The Expression of a Subset of Aging and Antiaging Markers Following the Chondrogenic and Osteogenic Differentiation of Mesenchymal Stem Cells of Placental Origin
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture MSCs
2.2. Flow Cytometry
2.3. Colony-Forming Unit (CFU) Assay
2.4. Osteogenic, Chondrogenic, and Adipogenic Induction
2.5. RT-qPCR
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic Differentiation of Mesenchymal Stem Cells: Challenges and Unfulfilled Expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, C.; Choi, Y.S.; Kim, M.; Park, C.; Suh, Y. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: Implication to age-associated bone diseases and defects. Mech. Ageing Dev. 2012, 133, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Wong, X.; Melendez-Perez, A.J.; Reddy, K.L. The Nuclear Lamina. Cold Spring Harb. Perspect. Biol. 2022, 14, a040113. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, D.; Gray, H.L.; Sammak, P.J.; Schatten, G.P.; Csoka, A.B. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. Stem Cells 2006, 24, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.H.; Kang Sm Lee, S.J.; Woo, T.G.; Oh, A.Y.; Park, S.; Ha, N.-C.; Park, B.-J. p53 induces senescence through Lamin A/C stabilization-mediated nuclear deformation. Cell Death Dis. 2019, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Malashicheva, A.; Perepelina, K. Diversity of Nuclear Lamin A/C Action as a Key to Tissue-Specific Regulation of Cellular Identity in Health and Disease. Front. Cell Dev. Biol. 2021, 9, 761469. [Google Scholar] [CrossRef] [PubMed]
- Malashicheva, A.; Bogdanova, M.; Zabirnyk, A.; Smolina, N.; Ignatieva, E.; Freilikhman, O.; Fedorov, A.; Dmitrieva, R.; Sjöberg, G.; Sejersen, T.; et al. Various lamin A/C mutations alter expression profile of mesenchymal stem cells in mutation specific manner. Mol. Genet. Metab. 2015, 115, 118–127. [Google Scholar] [CrossRef]
