Regeneration of Propriospinal Axons in Rat Transected Spinal Cord Injury through a Growth-Promoting Pathway Constructed by Schwann Cells Overexpressing GDNF
Abstract
1. Introduction
2. Method and Materials
2.1. Generation of Purified Schwann Cells (SCs)
2.2. Transduction of SC In Vitro
2.3. Animal Groups and Exclusion Criteria
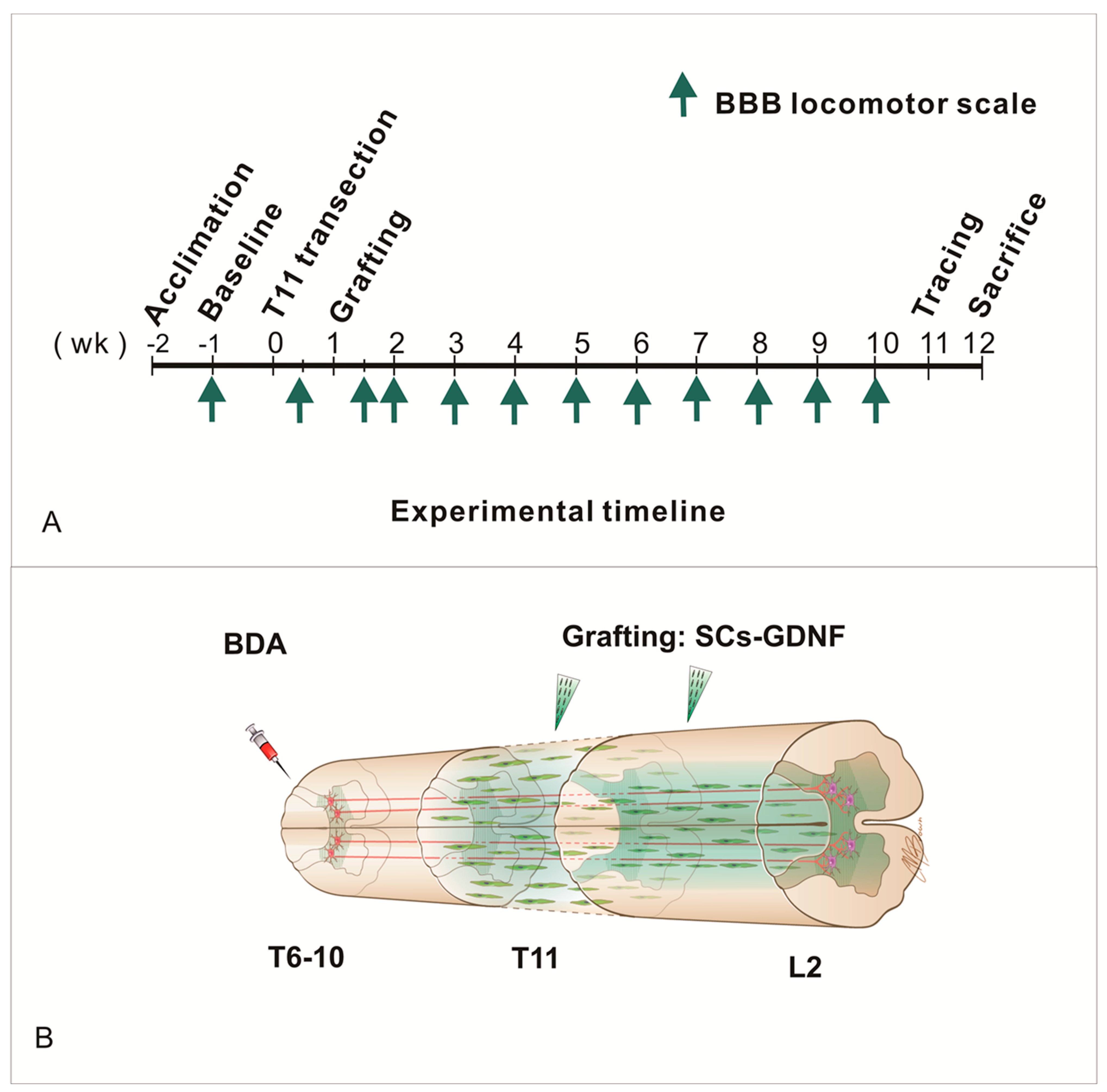
2.4. Thoracic Spinal Cord Transection Injury and Cell Transplantation
2.5. Behavior Assessments
2.6. Anterograde Tracing
2.7. Immunohistochemistry
2.8. Electron Microscopy
2.9. Quantification of BDA-Labeled Axons
2.10. Assessments of GFAP Immunoreactivity In Vivo
2.11. Statistical Analysis
3. Results
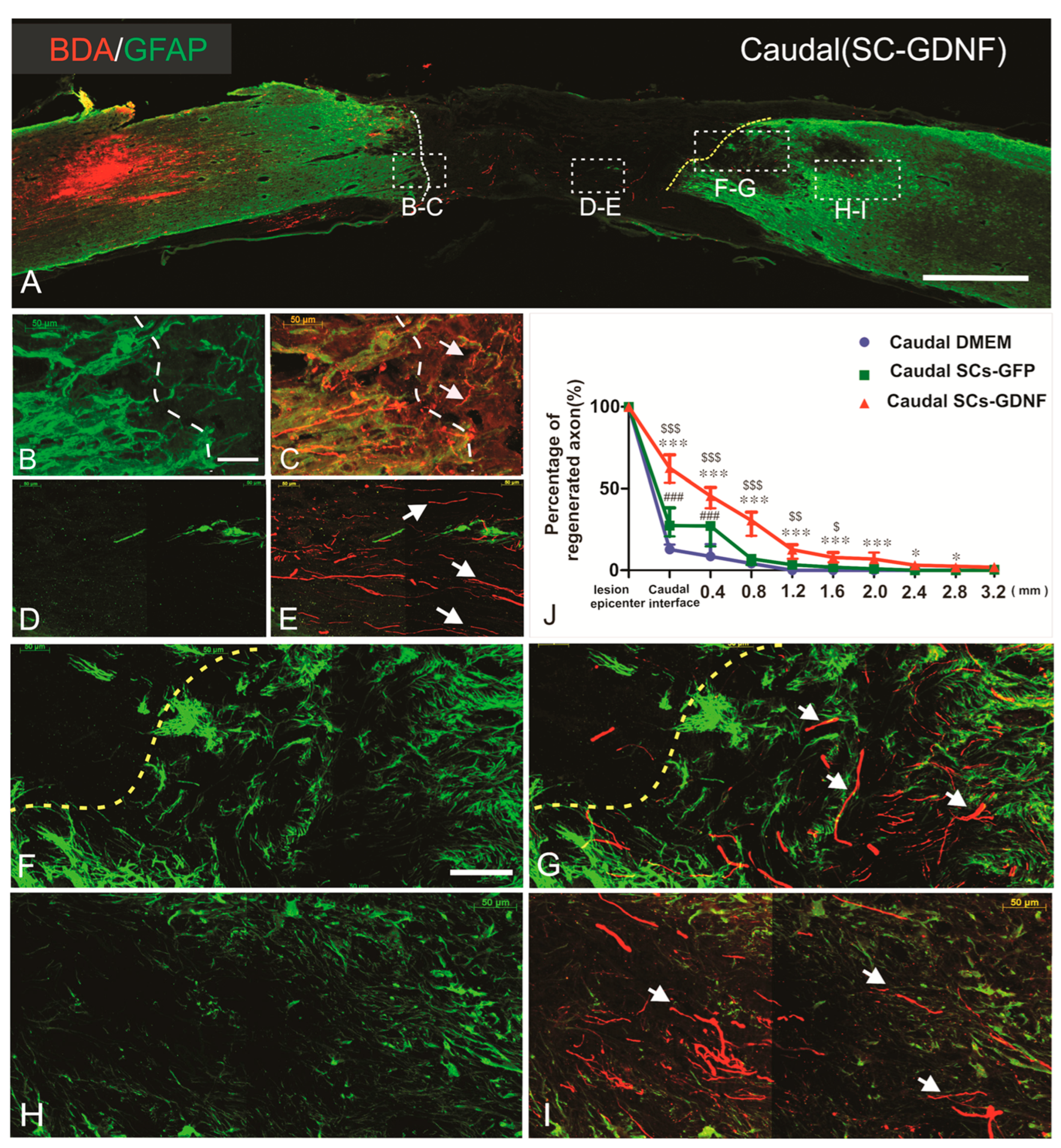
3.1. Regenerated Axons Formed Synapse on Caudal Host Spinal Cord
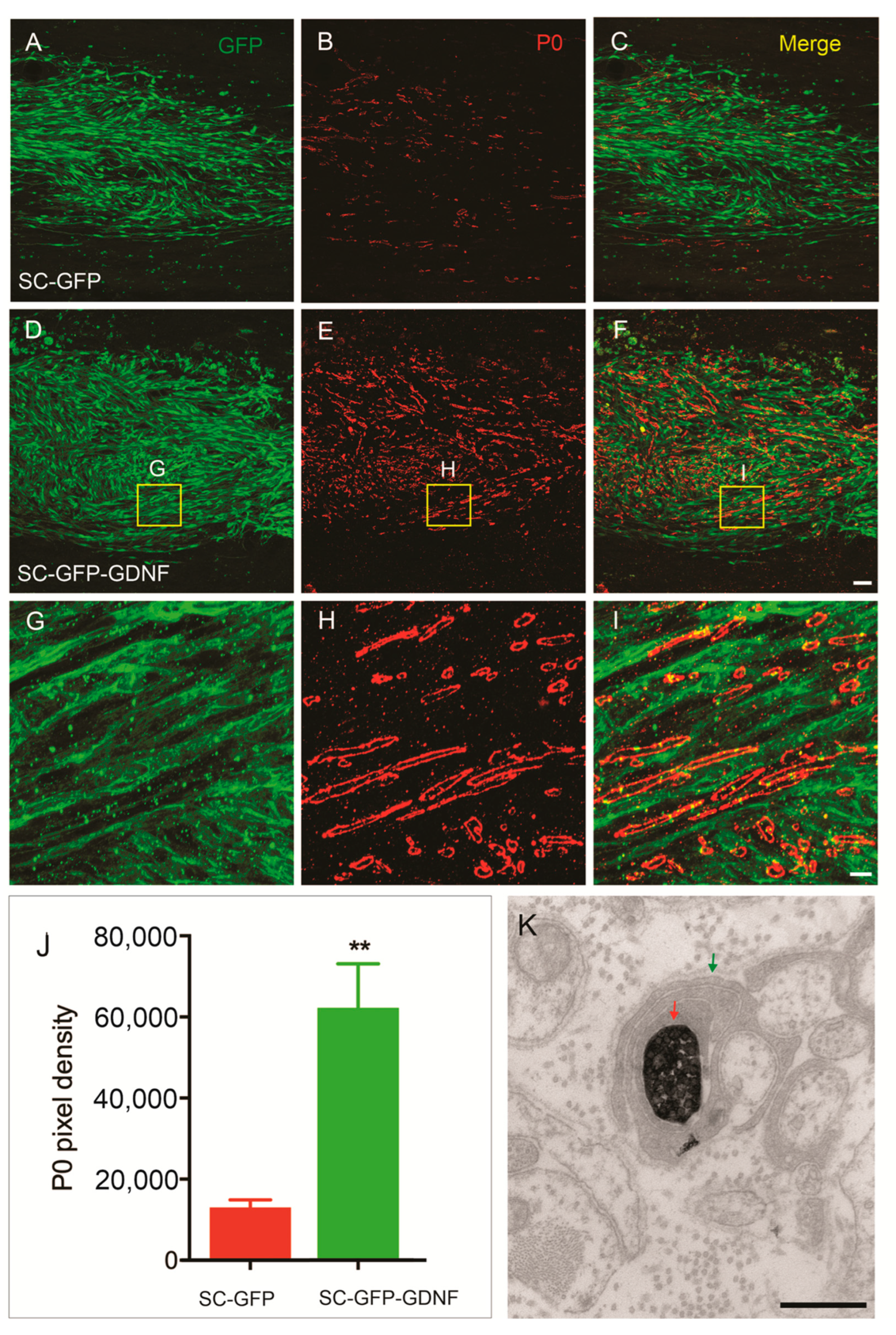
3.2. Remyelination Was Enhanced by SC Overexpressing GDNF
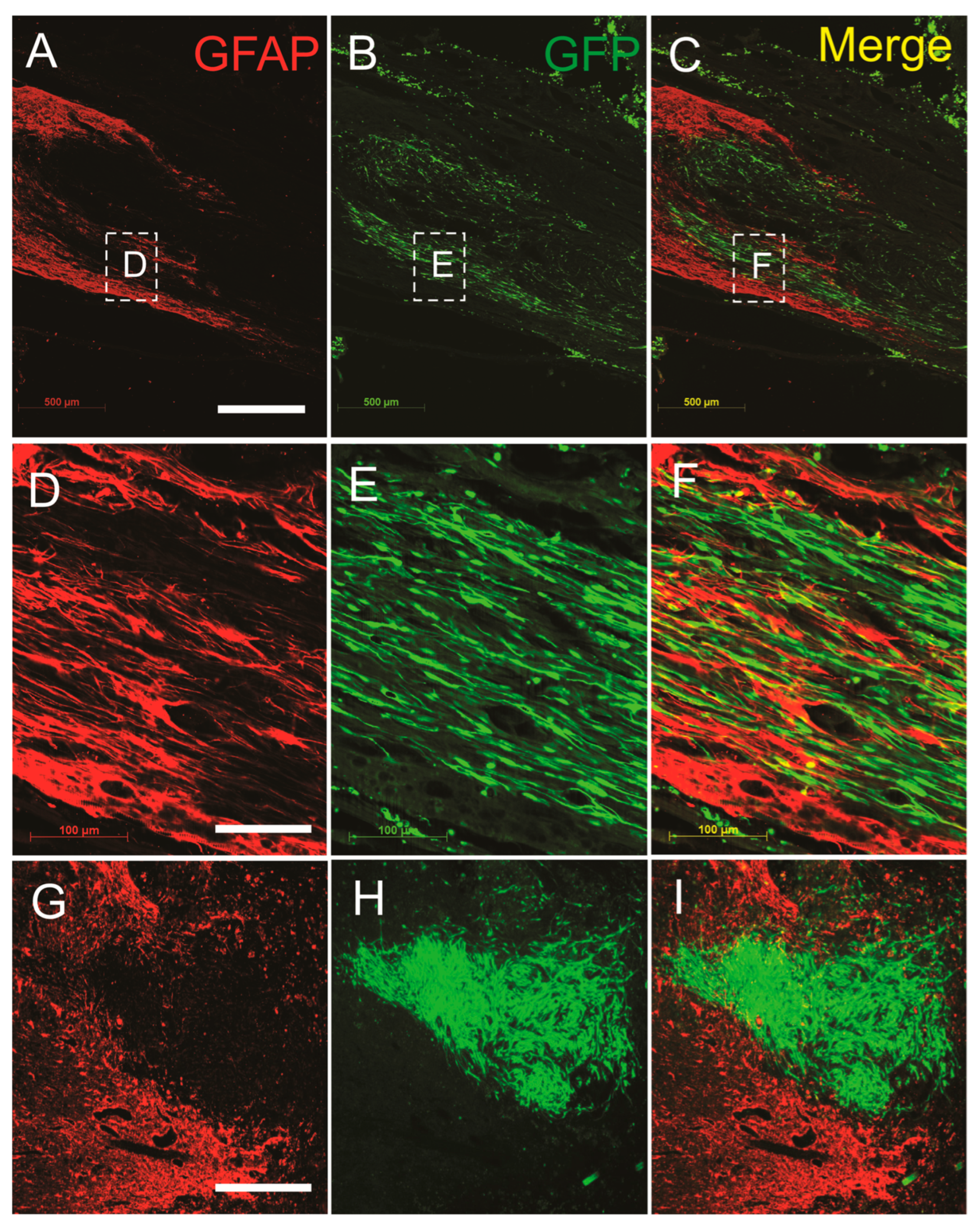
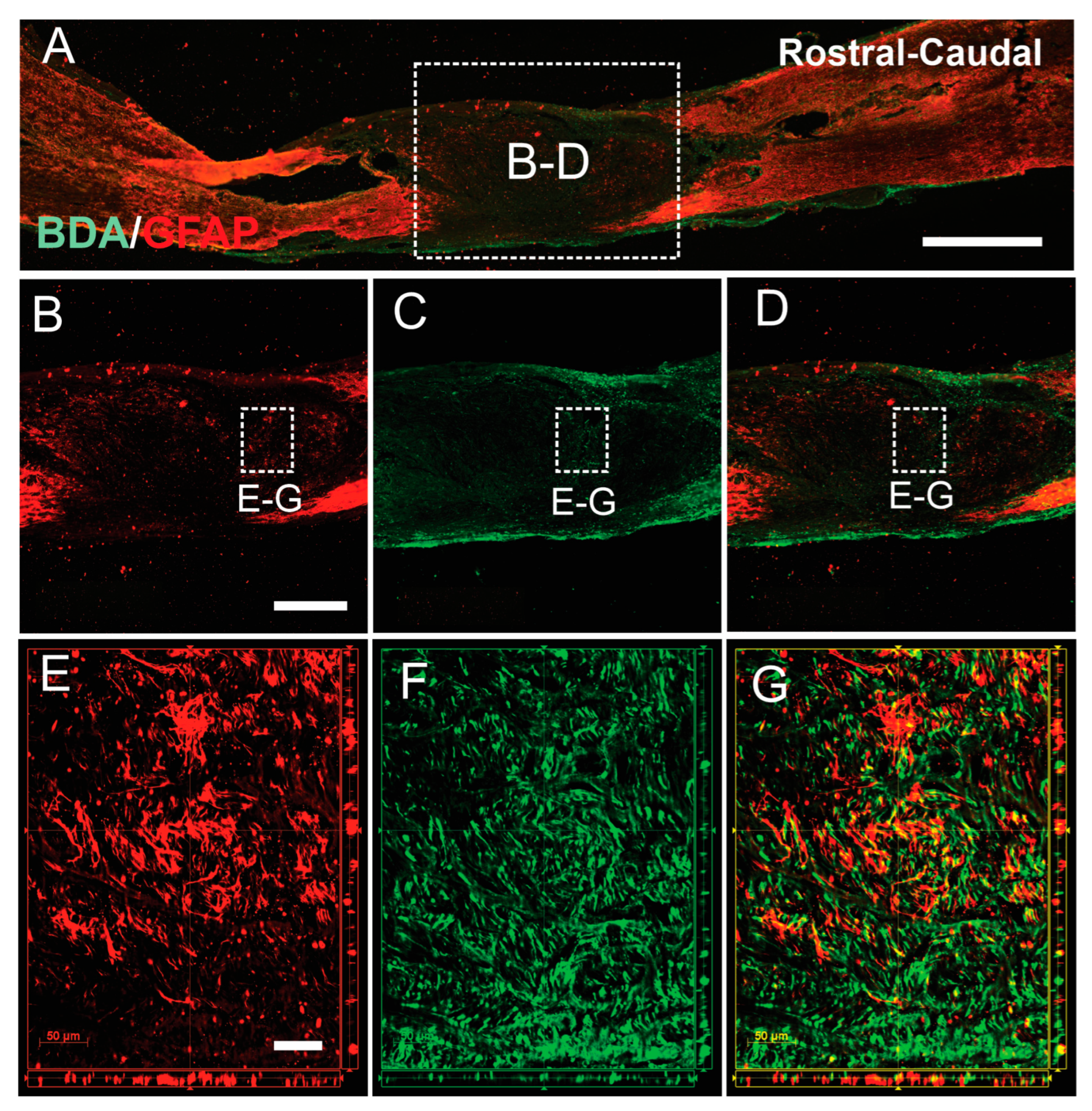
3.3. SCs-GDNF Remodeled Astrocytic Responses at the Caudal Graft–Host Interfaces
3.4. SCs-GDNF Promoted Migration of Host Astrocytes into the SCs-GDNF-Filled Lesion Gap Where They Are Closely Associated with Regenerated dPST Axons
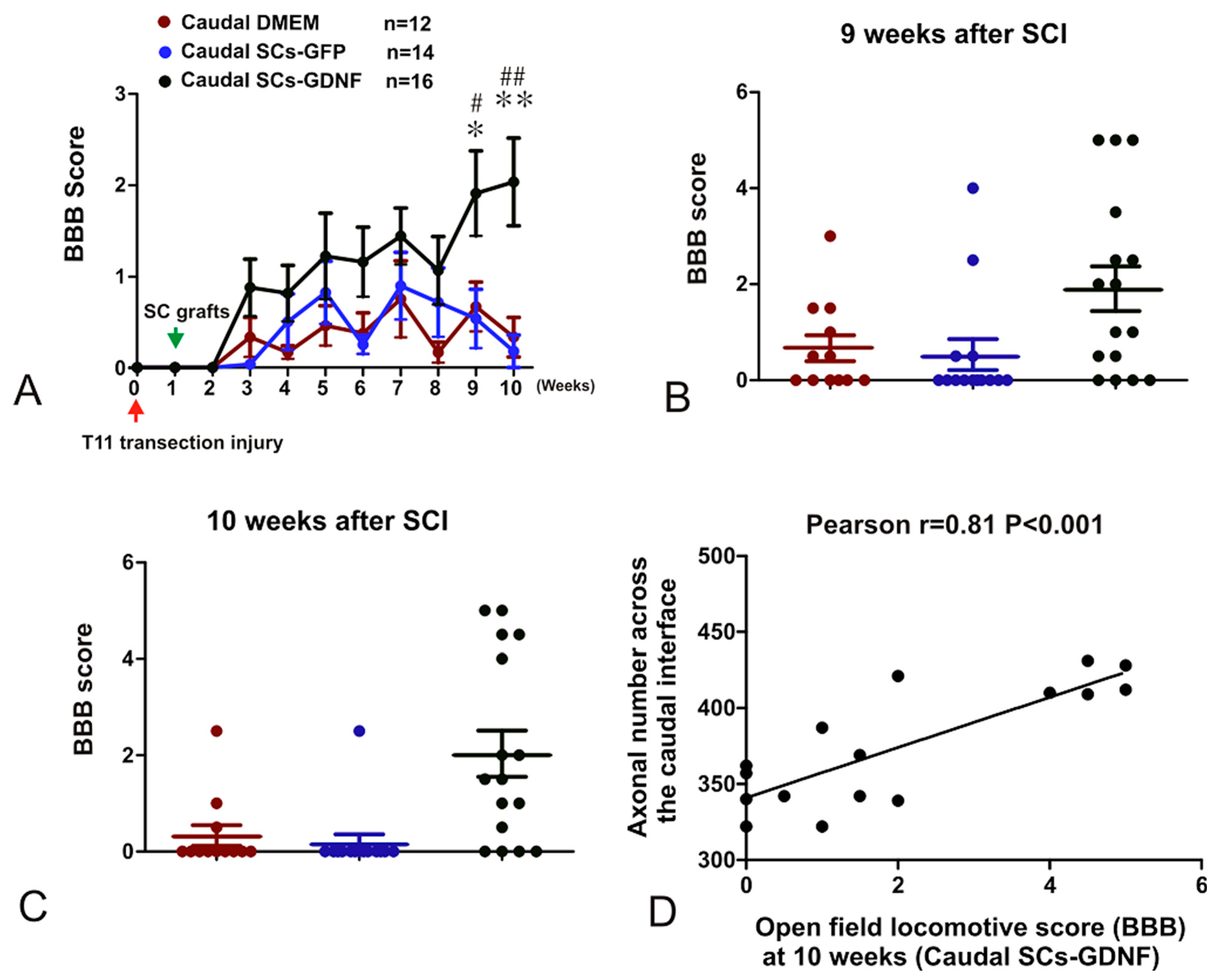
3.5. The Constructed SCs-GDNF Growth-Promoting Pathway Promoted Improved Hindlimb Locomotor Function
4. Discussion
4.1. GDNF Modulate the Interaction between Grafted SCs and Host Astrocytes
4.2. Astrocytes within GDNF Territory Display a Permissive Character for Axonal Regeneration
4.3. Limitation of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, M.A.; O’Shea, T.M.; Burda, J.E.; Ao, Y.; Barlatey, S.L.; Bernstein, A.M.; Kim, J.H.; James, N.D.; Rogers, A.; Kato, B.; et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 2018, 561, 396–400. [Google Scholar] [CrossRef]
- Sivasankaran, R.; Pei, J.; Wang, K.C.; Zhang, Y.P.; Shields, C.B.; Xu, X.M.; He, Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat. Neurosci. 2004, 7, 261–268. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Lu, C.C.; Kerman, R.; Steward, O.; Xu, X.M.; Zou, Y. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J. Neurosci. 2008, 28, 8376–8382. [Google Scholar] [CrossRef]
- Varadarajan, S.G.; Hunyara, J.L.; Hamilton, N.R.; Kolodkin, A.L.; Huberman, A.D. Central nervous system regeneration. Cell 2022, 185, 77–94. [Google Scholar] [CrossRef]
