The Proangiogenic Effects of Melanoma-Derived Ectosomes Are Mediated by αvβ5 Integrin Rather than αvβ3 Integrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Other Reagents
2.2. Cell Lines
2.3. Ectosome Isolation
2.4. Assessment of Ectosome Sample Purity
2.5. Incorporation of Ectosomes Derived from Melanoma Cell Lines and Melanocytes by Recipient Endothelial Cells
2.6. Assessment of Total Protein Expression of αvβ3 and αvβ5 Integrins in Melanoma Cell Lines, Melanocytes and Ectosomes Derived from Them, and in Endothelial Cells after Incubation with Ectosomes
2.7. Assessment of Surface Expression of αvβ3 and αvβ5 Integrins in Endothelial Cells after Incubation with Ectosomes
2.8. Analysis of Gene Expression for αv, β3 and β5 Integrin Subunits in Endothelial Cells after Incubation with Ectosomes
2.9. Analysis of Endothelial Cell Viability after Incubation with Ectosomes
2.10. Analysis of Migratory Properties Endothelial Cells after Incubation with Ectosomes
2.11. Analysis of Tube-Formation Potential of Endothelial Cells after Incubation with Ectosomes
2.12. Analysis of TNF-α and VEGF Gene and Protein Expression, and Their Secretion by Endothelial Cells after Incubation with Ectosomes
2.13. Statistical Analysis
3. Results
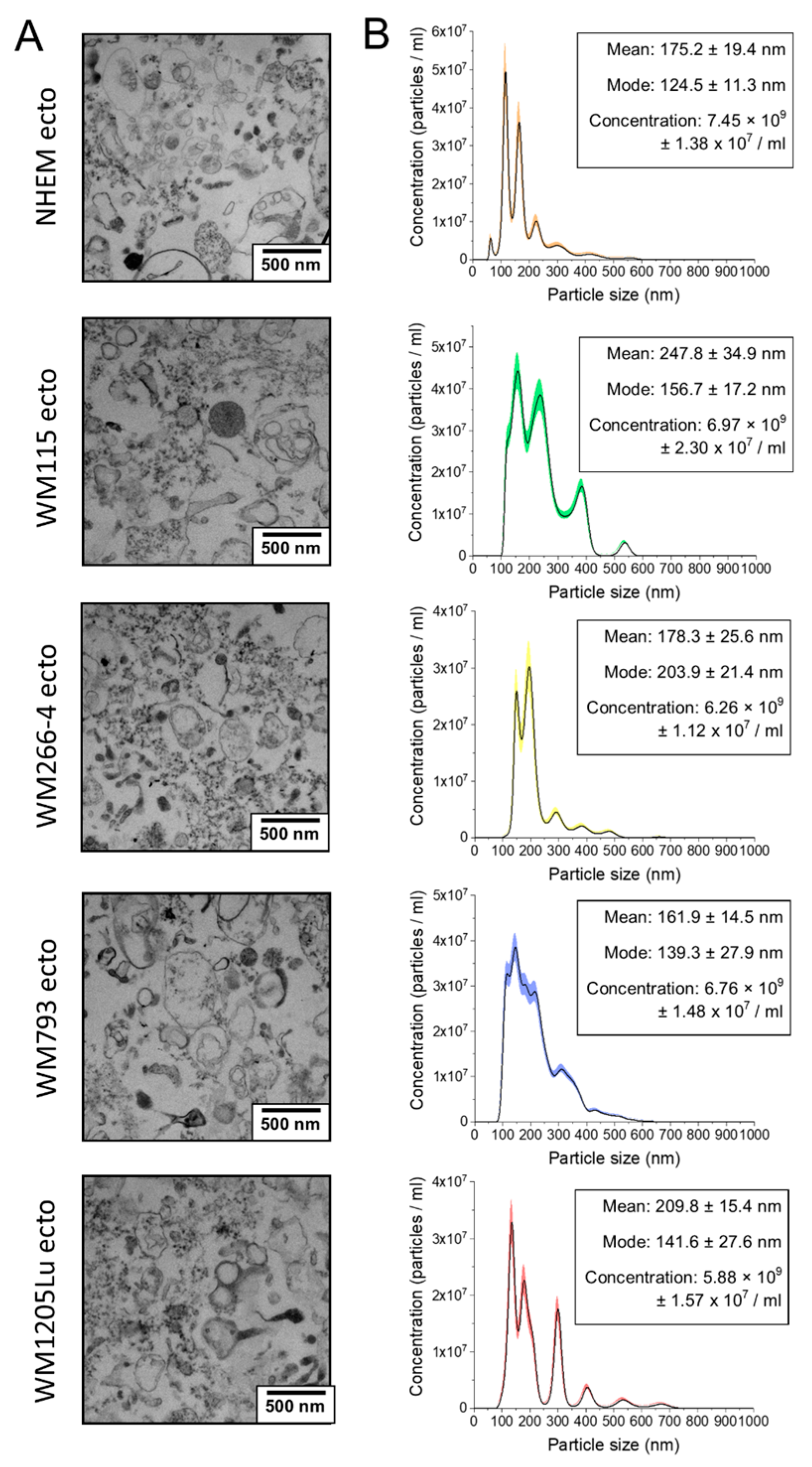
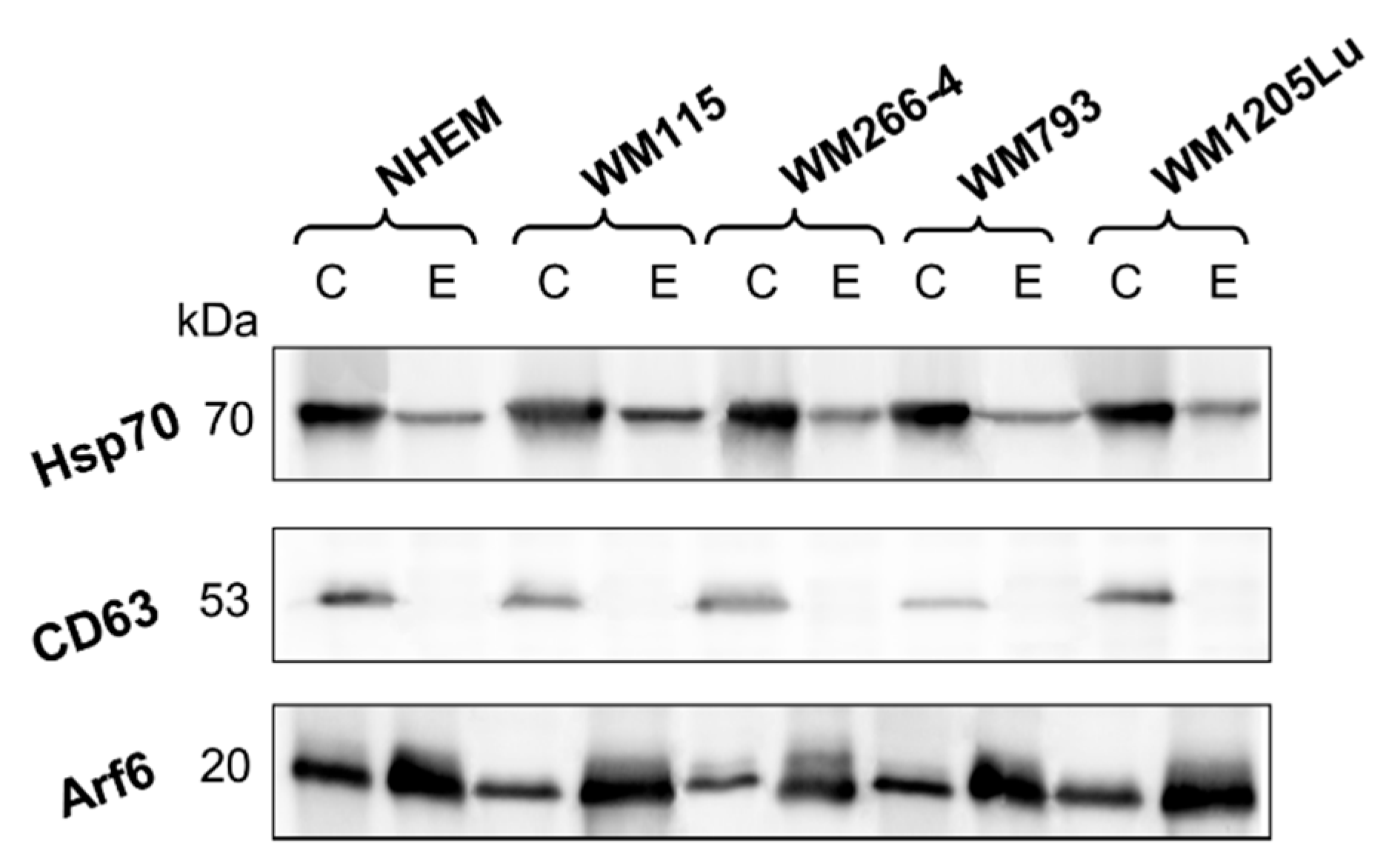
3.1. Assessment of Ectosome Sample Purity
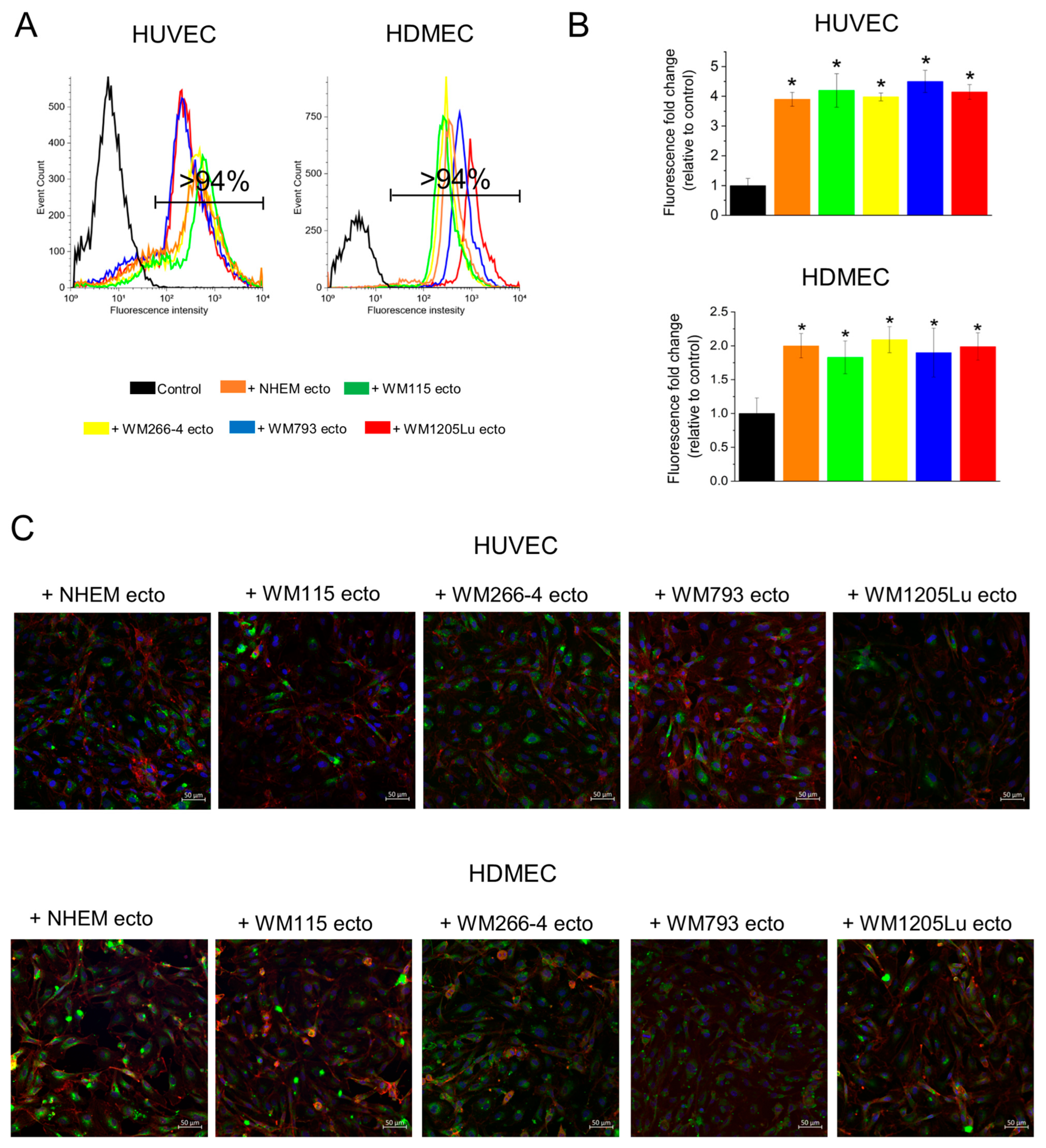
3.2. Incorporation of Melanoma- and Melanocyte-Derived Fluorescently Stained Ectosomes by Recipient Endothelial Cells
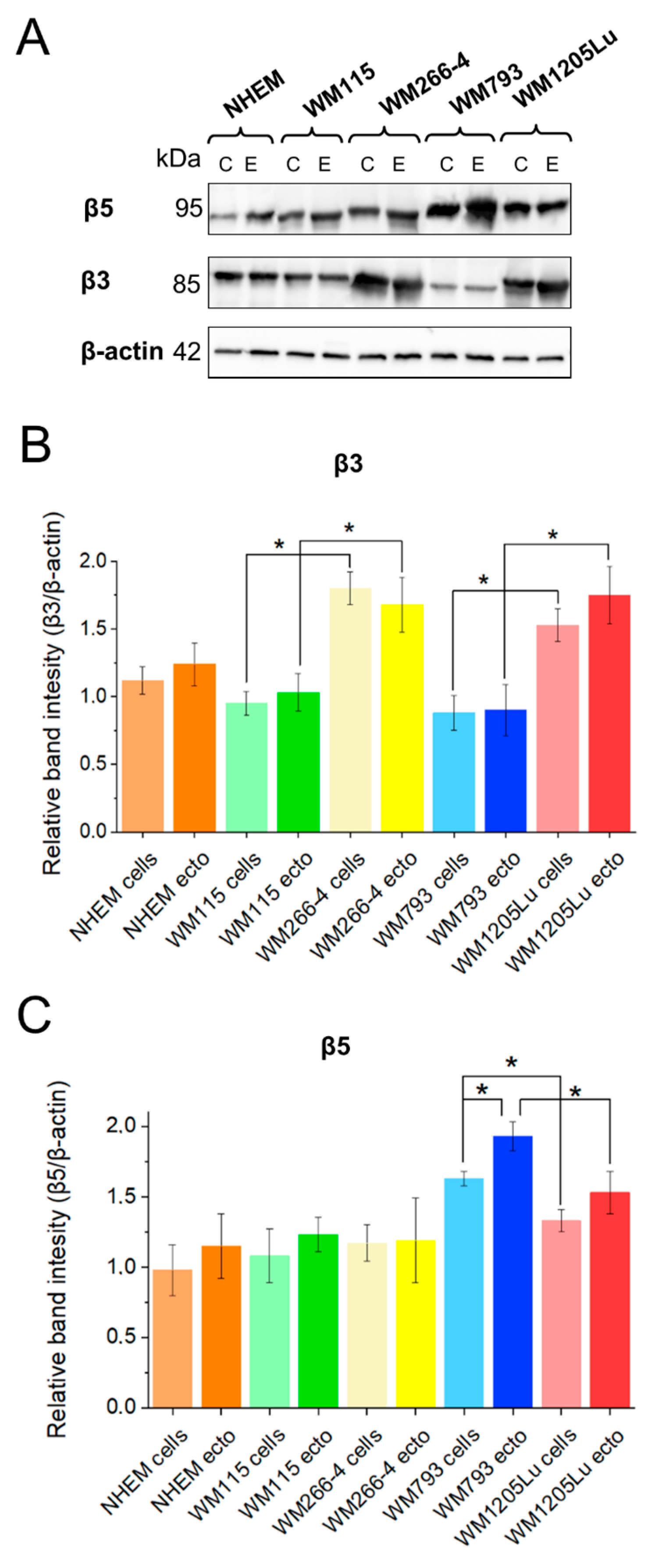
3.3. Analysis of αvβ3 and αvβ5 Integrin Protein Expression Levels in Melanoma Cells, Melanocytes, and Their Released Ectosomes
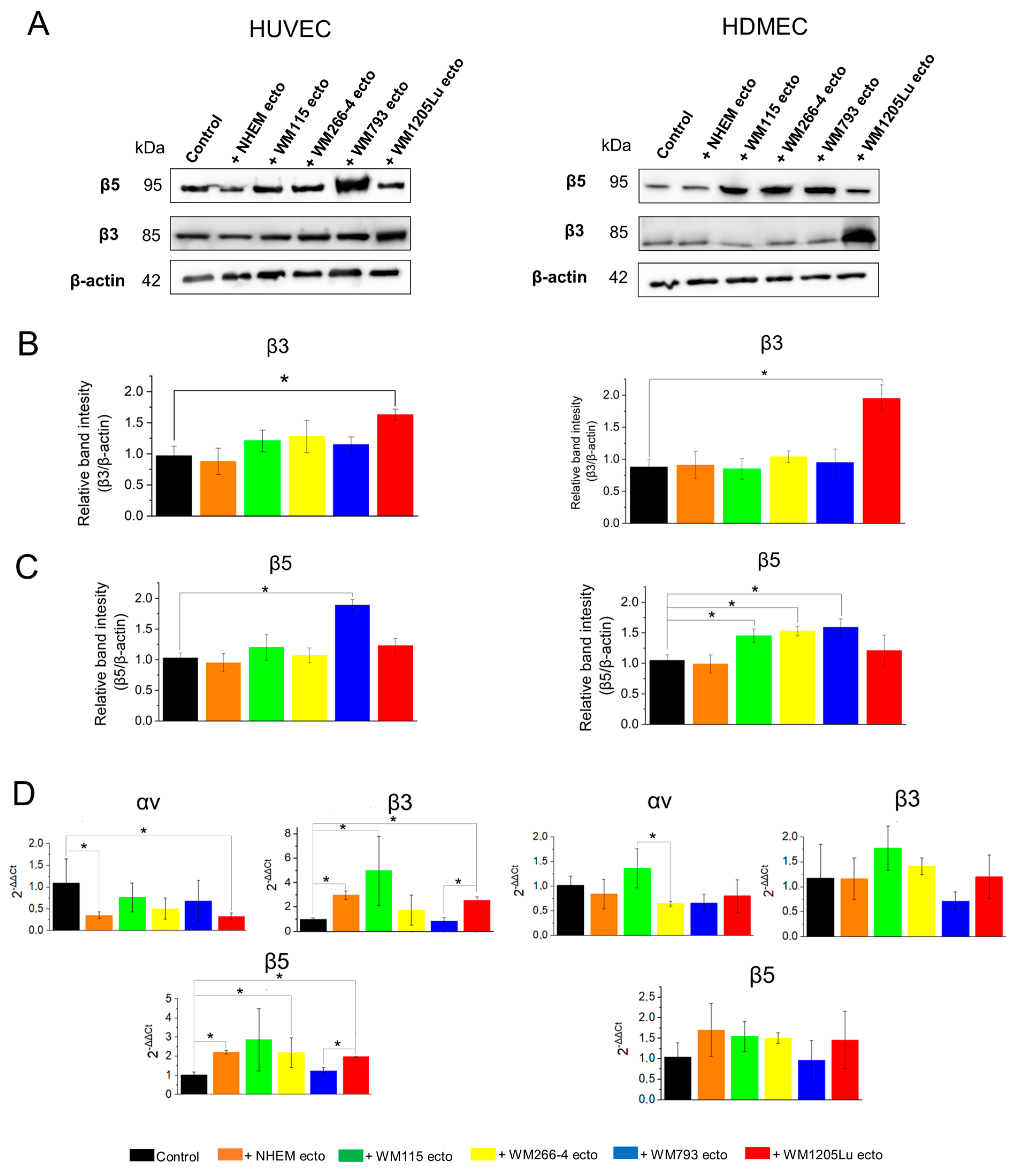
