Enzyme Is the Name—Adapter Is the Game
Abstract
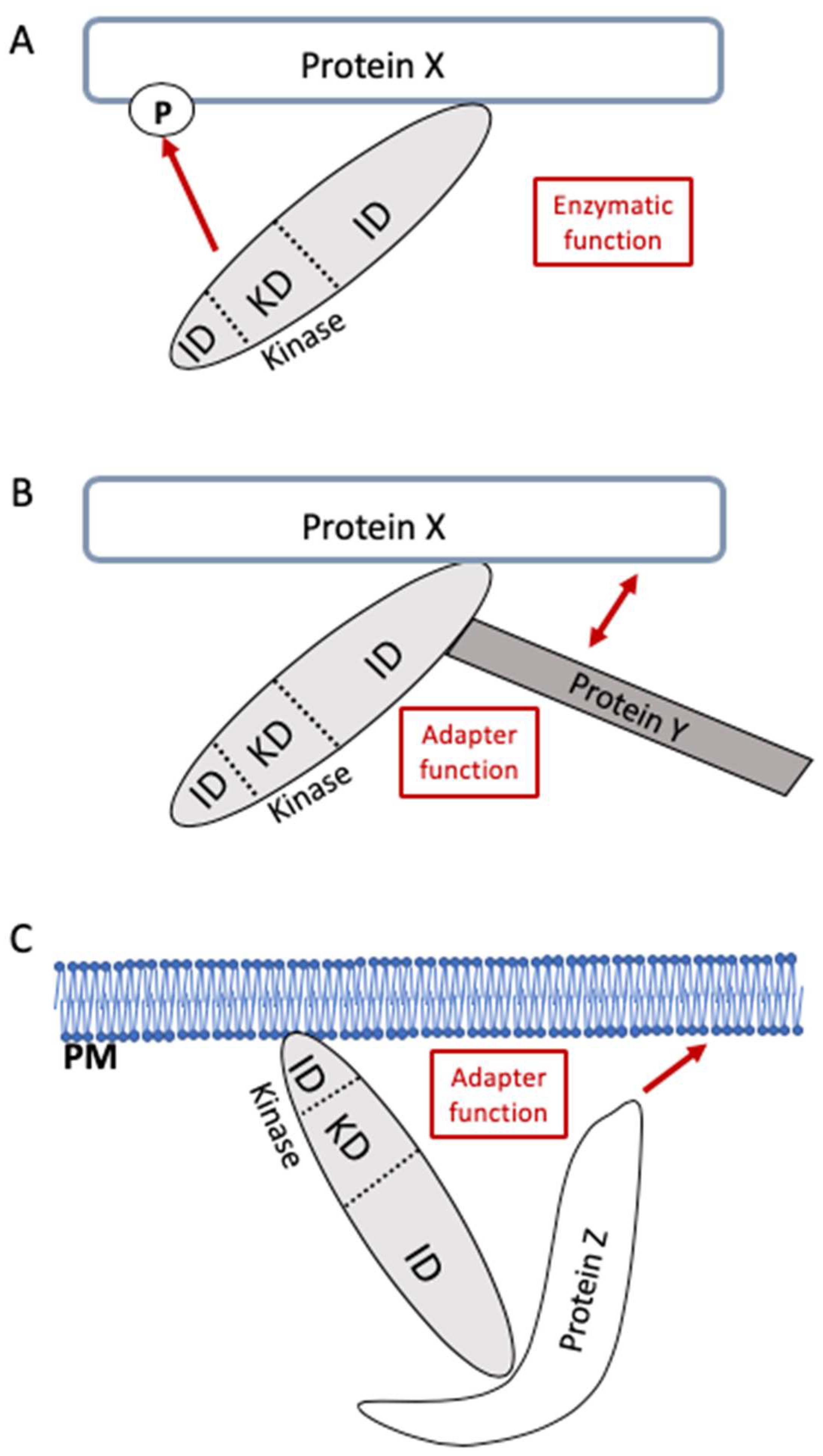
1. Introduction
2. Bruton’s Tyrosine Kinase (BTK) Exerts Important Enzymatic as Well as Adapter Functions in Immune Cells
3. The p110γ Isotype of Phosphatidylinositol 3-Kinase (PI3K) Enzymatically Controls Chemotaxis and Uses Its Adapter Function for Regulating cAMP Levels
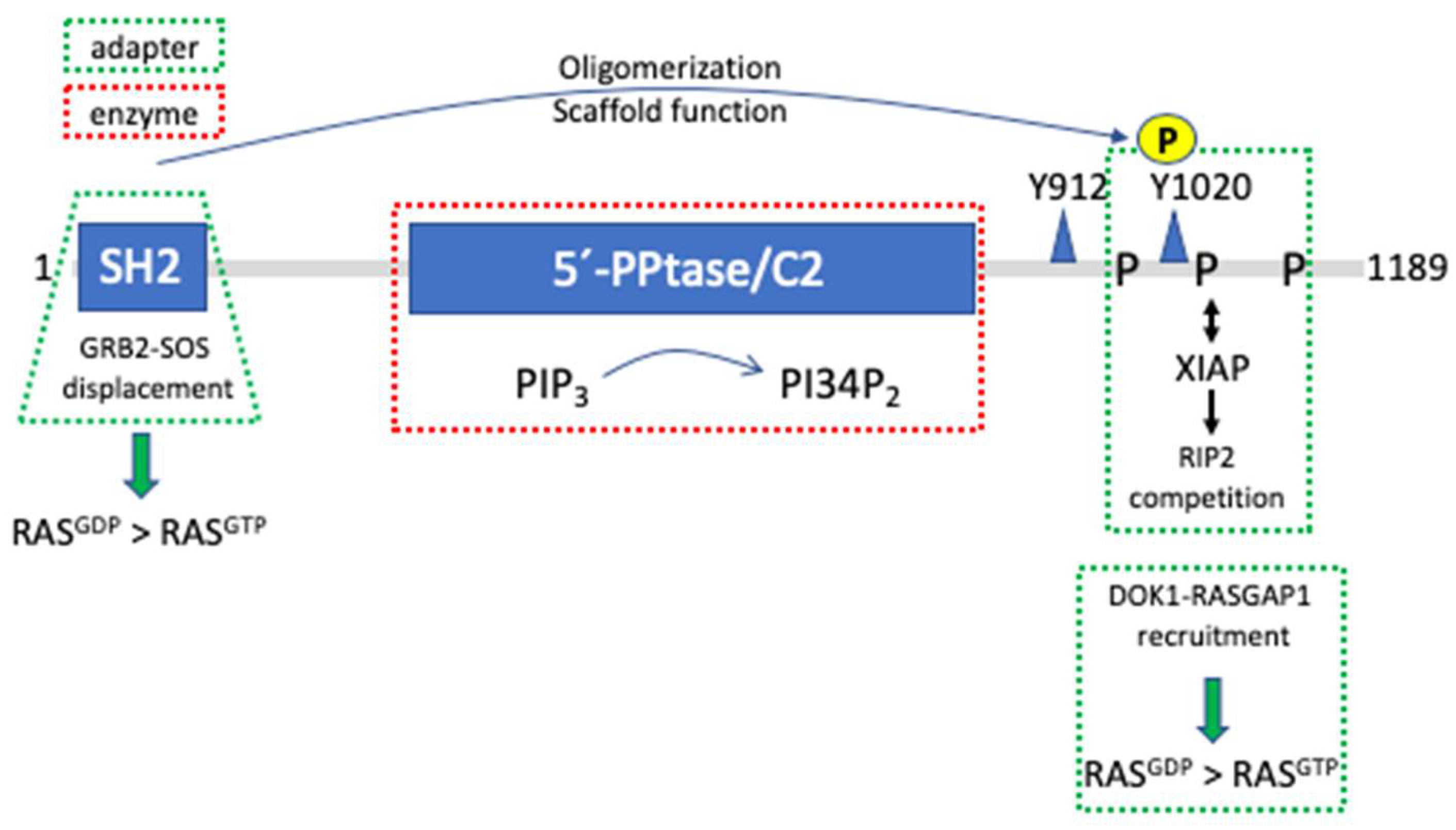
4. The Hemopoietic Lipid Phosphatase SH2-Containing Inositol Phosphatase 1 (SHIP1) Controls RAS Activity by Means of Differential Adapter Functions
5. Examples from Receptor Tyrosine Kinase (RTK)-MAPK Pathways
5.1. The Pseudokinase HER3/ErbB3 Contributes to Activation of EGFR Family Members in a Kinase-Independent Manner
5.2. The Tyrosine Phosphatase SHP2 Exerts Signaling Functions Independent of Its Catalytic Activity
5.3. Rapidly Accelerated Fibrosarcoma (RAF) and Kinase Suppressor of RAS (KSR) Proteins: Moonlighting and Allostery
| Protein | Role as Enzyme | Role as Adapter | Ref. |
|---|---|---|---|
| BTK | Phosphorylation/activation of PLCγ | Tumor suppressor function Membrane recruitment of PIP5Ks causing PI45P2 production | [16,17,18] |
| ITK | Phosphorylation/activation of PLCγ | TCR/CD3-triggered actin polymerization | [30] |
| PI3K (p110γ) | Phosphorylation of PI45P2 to yield PIP3 Regulation of leukocyte migration and inflammation | Constitutive interaction with PDE3B and promotion of PDE3B activity (cAMP hydrolysis) in cardiomyocytes | [34,37] |
| SHIP1 | Hydrolysis of PIP3 to yield PI34P2 Inhibition of PKB activation and Ca2+ mobilization upon BCR-FcγRIIB crosslinking | Attenuation of RAS activation by GRB2-SOS competition Inhibition of RAS by DOK1-RASGAP1 recruitment Attenuation of NOD2-induced NFκB activation by interacting with XIAP | [38,44,45,47,49,50] |
| HER3/ ErbB3 | Naturally inactive kinase (pseudokinase) or kinase with low intrinsic enzymatic activity | Allosteric transactivator of catalytically competent EGFR family members, most notably HER2/ErbB2; adaptor, phospho-tyrosine residues as docking sites for PI3K recruitment | [73,74,76] |
| SHP2 | Protein tyrosine phosphatase | Protection of phosphotyrosine residues by tandem SH2 domain against dephosphorylation | [87] |
| RAF1 | Protein serine/threonine kinase | Various adaptor functions, see text for details | [90,91,92,93,94,95] |
| KSR1 | Naturally inactive kinase (pseudokinase) or kinase with low intrinsic enzymatic activity | Scaffolding functions for the RAF/MEK/ERK pathway; allosteric transactivator for BRAF | [100] |
6. Pharmacological Implications
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pawson, T.; Nash, P. Assembly of Cell Regulatory Systems through Protein Interaction Domains. Science 2003, 300, 445–452. [Google Scholar] [CrossRef]
- Campos Alonso, M.; Knobeloch, K.P. In the Moonlight: Non-Catalytic Functions of Ubiquitin And Ubiquitin-Like Proteases. Front. Mol. Biosci. 2024, 11, 1349509. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Chen, J.; Cooke, E.W.; Subuddhi, A.; Roodman, E.T.; Chen, F.X.; Cao, K. Demethylase-Independent Roles of LSD1 in Regulating Enhancers and Cell Fate Transition. Nat. Commun. 2023, 14, 4944. [Google Scholar] [CrossRef] [PubMed]
- Bruton, O.C. Agammaglobulinemia. Pediatrics 1952, 9, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.I. From Identification of the BTK Kinase to Effective Management of Leukemia. Oncogene 2017, 36, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.D.; Lawton, A.R.; Bockman, D.E. Agammaglobulinaemia with B Lymphocytes. Specific Defect of Plasma-Cell Differentiation. Lancet 1971, 2, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Naor, D.; Bentwich, Z.; Cividalli, G. Inability of Peripheral Lymphoid Cells of Agammaglobulinaemic Patients to Bind Radioiodinated Albumins. Aust. J. Exp. Biol. Med. Sci. 1969, 47, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Noordzij, J.G.; de Bruin-Versteeg, S.; Comans-Bitter, W.M.; Hartwig, N.G.; Hendriks, R.W.; de Groot, R.; van Dongen, J.J. Composition of Precursor B-Cell Compartment in Bone Marrow from Patients with X-Linked Agammaglobulinemia Compared with Healthy Children. Pediatr. Res. 2002, 51, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, S.; Saffran, D.C.; Rawlings, D.J.; Parolini, O.; Allen, R.C.; Klisak, I.; Sparkes, R.S.; Kubagawa, H.; Mohandas, T.; Quan, S.; et al. Deficient Expression of a B Cell Cytoplasmic Tyrosine Kinase in Human X-Linked Agammaglobulinemia. Cell 1993, 72, 279–290. [Google Scholar] [CrossRef]