- Schaeffer, J.; Vilallongue, N.; Belin, S.; Nawabi, H. Axon guidance in regeneration of the mature central. Neural Regen. Res. 2023, 18, 2665–2666. [Google Scholar] [CrossRef]
- Yiu, G.; He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Liu, C.; Han, X.; Gu, X.; Zhou, S. Deciphering glial scar after spinal cord injury. Burn. Trauma. 2021, 9, tkab035. [Google Scholar] [CrossRef]
- Clifford, T.; Finkel, Z.; Rodriguez, B.; Joseph, A.; Cai, L. Current Advancements in Spinal Cord Injury Research-Glial Scar Formation and Neural Regeneration. Cells 2023, 12, 853. [Google Scholar] [CrossRef]
- Bunge, M.B. Novel combination strategies to repair the injured mammalian spinal cord. J. Spinal Cord. Med. 2008, 31, 262–269. [Google Scholar] [CrossRef]
- Fortun, J.; Hill, C.E.; Bunge, M.B. Combinatorial strategies with Schwann cell transplantation to improve repair of the injured spinal cord. Neurosci. Lett. 2009, 456, 124–132. [Google Scholar] [CrossRef]
- Olson, L. Combinatory treatments needed for spinal cord injury. Exp. Neurol. 2013, 248, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, S.; Yang, M. Recent progress and challenges in the treatment of spinal cord injury. Protein Cell 2023, 14, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Tuszynski, M.H. Regulation of axonal regeneration after mammalian spinal cord injury. Nat. Rev. Mol. Cell Biol. 2023, 24, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.M.; Bradke, F. Therapeutic repair for spinal cord injury: Combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020, 12, e11505. [Google Scholar] [CrossRef] [PubMed]
- Pearse, D.D.; Pereira, F.C.; Marcillo, A.E.; Bates, M.L.; Berrocal, Y.A.; Filbin, M.T.; Bunge, M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004, 10, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.X.; Deng, P.; Ruan, Y.; Xu, Z.C.; Liu, N.K.; Wen, X.; Smith, G.M.; Xu, X.M. A novel growth-promoting pathway formed by GDNF-overexpressing Schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury. J. Neurosci. 2013, 33, 5655–5667. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Blesch, A.; Graham, L.; Wang, Y.; Samara, R.; Banos, K.; Haringer, V.; Havton, L.; Weishaupt, N.; Bennett, D.; et al. Motor axonal regeneration after partial and complete spinal cord transection. J. Neurosci. 2012, 32, 8208–8218. [Google Scholar] [CrossRef] [PubMed]