3.4. Changes in αvβ3 and αvβ5 Integrin Protein and Gene Expression in Endothelial Cells Following Incubation with Melanoma/Melanocyte-Derived Ectosomes
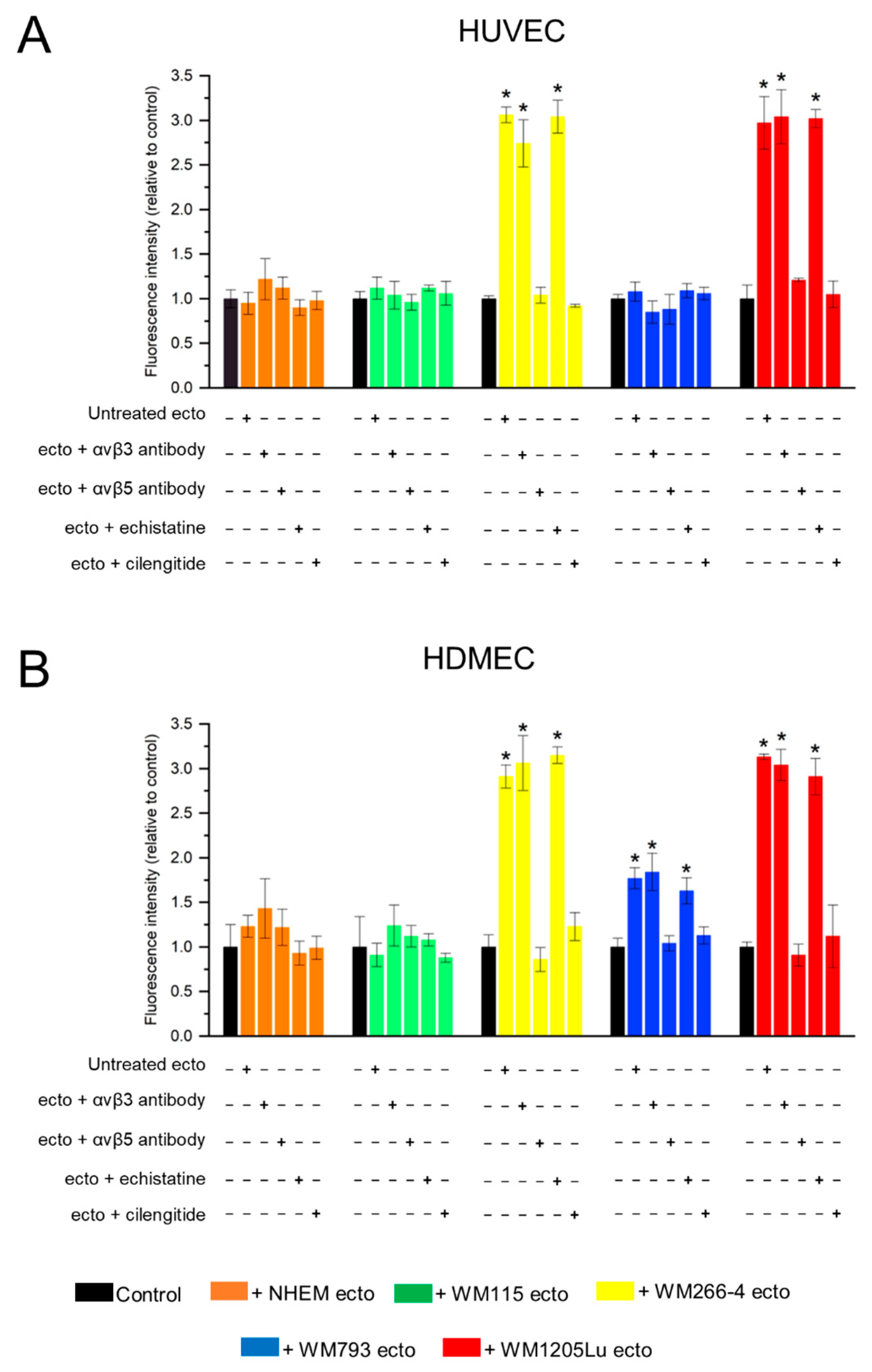
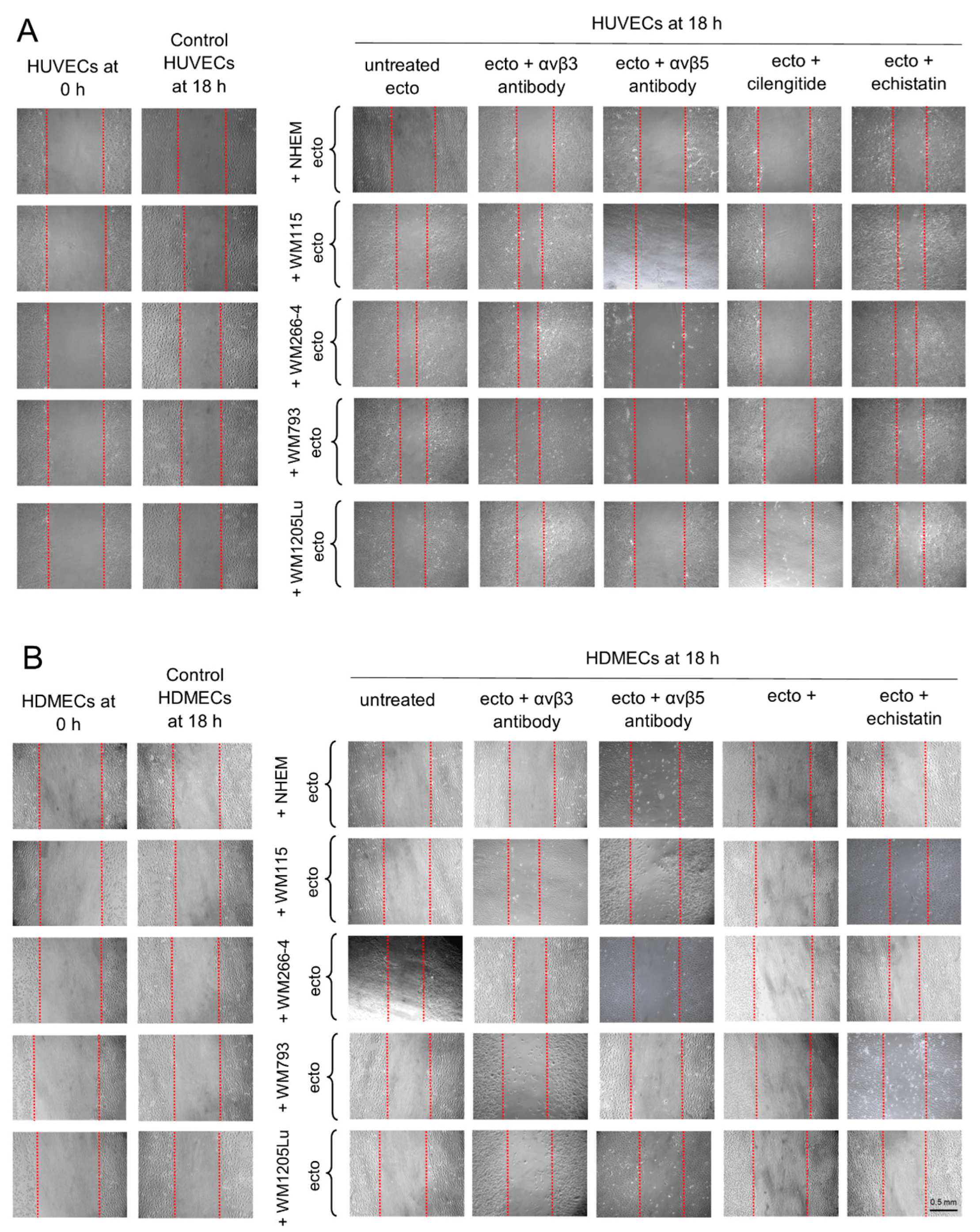
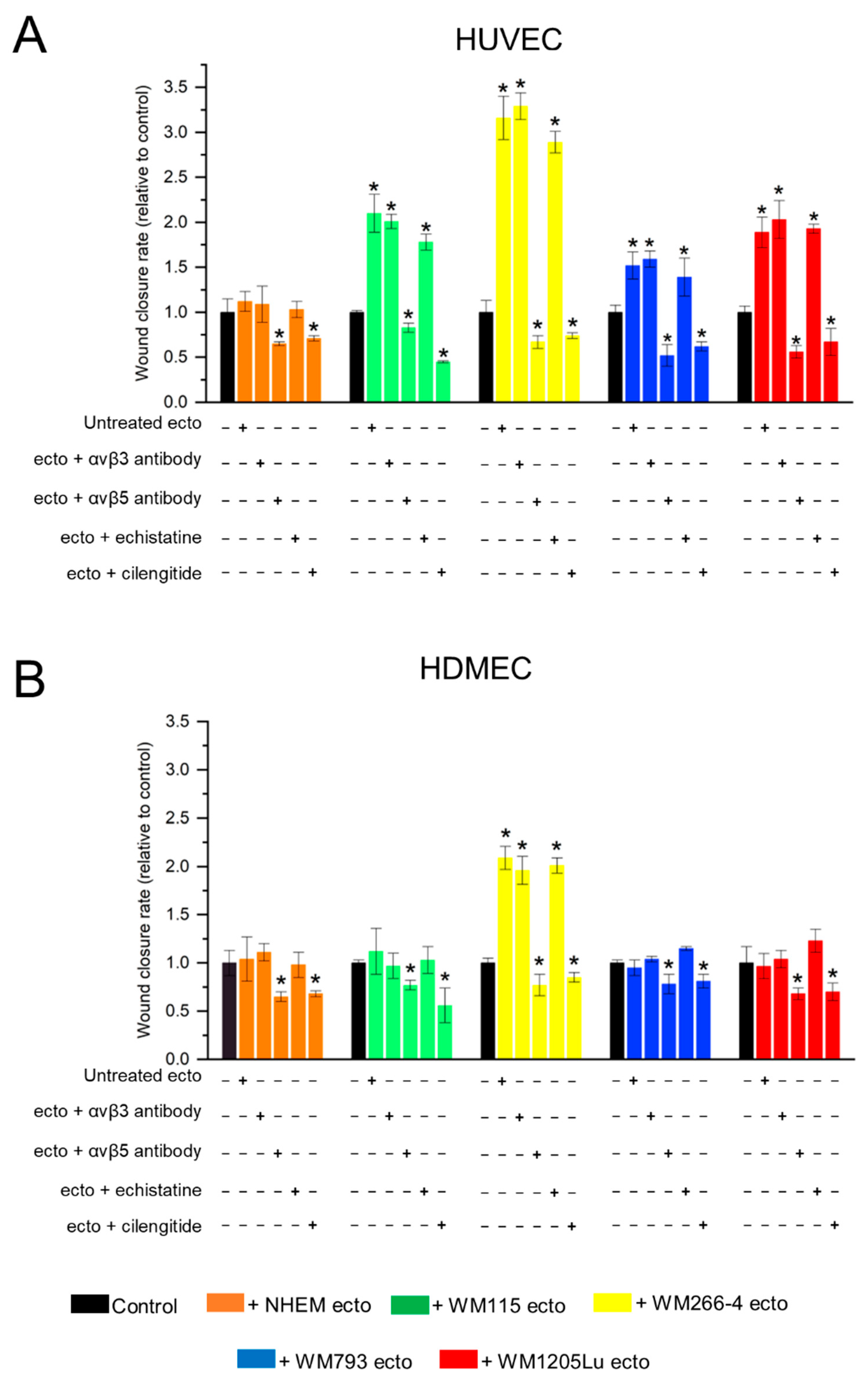
3.5. Functional Impact of Melanoma/Melanocyte-Derived Ectosomes Carrying αvβ3 and αvβ5 Integrins on Endothelial Cells Viability and Migration
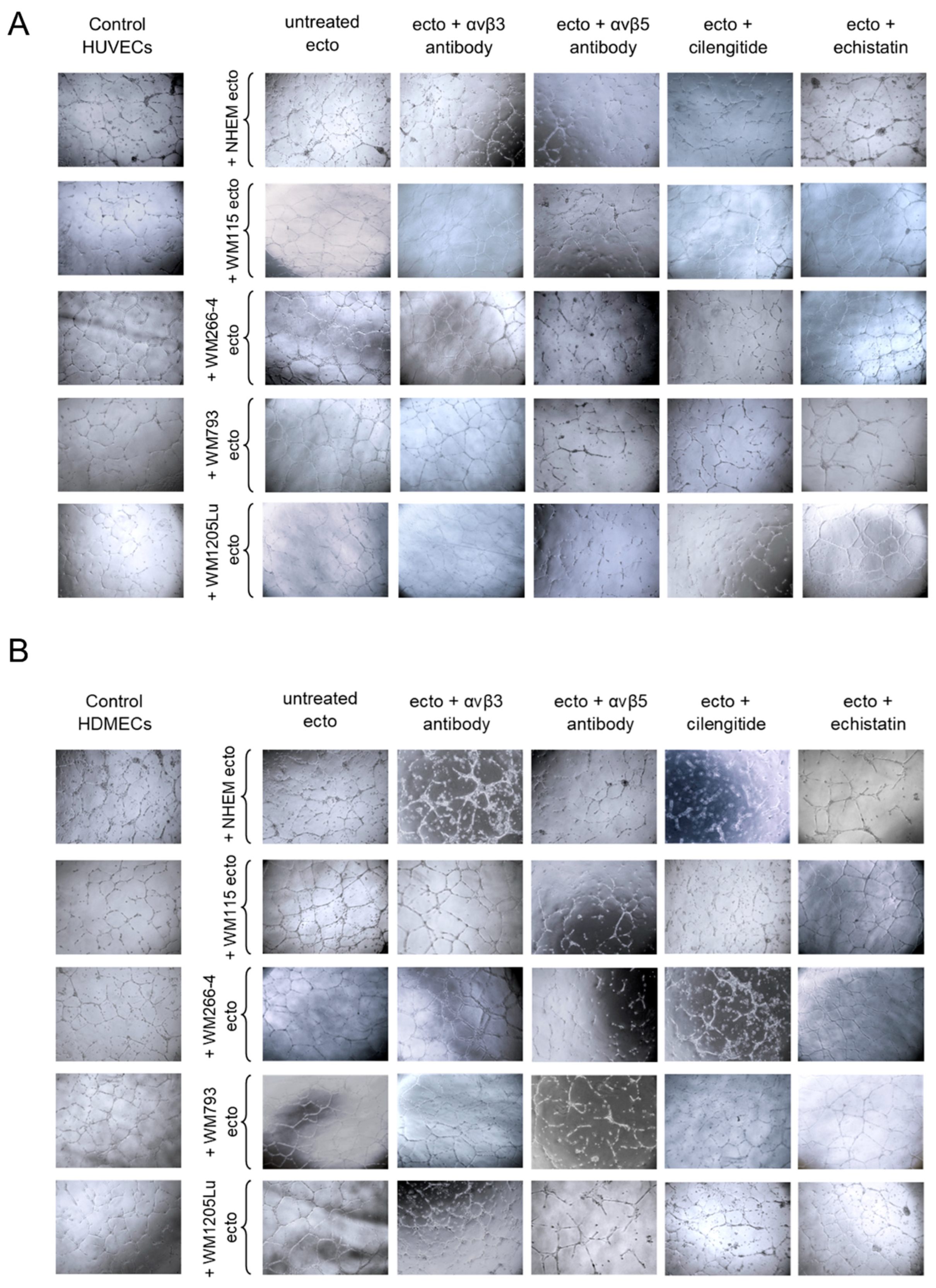
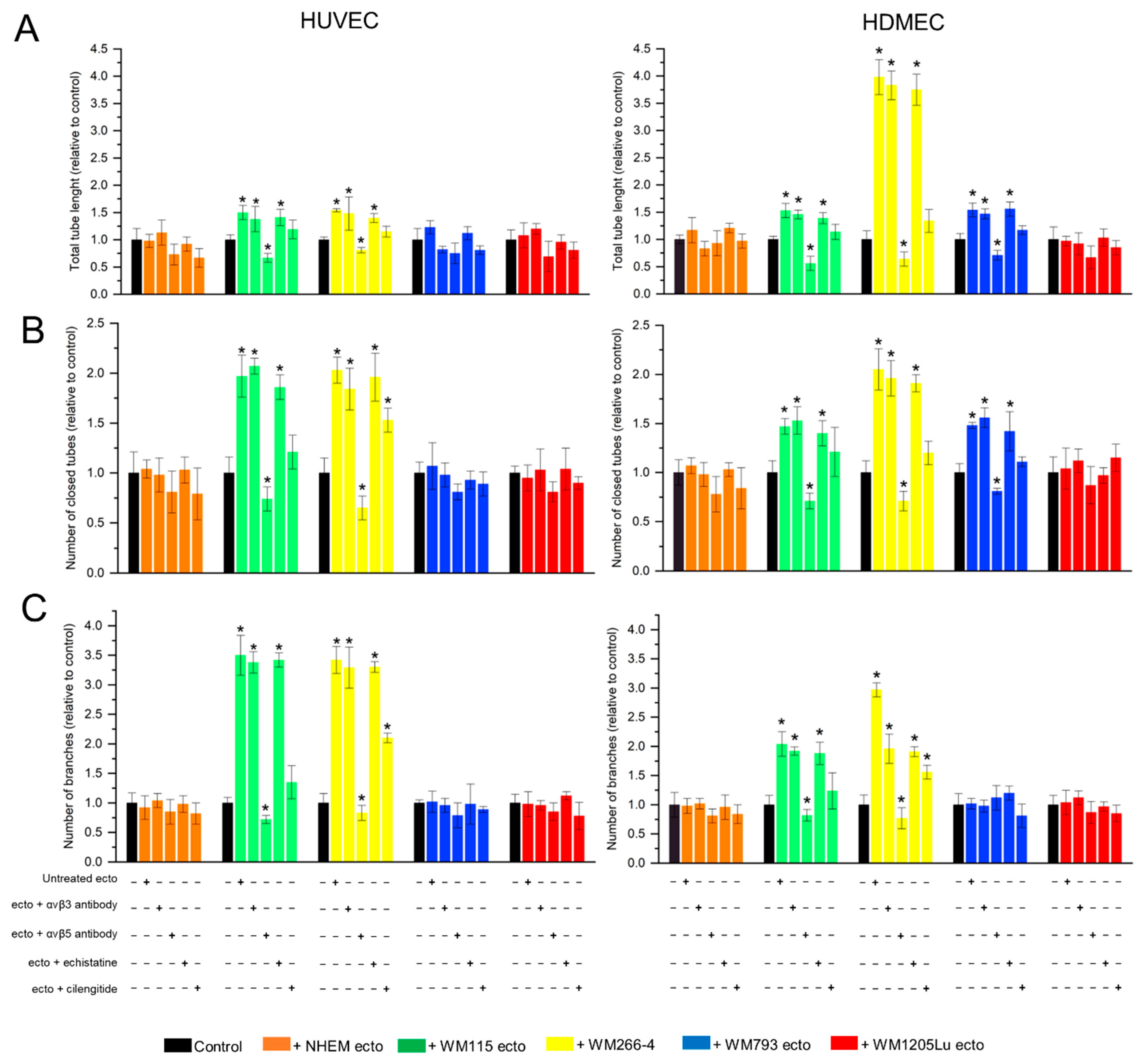
3.6. Functional Impact of Melanoma/Melanocyte-Derived Ectosomes Carrying αvβ3 and αvβ5 Integrins on the Potential for Tube Formation by Endothelial Cells
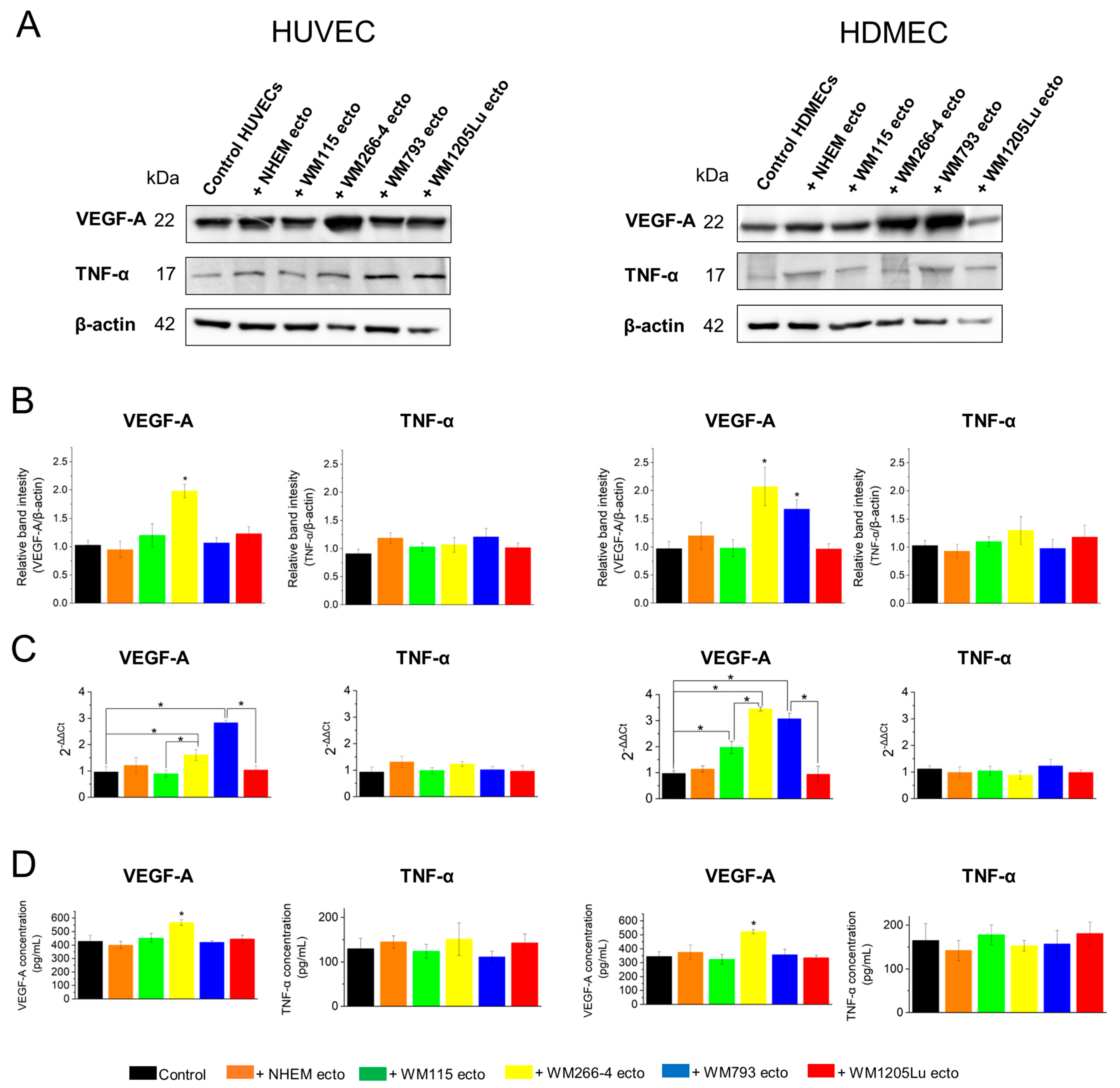
3.7. Evaluation of Activation of Proangiogenic Signaling Pathways (αvβ3 Integrin/TNF-α and αvβ5 Integrin/VEGF) in Endothelial Cells after Incubation with Ectosomes
4. Discussion
4.1. The Role and Expression of αvβ3 and αvβ5 Integrins in Melanoma Cells and Melanoma-Derived Ectosomes
4.2. Transfer of αvβ3 and αvβ5 Integrins via Melanoma-Derived Ectosomes Is Associated with Alterations in αvβ3/αvβ5 Integrin Protein and Gene Expression in Recipient Endothelial Cells
4.3. Melanoma-Derived Ectosomes Bearing αvβ3 and αvβ5 Integrins Exert Proangiogenic Effect on Recipient Endothelial Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonyak, M.A.; Li, B.; Boroughs, L.K.; Johnson, J.L.; Druso, J.E.; Bryant, K.L.; Holowka, D.A.; Cerione, R.A. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc. Natl. Acad. Sci. USA 2011, 108, 4852–4857. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Würdinger, T.; Van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, M.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Szajnik, M.; Czystowska, M.; Szczepanski, M.J.; Mandapathil, M.; Whiteside, T.L. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS ONE 2010, 5, e11469. [Google Scholar] [CrossRef] [PubMed]
