A Camelid-Derived STAT-Specific Nanobody Inhibits Neuroinflammation and Ameliorates Experimental Autoimmune Encephalomyelitis (EAE)
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Reagents
2.2. Experimental Autoimmune Encephalitis (EAE)
2.3. Adoptive Transfer
2.4. Preparation of Single Cell Suspension of CNS Tissues, Draining Lymph Nodes and Spleen
2.5. Intracellular Cytokine Staining and Flow Cytometry Analysis
2.6. Immunohistochemistry (IHC)
2.7. Cells and Cell Culture
2.8. Cell Proliferation Assays
2.9. Molecular Modeling to Identify Binding Interactions between SBT-100 and STATs
2.10. Statistics
3. Results
3.1. Characterization of the Miniature SBT-100 Nanobody
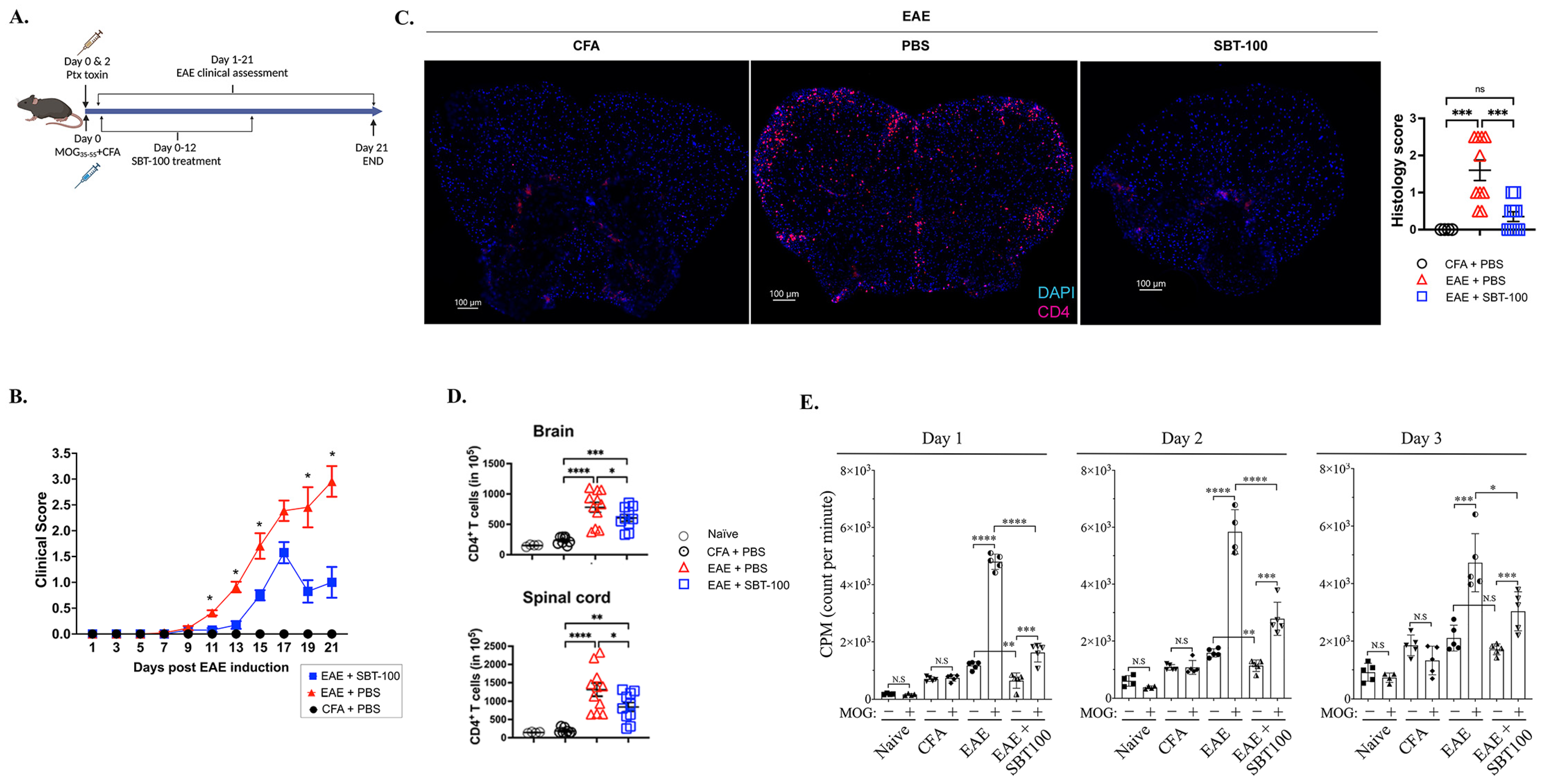
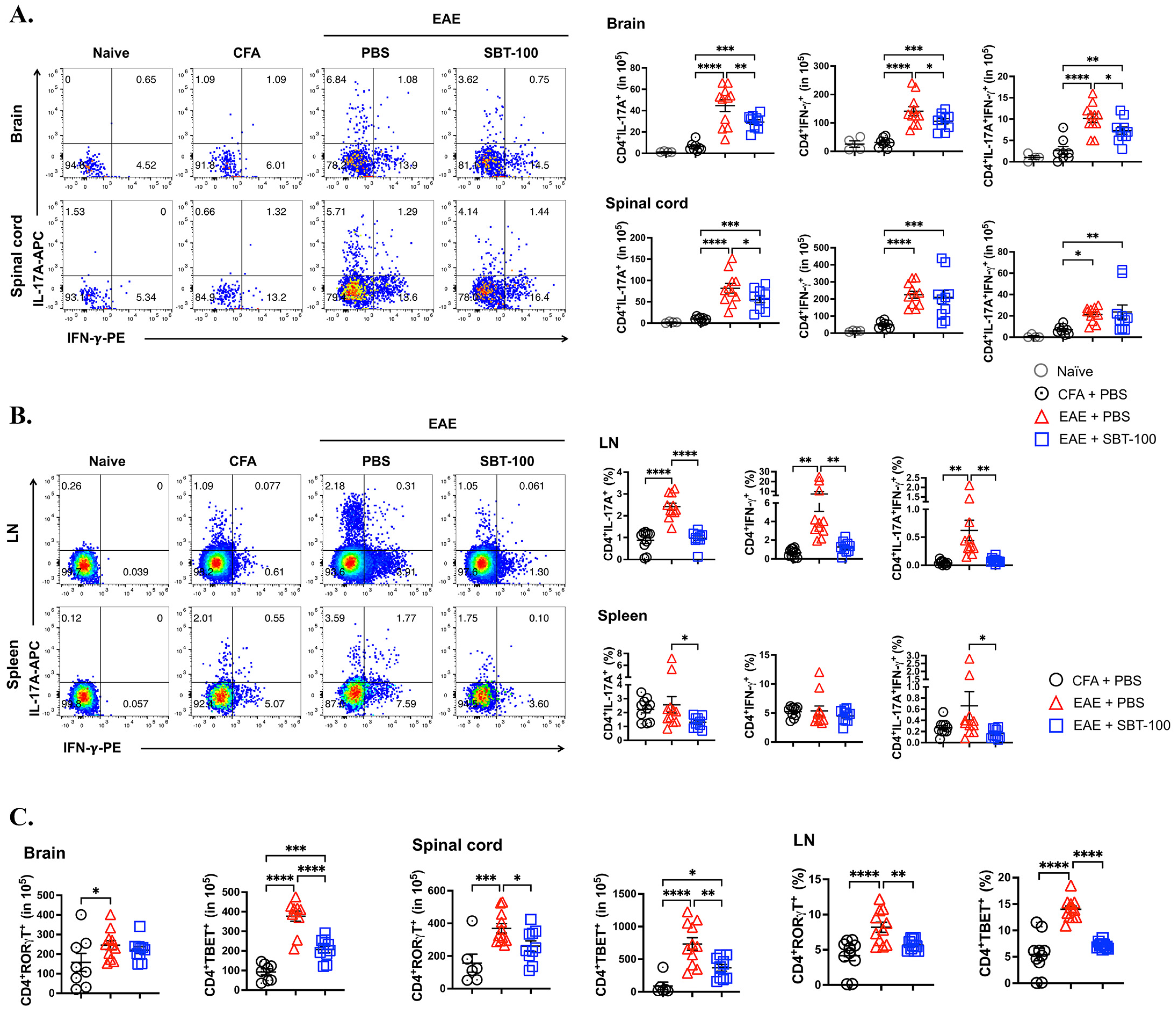
3.2. SBT-100 Ameliorates EAE by Suppressing Inflammation in the Brain and Spinal Cord
3.3. SBT-100 Mediated Targeting of STATs Is Effective in Suppressing cEAE and aEAE
3.4. Lymphocytes from SBT-100-treated EAE Mice Have Reduced Capacity to Transfer EAE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, D.E.; Darnell, J.E., Jr. Stats: Transcriptional control and biological impact. Nat. Rev. 2002, 3, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Egwuagu, C.E.; Larkin, J., III. Therapeutic targeting of STAT pathways in CNS autoimmune diseases. JAK-STAT 2013, 2, e24134. [Google Scholar] [CrossRef] [PubMed]
- Egwuagu, C.E. STAT3 in CD4+ T helper cell differentiation and inflammatory diseases. Cytokine 2009, 47, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Ren, Z.; Parker, G.N.; Sondermann, H.; Pastorello, M.A.; Wang, W.; McMurray, J.S.; Demeler, B.; Darnell, J.E.; Chen, X., Jr. Structural