- Kanno, H.; Pressman, Y.; Moody, A.; Berg, R.; Muir, E.M.; Rogers, J.H.; Ozawa, H.; Itoi, E.; Pearse, D.D.; Bunge, M.B. Combination of engineered Schwann cell grafts to secrete neurotrophin and chondroitinase promotes axonal regeneration and locomotion after spinal cord injury. J. Neurosci. 2014, 34, 1838–1855. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lin, C.Y.; Jiang, H.H.; Depaul, M.; Lin, V.W.; Silver, J. Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J. Neurosci. 2013, 33, 10591–10606. [Google Scholar] [CrossRef] [PubMed]
- Fouad, K.; Schnell, L.; Bunge, M.B.; Schwab, M.E.; Liebscher, T.; Pearse, D.D. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J. Neurosci. 2005, 25, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Guenard, V.; Kleitman, N.; Aebischer, P.; Bunge, M.B. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp. Neurol. 1995, 134, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.H.; Shum, D.K.; Li, H.; Pei, J.; Lui, Y.Y.; Wirthlin, L.; Chan, Y.S.; Xu, X.M. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J. 2004, 18, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Rodemer, W.; Hu, J.; Selzer, M.E.; Shifman, M.I. Heterogeneity in the regenerative abilities of central nervous system axons within species: Why do some neurons regenerate better than others? Neural Regen. Res. 2020, 15, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.; Janowitz, H.; Dougherty, S.E. Neuronal Redevelopment and the Regeneration of Neuromodulatory Axons in the Adult Mammalian Central Nervous System. Front. Cell Neurosci. 2022, 16, 872501. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.C.; He, Z.; Jacobi, A. Axon Regeneration: A Subcellular Extension in Multiple Dimensions. Cold Spring Harb. Perspect. Biol. 2022, 14, a040923. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Ribeiro, F.F.; Sebastiao, A.M.; Muir, E.M.; Vaz, S.H. Bridging the gap of axonal regeneration in the central nervous system: A state of the art review on central axonal regeneration. Front. Neurosci. 2022, 16, 1003145. [Google Scholar] [CrossRef]
- Xu, X.M.; Guenard, V.; Kleitman, N.; Bunge, M.B. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J. Comp. Neurol. 1995, 351, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Zhang, S.X.; Li, H.; Aebischer, P.; Bunge, M.B. Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur. J. Neurosci. 1999, 11, 1723–1740. [Google Scholar] [CrossRef]