- Vetrie, D.; Vorechovsky, I.; Sideras, P.; Holland, J.; Davies, A.; Flinter, F.; Hammarstrom, L.; Kinnon, C.; Levinsky, R.; Bobrow, M.; et al. The Gene Involved in X-Linked Agammaglobulinaemia Is a Member of the Src Family of Protein-Tyrosine Kinases. Nature 1993, 361, 226–233. [Google Scholar] [CrossRef]
- Wicker, L.S.; Scher, I. X-Linked Immune Deficiency (xid) of CBA/N Mice. Curr. Top Microbiol. Immunol. 1986, 124, 87–101. [Google Scholar] [CrossRef]
- Khan, W.N.; Alt, F.W.; Gerstein, R.M.; Malynn, B.A.; Larsson, I.; Rathbun, G.; Davidson, L.; Muller, S.; Kantor, A.B.; Herzenberg, L.A.; et al. Defective B Cell Development and Function in Btk-Deficient Mice. Immunity 1995, 3, 283–299. [Google Scholar] [CrossRef] [PubMed]
- de Weers, M.; Mensink, R.G.; Kraakman, M.E.; Schuurman, R.K.; Hendriks, R.W. Mutation Analysis of the Bruton’s Tyrosine Kinase Gene in X-Linked Agammaglobulinemia: Identification of a Mutation Which Affects the Same Codon as Is Altered in Immunodeficient Xid Mice. Hum. Mol. Genet. 1994, 3, 161–166. [Google Scholar] [CrossRef]
- Mohamed, A.J.; Yu, L.; Backesjo, C.M.; Vargas, L.; Faryal, R.; Aints, A.; Christensson, B.; Berglof, A.; Vihinen, M.; Nore, B.F.; et al. Bruton’s Tyrosine Kinase (Btk): Function, Regulation, and Transformation with Special Emphasis on the PH Domain. Immunol. Rev. 2009, 228, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Koretzky, G.A.; Abtahian, F.; Silverman, M.A. SLP76 and SLP65: Complex Regulation of Signalling in Lymphocytes and Beyond. Nat. Rev. Immunol. 2006, 6, 67–78. [Google Scholar] [CrossRef]
- Middendorp, S.; Dingjan, G.M.; Maas, A.; Dahlenborg, K.; Hendriks, R.W. Function of Bruton’s Tyrosine Kinase during B Cell Development Is Partially Independent of Its Catalytic Activity. J. Immunol. 2003, 171, 5988–5996. [Google Scholar] [CrossRef] [PubMed]
- Takata, M.; Kurosaki, T. A Role for Bruton’s Tyrosine Kinase in B Cell Antigen Receptor-Mediated Activation of Phospholipase C-Gamma 2. J. Exp. Med. 1996, 184, 31–40. [Google Scholar] [CrossRef]
- Saito, K.; Tolias, K.F.; Saci, A.; Koon, H.B.; Humphries, L.A.; Scharenberg, A.; Rawlings, D.J.; Kinet, J.P.; Carpenter, C.L. BTK Regulates PtdIns-4,5-P2 Synthesis: Importance for Calcium Signaling and PI3K Activity. Immunity 2003, 19, 669–678. [Google Scholar] [CrossRef]
- Flemming, A.; Brummer, T.; Reth, M.; Jumaa, H. The Adaptor protein SLP-65 acts as a tumor suppressor that limits pre-B cell expansion. Nat. Immunol. 2003, 4, 38–43. [Google Scholar] [CrossRef]
- Jumaa, H.; Bossaller, L.; Portugal, K.; Storch, B.; Lotz, M.; Flemming, A.; Schrappe, M.; Postila, V.; Riikonen, P.; Pelkonen, J.; et al. Deficiency of the adaptor SLP-65 in pre-B-cell acute lymphoblastic leukaemia. Nature 2003, 423, 452–456. [Google Scholar] [CrossRef]
- Kersseboom, R.; Middendorp, S.; Dingjan, G.M.; Dahlenborg, K.; Reth, M.; Jumaa, H.; Hendriks, R.W. Bruton’s tyrosine kinase cooperates with the B cell linker protein SLP-65 as a tumor suppressor in Pre-B cells. J. Exp. Med. 2003, 198, 91–98. [Google Scholar] [CrossRef]
- Middendorp, S.; Zijlstra, A.J.; Kersseboom, R.; Dingjan, G.M.; Jumaa, H.; Hendriks, R.W. Tumor suppressor function of Bruton tyrosine kinase is independent of its catalytic activity. Blood 2005, 105, 259–265. [Google Scholar] [CrossRef]
- Varnai, P.; Rother, K.I.; Balla, T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton’s tyrosine kinase pleckstrin homology domain visualized in single living cells. J. Biol. Chem. 1999, 274, 10983–10989. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsuda, S.; Terauchi, Y.; Fujiwara, M.; Ohteki, T.; Asano, T.; Behrens, T.W.; Kouro, T.; Takatsu, K.; Kadowaki, T.; et al. PI3K and Btk differentially regulate B cell antigen receptor-mediated signal transduction. Nat. Immunol. 2003, 4, 280–286. [Google Scholar] [CrossRef]
- Zorn, C.N.; Simonowski, A.; Huber, M. Stimulus strength determines the BTK-dependence of the SHIP1-deficient phenotype in IgE/antigen-triggered mast cells. Sci. Rep. 2018, 8, 15467. [Google Scholar] [CrossRef]
- Tkaczyk, C.; Beaven, M.A.; Brachman, S.M.; Metcalfe, D.D.; Gilfillan, A.M. The phospholipase C gamma 1-dependent pathway of Fc epsilon RI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase. J. Biol. Chem. 2003, 278, 48474–48484. [Google Scholar] [CrossRef]
- Wang, Q.; Vogan, E.M.; Nocka, L.M.; Rosen, C.E.; Zorn, J.A.; Harrison, S.C.; Kuriyan, J. Autoinhibition of Bruton’s tyrosine kinase (Btk) and activation by soluble inositol hexakisphosphate. Elife 2015, 4, e06074. [Google Scholar] [CrossRef]