bases of unphosphorylated STAT1 association and receptor binding. Mol. Cell 2005, 17, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Vinkemeier, U. Nucleocytoplasmic shuttling of STAT transcription factors. Eur. J. Biochem. FEBS 2004, 271, 4606–4612. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, J.W.; Kidd, D.; Moseley, I.F.; Thompson, A.J.; MacManus, D.G.; Compston, D.A.; McDonald, W.I.; Miller, D.H. Spinal MRI in patients with suspected multiple sclerosis and negative brain MRI. Brain 1996, 119 Pt 3, 709–714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nociti, V.; Cianfoni, A.; Mirabella, M.; Caggiula, M.; Frisullo, G.; Patanella, A.K.; Sancricca, C.; Angelucci, F.; Tonali, P.A.; Batocchi, A.P. Clinical characteristics, course and prognosis of spinal multiple sclerosis. Spinal Cord. 2005, 43, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.R.; Golumbek, P.T.; Sim, J.; Dorsey, D.; Russell, J.H. Regional CNS responses to IFN-gamma determine lesion localization patterns during EAE pathogenesis. J. Exp. Med. 2008, 205, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Stoolman, J.S.; Duncker, P.C.; Huber, A.K.; Giles, D.A.; Washnock-Schmid, J.M.; Soulika, A.M.; Segal, B.M. An IFNgamma/CXCL2 regulatory pathway determines lesion localization during EAE. J. Neuroinflamm. 2018, 15, 208. [Google Scholar] [CrossRef]
- Stoolman, J.S.; Duncker, P.C.; Huber, A.K.; Segal, B.M. Site-specific chemokine expression regulates central nervous system inflammation and determines clinical phenotype in autoimmune encephalomyelitis. J. Immunol. 2014, 193, 564–570. [Google Scholar] [CrossRef]