- Xu, X.M.; Chen, A.; Guenard, V.; Kleitman, N.; Bunge, M.B. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J. Neurocytol. 1997, 26, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.R.; Henao, M.; Pearse, D.D.; Bunge, M.B. Permissive schwann cell graft/spinal cord interfaces for axon regeneration. Cell Transplant. 2015, 24, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.V.; Deng, L.; Xu, X.M. Human Schwann Cell Transplantation for Spinal Cord Injury: Prospects and Challenges in Translational Medicine. Front. Cell Neurosci. 2021, 15, 690894. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Hu, D.; Chen, J.; Wang, Q.; Zhang, Y.; Qi, C.; Yu, T. Repair of the Injured Spinal Cord by Schwann Cell Transplantation. Front. Neurosci. 2022, 16, 800513. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Pearse, D.D. Schwann Cell-Derived Exosomal Vesicles: A Promising Therapy for the Injured Spinal Cord. Int. J. Mol. Sci. 2023, 24, 17317. [Google Scholar] [CrossRef] [PubMed]
- Hopf, A.; Schaefer, D.J.; Kalbermatten, D.F.; Guzman, R.; Madduri, S. Schwann Cell-Like Cells: Origin and Usability for Repair and Regeneration of the Peripheral and Central Nervous System. Cells 2020, 9, 1990. [Google Scholar] [CrossRef] [PubMed]
- Plant, G.W.; Bates, M.L.; Bunge, M.B. Inhibitory proteoglycan immunoreactivity is higher at the caudal than the rostral Schwann cell graft-transected spinal cord interface. Mol. Cell. Neurosci. 2001, 17, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W. The Struggle to Make CNS Axons Regenerate: Why Has It Been so Difficult? Neurochem. Res. 2020, 45, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.L.; Oudega, M.; Bunge, M.B.; Tuszynski, M.H. Neurotrophic factors, cellular bridges and gene therapy for spinal cord injury. J. Physiol. 2001, 533, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Akram, R.; Anwar, H.; Javed, M.S.; Rasul, A.; Imran, A.; Malik, S.A.; Raza, C.; Khan, I.U.; Sajid, F.; Iman, T.; et al. Axonal Regeneration: Underlying Molecular Mechanisms and Potential Therapeutic Targets. Biomedicines 2022, 10, 3186. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, C.; Zhao, L.; Dai, X.; Yi, S. Unleashing Axonal Regeneration Capacities: Neuronal and Non-neuronal Changes After Injuries to Dorsal Root Ganglion Neuron Central and Peripheral Axonal Branches. Mol. Neurobiol. 2024, 61, 423–433. [Google Scholar] [CrossRef]
- Lu, P.; Yang, H.; Jones, L.L.; Filbin, M.T.; Tuszynski, M.H. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J. Neurosci. 2004, 24, 6402–6409. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Jones, L.; Tuszynski, M.H.; Blesch, A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J. Neurosci. 2006, 26, 9713–9721. [Google Scholar] [CrossRef] [PubMed]