- Timofeeva, N.; Gandhi, V. Ibrutinib combinations in CLL therapy: Scientific rationale and clinical results. Blood Cancer J. 2021, 11, 79. [Google Scholar] [CrossRef]
- Dhami, K.; Chakraborty, A.; Gururaja, T.L.; Cheung, L.W.; Sun, C.; DeAnda, F.; Huang, X. Kinase-deficient BTK mutants confer ibrutinib resistance through activation of the kinase HCK. Sci. Signal. 2022, 15, eabg5216. [Google Scholar] [CrossRef]
- Grasis, J.A.; Browne, C.D.; Tsoukas, C.D. Inducible T cell tyrosine kinase regulates actin-dependent cytoskeletal events induced by the T cell antigen receptor. J. Immunol. 2003, 170, 3971–3976. [Google Scholar] [CrossRef]
- Marone, R.; Cmiljanovic, V.; Giese, B.; Wymann, M.P. Targeting phosphoinositide 3-kinase: Moving towards therapy. Biochim. Biophys. Acta 2008, 1784, 159–185. [Google Scholar] [CrossRef]
- Stoyanov, B.; Volinia, S.; Hanck, T.; Rubio, I.; Loubtchenkov, M.; Malek, D.; Stoyanova, S.; Vanhaesebroeck, B.; Dhand, R.; Nurnberg, B.; et al. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science 1995, 269, 690–693. [Google Scholar] [CrossRef]
- Bondeva, T.; Pirola, L.; Bulgarelli-Leva, G.; Rubio, I.; Wetzker, R.; Wymann, M.P. Bifurcation of lipid and protein kinase signals of PI3Kgamma to the protein kinases PKB and MAPK. Science 1998, 282, 293–296. [Google Scholar] [CrossRef]
- Hirsch, E.; Katanaev, V.L.; Garlanda, C.; Azzolino, O.; Pirola, L.; Silengo, L.; Sozzani, S.; Mantovani, A.; Altruda, F.; Wymann, M.P. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 2000, 287, 1049–1053. [Google Scholar] [CrossRef]
- Crackower, M.A.; Oudit, G.Y.; Kozieradzki, I.; Sarao, R.; Sun, H.; Sasaki, T.; Hirsch, E.; Suzuki, A.; Shioi, T.; Irie-Sasaki, J.; et al. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell 2002, 110, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Brittsan, A.G.; Kranias, E.G. Phospholamban and cardiac contractile function. J. Mol. Cell. Cardiol. 2000, 32, 2131–2139. [Google Scholar] [CrossRef]
- Patrucco, E.; Notte, A.; Barberis, L.; Selvetella, G.; Maffei, A.; Brancaccio, M.; Marengo, S.; Russo, G.; Azzolino, O.; Rybalkin, S.D.; et al. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell 2004, 118, 375–387. [Google Scholar] [CrossRef]
- Huber, M.; Helgason, C.D.; Damen, J.E.; Scheid, M.P.; Duronio, V.; Lam, V.; Humphries, R.K.; Krystal, G. The role of the SRC homology 2-containing inositol 5’-phosphatase in Fc epsilon R1-induced signaling. Curr. Top. Microbiol. Immunol. 1999, 244, 29–41. [Google Scholar]
- Dyson, J.M.; Fedele, C.G.; Davies, E.M.; Becanovic, J.; Mitchell, C.A. Phosphoinositide phosphatases: Just as important as the kinases. Subcell. Biochem. 2012, 58, 215–279. [Google Scholar] [CrossRef]
- Cheung, S.M.; Kornelson, J.C.; Al-Alwan, M.; Marshall, A.J. Regulation of phosphoinositide 3-kinase signaling by oxidants: Hydrogen peroxide selectively enhances immunoreceptor-induced recruitment of phosphatidylinositol (3,4) bisphosphate-binding PH domain proteins. Cell. Signal. 2007, 19, 902–912. [Google Scholar] [CrossRef]
- Conde, C.; Gloire, G.; Piette, J. Enzymatic and non-enzymatic activities of SHIP-1 in signal transduction and cancer. Biochem. Pharmacol. 2011, 82, 1320–1334. [Google Scholar] [CrossRef]
- Ong, C.J.; Ming-Lum, A.; Nodwell, M.; Ghanipour, A.; Yang, L.; Williams, D.E.; Kim, J.; Demirjian, L.; Qasimi, P.; Ruschmann, J.; et al. Small-molecule agonists of SHIP1 inhibit the phosphoinositide 3-kinase pathway in hematopoietic cells. Blood 2007, 110, 1942–1949. [Google Scholar] [CrossRef]
- Kalesnikoff, J.; Sly, L.M.; Hughes, M.R.; Buchse, T.; Rauh, M.J.; Cao, L.P.; Lam, V.; Mui, A.; Huber, M.; Krystal, G. The role of SHIP in cytokine-induced signaling. Rev. Physiol. Biochem. Pharmacol. 2003, 149, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Isnardi, I.; Bruhns, P.; Bismuth, G.; Fridman, W.H.; Daeron, M. The SH2 domain-containing inositol 5-phosphatase SHIP1 is recruited to the intracytoplasmic domain of human FcgammaRIIB and is mandatory for negative regulation of B cell activation. Immunol. Lett. 2006, 104, 156–165. [Google Scholar] [CrossRef]
- Ono, M.; Bolland, S.; Tempst, P.; Ravetch, J.V. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature 1996, 383, 263–266. [Google Scholar] [CrossRef]
- Harmer, S.L.; DeFranco, A.L. The src homology domain 2-containing inositol phosphatase SHIP forms a ternary complex with Shc and Grb2 in antigen receptor-stimulated B lymphocytes. J. Biol. Chem. 1999, 274, 12183–12191. [Google Scholar] [CrossRef]
