CD3ζ-Mediated Signaling Protects Retinal Ganglion Cells in Glutamate Excitotoxicity of the Retina
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Intraocular Injection of N-methyl-d-Aspartic Acid (NMDA) and Inhibitors
2.3. Primary Antibodies
2.4. Preparation of Retinal Whole-Mounts and Retinal Section for Antibody Staining
2.5. Confocal Laser Scanning Microscopy and Image Sampling
2.6. Statistical Analysis
3. Results
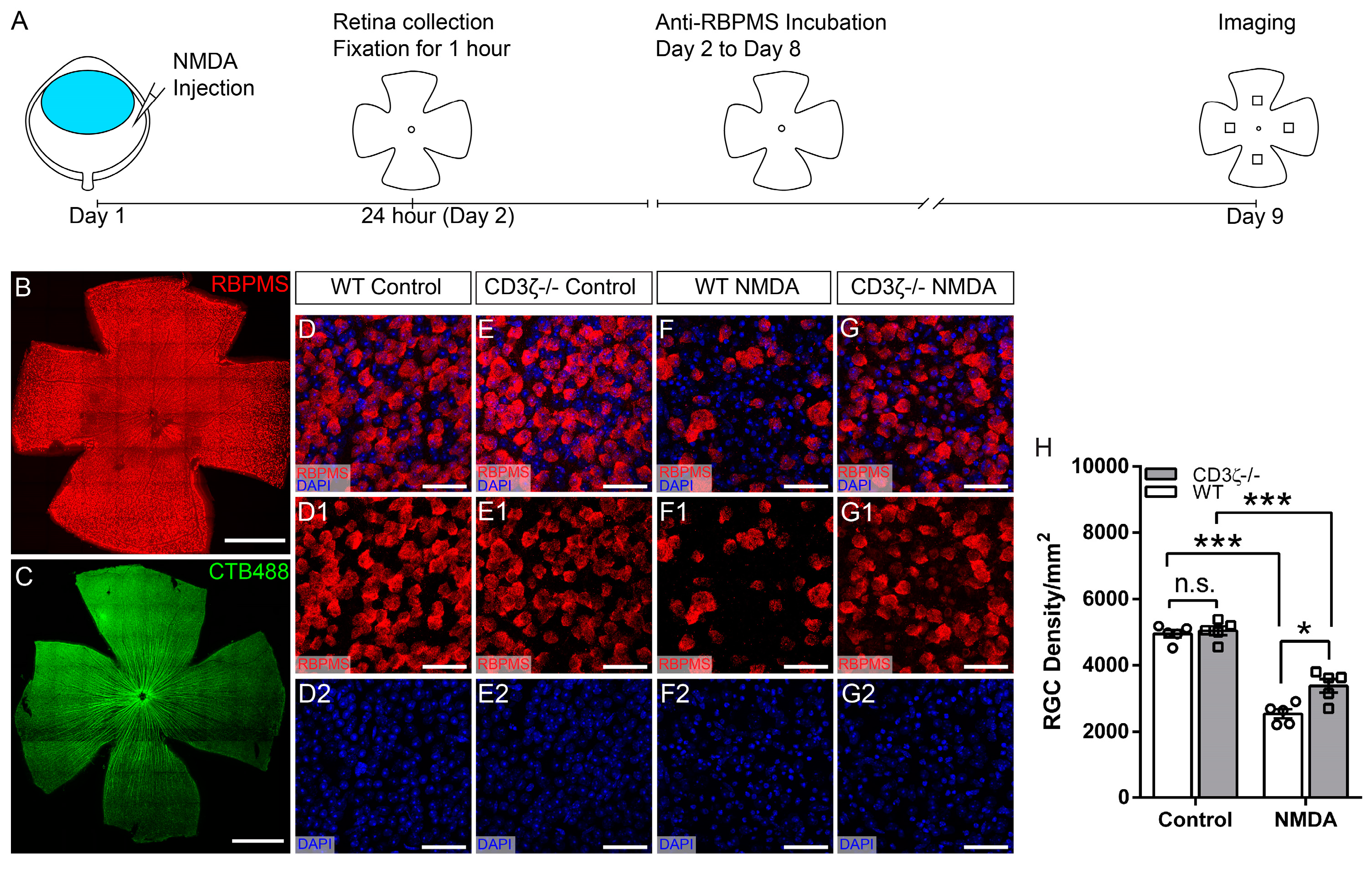
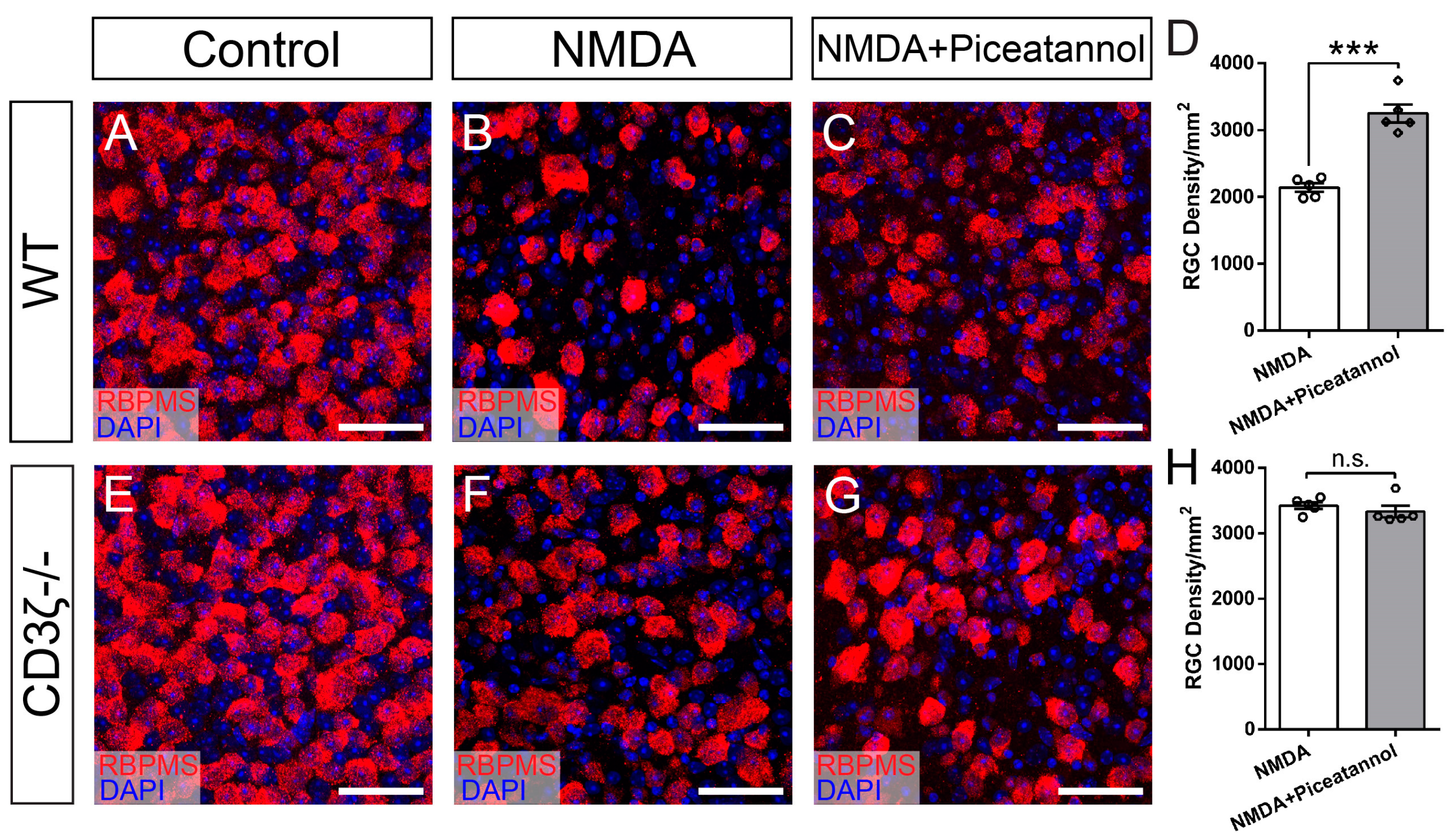
3.1. CD3ζ Mutation Reduces RGC Death in Glutamate Excitotoxicity
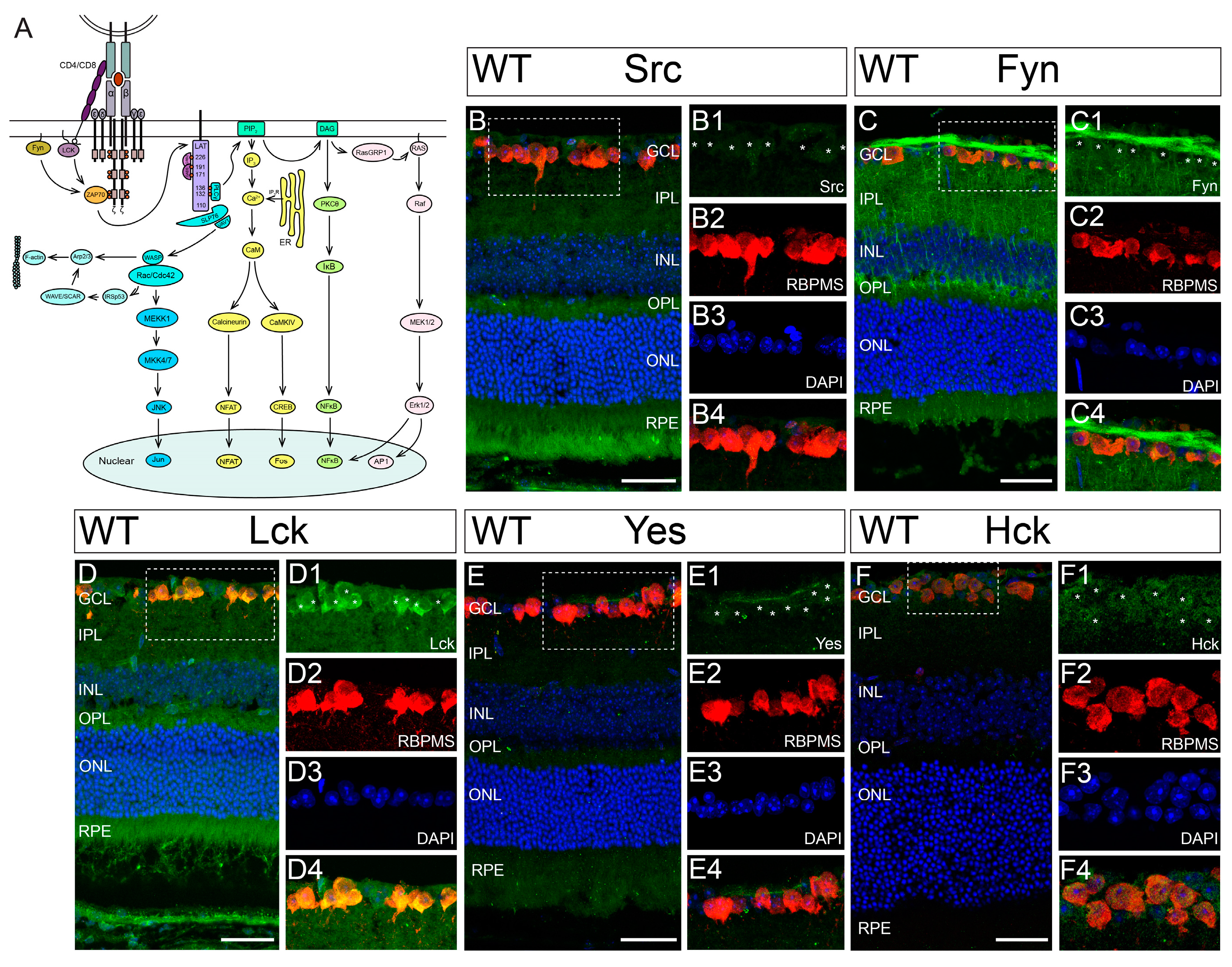
3.2. Hck Is Expressed by RGCs in Mouse Retina
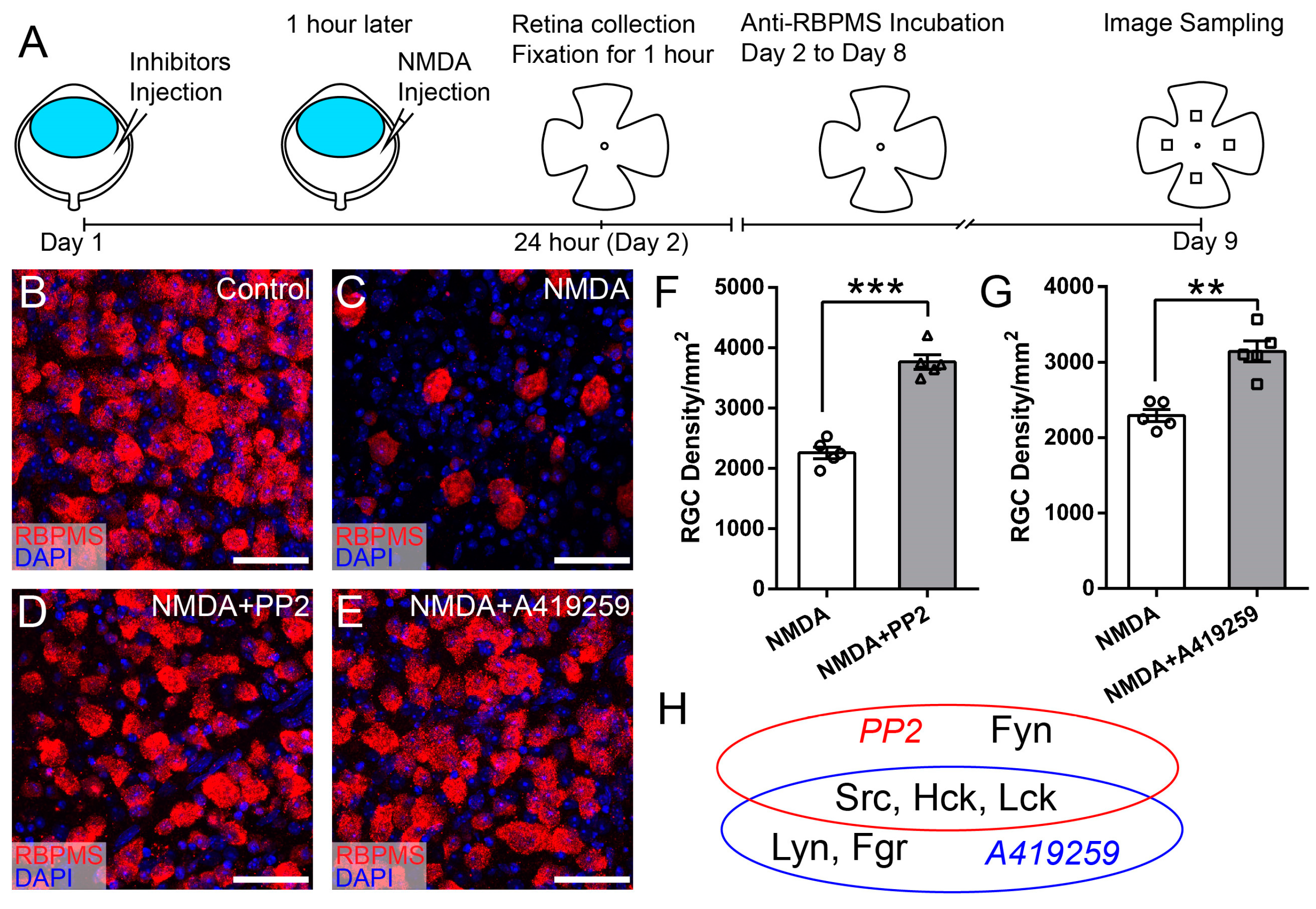
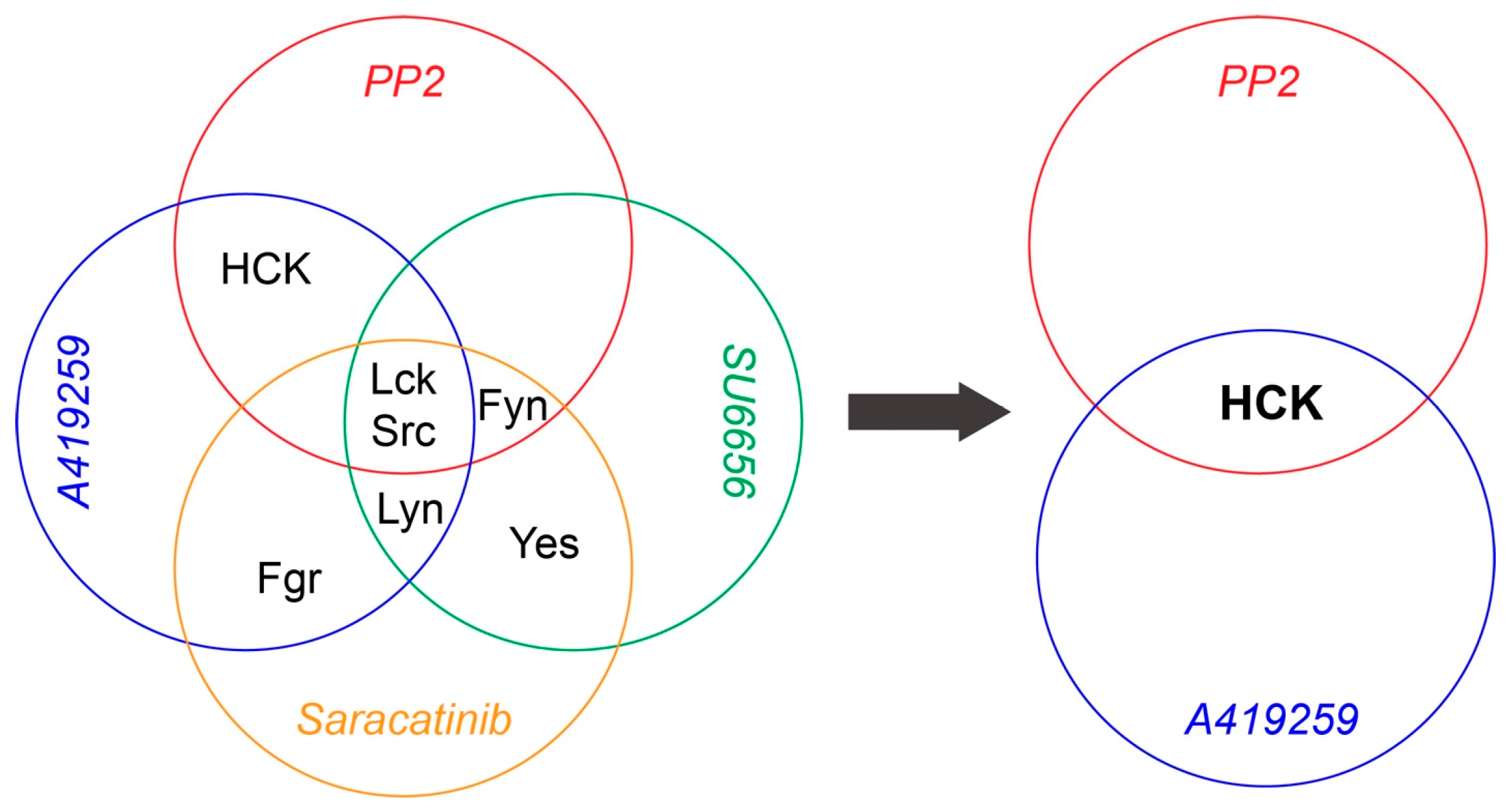