- Tom, V.J.; Houle, J.D. Intraspinal microinjection of chondroitinase ABC following injury promotes axonal regeneration out of a peripheral nerve graft bridge. Exp. Neurol. 2008, 211, 315–319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blits, B.; Oudega, M.; Boer, G.J.; Bartlett Bunge, M.; Verhaagen, J. Adeno-associated viral vector-mediated neurotrophin gene transfer in the injured adult rat spinal cord improves hind-limb function. Neuroscience 2003, 118, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Kiyoshi, C.; Tedeschi, A. Axon growth and synaptic function: A balancing act for axonal regeneration and neuronal circuit formation in CNS trauma and disease. Dev. Neurobiol. 2020, 80, 277–301. [Google Scholar] [CrossRef]
- Garcia, A.L.; Udeh, A.; Kalahasty, K.; Hackam, A.S. A growing field: The regulation of axonal regeneration by Wnt signaling. Neural Regen. Res. 2018, 13, 43–52. [Google Scholar] [CrossRef]
- Deng, L.X.; Hu, J.; Liu, N.; Wang, X.; Smith, G.M.; Wen, X.; Xu, X.M. GDNF modifies reactive astrogliosis allowing robust axonal regeneration through Schwann cell-seeded guidance channels after spinal cord injury. Exp. Neurol. 2011, 229, 238–250. [Google Scholar] [CrossRef]
- Levi, A.D.; Dancausse, H.; Li, X.; Duncan, S.; Horkey, L.; Oliviera, M. Peripheral nerve grafts promoting central nervous system regeneration after spinal cord injury in the primate. J. Neurosurg. 2002, 96, 197–205. [Google Scholar] [CrossRef]
- Steward, O.; Sharp, K.; Selvan, G.; Hadden, A.; Hofstadter, M.; Au, E.; Roskams, J. A re-assessment of the consequences of delayed transplantation of olfactory lamina propria following complete spinal cord transection in rats. Exp. Neurol. 2006, 198, 483–499. [Google Scholar] [CrossRef]
- Kwiecien, J.M. Barriers to axonal regeneration after spinal cord injury: A current perspective. Neural Regen. Res. 2022, 17, 85–86. [Google Scholar] [CrossRef]
- Punjani, N.; Deska-Gauthier, D.; Hachem, L.D.; Abramian, M.; Fehlings, M.G. Neuroplasticity and regeneration after spinal cord injury. N. Am. Spine Soc. J. 2023, 15, 100235. [Google Scholar] [CrossRef]
- Hashimoto, S.; Nagoshi, N.; Nakamura, M.; Okano, H. Regenerative medicine strategies for chronic complete spinal cord injury. Neural Regen. Res. 2024, 19, 818–824. [Google Scholar] [CrossRef]