- Tridandapani, S.; Chacko, G.W.; Van Brocklyn, J.R.; Coggeshall, K.M. Negative signaling in B cells causes reduced Ras activity by reducing Shc-Grb2 interactions. J. Immunol. 1997, 158, 1125–1132. [Google Scholar] [CrossRef]
- Tridandapani, S.; Pradhan, M.; LaDine, J.R.; Garber, S.; Anderson, C.L.; Coggeshall, K.M. Protein interactions of Src homology 2 (SH2) domain-containing inositol phosphatase (SHIP): Association with Shc displaces SHIP from FcgammaRIIb in B cells. J. Immunol. 1999, 162, 1408–1414. [Google Scholar] [CrossRef]
- Liu, Q.; Oliveira-Dos-Santos, A.J.; Mariathasan, S.; Bouchard, D.; Jones, J.; Sarao, R.; Kozieradzki, I.; Ohashi, P.S.; Penninger, J.M.; Dumont, D.J. The inositol polyphosphate 5-phosphatase ship is a crucial negative regulator of B cell antigen receptor signaling. J. Exp. Med. 1998, 188, 1333–1342. [Google Scholar] [CrossRef]
- Tamir, I.; Stolpa, J.C.; Helgason, C.D.; Nakamura, K.; Bruhns, P.; Daeron, M.; Cambier, J.C. The RasGAP-binding protein p62dok is a mediator of inhibitory FcgammaRIIB signals in B cells. Immunity 2000, 12, 347–358. [Google Scholar] [CrossRef]
- Ott, V.L.; Tamir, I.; Niki, M.; Pandolfi, P.P.; Cambier, J.C. Downstream of kinase, p62(dok), is a mediator of Fc gamma IIB inhibition of Fc epsilon RI signaling. J. Immunol. 2002, 168, 4430–4439. [Google Scholar] [CrossRef]
- Tsujishita, Y.; Guo, S.; Stolz, L.E.; York, J.D.; Hurley, J.H. Specificity determinants in phosphoinositide dephosphorylation: Crystal structure of an archetypal inositol polyphosphate 5-phosphatase. Cell 2001, 105, 379–389. [Google Scholar] [CrossRef]
- Mukherjee, O.; Weingarten, L.; Padberg, I.; Pracht, C.; Sinha, R.; Hochdorfer, T.; Kuppig, S.; Backofen, R.; Reth, M.; Huber, M. The SH2-domain of SHIP1 interacts with the SHIP1 C-terminus: Impact on SHIP1/Ig-alpha interaction. Biochim. Biophys. Acta 2012, 1823, 206–214. [Google Scholar] [CrossRef]
- Havrylov, S.; Redowicz, M.J.; Buchman, V.L. Emerging roles of Ruk/CIN85 in vesicle-mediated transport, adhesion, migration and malignancy. Traffic 2010, 11, 721–731. [Google Scholar] [CrossRef]
- Kowanetz, K.; Husnjak, K.; Holler, D.; Kowanetz, M.; Soubeyran, P.; Hirsch, D.; Schmidt, M.H.H.; Pavelic, K.; De Camilli, P.; Randazzo, P.A.; et al. CIN85 associates with multiple effectors controlling intracellular trafficking of epidermal growth factor receptors. Mol. Biol. Cell 2004, 15, 3155–3166. [Google Scholar] [CrossRef]
- Buchse, T.; Horras, N.; Lenfert, E.; Krystal, G.; Korbel, S.; Schumann, M.; Krause, E.; Mikkat, S.; Tiedge, M. CIN85 interacting proteins in B cells-specific role for SHIP-1. Mol. Cell. Proteom. 2011, 10, M110-006239. [Google Scholar] [CrossRef]
- Kuhn, J.; Wong, L.E.; Pirkuliyeva, S.; Schulz, K.; Schwiegk, C.; Funfgeld, K.G.; Keppler, S.; Batista, F.D.; Urlaub, H.; Habeck, M.; et al. The adaptor protein CIN85 assembles intracellular signaling clusters for B cell activation. Sci. Signal. 2016, 9, ra66. [Google Scholar] [CrossRef]
- Conde, C.; Rambout, X.; Lebrun, M.; Lecat, A.; Di Valentin, E.; Dequiedt, F.; Piette, J.; Gloire, G.; Legrand, S. The inositol phosphatase SHIP-1 inhibits NOD2-induced NF-kappaB activation by disturbing the interaction of XIAP with RIP2. PLoS ONE 2012, 7, e41005. [Google Scholar] [CrossRef]
- Lu, C.; Wang, A.; Dorsch, M.; Tian, J.; Nagashima, K.; Coyle, A.J.; Jaffee, B.; Ocain, T.D.; Xu, Y. Participation of Rip2 in lipopolysaccharide signaling is independent of its kinase activity. J. Biol. Chem. 2005, 280, 16278–16283. [Google Scholar] [CrossRef]
- Reiterer, V.; Eyers, P.A.; Farhan, H. Day of the dead: Pseudokinases and pseudophosphatases in physiology and disease. Trends Cell Biol. 2014, 24, 489–505. [Google Scholar] [CrossRef]
- Yarden, Y.; Pines, G. The ERBB network: At last, cancer therapy meets systems biology. Nat. Rev. Cancer 2012, 12, 553–563. [Google Scholar] [CrossRef]
- Baker, S.J.; Rane, S.G.; Reddy, E.P. Hematopoietic cytokine receptor signaling. Oncogene 2007, 26, 6724–6737. [Google Scholar] [CrossRef]
- Reth, M.; Brummer, T. Feedback regulation of lymphocyte signalling. Nat. Rev. Immunol. 2004, 4, 269–277. [Google Scholar] [CrossRef]
- Harkiolaki, M.; Tsirka, T.; Lewitzky, M.; Simister, P.C.; Joshi, D.; Bird, L.E.; Jones, E.Y.; O’Reilly, N.; Feller, S.M. Distinct binding modes of two epitopes in Gab2 that interact with the SH3C domain of Grb2. Structure 2009, 17, 809–822. [Google Scholar] [CrossRef]
- Wohrle, F.U.; Daly, R.J.; Brummer, T. Function, regulation and pathological roles of the Gab/DOS docking proteins. Cell Commun. Signal. 2009, 7, 22. [Google Scholar] [CrossRef]