- Taraboletti, G.; D’Ascenzo, S.; Giusti, I.; Marchetti, D.; Borsotti, P.; Millimaggi, D.; Giavazzi, R.; Pavan, A.; Dolo, V. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia 2006, 8, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Millimaggi, D.; Mari, M.; D’Ascenzo, S.; Carosa, E.; Jannini, E.A.; Zucker, S.; Carta, G.; Pavan, A.; Dolo, V. Tumor vesicle associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia 2007, 9, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, X.J.; Zeng, C.; Wu, P.H.; Wang, H.X.; Chen, Z.C.; Li, Q.B. Microvesicles secreted from human multiple myeloma cells promote angiogenesis. Acta Pharmacol. Sin. 2014, 35, 230–238. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Kerbel, R.S.; Allison, A.C.; Rak, J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc. Natl. Acad. Sci. USA 2009, 106, 3794–3799. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Lee, H.M.; Lee, T.H.; Kang, C.; Kleinman, H.K.; Gho, Y.S. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res. 2002, 62, 6312–6317. [Google Scholar] [PubMed]
- Yamada, N.; Tsujimura, N.; Kumazaki, M.; Shinohara, H.; Taniguchi, K.; Nakagawa, Y.; Naoe, T.; Akao, Y. Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochim. Biophys. Acta 2014, 1839, 1256–1272. [Google Scholar] [CrossRef] [PubMed]
- Munster, M.; Fremder, E.; Miller, V.; Ben-Tsedek, N.; Davidi, S.; Scherer, S.J.; Shaked, Y. Anti-VEGF-A affects the angiogenic properties of tumor-derived microparticles. PLoS ONE 2014, 9, e95983. [Google Scholar] [CrossRef]
- Fedele, C.; Singh, A.; Zerlanko, B.J.; Iozzo, R.V.; Languino, L.R. The αvβ6 integrin is transferred intercellularly via exosomes. J. Biol. Chem. 2015, 290, 4545–4551. [Google Scholar] [CrossRef]