- Pierson, E.; Simmons, S.B.; Castelli, L.; Goverman, J.M. Mechanisms regulating regional localization of inflammation during CNS autoimmunity. Immunol. Rev. 2012, 248, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Mbanefo, E.C.; Yan, M.; Kang, M.; Alhakeem, S.A.; Jittayasothorn, Y.; Yu, C.-R.; Parihar, A.; Singh, S.; Egwuagu, C.E. STAT3-Specific Single Domain Nanobody Inhibits Expansion of Pathogenic Th17 Responses and Suppresses Uveitis in Mice. Front. Immunol. 2021, 12, 724609. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Murillo, G.; Chen, D.; Parihar, A.S.; Mehta, R.G. Suppression of Breast Cancer Cell Proliferation by Selective Single-Domain Antibody for Intracellular STAT3. Breast Cancer 2018, 12, 1178223417750858. [Google Scholar] [CrossRef] [PubMed]
- Thakker, P.; Leach, M.W.; Kuang, W.; Benoit, S.E.; Leonard, J.P.; Marusic, S. IL-23 is critical in the induction but not in the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 2007, 178, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Sternberg, M.J. Protein structure prediction on the Web: A case study using the Phyre server. Nat. Protoc. 2009, 4, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, D.; Zhou, P.; Li, B.; Huang, S.Y. HDOCK: A web server for protein-protein and protein-DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res. 2017, 45, W365–W373. [Google Scholar] [CrossRef]
- Yan, Y.; Tao, H.; He, J.; Huang, S.Y. The HDOCK server for integrated protein-protein docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef]
- Schrödinger, L.; DeLano, W. PyMOL. Volume 2020. 2023. Available online: http://www.pymol.org/pymol (accessed on 10 December 2023).