- Jeon, H.; Tkacik, E.; Eck, M.J. Signaling from RAS to RAF: The Molecules and Their Mechanisms. Annu. Rev. Biochem. 2024, 93. [Google Scholar] [CrossRef]
- Hu, J.; Stites, E.C.; Yu, H.; Germino, E.A.; Meharena, H.S.; Stork, P.J.S.; Kornev, A.P.; Taylor, S.S.; Shaw, A.S. Allosteric activation of functionally asymmetric RAF kinase dimers. Cell 2013, 154, 1036–1046. [Google Scholar] [CrossRef]
- Brummer, T.; McInnes, C. RAF kinase dimerization: Implications for drug discovery and clinical outcomes. Oncogene 2020, 39, 4155–4169. [Google Scholar] [CrossRef]
- Hanrahan, A.J.; Chen, Z.; Rosen, N.; Solit, D.B. BRAF—A tumour-agnostic drug target with lineage-specific dependencies. Nat. Rev. Clin. Oncol. 2024, 21, 224–247. [Google Scholar] [CrossRef]
- Yaktapour, N.; Meiss, F.; Mastroianni, J.; Zenz, T.; Andrlova, H.; Mathew, N.R.; Claus, R.; Hutter, B.; Frohling, S.; Brors, B.; et al. BRAF inhibitor-associated ERK activation drives development of chronic lymphocytic leukemia. J. Clin. Investig. 2014, 124, 5074–5084. [Google Scholar] [CrossRef]
- Cadranel, J.; Liu, S.V.; Duruisseaux, M.; Branden, E.; Goto, Y.; Weinberg, B.A.; Heining, C.; Schlenk, R.F.; Cheema, P.; Jones, M.R.; et al. Therapeutic Potential of Afatinib in NRG1 Fusion-Driven Solid Tumors: A Case Series. Oncologist 2021, 26, 7–16. [Google Scholar] [CrossRef]
- Citri, A.; Skaria, K.B.; Yarden, Y. The deaf and the dumb: The biology of ErbB-2 and ErbB-3. Exp. Cell Res. 2003, 284, 54–65. [Google Scholar] [CrossRef]
- Kovacs, E.; Zorn, J.A.; Huang, Y.; Barros, T.; Kuriyan, J. A structural perspective on the regulation of the epidermal growth factor receptor. Annu. Rev. Biochem. 2015, 84, 739–764. [Google Scholar] [CrossRef]
- Kung, J.E.; Jura, N. Structural Basis for the Non-catalytic Functions of Protein Kinases. Structure 2016, 24, 7–24. [Google Scholar] [CrossRef]
- Trenker, R.; Diwanji, D.; Jura, N. Mutant HER2 needs mutant HER3 to be an effective oncogene. Cell Rep. Med. 2021, 2, 100361. [Google Scholar] [CrossRef]
- Schulze, W.X.; Deng, L.; Mann, M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol. Syst. Biol. 2005, 1, 2005-0008. [Google Scholar] [CrossRef]
- Koivu, M.K.A.; Chakroborty, D.; Airenne, T.T.; Johnson, M.S.; Kurppa, K.J.; Elenius, K. Trans-activating mutations of the pseudokinase ERBB3. Oncogene 2024, 43, 2253–2265. [Google Scholar] [CrossRef]
- Sodir, N.M.; Pathria, G.; Adamkewicz, J.I.; Kelley, E.H.; Sudhamsu, J.; Merchant, M.; Chiarle, R.; Maddalo, D. SHP2: A Pleiotropic Target at the Interface of Cancer and Its Microenvironment. Cancer Discov. 2023, 13, 2339–2355. [Google Scholar] [CrossRef]
- Lu, W.; Gong, D.; Bar-Sagi, D.; Cole, P.A. Site-specific incorporation of a phosphotyrosine mimetic reveals a role for tyrosine phosphorylation of SHP-2 in cell signaling. Mol. Cell 2001, 8, 759–769. [Google Scholar] [CrossRef]
- Wei, W.; Geer, M.J.; Guo, X.; Dolgalev, I.; Sanjana, N.E.; Neel, B.G. Genome-wide CRISPR/Cas9 screens reveal shared and cell-specific mechanisms of resistance to SHP2 inhibition. J. Exp. Med. 2023, 220, e20221563. [Google Scholar] [CrossRef]
- Bunda, S.; Burrell, K.; Heir, P.; Zeng, L.; Alamsahebpour, A.; Kano, Y.; Raught, B.; Zhang, Z.Y.; Zadeh, G.; Ohh, M. Inhibition of SHP2-mediated dephosphorylation of Ras suppresses oncogenesis. Nat. Commun. 2015, 6, 8859. [Google Scholar] [CrossRef]
- Bentires-Alj, M.; Paez, J.G.; David, F.S.; Keilhack, H.; Halmos, B.; Naoki, K.; Maris, J.M.; Richardson, A.; Bardelli, A.; Sugarbaker, D.J.; et al. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 2004, 64, 8816–8820. [Google Scholar] [CrossRef]
- Aoki, Y.; Niihori, T.; Narumi, Y.; Kure, S.; Matsubara, Y. The RAS/MAPK syndromes: Novel roles of the RAS pathway in human genetic disorders. Hum. Mutat. 2008, 29, 992–1006. [Google Scholar] [CrossRef]
- Mai, T.T.; Lito, P. A treatment strategy for KRAS-driven tumors. Nat. Med. 2018, 24, 902–904. [Google Scholar] [CrossRef]
- Guo, W.; Xu, Q. Phosphatase-independent functions of SHP2 and its regulation by small molecule compounds. J. Pharmacol. Sci. 2020, 144, 139–146. [Google Scholar] [CrossRef]
- Stewart, R.A.; Sanda, T.; Widlund, H.R.; Zhu, S.; Swanson, K.D.; Hurley, A.D.; Bentires-Alj, M.; Fisher, D.E.; Kontaridis, M.I.; Look, A.T.; et al. Phosphatase-dependent and -independent functions of Shp2 in neural crest cells underlie LEOPARD syndrome pathogenesis. Dev. Cell 2010, 18, 750–762. [Google Scholar] [CrossRef]
