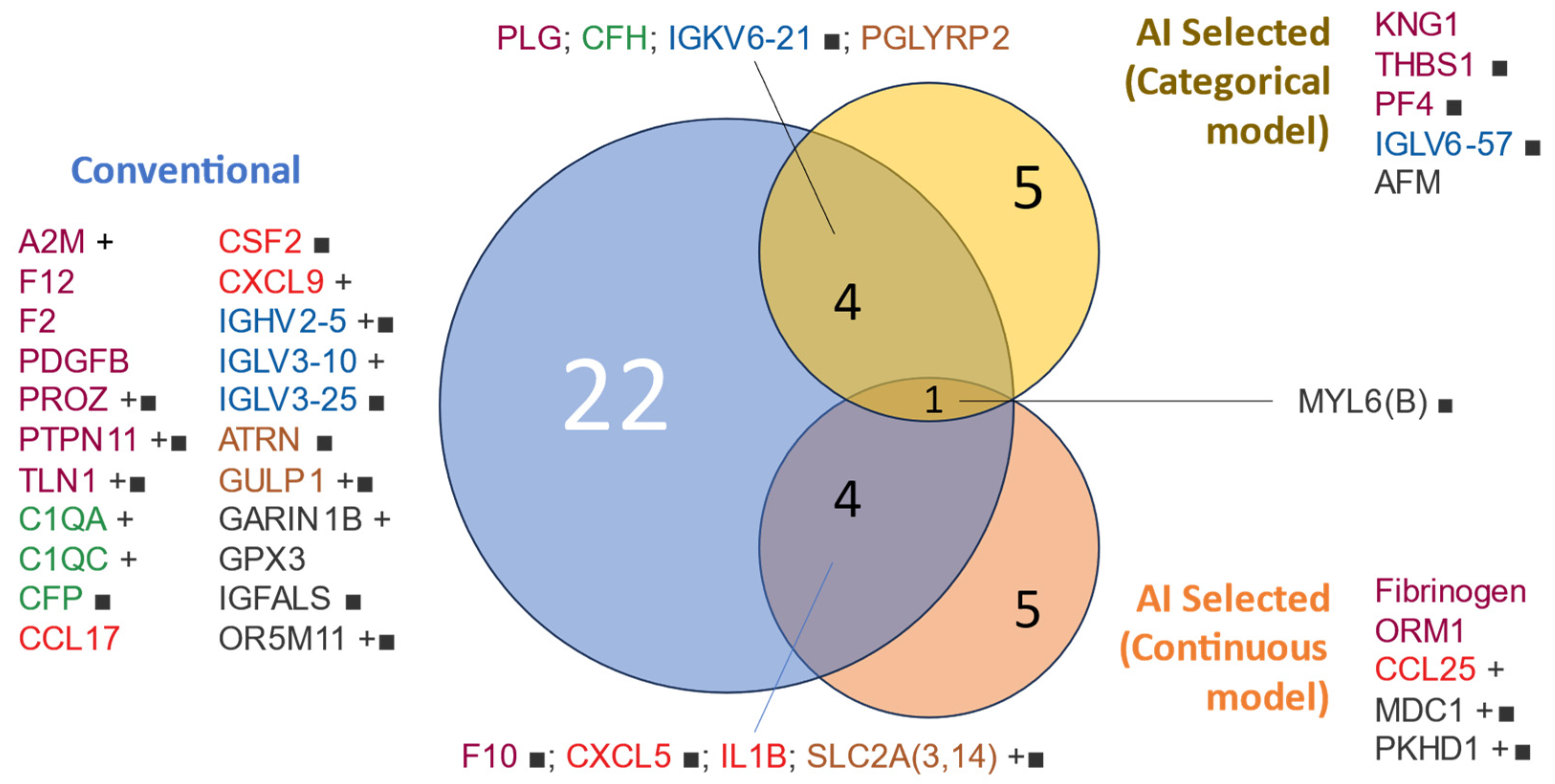
| COPD 4-Year Survivors (n = 23) | COPD 4-Year Non-Survivors (n = 11) | |
|---|---|---|
| General Characteristics | ||
| Age, year | 67 ± 9 | 72 ± 7 |
| Males, n (% in the group) | 15 (65%) | 9 (82%) |
| BMI, kg/m2 | 25.4 ± 6.6 | 25.0 ± 6.5 |
| Exacerbator profile | ||
| FE, n (% in the group) | 7 (30%) | 7 (64%) |
| AE last year, n | 1.7 ± 1.8 | 2.4 ± 3.8 |
| Smoking status | ||
| Current, n (%) | 6 (26%) | 4 (36%) |
| Ex-smoker, n (%) | 17 (74%) | 7 (64%) |
| Pack/year smoking | 52.2 ± 24.2 | 54.0 ± 20.6 |
| Lung Function | ||
| Post-BD FEV1, % pred | 42 ± 15 | 42 ± 15 |
| Post-BD FEV1/FVC, % pred | 49 ± 12 | 44 ± 9 |
| DLco, %pred | 48 ± 20 | 44 ± 13 |
| GOLD Stages | ||
| I-II, n (% in the group) | 5 (22%) | 4 (36%) |
| III-IV, n (% in the group) | 18 (78%) | 7 (64%) |
| A-B, n (% in the group) | 7 (30%) | 2 (18%) |
| E, n (% in the group) | 16 (70%) | 9 (82%) |
| Conventional Blood Analysis | ||
| Leucocytes, /µL | 8763 ± 2673 | 8313 ± 2673 |
| Neutrophils, /µL | 5627 ± 2333 | 5795 ± 2302 |
| Eosinophils, /µL | 259 ± 240 | 170 ± 123 |
| CRP, mg/dL | 0.8 ± 1.4 | 1.0 ± 1.1 |
| Fibrinogen, mg/dL | 211 ± 57 | 203 ± 37 |
| Protein/ Ig Fraction | Protein Name | Functional Classification | %Δ | p-Value |
|---|---|---|---|---|
| A2M | Alpha-2-macroglobulin | Hemostasis | 26.105 | 0.024 |
| F12 | Coagulation factor XII | Hemostasis | −27.265 | 0.038 |
| F2 | Prothrombin | Hemostasis | −14.521 | 0.046 |
| PDGFB | Platelet-derived growth factor subunit B | Hemostasis | −69.182 | 0.015 |
| PLG | Plasminogen | Hemostasis | −20.748 | 0.017 |
| C1QA | Complement C1q subcomponent subunit A | Complement cascade | 18.952 | 0.045 |
| C1QC | Complement C1q subcomponent subunit C | Complement cascade | 21.426 | 0.032 |
| CFH | Complement factor H | Complement cascade | −17.151 | 0.022 |
| CCL17 | C-C motif chemokine 17 | Cytokine | −63.547 | 0.035 |
| CXCL9 | C-X-C motif chemokine 9 | Cytokine | 85.719 | 0.029 |
| IL1B | Interleukin-1 beta | Cytokine | −73.025 | 0.003 |
| IGLV3-10 | Immunoglobulin lambda variable 3-10 | Adaptive immunity | 53.784 | 0.046 |
| PGLYRP2 | N-acetylmuramoyl-L-alanine amidase | Other immune-related pathways | −25.314 | 0.018 |
| GARIN1B | Golgi-associated RAB2 interactor protein 1B | Orphan | 54.938 | 0.021 |
| GPX3 | Glutathione peroxidase 3 | Orphan | −28.710 | 0.050 |
| Protein/ Ig Fraction | Protein Name | Functional Classification | MCC | p-Value |
|---|---|---|---|---|
| F10 | Coagulation factor X | Hemostasis | −0.403 | 0.022 |
| PROZ | Vitamin K-dependent protein Z | Hemostasis | 0.357 | 0.041 |