- Contois, L.W.; Akalu, A.; Caron, J.M.; Tweedie, E.; Cretu, A.; Henderson, T.; Liaw, L.; Friesel, R.; Vary, C.; Brooks, P.C. Inhibition of tumor-associated αvβ3 integrin regulates the angiogenic switch by enhancing expression of IGFBP-4 leading to reduced melanoma growth and angiogenesis in vivo. Angiogenesis 2015, 18, 31–46. [Google Scholar] [CrossRef]
- Singh, A.; Fedele, C.; Lu, H.; Nevalainen, M.T.; Keen, J.H.; Languino, L.R. Exosome-mediated transfer of αvβ3 integrin from tumorigenic to nontumorigenic cells promotes a migratory phenotype. Mol. Cancer Res. 2016, 14, 1136–1146. [Google Scholar] [CrossRef]
- Friedlander, M.; Brooks, P.C.; Shaffer, R.W.; Kincaid, C.M.; Varner, J.A.; Cheresh, D.A. Definition of two angiogenic pathways by distinct alpha v integrins. Science 1995, 270, 1500–1502. [Google Scholar] [CrossRef]
- Herlyn, M.; Balaban, G.; Bennicelli, J.; Guerry, D., IV; Halaban, R.; Herlyn, D.; Elder, D.E.; Maul, G.G.; Steplewski, Z.; Nowell, P.C.; et al. Primary melanoma cells of the vertical growth phase: Similarities to metastatic cells. J. Natl. Cancer Inst. 1985, 74, 283–289. [Google Scholar]
- Cornil, I.; Theodorescu, D.; Man, S.; Herlyn, M.; Jambrosic, J.; Kerbel, R.S. Fibroblast cell interactions with human melanoma cells affect tumor cell growth as a function of tumor progression. Proc. Natl. Acad. Sci. USA 1991, 88, 6028–6032. [Google Scholar] [CrossRef]
- Juhasz, I.; Albelda, S.M.; Elder, D.E.; Murphy, G.F.; Adachi, K.; Herlyn, D.; Valyi-Nagy, I.T.; Herlyn, M. Growth and invasion of human melanomas in human skin grafted to immunodeficient mice. Am. J. Pathol. 1993, 143, 528–537. [Google Scholar]