- Vemulapalli, V.; Chylek, L.A.; Erickson, A.; Pfeiffer, A.; Gabriel, K.H.; LaRochelle, J.; Subramanian, K.; Cao, R.; Stegmaier, K.; Mohseni, M.; et al. Time-resolved phosphoproteomics reveals scaffolding and catalysis-responsive patterns of SHP2-dependent signaling. Elife 2021, 10, e64251. [Google Scholar] [CrossRef]
- Lin, C.C.; Suen, K.M.; Jeffrey, P.A.; Wieteska, L.; Lidster, J.A.; Bao, P.; Curd, A.P.; Stainthorp, A.; Seiler, C.; Koss, H.; et al. Receptor tyrosine kinases regulate signal transduction through a liquid-liquid phase separated state. Mol. Cell 2022, 82, 1089–1106 e1012. [Google Scholar] [CrossRef]
- Desideri, E.; Cavallo, A.L.; Baccarini, M. Alike but Different: RAF Paralogs and Their Signaling Outputs. Cell 2015, 161, 967–970. [Google Scholar] [CrossRef]
- Riaud, M.; Maxwell, J.; Soria-Bretones, I.; Dankner, M.; Li, M.; Rose, A.A.N. The role of CRAF in cancer progression: From molecular mechanisms to precision therapies. Nat. Rev. Cancer 2024, 24, 105–122. [Google Scholar] [CrossRef]
- Dorard, C.; Madry, C.; Buhard, O.; Toifl, S.; Didusch, S.; Ratovomanana, T.; Letourneur, Q.; Dolznig, H.; Garnett, M.J.; Duval, A.; et al. RAF1 contributes to cell proliferation and STAT3 activation in colorectal cancer independently of microsatellite and KRAS status. Oncogene 2023, 42, 1649–1660. [Google Scholar] [CrossRef]
- Nguyen, L.K.; Matallanas, D.G.; Romano, D.; Kholodenko, B.N.; Kolch, W. Competing to coordinate cell fate decisions: The MST2-Raf-1 signaling device. Cell Cycle 2015, 14, 189–199. [Google Scholar] [CrossRef]
- O’Neill, E.; Rushworth, L.; Baccarini, M.; Kolch, W. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science 2004, 306, 2267–2270. [Google Scholar] [CrossRef]
- Chen, J.; Fujii, K.; Zhang, L.; Roberts, T.; Fu, H. Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism. Proc. Natl. Acad. Sci. USA 2001, 98, 7783–7788. [Google Scholar] [CrossRef]
- Varga, A.; Ehrenreiter, K.; Aschenbrenner, B.; Kocieniewski, P.; Kochanczyk, M.; Lipniacki, T.; Baccarini, M. RAF1/BRAF dimerization integrates the signal from RAS to ERK and ROKalpha. Sci. Signal. 2017, 10, eaai8482. [Google Scholar] [CrossRef]
- Sanclemente, M.; Nieto, P.; Garcia-Alonso, S.; Fernandez-Garcia, F.; Esteban-Burgos, L.; Guerra, C.; Drosten, M.; Caleiras, E.; Martinez-Torrecuadrada, J.; Santamaria, D.; et al. RAF1 kinase activity is dispensable for KRAS/p53 mutant lung tumor progression. Cancer Cell 2021, 39, 294–296. [Google Scholar] [CrossRef]
- Liu, Z.; Krstic, A.; Neve, A.; Casalou, C.; Rauch, N.; Wynne, K.; Cassidy, H.; McCann, A.; Kavanagh, E.; McCann, B.; et al. Kinase Suppressor of RAS 1 (KSR1) Maintains the Transformed Phenotype of BRAFV600E Mutant Human Melanoma Cells. Int. J. Mol. Sci. 2023, 24, 11821. [Google Scholar] [CrossRef]
- Kolch, W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 2005, 6, 827–837. [Google Scholar] [CrossRef]
- Roring, M.; Herr, R.; Fiala, G.J.; Heilmann, K.; Braun, S.; Eisenhardt, A.E.; Halbach, S.; Capper, D.; von Deimling, A.; Schamel, W.W.; et al. Distinct requirement for an intact dimer interface in wild-type, V600E and kinase-dead B-Raf signalling. EMBO J. 2012, 31, 2629–2647. [Google Scholar] [CrossRef]
- Lavoie, H.; Sahmi, M.; Maisonneuve, P.; Marullo, S.A.; Thevakumaran, N.; Jin, T.; Kurinov, I.; Sicheri, F.; Therrien, M. MEK drives BRAF activation through allosteric control of KSR proteins. Nature 2018, 554, 549–553. [Google Scholar] [CrossRef]
- Chessel, A.; De Croze, N.; Molina, M.D.; Taberner, L.; Dru, P.; Martin, L.; Lepage, T. RAS-independent ERK activation by constitutively active KSR3 in non-chordate metazoa. Nat. Commun. 2023, 14, 3970. [Google Scholar] [CrossRef]
- Rohrer, L.; Spohr, C.; Beha, C.; Griffin, R.; Braun, S.; Halbach, S.; Brummer, T. Analysis of RAS and drug induced homo- and heterodimerization of RAF and KSR1 proteins in living cells using split Nanoluc luciferase. Cell Commun. Signal. 2023, 21, 136. [Google Scholar] [CrossRef]
- Paniagua, G.; Jacob, H.K.C.; Brehey, O.; Garcia-Alonso, S.; Lechuga, C.G.; Pons, T.; Musteanu, M.; Guerra, C.; Drosten, M.; Barbacid, M. KSR induces RAS-independent MAPK pathway activation and modulates the efficacy of KRAS inhibitors. Mol. Oncol. 2022, 16, 3066–3081. [Google Scholar] [CrossRef]
- Heidorn, S.J.; Milagre, C.; Whittaker, S.; Nourry, A.; Niculescu-Duvas, I.; Dhomen, N.; Hussain, J.; Reis-Filho, J.S.; Springer, C.J.; Pritchard, C.; et al. Kinase-Dead BRAF and Oncogenic RAS Cooperate to Drive Tumor Progression through CRAF. Cell 2010, 140, 209–221. [Google Scholar] [CrossRef]