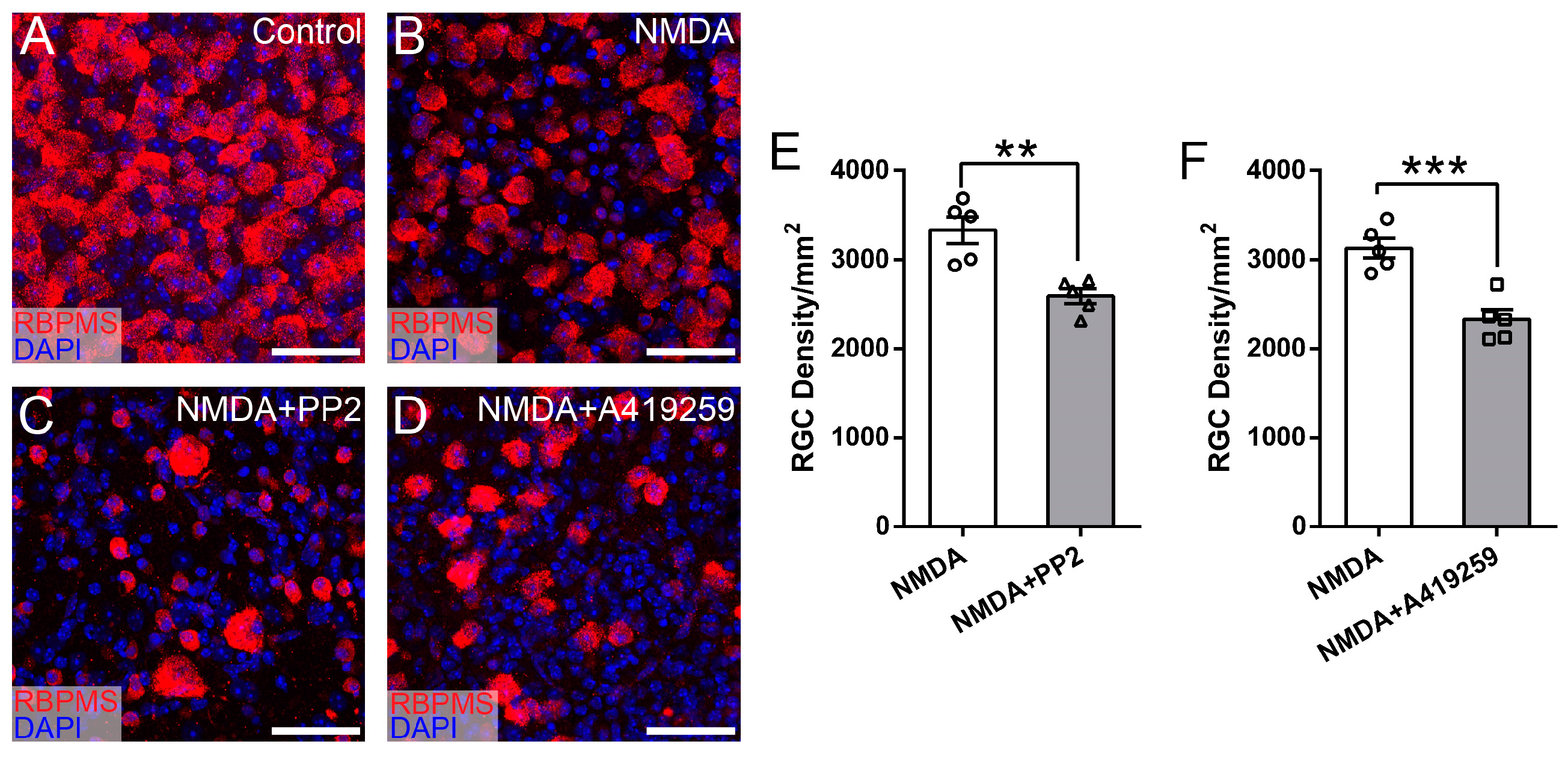
3.3. Selective Inhibition of Hck Increases RGC Survival in NMDA-Induced Excitotoxicity
3.4. Syk/Zap70 Family Kinases Are Expressed by RGCs in Mouse Retina and Mediate RGC Death
4. Discussion
4.1. How Does Glutamate Excitotoxicity Damage RGCs in Retinal Diseases?
4.2. How do SFKs Regulate NMDA-Induced RGC Death through the CD3ζ-Hck-Syk/Zap70 Signal Pathway?
4.3. What Are the Regulatory Mechanisms of CD3ζ-Mediated RGC Death in Retinal Diseases?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuehn, M.; Fingert, J.; Kwon, Y. Retinal Ganglion Cell Death in Glaucoma: Mechanisms and Neuroprotective Strategies. Ophthalmol. Clin. N. Am. 2005, 18, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G. Immune