- Sehgal, P.; Chaturvedi, P.; Kumaran, R.I.; Kumar, S.; Parnaik, V.K. Lamin A/C Haploinsufficiency Modulates the Differentiation Potential of Mouse Embryonic Stem Cells. PLoS ONE 2013, 8, e57891. [Google Scholar] [CrossRef]
- Naito, M.; Omoteyama, K.; Mikami, Y.; Takagi, M.; Takahashi, T. Suppression of lamin A/C by short hairpin RNAs promotes adipocyte lineage commitment in mesenchymal progenitor cell line, ROB-C26. Histochem. Cell Biol. 2012, 137, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, Y.; Keyimu, R.; Hao, J.; Zhao, Z.; Ye, R. The role of lamin A/C in mesenchymal stem cell differentiation. J. Physiol. Biochem. 2019, 75, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Busch, F.; Shayan, P.; Shakibaei, M. Sirtuin-1 (SIRT1) Is Required for Promoting Chondrogenic Differentiation of Mesenchymal Stem Cells. J. Biol. Chem. 2014, 289, 22048–22062. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Li, N.T.; Cheng, H.S.; Yen, M.L. Oxidative stress induces imbalance of adipogenic/osteoblastic lineage commitment in mesenchymal stem cells through decreasing SIRT1 functions. J. Cell. Mol. Med. 2018, 22, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Denu, R.A. SIRT3 Enhances Mesenchymal Stem Cell Longevity and Differentiation. Oxidative Med. Cell. Longev. 2017, 2017, 5841716. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A. SIRT7 in the aging process. Cell. Mol. Life Sci. 2022, 79, 297. [Google Scholar] [CrossRef] [PubMed]
- Raza, U.; Tang, X.; Liu, Z.; Liu, B. SIRT7: The seventh key to unlocking the mystery of aging. Physiol. Rev. 2023, 104, 253–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.E.M.; Zhang, W.; Ye, C.C.Y.; Gao, X.; Jiang, L.L.J.; Zhao, T.T.F.; Pan, Z.Z.J.; Xue, D.D.T. Knockdown of SIRT7 enhances the osteogenic differentiation of human bone marrow mesenchymal stem cells partly via activation of the Wnt/b-catenin signaling pathway. Cell Death Dis. 2017, 8, e3042. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.E.; Rai, R.; Khan, S.S.; Eren, M.; Ghosh, A.K. Plasminogen Activator Inhibitor-1 Is a Marker and a Mediator of Senescence. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1446–1452. [Google Scholar] [CrossRef]