- Nieto, P.; Ambrogio, C.; Esteban-Burgos, L.; Gomez-Lopez, G.; Blasco, M.T.; Yao, Z.; Marais, R.; Rosen, N.; Chiarle, R.; Pisano, D.G.; et al. A Braf kinase-inactive mutant induces lung adenocarcinoma. Nature 2017, 548, 239–243. [Google Scholar] [CrossRef]
- Yao, Z.; Yaeger, R.; Rodrik-Outmezguine, V.S.; Tao, A.; Torres, N.M.; Chang, M.T.; Drosten, M.; Zhao, H.; Cecchi, F.; Hembrough, T.; et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 2017, 548, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Hoefflin, R.; Geissler, A.L.; Fritsch, R.; Claus, R.; Wehrle, J.; Metzger, P.; Reiser, M.; Mehmed, L.; Fauth, L.; Heiland, D.H.; et al. Personalized Clinical Decision Making Through Implementation of a Molecular Tumor Board: A German Single-Center Experience. JCO Precis. Oncol. 2018, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.; Berry, D.; Heney, K.A.; Strong, C.; Ramsay, L.; Lajoie, M.; Alkallas, R.; Nguyen, T.T.; Thomson, C.; Ahanfeshar-Adams, M.; et al. Melanomas with concurrent BRAF non-p.V600 and NF1 loss-of-function mutations are targetable by BRAF/MEK inhibitor combination therapy. Cell Rep. 2022, 39, 110634. [Google Scholar] [CrossRef]
- Bollag, G.; Tsai, J.; Zhang, J.; Zhang, C.; Ibrahim, P.; Nolop, K.; Hirth, P. Vemurafenib: The first drug approved for BRAF-mutant cancer. Nat. Rev. Drug Discov. 2012, 11, 873–886. [Google Scholar] [CrossRef]
- Hunter, T. Treatment for chronic myelogenous leukemia: The long road to imatinib. J. Clin. Investig. 2007, 117, 2036–2043. [Google Scholar] [CrossRef]
- Kerr, D.L.; Haderk, F.; Bivona, T.G. Allosteric SHP2 inhibitors in cancer: Targeting the intersection of RAS, resistance, and the immune microenvironment. Curr. Opin. Chem. Biol. 2021, 62, 1–12. [Google Scholar] [CrossRef]
- Rea, D.; Hughes, T.P. Development of asciminib, a novel allosteric inhibitor of BCR-ABL1. Crit. Rev. Oncol. Hematol. 2022, 171, 103580. [Google Scholar] [CrossRef]
- Cook, S.J.; Tucker, J.A.; Lochhead, P.A. Small molecule ERK5 kinase inhibitors paradoxically activate ERK5 signalling: Be careful what you wish for…. Biochem. Soc. Trans. 2020, 48, 1859–1875. [Google Scholar] [CrossRef]
- Lochhead, P.A.; Tucker, J.A.; Tatum, N.J.; Wang, J.; Oxley, D.; Kidger, A.M.; Johnson, V.P.; Cassidy, M.A.; Gray, N.S.; Noble, M.E.M.; et al. Paradoxical activation of the protein kinase-transcription factor ERK5 by ERK5 kinase inhibitors. Nat. Commun. 2020, 11, 1383. [Google Scholar] [CrossRef] [PubMed]
- Herr, R.; Kohler, M.; Andrlova, H.; Weinberg, F.; Moller, Y.; Halbach, S.; Lutz, L.; Mastroianni, J.; Klose, M.; Bittermann, N.; et al. B-Raf Inhibitors Induce Epithelial Differentiation in BRAF-Mutant Colorectal Cancer Cells. Cancer Res. 2015, 75, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Viros, A.; Milagre, C.; Trunzer, K.; Bollag, G.; Spleiss, O.; Reis-Filho, J.S.; Kong, X.; Koya, R.C.; Flaherty, K.T.; et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N. Engl. J. Med. 2012, 366, 207–215. [Google Scholar] [CrossRef]
- Carlino, M.S.; Kwan, V.; Miller, D.K.; Saunders, C.A.; Yip, D.; Nagrial, A.M.; Tomlinson, J.; Grimmond, S.M.; Scolyer, R.A.; Kefford, R.F.; et al. New RAS-Mutant Pancreatic Adenocarcinoma With Combined BRAF and MEK Inhibition for Metastatic Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 33, e52–e56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Callahan, M.K.; Rampal, R.; Harding, J.J.; Klimek, V.M.; Chung, Y.R.; Merghoub, T.; Wolchok, J.D.; Solit, D.B.; Rosen, N.; Abdel-Wahab, O.; et al. Progression of RAS-mutant leukemia during RAF inhibitor treatment. N. Engl. J. Med. 2012, 367, 2316–2321. [Google Scholar] [CrossRef]
- Adamopoulos, C.; Ahmed, T.A.; Tucker, M.R.; Ung, P.M.U.; Xiao, M.; Karoulia, Z.; Amabile, A.; Wu, X.; Aaronson, S.A.; Ang, C.; et al. Exploiting Allosteric Properties of RAF and MEK Inhibitors to Target Therapy-Resistant Tumors Driven by Oncogenic BRAF Signaling. Cancer Discov. 2021, 11, 1716–1735. [Google Scholar] [CrossRef]
- Vasta, J.D.; Michaud, A.; Zimprich, C.A.; Beck, M.T.; Swiatnicki, M.R.; Zegzouti, H.; Thomas, M.R.; Wilkinson, J.; Crapster, J.A.; Robers, M.B. Protomer selectivity of type II RAF inhibitors within the RAS/RAF complex. Cell Chem. Biol. 2023, 30, 1354–1365 e1356. [Google Scholar] [CrossRef]
- Anastassiadis, T.; Deacon, S.W.; Devarajan, K.; Ma, H.; Peterson, J.R. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat. Biotechnol. 2011, 29, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.; Defnet, A.; Shapiro, P. Avoiding or Co-Opting ATP Inhibition: Overview of Type III, IV, V, and VI Kinase Inhibitors. In Next Generation Kinase Inhibitors: Moving Beyond the ATP Binding/Catalytic Sites; Shapiro, P., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 29–59. [Google Scholar] [CrossRef]