- Drożdż, A.; Kamińska, A.; Surman, M.; Gonet-Surówka, A.; Jach, R.; Huras, H.; Przybyło, M.; Stępień, E.Ł. Low-vacuum filtration as an alternative extracellular vesicle concentration method: A comparison with ultracentrifugation and differential centrifugation. Pharmaceutics 2020, 12, 872. [Google Scholar] [CrossRef]
- Surman, M.; Hoja-Łukowicz, D.; Szwed, S.; Drożdż, A.; Stępień, E.; Przybyło, M. Human melanoma-derived ectosomes are enriched with specific glycan epitopes. Life Sci. 2018, 207, 395–411. [Google Scholar] [CrossRef]
- Surman, M.; Kędracka-Krok, S.; Hoja-Łukowicz, D.; Jankowska, U.; Drożdż, A.; Stępień, E.Ł.; Przybyło, M. Mass Spectrometry-Based Proteomic Characterization of Cutaneous Melanoma Ectosomes Reveals the Presence of Cancer-Related Molecules. Int. J. Mol. Sci. 2020, 21, 2934. [Google Scholar] [CrossRef]
- Goodman, S.L.; Grote, H.J.; Wilm, C. Matched rabbit monoclonal antibodies against αv-series integrins reveal a novel αvβ3-LIBS epitope, and permit routine staining of archival paraffin samples of human tumors. Biol. Open 2012, 1, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Maltsev, O.V.; Cavalcanti-Adam, E.A.; Zarka, R.; Reuning, U.; Notni, J.; Wester, H.J.; Mas-Moruno, C.; et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci. Rep. 2017, 7, 39805. [Google Scholar] [CrossRef] [PubMed]
- Hieken, T.J.; Ronan, S.G.; Farolan, M.; Shilkaitis, A.L.; Das Gupta, T.K. Molecular prognostic markers in intermediate-thickness cutaneous malignant melanoma. Cancer 1999, 85, 375–382. [Google Scholar] [CrossRef]
- Danen, E.H.; Jansen, K.F.; Van Kraats, A.A.; Cornelissen, I.M.; Ruiter, D.J.; Van Muijen, G.N. Alpha v-integrins in human melanoma: Gain of alpha v beta 3 and loss of alpha v beta 5 are related to tumor progression in situ but not to metastatic capacity of cell lines in nude mice. Int. J. Cancer 1995, 61, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Mittelbronn, M.; Warth, A.; Meyermann, R.; Goodman, S.; Weller, M. Expression of integrins αvβ3 and αvβ5 and their ligands in primary and secondary central nervous system neoplasms. Histol. Histopathol. 