- Takafuji, Y.; Tatsumi, K.; Ishida, M.; Kawao, N.; Okada, K.; Matsuo, O.; Kaji, H. Plasminogen activator inhibitor-1 deficiency suppresses osteoblastic differentiation of mesenchymal stem cells in mice. J. Cell. Physiol. 2019, 234, 9687–9697. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Incalzi, R.A. Plasminogen Activator Inhibitor-1 (PAI-1): A Key Factor Linking Fibrinolysis and Age-Related Subclinical and Clinical Conditions. Cardiovasc. Ther. 2010, 28, e72–e91. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Takeshita, K.; Shimokawa, T.; Yi, H.; Isobe, K.I.; Loskutoff, D.J.; Saito, H. Plasminogen activator inhibitor-1 is a major stress-regulated gene: Implications for stress-induced thrombosis in aged individuals. Proc. Natl. Acad. Sci. USA 2002, 99, 890–895. [Google Scholar] [CrossRef]
- Copland, I.B.; Lord-Dufour, S.; Cuerquis, J.; Coutu, D.L.; Annabi, B.; Wang, E.; Galipeau, J. Improved autograft survival of mesenchymal stromal cells by plasminogen activator inhibitor 1 inhibition. Stem Cells 2009, 27, 467–477. [Google Scholar] [CrossRef]
- Miao, S.B.; Xie, X.L.; Yin, Y.J.; Zhao, L.L.; Zhang, F.; Shu, Y.-N.; Chen, R.; Chen, P.; Dong, L.-H.; Lin, Y.-L.; et al. Accumulation of Smooth Muscle 22a Protein Accelerates Senescence of Vascular Smooth Muscle Cells via Stabilization of p53 In Vitro and In Vivo. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1849–1859. [Google Scholar] [CrossRef]