- Taylor, S.S.; Shaw, A.S.; Kannan, N.; Kornev, A.P. Integration of signaling in the kinome: Architecture and regulation of the alphaC Helix. Biochim. Biophys. Acta 2015, 1854 Pt B, 1567–1574. [Google Scholar] [CrossRef]
- Karoulia, Z.; Gavathiotis, E.; Poulikakos, P.I. New perspectives for targeting RAF kinase in human cancer. Nat. Rev. Cancer 2017, 17, 676–691. [Google Scholar] [CrossRef]
- Lee, P.Y.; Yeoh, Y.; Low, T.Y. A recent update on small-molecule kinase inhibitors for targeted cancer therapy and their therapeutic insights from mass spectrometry-based proteomic analysis. FEBS J. 2023, 290, 2845–2864. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, B.; Rigbolt, K.T.; Roring, M.; Herr, R.; Kaeser-Pebernard, S.; Gretzmeier, C.; Murphy, R.F.; Brummer, T.; Dengjel, J. Discrete cytosolic macromolecular BRAF complexes exhibit distinct activities and composition. EMBO J. 2017, 36, 646–663. [Google Scholar] [CrossRef] [PubMed]
- Gunderwala, A.Y.; Nimbvikar, A.A.; Cope, N.J.; Li, Z.; Wang, Z. Development of Allosteric BRAF Peptide Inhibitors Targeting the Dimer Interface of BRAF. ACS Chem. Biol. 2019, 14, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Beneker, C.M.; Rovoli, M.; Kontopidis, G.; Roring, M.; Galda, S.; Braun, S.; Brummer, T.; McInnes, C. Design and Synthesis of Type-IV Inhibitors of BRAF Kinase That Block Dimerization and Overcome Paradoxical MEK/ERK Activation. J. Med. Chem. 2019, 62, 3886–3897. [Google Scholar] [CrossRef] [PubMed]
- Posternak, G.; Tang, X.; Maisonneuve, P.; Jin, T.; Lavoie, H.; Daou, S.; Orlicky, S.; Goullet de Rugy, T.; Caldwell, L.; Chan, K.; et al. Functional characterization of a PROTAC directed against BRAF mutant V600E. Nat. Chem. Biol. 2020, 16, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Alabi, S.; Jaime-Figueroa, S.; Yao, Z.; Gao, Y.; Hines, J.; Samarasinghe, K.T.G.; Vogt, L.; Rosen, N.; Crews, C.M. Mutant-selective degradation by BRAF-targeting PROTACs. Nat. Commun. 2021, 12, 920. [Google Scholar] [CrossRef]
- Siva Sankar, D.; Dengjel, J. Protein complexes and neighborhoods driving autophagy. Autophagy 2021, 17, 2689–2705. [Google Scholar] [CrossRef]
- Wojnowski, L.; Stancato, L.F.; Larner, A.C.; Rapp, U.R.; Zimmer, A. Overlapping and specific functions of Braf and Craf-1 proto-oncogenes during mouse embryogenesis. Mech. Dev. 2000, 91, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Galabova-Kovacs, G.; Catalanotti, F.; Matzen, D.; Reyes, G.X.; Zezula, J.; Herbst, R.; Silva, A.; Walter, I.; Baccarini, M. Essential role of B-Raf in oligodendrocyte maturation and myelination during postnatal central nervous system development. J. Cell Biol. 2008, 180, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Mikula, M.; Schreiber, M.; Husak, Z.; Kucerova, L.; Ruth, J.; Wieser, R.; Zatloukal, K.; Beug, H.; Wagner, E.F.; Baccarini, M. Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf-1 gene. EMBO J. 2001, 20, 1952–1962. [Google Scholar] [CrossRef] [PubMed]
- Köhler, M.; Röring, M.; Schorch, B.; Heilmann, K.; Stickel, N.; Fiala, G.J.; Schmitt, L.C.; Braun, S.; Ehrenfeld, S.; Uhl, F.M.; et al. Activation loop phosphorylation regulates B-Raf in vivo and transformation by B-Raf mutants. EMBO J. 2016, 35, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Huser, M.; Luckett, J.; Chiloeches, A.; Mercer, K.; Iwobi, M.; Giblett, S.; Sun, X.M.; Brown, J.; Marais, R.; Pritchard, C. MEK kinase activity is not necessary for Raf-1 function. EMBO J. 2001, 20, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Kamata, T.; Hussain, J.; Giblett, S.; Hayward, R.; Marais, R.; Pritchard, C. BRAF inactivation drives aneuploidy by deregulating CRAF. Cancer Res. 2010, 70, 8475–8486. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huber, M.; Brummer, T. Enzyme Is the Name—Adapter Is the Game. Cells 2024, 13, 1249. https://doi.org/10.3390/cells13151249
Huber M, Brummer T. Enzyme Is the Name—Adapter Is the Game. Cells. 2024; 13(15):1249. https://doi.org/10.3390/cells13151249
Chicago/Turabian StyleHuber, Michael, and Tilman Brummer. 2024. "Enzyme Is the Name—Adapter Is the Game" Cells 13, no. 15: 1249. https://doi.org/10.3390/cells13151249
APA StyleHuber, M., & Brummer, T. (2024). Enzyme Is the Name—Adapter Is the Game. Cells, 13(15), 1249. https://doi.org/10.3390/cells13151249