2013, 28, 749–758. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Fujita, Y.; Kato, T.; Mizutani, K.; Kameyama, K.; Tsumoto, H.; Miura, Y.; Deguchi, T.; Ito, M. Integrin α4 and vinculin contained in exosomes are potential markers for progression of prostate cancer associated with taxane-resistance. Int. J. Oncol. 2015, 47, 384–390. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. αV integrins in angiogenesis and cancer. Cold Spring Harb. Perspect. Med. 2011, 1, a006478. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.W.; Yu, Z.L.; Chen, G.; Jia, J. Extracellular vesicles in tumor angiogenesis and resistance to anti-angiogenic therapy. Cancer Sci. 2023, 114, 2739–2749. [Google Scholar] [CrossRef]
- Hsu, M.Y.; Shih, D.T.; Meier, F.E.; Van Belle, P.; Hsu, J.Y.; Elder, D.E.; Buck, C.A.; Herlyn, M. Adenoviral gene transfer of beta3 integrin subunit induces conversion from radial to vertical growth phase in primary human melanoma. Am. J. Pathol. 1998, 153, 1435–1442. [Google Scholar] [CrossRef]
- Marshall, J.F.; Rutherford, D.C.; Happerfield, L.; Hanby, A.; McCartney, A.C.; Newton-Bishop, J.; Hart, I.R. Comparative analysis of integrins in vitro and in vivo in uveal and cutaneous melanomas. Br. J. Cancer 1998, 77, 522–529. [Google Scholar] [CrossRef]
- Rocha, L.A.; Learmonth, D.A.; Sousa, R.A.; Salgado, A.J. αvβ3 and α5β1 integrin-specific ligands: From tumor angiogenesis inhibitors to vascularization promoters in regenerative medicine? Biotechnol. Adv. 2018, 36, 208–227. [Google Scholar] [CrossRef]