- Kim, T.R.; Lee, H.M.; Lee, S.Y.; Kim, E.J.; Kim, K.C.; Paik, S.G.; Cho, E.W.; Kim, I.G. SM22a-induced activation of p16INK4a/retinoblastoma pathway promotes cellular senescence caused by a subclinical dose of g-radiation and doxorubicin in HepG2 cells. Biochem. Biophys. Res. Commun. 2010, 400, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Abomaray, F.M.; Al Jumah, M.A.; Alsaad, K.O.; Jawdat, D.; Al Khaldi, A.; AlAskar, A.; Al Harthy, S.; Al Subayyil, A.; Khatlani, T.; Alawad, A.; et al. Phenotypic and Functional Characterization of Mesenchymal Stem/Multipotent Stromal Cells from Decidua Basalis of Human Term Placenta. Stem Cells Int. 2016, 2016, 5184601. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ventura Ferreira, M.S.; Bienert, M.; Müller, K.; Rath, B.; Goecke, T.; Opländer, C.; Braunschweig, T.; Mela, P.; Brümmendorf, T.H.; Beier, F.; et al. Comprehensive characterization of chorionic villi-derived mesenchymal stromal cells from human placenta. Stem Cell Res. Ther. 2018, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Barlow, S.; Brooke, G.; Chatterjee, K.; Price, G.; Pelekanos, R.; Rossetti, T.; Doody, M.; Venter, D.; Pain, S.; Gilshenan, K.; et al. Comparison of Human Placenta- and Bone Marrow-Derived Multipotent Mesenchymal Stem Cells. Stem Cells Dev. 2008, 17, 1095–1108. [Google Scholar] [CrossRef]
- Brooke, G.; Tong, H.; Levesque, J.P.; Atkinson, K. Molecular Trafficking Mechanisms of Multipotent Mesenchymal Stem Cells Derived from Human Bone Marrow and Placenta. Stem Cells Dev. 2008, 17, 929–940. [Google Scholar] [CrossRef]
- Mariotti, E.; Mirabelli, P.; Abate, G.; Schiattarella, M.; Martinelli, P.; Fortunato, G.; Di Noto, R.; Del Vecchio, L. Comparative Characteristics of Mesenchymal Stem Cells from Human Bone Marrow and Placenta: CD10, CD49d, and CD56 Make a Difference. Stem Cells Dev. 2008, 17, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, F.; Song, L.; Shu, Y.; Lin, Y.; Dong, L.; Nie, X.; Zhang, D.; Chen, P.; Han, M. Transcriptome profiling reveals that the SM22a-regulated molecular pathways contribute to vascular pathology. J. Mol. Cell. Cardiol. 