- Bareyre, F.M.; Kerschensteiner, M.; Raineteau, O.; Mettenleiter, T.C.; Weinmann, O.; Schwab, M.E. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 2004, 7, 269–277. [Google Scholar] [CrossRef]
- Courtine, G.; Song, B.; Roy, R.R.; Zhong, H.; Herrmann, J.E.; Ao, Y.; Qi, J.; Edgerton, V.R.; Sofroniew, M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008, 14, 69–74. [Google Scholar] [CrossRef]
- Hutson, T.H.; Di Giovanni, S. The translational landscape in spinal cord injury: Focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019, 15, 732–745. [Google Scholar] [CrossRef]
- Martin, J.H. Neuroplasticity of spinal cord injury and repair. Handb. Clin. Neurol. 2022, 184, 317–330. [Google Scholar] [CrossRef]
- Tuszynski, M.H.; Steward, O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron 2012, 74, 777–791. [Google Scholar] [CrossRef]
- Fawcett, J.W.; Curt, A.; Steeves, J.D.; Coleman, W.P.; Tuszynski, M.H.; Lammertse, D.; Bartlett, P.F.; Blight, A.R.; Dietz, V.; Ditunno, J.; et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007, 45, 190–205. [Google Scholar] [CrossRef]
- Wang, T.Y.; Park, C.; Zhang, H.; Rahimpour, S.; Murphy, K.R.; Goodwin, C.R.; Karikari, I.O.; Than, K.D.; Shaffrey, C.I.; Foster, N.; et al. Management of Acute Traumatic Spinal Cord Injury: A Review of the Literature. Front. Surg. 2021, 8, 698736. [Google Scholar] [CrossRef]
- Abdellatif, A.A.; Pelt, J.L.; Benton, R.L.; Howard, R.M.; Tsoulfas, P.; Ping, P.; Xu, X.M.; Whittemore, S.R. Gene delivery to the spinal cord: Comparison between lentiviral, adenoviral, and retroviral vector delivery systems. J. Neurosci. Res. 2006, 84, 553–567. [Google Scholar] [CrossRef]
- Hou, S.; Tom, V.J.; Graham, L.; Lu, P.; Blesch, A. Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection. J. Neurosci. 2013, 33, 17138–17149. [Google Scholar] [CrossRef]
- Cote, M.P.; Amin, A.A.; Tom, V.J.; Houle, J.D. Peripheral nerve grafts support regeneration after spinal cord injury. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2011, 8, 294–303. [Google Scholar] [CrossRef]
- Bamber, N.I.; Li, H.; Lu, X.; Oudega, M.; Aebischer, P.; Xu, X.M. Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur. J. Neurosci. 2001, 13, 257–268. [Google Scholar]
- Cai, D.; Shen, Y.; De Bellard, M.; Tang, S.; Filbin, M.T. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron 1999, 22, 89–101. [Google Scholar] [CrossRef]