Regulation toward Immunomodulation for Neuroprotection in Glaucoma. Curr. Opin. Pharmacol. 2013, 13, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.H.; Fingert, J.H.; Kuehn, M.H.; Alward, W.L. Primary Open-Angle Glaucoma. N. Engl. J. Med. 2009, 360, 1113–1124. [Google Scholar] [CrossRef]
- Evangelho, K.; Mogilevskaya, M.; Losada-Barragan, M.; Vargas-Sanchez, J.K. Pathophysiology of Primary Open-Angle Glaucoma from a Neuroinflammatory and Neurotoxicity Perspective: A Review of the Literature. Int. Ophthalmol. 2019, 39, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Sumino, A.; Kajioka, D.; Shibagaki, F.; Yamamuro, A.; Yoshioka, Y.; Maeda, S. Apelin Protects against NMDA-Induced Retinal Neuronal Death via an APJ Receptor by Activating Akt and ERK1/2, and Suppressing TNF-α Expression in Mice. J. Pharmacol. Sci. 2017, 133, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Liberatore, F.; Bucci, D.; Mascio, G.; Madonna, M.; Di Pietro, P.; Beneventano, M.; Puliti, A.M.; Battaglia, G.; Bruno, V.; Nicoletti, F.; et al. Permissive Role for mGlu1 Metabotropic Glutamate Receptors in Excitotoxic Retinal Degeneration. Neuroscience 2017, 363, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The Molecular Basis of Retinal Ganglion Cell Death in Glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.-D.; Chen, J.; Li, F.; Gao, F.; Wu, J.; Miao, Y.; Wang, Z. Enhanced Expression of NR2B Subunits of NMDA Receptors in the Inherited Glaucomatous DBA/2J Mouse Retina. Neural. Plast. 