2014, 72, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yang, M.; Jiang, H.; Ju, D.; Zheng, J.P.; Xu, Z.; Liao, T.-D.; Li, L. Arterial injury promotes medial chondrogenesis in Sm22 knockout mice. Cardiovasc. Res. 2011, 90, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yang, L.; Chang, H.; Dai, G.; Wang, F.; Duan, X.; Guo, L.; Zhang, Y.; Chen, G. Wnt/beta-catenin signaling regulates the proliferation and differentiation of mesenchymal progenitor cells through the p53 pathway. PLoS ONE 2014, 9, e97283. [Google Scholar]
- Hashimoto, S.; Nishiyama, T.; Hayashi, S.; Fujishiro, T.; Takebe, K.; Kanzaki, N.; Kuroda, R.; Kurosaka, M. Role of p53 in human chondrocyte apoptosis in response to shear strain. Arthritis Rheum 2009, 60, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Tan, X.; Xie, Z.; Yu, J.; Li, P.; Lin, X.; Ouyang, S.; Liu, Z.; Hou, Q.; Xie, N.; et al. p53: A Key Target in the Development of Osteoarthritis. Mol. Biotechnol. 2024, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Diekman, B.O.; Sessions, G.A.; Collins, J.A.; Knecht, A.K.; Strum, S.L.; Mitin, N.K.; Carlson, C.S.; Loeser, R.F.; Sharpless, N.E. Expression of p16INK4a is a biomarker of chondrocyte aging but does not cause osteoarthritis. Aging Cell 2018, 17, e12771. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; de Castro, L.F.; Shin, M.H.; Dubois, W.; Yang, H.H.; Jiang, S.; Mishra, P.J.; Ren, L.; Gou, H.; Lal, A.; et al. p53 Loss Increases the Osteogenic Differentiation of Bone Marrow Stromal Cells. Stem Cells 2015, 33, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kua, H.-Y.; Hu, Y.; Guo, K.; Zeng, Q.; Wu, Q.; Ng, H.-H.; Karsenty, G.; de Crombrugghe, B.; Yeh, J.; et al. p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J. Cell Biol. 2005, 172, 115–125. [Google Scholar] [CrossRef]
- González-Cruz, R.D.; Dahl, K.N.; Darling, E.M. The Emerging Role of Lamin C as an Important LMNA Isoform in Mechanophenotype. Front. Cell Dev. Biol. 2018, 6, 151. [Google Scholar] [CrossRef]