- Chan, J.R.; Cosgaya, J.M.; Wu, Y.J.; Shooter, E.M. Neurotrophins are key mediators of the myelination program in the peripheral nervous system. Proc. Natl. Acad. Sci. USA 2001, 98, 14661–14668. [Google Scholar] [CrossRef]
- Sharma, H.S. A select combination of neurotrophins enhances neuroprotection and functional recovery following spinal cord injury. Ann. N. Y. Acad. Sci. 2007, 1122, 95–111. [Google Scholar] [CrossRef]
- Kalb, R. The protean actions of neurotrophins and their receptors on the life and death of neurons. Trends Neurosci. 2005, 28, 5–11. [Google Scholar] [CrossRef]
- Iannotti, C.; Li, H.; Yan, P.; Lu, X.; Wirthlin, L.; Xu, X.M. Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury. Exp. Neurol. 2003, 183, 379–393. [Google Scholar] [CrossRef]
- Fraher, J.P. Axon-glial relationships in early CNS-PNS transitional zone development: An ultrastructural study. J. Neurocytol. 1997, 26, 41–52. [Google Scholar] [CrossRef]
- Golding, J.; Shewan, D.; Cohen, J. Maturation of the mammalian dorsal root entry zone—From entry to no entry. Trends Neurosci. 1997, 20, 303–308. [Google Scholar] [CrossRef]
- Baron-Van Evercooren, A.; Clerin-Duhamel, E.; Lapie, P.; Gansmuller, A.; Lachapelle, F.; Gumpel, M. The fate of Schwann cells transplanted in the brain during development. Dev. Neurosci. 1992, 14, 73–84. [Google Scholar] [CrossRef]
- Sims, T.J.; Durgun, M.B.; Gilmore, S.A. Transplantation of sciatic nerve segments into normal and glia-depleted spinal cords. Exp. Brain Res. 1999, 125, 495–501. [Google Scholar] [CrossRef]
- Santos-Silva, A.; Fairless, R.; Frame, M.C.; Montague, P.; Smith, G.M.; Toft, A.; Riddell, J.S.; Barnett, S.C. FGF/heparin differentially regulates Schwann cell and olfactory ensheathing cell interactions with astrocytes: A role in astrocytosis. J. Neurosci. 2007, 27, 7154–7167. [Google Scholar] [CrossRef]
- Fitch, M.T.; Silver, J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 2008, 209, 294–301. [Google Scholar] [CrossRef] [PubMed]
- White, R.E.; Rao, M.; Gensel, J.C.; McTigue, D.M.; Kaspar, B.K.; Jakeman, L.B. Transforming growth factor alpha transforms astrocytes to a growth-supportive phenotype after spinal cord injury. J. Neurosci. 2011, 31, 15173–15187. [Google Scholar] [CrossRef]
- White, R.E.; Yin, F.Q.; Jakeman, L.B. TGF-alpha increases astrocyte invasion and promotes axonal growth into the lesion following spinal cord injury in mice. Exp. Neurol. 2008, 214, 10–24. [Google Scholar] [CrossRef]
