Daily Light Onset and Plasma Membrane Tethers Regulate Mitochondria Redistribution within the Retinal Pigment Epithelium
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Tissue
2.2. Mouse Tissue
2.3. Transmission Electron Microscopy
2.4. Electron Tomography
3. Results
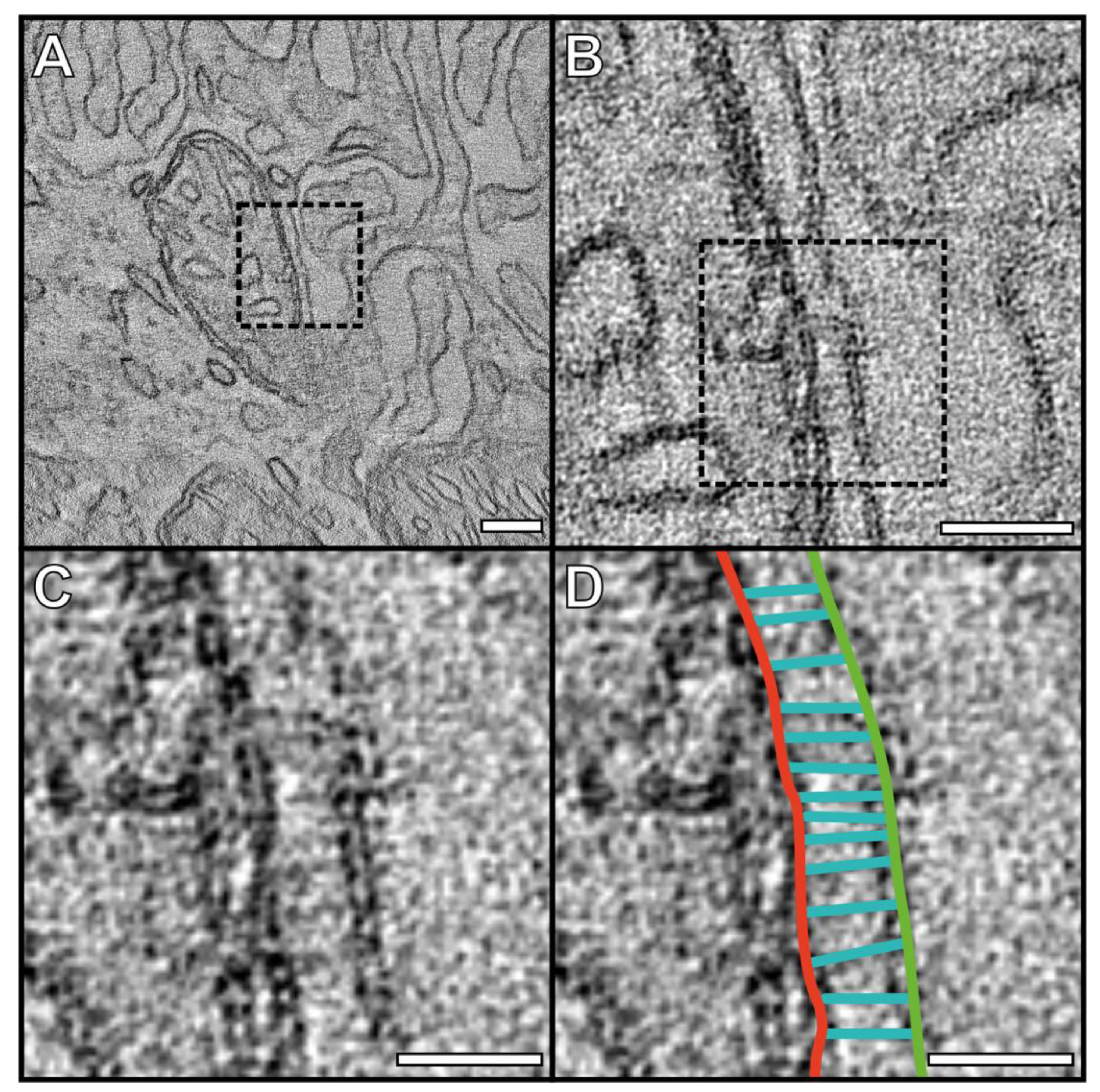
3.1. Mitochondria Are Tethered to the Basal and Lateral Plasma Membrane of the RPE
3.2. After Light Onset, Mitochondria Redistribute from Basal to Apical RPE
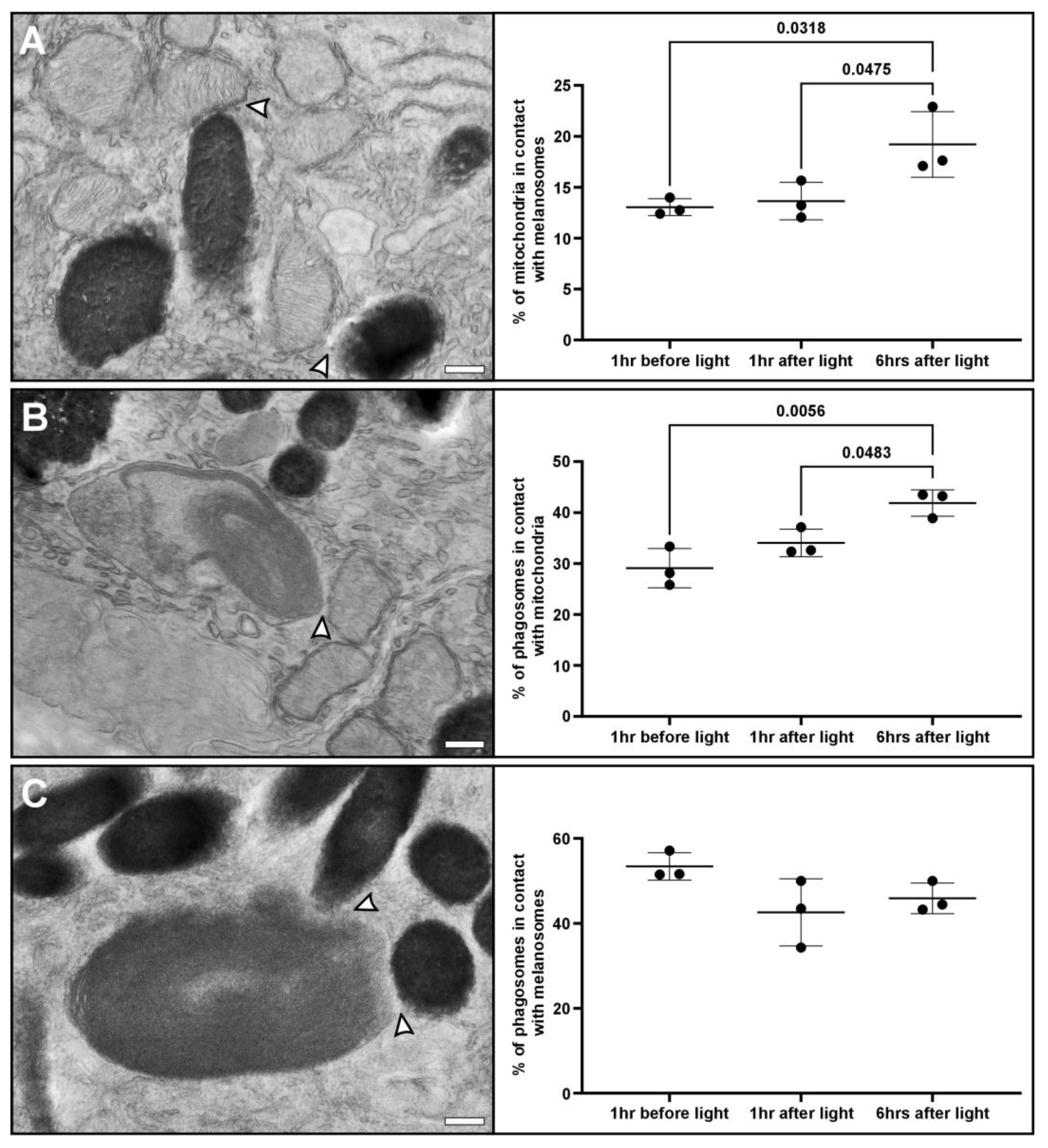
3.3. Redistribution of Mitochondria to the Apical Region Is Accompanied by Increased Interaction with Melanosomes and Phagosomes
3.4. Mitochondrial Positioning, but Not the Tethering of Mitochondria to the Plasma Membrane, Is Affected in the RPE of Choroideremia Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berkowitz, B.A. Preventing Diabetic Retinopathy by Mitigating Subretinal Space Oxidative Stress In Vivo. Vis. Neurosci. 2020, 37, E002. [Google Scholar] [CrossRef]
- Fisher, C.R.; Ferrington, D.A. Perspective on AMD Pathobiology: A Bioenergetic Crisis in the RPE. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD41–AMD47. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.R.; Shaaeli, A.A.; Ebeling, M.C.; Montezuma, S.R.; Ferrington, D.A. Investigating Mitochondrial Fission, Fusion, and Autophagy in Retinal Pigment Epithelium from Donors with Age-Related Macular Degeneration. Sci. Rep. 2022, 12, 21725. [Google Scholar] [CrossRef] [PubMed]
- Ferrington, D.A.; Kapphahn, R.J.; Leary, M.M.; Atilano, S.R.; Terluk, M.R.; Karunadharma, P.; Chen, G.K.J.; Ratnapriya, R.; Swaroop, A.; Montezuma, S.R.; et al. Increased Retinal MtDNA Damage in the CFH Variant Associated with Age-Related Macular Degeneration. Exp. Eye Res. 2016, 145, 269–277. [Google Scholar] [CrossRef]
- Karunadharma, P.P.; Nordgaard, C.L.; Olsen, T.W.; Ferrington, D.A. Mitochondrial DNA Damage as a Potential Mechanism for Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5470–5479. [Google Scholar] [CrossRef]
- Zhao, C.; Yasumura, D.; Li, X.; Matthes, M.; Lloyd, M.; Nielsen, G.; Ahern, K.; Snyder, M.; Bok, D.; Dunaief, J.L.; et al. MTOR-Mediated Dedifferentiation of the Retinal Pigment Epithelium Initiates Photoreceptor Degeneration in Mice. J. Clin. Investig. 2011, 121, 369–383. [Google Scholar] [CrossRef]