- Al-Saaidi, R.; Bross, P. Do lamin A and lamin C have unique roles? Chromosoma 2015, 124, 1–12. [Google Scholar] [CrossRef]
- González-Cruz, R.D.; Sadick, J.S.; Fonseca, V.C.; Darling, E.M. Nuclear Lamin Protein C Is Linked to Lineage-Specific, Whole-Cell Mechanical Properties. Cell. Mol. Bioeng. 2018, 11, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Cenni, V.; Capanni, C.; Mattioli, E.; Schena, E.; Squarzoni, S.; Bacalini, M.G.; Garagnani, P.; Salvioli, S.; Franceschi, C.; Lattanzi, G. Lamin A involvement in ageing processes. Ageing Res. Rev. 2020, 62, 101073. [Google Scholar] [CrossRef]
- Akter, R.; Rivas, D.; Geneau, G.; Drissi, H.; Duque, G. Effect of Lamin A/C Knockdown on Osteoblast Differentiation and Function. J. Bone Miner. Res. 2009, 24, 283–293. [Google Scholar] [CrossRef]
- Khan, H.; Mafi, P.; Mafi, R.; Khan, W. The Effects of Ageing on Differentiation and Characterisation of Human Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2018, 13, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.; Rodríguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Law, S.F.; Chandra, A. Bone Aging, Cellular Senescence, and Osteoporosis. JBMR Plus 2021, 5, e10488. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, J.C.; Yi, N.; Roy, M.; Hendrickson, D.G.; Kelley, D.R. Differentiation reveals latent features of aging and an energy barrier in murine myogenesis. Cell Rep. 2021, 35, 109046. [Google Scholar] [CrossRef]
- Remark, L.H.; Leclerc, K.; Ramsukh, M.; Lin, Z.; Lee, S.; Dharmalingam, B.; Gillinov, L.; Nayak, V.V.; El Parente, P.; Sambon, M.; et al. Loss of Notch signaling in skeletal stem cells enhances bone formation with aging. Bone Res. 2023, 11, 50. [Google Scholar] [CrossRef]
- Kim, H.N.; Ponte, F.; Warren, A.; Ring, R.; Iyer, S.; Han, L.; Almeida, M. A decrease in NAD+ contributes to the loss of osteoprogenitors and bone mass with aging. NPJ Aging Mech. Dis. 2021, 7, 8. [Google Scholar] [CrossRef]