| PTPN11 | Tyrosine-protein phosphatase non-receptor type 11 | Hemostasis | 0.506 | 0.004 |
| TLN1 | Talin-1 | Hemostasis | 0.346 | 0.048 |
| CFP | Properdin | Complement cascade | −0.403 | 0.022 |
| CSF2 | Granulocyte–macrophage colony-stimulating factor | Cytokine | −0.381 | 0.033 |
| CXCL5 | C-X-C motif chemokine 5 | Cytokine | −0.403 | 0.022 |
| IGHV2-5 | Immunoglobulin heavy variable 2-5 | Adaptive immunity | 0.346 | 0.048 |
| IGKV6-21 | Immunoglobulin kappa variable 6-21 | Adaptive immunity | −0.358 | 0.034 |
| IGLV3-25 | Immunoglobulin lambda variable 3-25 | Adaptive immunity | −0.346 | 0.048 |
| ATRN | Attractin | Other immune-related pathways | −0.451 | 0.016 |
| GULP1 | PTB domain-containing engulfment adapter protein 1 | Other immune-related pathways | 0.357 | 0.041 |
| SLC2A(3,14) | Solute carrier family 2, facilitated glucose transporter member 13 and/or 14 | Other immune-related pathways | 0.471 | 0.007 |
| IGFALS | Insulin-like growth factor-binding protein complex acid labile subunit | Orphan | −0.403 | 0.022 |
| MYL6(B) | Myosin light polypeptide 6 or chain 6b | Orphan | −0.384 | 0.027 |
| OR5M11 | Olfactory receptor 5M11 | Orphan | 0.403 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enríquez-Rodríguez, C.J.; Casadevall, C.; Faner, R.; Pascual-Guardia, S.; Castro-Acosta, A.; López-Campos, J.L.; Peces-Barba, G.; Seijo, L.; Caguana-Vélez, O.A.; Monsó, E.; et al. A Pilot Study on Proteomic Predictors of Mortality in Stable COPD. Cells 2024, 13, 1351. https://doi.org/10.3390/cells13161351
Enríquez-Rodríguez CJ, Casadevall C, Faner R, Pascual-Guardia S, Castro-Acosta A, López-Campos JL, Peces-Barba G, Seijo L, Caguana-Vélez OA, Monsó E, et al. A Pilot Study on Proteomic Predictors of Mortality in Stable COPD. Cells. 2024; 13(16):1351. https://doi.org/10.3390/cells13161351
Chicago/Turabian StyleEnríquez-Rodríguez, Cesar Jessé, Carme Casadevall, Rosa Faner, Sergi Pascual-Guardia, Ady Castro-Acosta, José Luis López-Campos, Germán Peces-Barba, Luis Seijo, Oswaldo Antonio Caguana-Vélez, Eduard Monsó, and et al. 2024. "A Pilot Study on Proteomic Predictors of Mortality in Stable COPD" Cells 13, no. 16: 1351. https://doi.org/10.3390/cells13161351
APA StyleEnríquez-Rodríguez, C. J., Casadevall, C., Faner, R., Pascual-Guardia, S., Castro-Acosta, A., López-Campos, J. L., Peces-Barba, G., Seijo, L., Caguana-Vélez, O. A., Monsó, E., Rodríguez-Chiaradia, D., Barreiro, E., Cosío, B. G., Agustí, A., Gea, J., & on behalf of the BIOMEPOC Group. (2024). A Pilot Study on Proteomic Predictors of Mortality in Stable COPD. Cells, 13(16), 1351. https://doi.org/10.3390/cells13161351