- Osman, C.; Voelker, D.R.; Langer, T. Making Heads or Tails of Phospholipids in Mitochondria. J. Cell Biol. 2011, 192, 7–16. [Google Scholar] [CrossRef]
- Rizzuto, R.; De Stefani, D.; Raffaello, A.; Mammucari, C. Mitochondria as Sensors and Regulators of Calcium Signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Bratic, A.; Larsson, N.G. The Role of Mitochondria in Aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef]
- Devin, A.; Rigoulet, M. Mechanisms of Mitochondrial Response to Variations in Energy Demand in Eukaryotic Cells. Am. J. Physiol. Cell Physiol. 2007, 292, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Sassano, M.L.; Felipe-Abrio, B.; Agostinis, P. ER-Mitochondria Contact Sites; A Multifaceted Factory for Ca2+ Signaling and Lipid Transport. Front. Cell Dev. Biol. 2022, 10, 988014. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.J.; Burgoyne, T.; Wavre-Shapton, S.T.; Tolmachova, T.; Seabra, M.C.; Futter, C.E. Remodeling of the Basal Labyrinth of Retinal Pigment Epithelial Cells with Osmotic Challenge, Age, and Disease. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.X.; Li, J.; Germer, C.J.; Lakkaraju, A. Analysis of Mitochondrial Dynamics and Function in the Retinal Pigment Epithelium by High-Speed High-Resolution Live Imaging. Front. Cell Dev. Biol. 2022, 10, 1044672. [Google Scholar] [CrossRef] [PubMed]
- Pollreisz, A.; Neschi, M.; Sloan, K.R.; Pircher, M.; Mittermueller, T.; Dacey, D.M.; Erfurth, U.S.; Curcio, C.A. Atlas of Human Retinal Pigment Epithelium Organelles Significant for Clinical Imaging. Investig. Ophthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Swarup, A.; Samuels, I.S.; Bell, B.A.; Han, J.Y.S.; Du, J.; Massenzio, E.; Abel, E.D.; Boesze-Battaglia, K.; Peachey, N.S.; Philp, N.J. Modulating GLUT1 Expression in Retinal Pigment Epithelium Decreases Glucose Levels in the Retina: Impact on Photoreceptors and Müller Glial Cells. Am. J. Physiol. Cell Physiol. 2019, 316, C121–C133. [Google Scholar] [CrossRef]
- Reichhart, N.; Strauß, O. Ion Channels and Transporters of the Retinal Pigment Epithelium. Exp. Eye Res. 2014, 126, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Seabra, M.C.; Mules, E.H.; Hume, A.N. Rab GTPases, Intracellular Traffic and Disease. Trends Mol. Med. 2002, 8, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Wavre-Shapton, S.T.; Tolmachova, T.; da Silva, M.L.; Futter, C.E.; Seabra, M.C. Conditional Ablation of the Choroideremia Gene Causes Age-Related Changes in Mouse Retinal Pigment Epithelium. PLoS ONE 2013, 8, e57769. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as Coordinators of Vesicle Traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Tolmachova, T.; Anders, R.; Abrink, M.; Bugeon, L.; Dallman, M.J.; Futter, C.E.; Ramalho, J.S.; Tonagel, F.; Tanimoto, N.; Seeliger, M.W.; et al. Independent Degeneration of Photoreceptors and Retinal Pigment Epithelium in Conditional Knockout Mouse Models of Choroideremia. J. Clin. Investig. 2006, 116, 386–394. [Google Scholar] [CrossRef]
- Grace, M.S.; Chiba, A.; Menaker, M. Circadian Control of Photoreceptor Outer Segment Membrane Turnover in Mice Genetically Incapable of Melatonin Synthesis. Vis. Neurosci. 1999, 16, 909–918. [Google Scholar] [CrossRef] [PubMed]
- LaVail, M.M. Rod Outer Segment Disk Shedding in Rat Retina: Relationship to Cyclic Lighting. Science 1976, 194, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- McBee, J.K.; Palczewski, K.; Baehr, W.; Pepperberg, D.R. Confronting Complexity: The Interlink of Phototransduction and Retinoid Metabolism in the Vertebrate Retina. Prog. Retin. Eye Res. 2001, 20, 469–529. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, L.; De Matteis, M.A.; Emr, S.; Giordano, F.; Hajnóczky, G.; Kornmann, B.; Lackner, L.L.; Levine, T.P.; Pellegrini, L.; Reinisch, K.; et al. Coming Together to Define Membrane Contact Sites. Nat. Commun. 2019, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Tolmachova, T.; Wavre-Shapton, S.T.; Barnard, A.R.; MacLaren, R.E.; Futter, C.E.; Seabra, M.C. Retinal Pigment Epithelium Defects Accelerate Photoreceptor Degeneration in Cell Type–Specific Knockout Mouse Models of Choroideremia. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4913–4920. [Google Scholar] [CrossRef] [PubMed]
- Meschede, I.P.; Ovenden, N.C.; Seabra, M.C.; Futter, C.E.; Votruba, M.; Cheetham, M.E.; Burgoyne, T. Symmetric Arrangement of Mitochondria: Plasma Membrane Contacts between Adjacent Photoreceptor Cells Regulated by Opa1. Proc. Natl. Acad. Sci. USA 2020, 117, 15684–15693. [Google Scholar] [CrossRef]
- Kremer, J.R.; Mastronarde, D.N.; McIntosh, J.R. Computer Visualization of Three-Dimensional Image Data Using IMOD. J. Struct. Biol. 1996, 116, 71–76. [Google Scholar] [CrossRef]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Bok, D. The Retinal Pigment Epithelium: A Versatile Partner in Vision. J. Cell Sci. Suppl. 1993, 17, 189–195. [Google Scholar] [CrossRef]