- Moerman, E.J.; Teng, K.; Lipschitz, D.A.; Lecka-Czernik, B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 2004, 3, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Bermeo, S.; Vidal, C.; Zhou, H.; Duque, G. Lamin A/C Acts as an Essential Factor in Mesenchymal Stem Cell Differentiation Through the Regulation of the Dynamics of the Wnt/β-Catenin Pathway. J. Cell. Biochem. 2015, 116, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Hadadeh, O.; Barruet, E.; Peiretti, F.; Verdier, M.; Bernot, D.; Hadjal, Y.; El Yazidi, C.; Robaglia-Schlupp, A.; De Paula, A.M.; Nègre, D.; et al. The plasminogen activation system modulates differently adipogenesis and myogenesis of embryonic stem cells. PLoS ONE 2012, 7, e49065. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sense (5′→3′) | Antisense (5′→3′) | Probe (5′→3′) | Accession Number | Amplicon Length (bp) | Melting Temperature (°C) |
|---|---|---|---|---|---|---|
| SIRT7 | ACTGCTTCAGAAAGGGAGA | CACAGTTCTGAGACACCACA | ACTGCTTCAGAAAGGGAGA | NM_016538.2 | 128 | 92.5 |
| LMNA | TGACTGTGGTTGAGGACGAC | GACACTGGAGGCAGAAGAGC | CGCTGAGTACAACCT | NM_170707.3 | 221 | 98.5 |
| LMNC | GTGGAAGGCACAGAACACCT | GCGGCGGCTACCACTCAC | AGATGACCTGCTCCATCACC | NM_005572.3 | 178 | 94.0 |
| LMNAΔ10 | AACTCCACTGGGGAAGGCTCC | GCTCCTGAGCCGCTGGCAGA | AGTACAACCTGCGCTCGCGC | NM_170708.3 | 131 | 98.0 |
| p16INK4a | GGGGGCACCAGAGGCAGT | GGTTGTGGCGGGGGCAGTT | NM_000077.4 | 159 | 92 | |
| p53 (TP53) | CCGGCGCACAGAGGAAGAGA | TGGGGAGAGGAGCTGGTGTTGT | NM_000546.5 | 108 | 92.5 | |
| PAI-1 | GTGTTTCAGCAGGTGGCGC | CCGGAACAGCCTGAAGAAGTG | NM_001386460.1 | 300 | 94.0 | |
| SM22α | TGGCGTGATTCTGAGCAA | CTGCCAAGCTGCCCAAGG | NM_001001522.2 | 239 | 92.5 | |
| Ubiquitin C | ACTACAACATCCAGAAAGAGTCCA | CCAGTCAGGGTCTTCACGAAG | NM_021009.6 | 85 | 88.0 | |
| RPL13 | AACAAGTTGAAGTACCTGGCTTTC | TGGTTTTGTGGGGCAGCATA | NM_012423.4 | 130 | 95.3 | |