- Tom, V.J.; Doller, C.M.; Malouf, A.T.; Silver, J. Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J. Neurosci. 2004, 24, 9282–9290. [Google Scholar] [CrossRef]
- Vaccarino, F.M.; Fagel, D.M.; Ganat, Y.; Maragnoli, M.E.; Ment, L.R.; Ohkubo, Y.; Schwartz, M.L.; Silbereis, J.; Smith, K.M. Astroglial cells in development, regeneration, and repair. Neuroscientist 2007, 13, 173–185. [Google Scholar] [CrossRef]
- Clarke, S.R.; Shetty, A.K.; Bradley, J.L.; Turner, D.A. Reactive astrocytes express the embryonic intermediate neurofilament nestin. Neuroreport 1994, 5, 1885–1888. [Google Scholar] [CrossRef]
- Menet, V.; Gimenez y Ribotta, M.; Chauvet, N.; Drian, M.J.; Lannoy, J.; Colucci-Guyon, E.; Privat, A. Inactivation of the glial fibrillary acidic protein gene, but not that of vimentin, improves neuronal survival and neurite growth by modifying adhesion molecule expression. J. Neurosci. 2001, 21, 6147–6158. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Zhang, S.; Khabbaz, A.; Cohen, K.L.; Zhang, Y.; Chakraborty, S.; Smith, G.M.; Wang, H.; Yadav, A.P.; Liu, N.; et al. Regeneration of Propriospinal Axons in Rat Transected Spinal Cord Injury through a Growth-Promoting Pathway Constructed by Schwann Cells Overexpressing GDNF. Cells 2024, 13, 1160. https://doi.org/10.3390/cells13131160
Du X, Zhang S, Khabbaz A, Cohen KL, Zhang Y, Chakraborty S, Smith GM, Wang H, Yadav AP, Liu N, et al. Regeneration of Propriospinal Axons in Rat Transected Spinal Cord Injury through a Growth-Promoting Pathway Constructed by Schwann Cells Overexpressing GDNF. Cells. 2024; 13(13):1160. https://doi.org/10.3390/cells13131160
Chicago/Turabian StyleDu, Xiaolong, Shengqi Zhang, Aytak Khabbaz, Kristen Lynn Cohen, Yihong Zhang, Samhita Chakraborty, George M. Smith, Hongxing Wang, Amol P. Yadav, Naikui Liu, and et al. 2024. "Regeneration of Propriospinal Axons in Rat Transected Spinal Cord Injury through a Growth-Promoting Pathway Constructed by Schwann Cells Overexpressing GDNF" Cells 13, no. 13: 1160. https://doi.org/10.3390/cells13131160
APA StyleDu, X., Zhang, S., Khabbaz, A., Cohen, K. L., Zhang, Y., Chakraborty, S., Smith, G. M., Wang, H., Yadav, A. P., Liu, N., & Deng, L. (2024). Regeneration of Propriospinal Axons in Rat Transected Spinal Cord Injury through a Growth-Promoting Pathway Constructed by Schwann Cells Overexpressing GDNF. Cells, 13(13), 1160. https://doi.org/10.3390/cells13131160