- Krishn, S.R.; Singh, A.; Bowler, N.; Duffy, A.N.; Friedman, A.; Fedele, C.; Kurtoglu, S.; Tripathi, S.K.; Wang, K.; Hawkins, A.; et al. Prostate cancer sheds the αvβ3 integrin in vivo through exosomes. Matrix Biol. 2019, 77, 41–57. [Google Scholar] [CrossRef]
- Altei, W.F.; Pachane, B.C.; Dos Santos, P.K.; Ribeiro, L.N.M.; Sung, B.H.; Weaver, A.M.; Selistre-de-Araújo, H.S. Inhibition of αvβ3 integrin impairs adhesion and uptake of tumor-derived small extracellular vesicles. Cell Commun. Signal. 2020, 18, 158. [Google Scholar] [CrossRef] [PubMed]
- Gutheil, J.C.; Campbell, T.N.; Pierce, P.R.; Watkins, J.D.; Huse, W.D.; Bodkin, D.J.; Cheresh, D.A. Targeted antiangiogenic therapy for cancer using Vitaxin: A humanized monoclonal antibody to the integrin ανβ3. Clin. Cancer Res. 2000, 6, 3056–3061. [Google Scholar] [PubMed]
- Nabors, L.B.; Mikkelsen, T.; Rosenfeld, S.S.; Hochberg, F.; Akella, N.S.; Fisher, J.D.; Cloud, G.A.; Zhang, Y.; Carson, K.; Wittemer, S.M.; et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J. Clin. Oncol. 2007, 25, 1651–1657. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Prieto, V.; Joseph, R.W.; Diwan, A.H.; Gallick, G.E.; Papadopoulos, N.E.; Bedikian, A.Y.; Camacho, L.H.; Hwu, P.; Ng, C.S.; et al. A randomized phase II study of cilengitide (EMD 121974) in patients with metastatic melanoma. Melanoma Res. 2012, 22, 294–301. [Google Scholar] [CrossRef]
- Ruffini, F.; Graziani, G.; Levati, L.; Tentori, L.; D’Atri, S.; Lacal, P.M. Cilengitide downmodulates invasiveness and vasculogenic mimicry of neuropilin 1 expressing melanoma cells through the inhibition of αvβ5 integrin. Int. J. Cancer 2015, 136, E545–E558. [Google Scholar] [CrossRef]

| Symbol | Gene Name (Assay ID, TaqMan Probes; Amplicon Size bp) | Location (GeneCards) | Description |