- Steinberg, R.H. Interactions between the Retinal Pigment Epithelium and the Neural Retina. Doc. Ophthalmol. 1985, 60, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Korkka, I.; Skottman, H.; Nymark, S. Heterogeneity of Potassium Channels in Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium. Stem Cells Transl. Med. 2022, 11, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kittredge, A.; Ward, N.; Ji, C.; Chen, S.; Yang, T. ATP Activates Bestrophin Ion Channels through Direct Interaction. Nat. Commun. 2018, 9, 3126. [Google Scholar] [CrossRef] [PubMed]
- Bantseev, V.; Youn, H.Y. Mitochondrial “Movement” and Lens Optics Following Oxidative Stress from UV-B Irradiation. Ann. N. Y. Acad. Sci. 2006, 1091, 17–33. [Google Scholar] [CrossRef]
- Youn, H.Y.; Cullen, A.P.; Chou, B.R.; Sivak, J.G. Phototoxicity of Ultraviolet (UV) Radiation: Evaluation of UV-Blocking Efficiency of Intraocular Lens (IOL) Materials Using Retinal Cell Culture and In Vitro Bioassays. Open Toxicol. J. 2010, 4, 13–20. [Google Scholar] [CrossRef]
- Lucas, M.S.; Günthert, M.; Bittermann, A.G.; de Marco, A.; Wepf, R. Correlation of Live-Cell Imaging with Volume Scanning Electron Microscopy. Methods Cell Biol. 2017, 140, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Fermie, J.; Liv, N.; ten Brink, C.; van Donselaar, E.G.; Müller, W.H.; Schieber, N.L.; Schwab, Y.; Gerritsen, H.C.; Klumperman, J. Single Organelle Dynamics Linked to 3D Structure by Correlative Live-Cell Imaging and 3D Electron Microscopy. Traffic 2018, 19, 354–369. [Google Scholar] [CrossRef]
- Daniele, T.; Hurbain, I.; Vago, R.; Casari, G.; Raposo, G.; Tacchetti, C.; Schiaffino, M.V. Mitochondria and Melanosomes Establish Physical Contacts Modulated by Mfn2 and Involved in Organelle Biogenesis. Curr. Biol. 2014, 24, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Handa, J.T. How Does the Macula Protect Itself from Oxidative Stress? Mol. Asp. Med. 2012, 33, 418–435. [Google Scholar] [CrossRef]
- Blasiak, J.; Glowacki, S.; Kauppinen, A.; Kaarniranta, K. Mitochondrial and Nuclear DNA Damage and Repair in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2013, 14, 2996–3010. [Google Scholar] [CrossRef]