| Cyclophilin A | CCCACCGTGTTCTTCGACAT | TTTCTGCTGTCTTTGGGACCTT | NM_021130.5 | 94 | 92.0 | |
| GAPDH | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA | NM_002046.7 | 452 | 95.5 |
| Chondrogenic Lineage | Osteogenic Lineage | Adipogenic Lineage | |
|---|---|---|---|
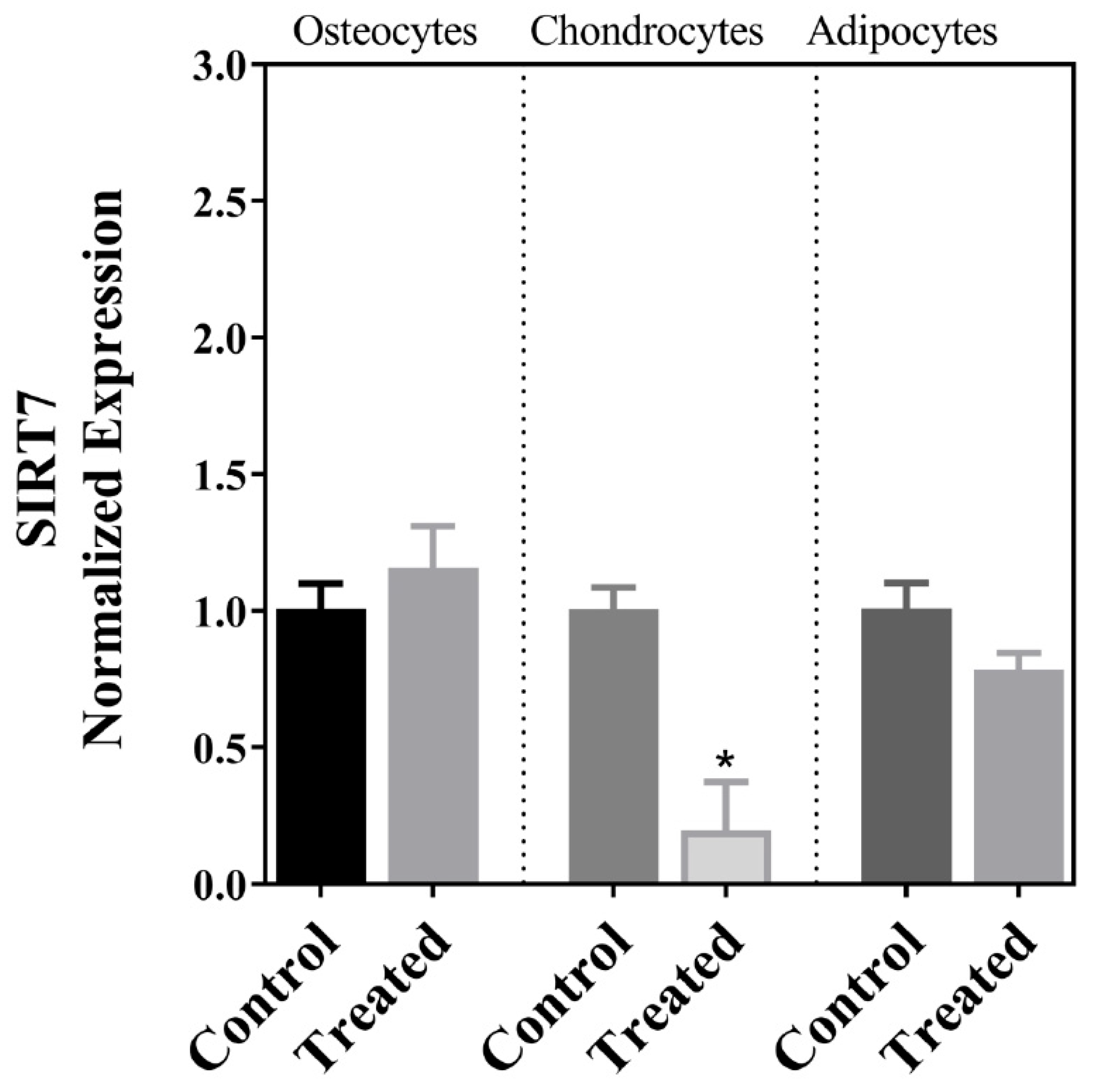
| SIRT7 | ↓ | ↔ | ↔ |
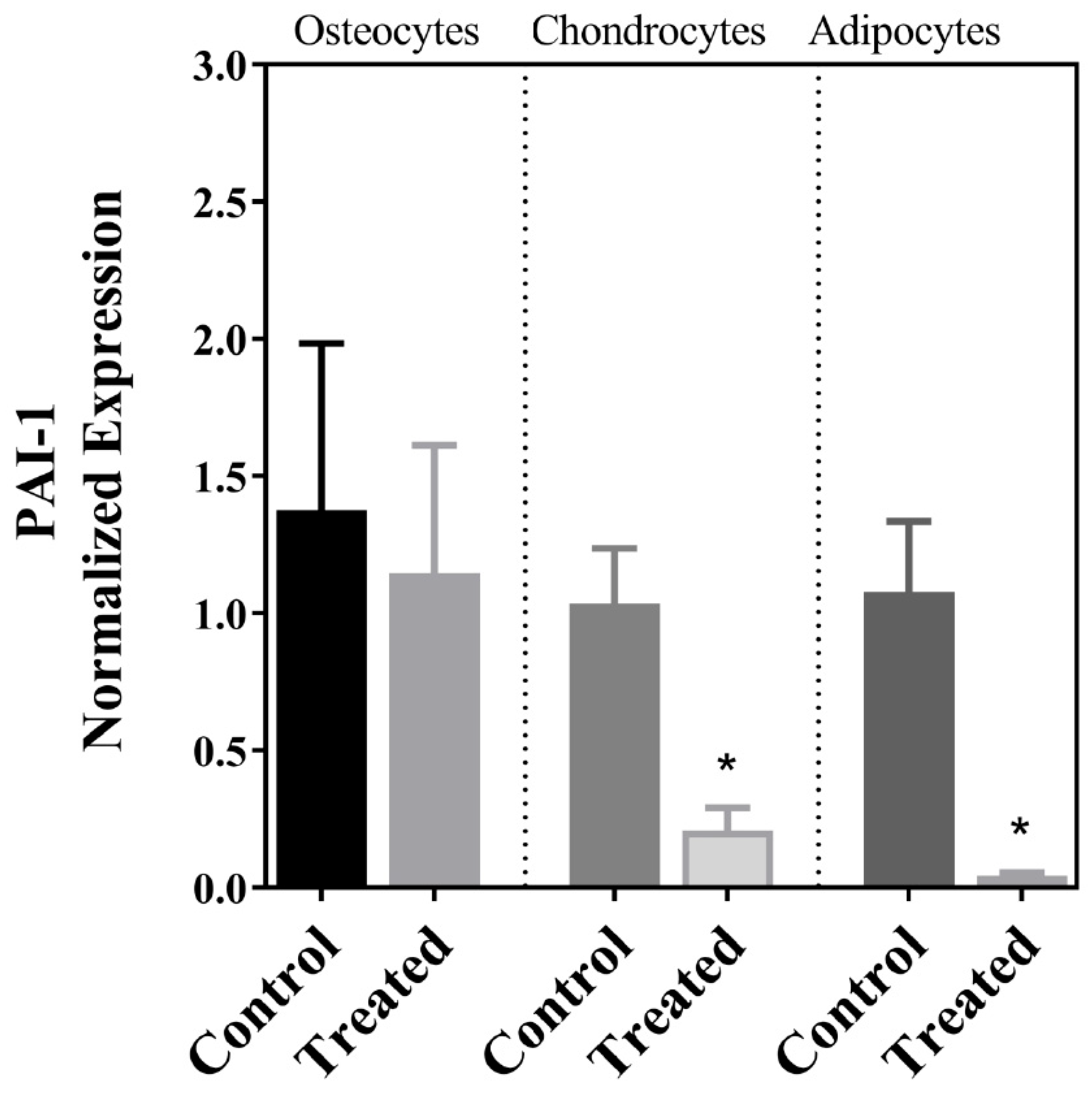
| PAI-1 | ↓ | ↔ | ↓ |
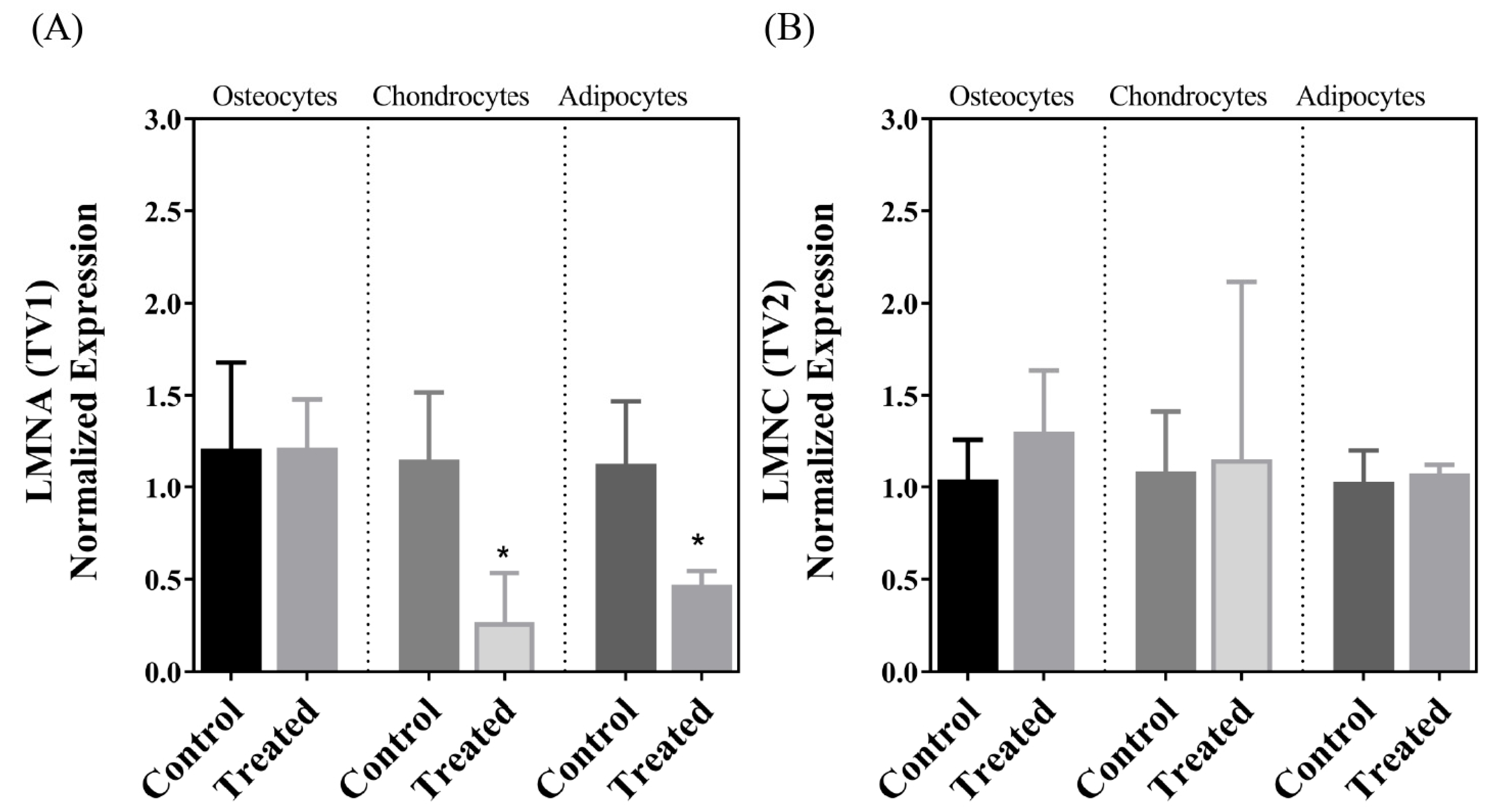
| LMNA | ↓ | ↔ | ↓ |
| LMNC | ↔ | ↔ | ↔ |
| LMNAΔ10 | ND | ND | ND |
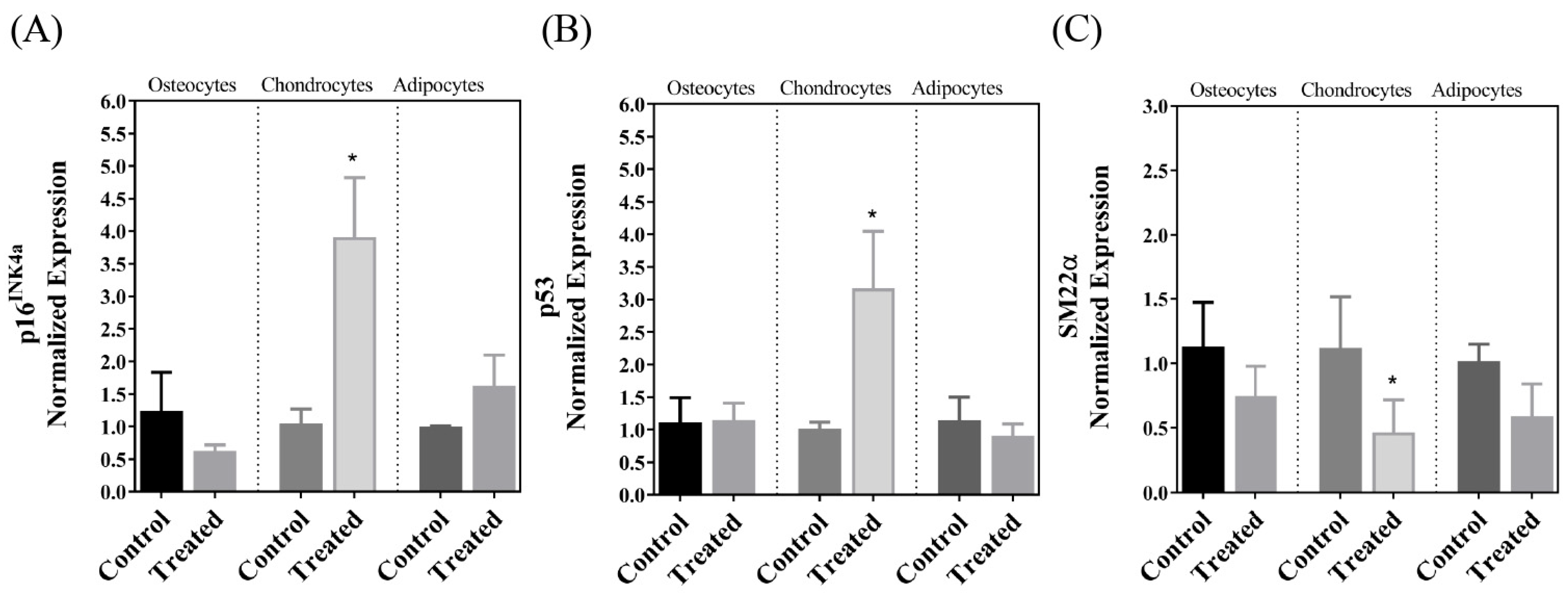
| p16INK4a | ↑ | ↔ | ↔ |
| p53 (TP53) | ↑ | ↔ | ↔ |
| SM22α | ↓ | ↔ | ↔ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhra, M.; Magableh, A.M.; Samhan, L.M.; Fatani, L.M.; Qasem, R.J.; Aljada, A. The Expression of a Subset of Aging and Antiaging Markers Following the Chondrogenic and Osteogenic Differentiation of Mesenchymal Stem Cells of Placental Origin. Cells 2024, 13, 1022. https://doi.org/10.3390/cells13121022
Zhra M, Magableh AM, Samhan LM, Fatani LM, Qasem RJ, Aljada A. The Expression of a Subset of Aging and Antiaging Markers Following the Chondrogenic and Osteogenic Differentiation of Mesenchymal Stem Cells of Placental Origin. Cells. 2024; 13(12):1022. https://doi.org/10.3390/cells13121022
Chicago/Turabian StyleZhra, Mahmoud, Ahmad M. Magableh, Lara M. Samhan, Lein M. Fatani, Rani J. Qasem, and Ahmad Aljada. 2024. "The Expression of a Subset of Aging and Antiaging Markers Following the Chondrogenic and Osteogenic Differentiation of Mesenchymal Stem Cells of Placental Origin" Cells 13, no. 12: 1022. https://doi.org/10.3390/cells13121022
APA StyleZhra, M., Magableh, A. M., Samhan, L. M., Fatani, L. M., Qasem, R. J., & Aljada, A. (2024). The Expression of a Subset of Aging and Antiaging Markers Following the Chondrogenic and Osteogenic Differentiation of Mesenchymal Stem Cells of Placental Origin. Cells, 13(12), 1022. https://doi.org/10.3390/cells13121022