|---|---|---|---|
| Housekeeping gene: | |||
| YWHAZ | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (Hs01122444_g; 120 bp) | 8q22.3 | Mediator of various signaling pathways |
| Target genes: | |||
| ITGAV | integrin subunit alpha V (Hs00233808_m1; 64 bp) | 2q32.1 | Cell adhesion receptor |
| ITGB3 | integrin subunit beta 3 (Hs01001469_m1; 59 bp) | 7q21.32 | Cell adhesion receptor |
| ITGB5 | integrin subunit beta 5 (Hs00174435_m1; 78 bp) | 3q21.2 | Cell adhesion receptor |
| VEGFA | vascular endothelial growth factor A (Hs00900055_m1; 59 bp) | 6p21.1 | Angiogenesis inducer |
| TNFA | tumor necrosis factor-alpha (Hs00174128_m1; 80 bp) | 6p21.33 | Proinflammatory cytokine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surman, M.; Wilczak, M.; Bzowska, M.; Tylko, G.; Przybyło, M. The Proangiogenic Effects of Melanoma-Derived Ectosomes Are Mediated by αvβ5 Integrin Rather than αvβ3 Integrin. Cells 2024, 13, 1336. https://doi.org/10.3390/cells13161336
Surman M, Wilczak M, Bzowska M, Tylko G, Przybyło M. The Proangiogenic Effects of Melanoma-Derived Ectosomes Are Mediated by αvβ5 Integrin Rather than αvβ3 Integrin. Cells. 2024; 13(16):1336. https://doi.org/10.3390/cells13161336
Chicago/Turabian StyleSurman, Magdalena, Magdalena Wilczak, Małgorzata Bzowska, Grzegorz Tylko, and Małgorzata Przybyło. 2024. "The Proangiogenic Effects of Melanoma-Derived Ectosomes Are Mediated by αvβ5 Integrin Rather than αvβ3 Integrin" Cells 13, no. 16: 1336. https://doi.org/10.3390/cells13161336
APA StyleSurman, M., Wilczak, M., Bzowska, M., Tylko, G., & Przybyło, M. (2024). The Proangiogenic Effects of Melanoma-Derived Ectosomes Are Mediated by αvβ5 Integrin Rather than αvβ3 Integrin. Cells, 13(16), 1336. https://doi.org/10.3390/cells13161336