- Reyes-Reveles, J.; Dhingra, A.; Alexander, D.; Bragin, A.; Philp, N.J.; Boesze-Battaglia, K. Phagocytosis-Dependent Ketogenesis in Retinal Pigment Epithelium. J. Biol. Chem. 2017, 292, 8038–8047. [Google Scholar] [CrossRef] [PubMed]
- Wavre-Shapton, S.T.; Meschede, I.P.; Seabra, M.C.; Futter, C.E. Phagosome Maturation during Endosome Interaction Revealed by Partial Rhodopsin Processing in Retinal Pigment Epithelium. J. Cell Sci. 2014, 127, 3852–3861. [Google Scholar] [CrossRef]
- Lyu, Y.; Tschulakow, A.V.; Wang, K.; Brash, D.E.; Schraermeyer, U. Chemiexcitation and Melanin in Photoreceptor Disc Turnover and Prevention of Macular Degeneration. Proc. Natl. Acad. Sci. USA 2023, 120, e2216935120. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.; Gilady, S.Y.; B Fitzsimmons, R.E.; Benson, M.D.; Lynes, E.M.; Gesson, K.; Alto, N.M.; Strack, S.; Scott, J.D.; Simmen, T. Rab32 Modulates Apoptosis Onset and Mitochondria-Associated Membrane (MAM) Properties. J. Biol. Chem. 2010, 285, 31590–31602. [Google Scholar] [CrossRef]
- Alto, N.M.; Soderling, J.; Scott, J.D. Rab32 Is an A-Kinase Anchoring Protein and Participates in Mitochondrial Dynamics. J. Cell Biol. 2002, 158, 659–668. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neto, M.V.; De Rossi, G.; Berkowitz, B.A.; Seabra, M.C.; Luthert, P.J.; Futter, C.E.; Burgoyne, T. Daily Light Onset and Plasma Membrane Tethers Regulate Mitochondria Redistribution within the Retinal Pigment Epithelium. Cells 2024, 13, 1100. https://doi.org/10.3390/cells13131100
Neto MV, De Rossi G, Berkowitz BA, Seabra MC, Luthert PJ, Futter CE, Burgoyne T. Daily Light Onset and Plasma Membrane Tethers Regulate Mitochondria Redistribution within the Retinal Pigment Epithelium. Cells. 2024; 13(13):1100. https://doi.org/10.3390/cells13131100
Chicago/Turabian StyleNeto, Matilde V., Giulia De Rossi, Bruce A. Berkowitz, Miguel C. Seabra, Philip J. Luthert, Clare E. Futter, and Thomas Burgoyne. 2024. "Daily Light Onset and Plasma Membrane Tethers Regulate Mitochondria Redistribution within the Retinal Pigment Epithelium" Cells 13, no. 13: 1100. https://doi.org/10.3390/cells13131100
APA StyleNeto, M. V., De Rossi, G., Berkowitz, B. A., Seabra, M. C., Luthert, P. J., Futter, C. E., & Burgoyne, T. (2024). Daily Light Onset and Plasma Membrane Tethers Regulate Mitochondria Redistribution within the Retinal Pigment Epithelium. Cells, 13(13), 1100. https://doi.org/10.3390/cells13131100