- Oh, H.M.; Yu, C.R.; Dambuza, I.; Marrero, B.; Egwuagu, C.E. STAT3 protein interacts with Class O Forkhead transcription factors in the cytoplasm and regulates nuclear/cytoplasmic localization of FoxO1 and FoxO3a proteins in CD4(+) T cells. J. Biol. Chem. 2012, 287, 30436–30443. [Google Scholar] [CrossRef]
- Oh, H.M.; Yu, C.R.; Golestaneh, N.; Amadi-Obi, A.; Lee, Y.S.; Eseonu, A.; Mahdi, R.M.; Egwuagu, C.E. STAT3 protein promotes T-cell survival and inhibits interleukin-2 production through up-regulation of Class O Forkhead transcription factors. J. Biol. Chem. 2011, 286, 30888–30897. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Kaisho, T.; Yoshida, N.; Takeda, J.; Kishimoto, T.; Akira, S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: Generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 1998, 161, 4652–4660. [Google Scholar] [CrossRef] [PubMed]
- Nishibori, T.; Tanabe, Y.; Su, L.; David, M. Impaired development of CD4+ CD25+ regulatory T cells in the absence of STAT1: Increased susceptibility to autoimmune disease. J. Exp. Med. 2004, 199, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lee, Y.S.; Yu, C.R.; Egwuagu, C.E. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J. Immunol. 2008, 180, 6070–6076. [Google Scholar] [CrossRef]
- Griffin, J.D.; Spertini, O.; Ernst, T.J.; Belvin, M.P.; Levine, H.B.; Kanakura, Y.; Tedder, T.F. Granulocyte-macrophage colony-stimulating factor and other cytokines regulate surface expression of the leukocyte adhesion molecule-1 on human neutrophils, monocytes, and their precursors. J. Immunol. 1990, 145, 576–584. [Google Scholar] [CrossRef]
- El-Behi, M.; Ciric, B.; Dai, H.; Yan, Y.; Cullimore, M.; Safavi, F.; Zhang, G.X.; Dittel, B.N.; Rostami, A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 2011, 12, 568–575. [Google Scholar] [CrossRef]
- Codarri, L.; Gyülvészi, G.; Tosevski, V.; Hesske, L.; Fontana, A.; Magnenat, L.; Suter, T.; Becher, B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011, 12, 560–567. [Google Scholar] [CrossRef]
- King, I.L.; Kroenke, M.A.; Segal, B.M. GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J. Exp. Med. 2010, 207, 953–961. [Google Scholar] [CrossRef]
- Darnell, J.E., Jr. STATs and gene regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef]
- Dang, C.; Lu, Y.; Chen, X.; Li, Q. Baricitinib Ameliorates Experimental Autoimmune Encephalomyelitis by Modulating the Janus Kinase/Signal Transducer and Activator of Transcription Signaling Pathway. Front. Immunol. 2021, 12, 650708. [Google Scholar] [CrossRef]
- Liu, Y.; Holdbrooks, A.T.; De Sarno, P.; Rowse, A.L.; Yanagisawa, L.L.; McFarland, B.C.; Harrington, L.E.; Raman, C.; Sabbaj, S.; Benveniste, E.N.; et al. Therapeutic efficacy of suppressing the Jak/STAT pathway in multiple models of experimental autoimmune encephalomyelitis. J. Immunol. 2014, 192, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Massoud, F.; Ismail, I.; Al-Hashel, J.Y.; Abboud, H. CNS demyelination during tofacitinib therapy: First report. Mult. Scler. Relat. Disord. 2020, 46, 102568. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbanefo, E.C.; Seifert, A.; Yadav, M.K.; Yu, C.-R.; Nagarajan, V.; Parihar, A.; Singh, S.; Egwuagu, C.E. A Camelid-Derived STAT-Specific Nanobody Inhibits Neuroinflammation and Ameliorates Experimental Autoimmune Encephalomyelitis (EAE). Cells 2024, 13, 1042. https://doi.org/10.3390/cells13121042
Mbanefo EC, Seifert A, Yadav MK, Yu C-R, Nagarajan V, Parihar A, Singh S, Egwuagu CE. A Camelid-Derived STAT-Specific Nanobody Inhibits Neuroinflammation and Ameliorates Experimental Autoimmune Encephalomyelitis (EAE). Cells. 2024; 13(12):1042. https://doi.org/10.3390/cells13121042
Chicago/Turabian StyleMbanefo, Evaristus C., Allison Seifert, Manoj Kumar Yadav, Cheng-Rong Yu, Vijayaraj Nagarajan, Ashutosh Parihar, Sunanda Singh, and Charles E. Egwuagu. 2024. "A Camelid-Derived STAT-Specific Nanobody Inhibits Neuroinflammation and Ameliorates Experimental Autoimmune Encephalomyelitis (EAE)" Cells 13, no. 12: 1042. https://doi.org/10.3390/cells13121042
APA StyleMbanefo, E. C., Seifert, A., Yadav, M. K., Yu, C.-R., Nagarajan, V., Parihar, A., Singh, S., & Egwuagu, C. E. (2024). A Camelid-Derived STAT-Specific Nanobody Inhibits Neuroinflammation and Ameliorates Experimental Autoimmune Encephalomyelitis (EAE). Cells, 13(12), 1042. https://doi.org/10.3390/cells13121042