2013, 2013, 670254. [Google Scholar] [CrossRef] [PubMed]
- Christensen, I.; Lu, B.; Yang, N.; Huang, K.; Wang, P.; Tian, N. The Susceptibility of Retinal Ganglion Cells to Glutamatergic Excitotoxicity Is Type-Specific. Front. Neurosci. 2019, 13, 219. [Google Scholar] [CrossRef]
- Bessero, A.-C.; Clarke, P.G.H. Neuroprotection for Optic Nerve Disorders. Curr. Opin. Neurol. 2010, 23, 10–15. [Google Scholar] [CrossRef]
- Lebrun-Julien, F.; Di Polo, A. Molecular and Cell-Based Approaches for Neuroprotection in Glaucoma. Optom. Vis. Sci. 2008, 85, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.L.; Torero Ibad, R.; Rheey, J.; Castagner, F.; Prochiantz, A.; Moya, K.L. Cell Non-Autonomous Functions of Homeoproteins in Neuroprotection in the Brain. FEBS Lett. 2011, 585, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.R.; Thams, S.; Lidman, O.; Piehl, F.; Cullheim, S. A Role for MHC Class I Molecules in Synaptic Plasticity and Regeneration of Neurons after Axotomy. Proc. Natl. Acad. Sci. USA 2004, 101, 17843–17848. [Google Scholar] [CrossRef] [PubMed]
- Huh, G.S.; Boulanger, L.M.; Du, H.; Riquelme, P.A.; Brotz, T.M.; Shatz, C.J. Functional Requirement for Class I MHC in CNS Development and Plasticity. Science 2000, 290, 2155–2159. [Google Scholar] [CrossRef] [PubMed]
- Syken, J. PirB Restricts Ocular-Dominance Plasticity in Visual Cortex. Science 2006, 313, 1795–1800. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.J.; Huang, Y.H.; Datwani, A.; Shatz, C.J. H2-Kb and H2-Db Regulate Cerebellar Long-Term Depression and Limit Motor Learning. Proc. Natl. Acad. Sci. USA 2009, 106, 6784–6789. [Google Scholar] [CrossRef] [PubMed]
- Thams, S.; Brodin, P.; Plantman, S.; Saxelin, R.; Kärre, K.; Cullheim, S. Classical Major Histocompatibility Complex Class I Molecules in Motoneurons: New Actors at the Neuromuscular Junction. J. Neurosci. 2009, 29, 13503–13515. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Miranda, C.J.; Braun, L.; Meyer, K.; Frakes, A.E.; Ferraiuolo, L.; Likhite, S.; Bevan, A.K.; Foust, K.D.; McConnell, M.J.; et al. Major Histocompatibility Complex Class I Molecules Protect Motor Neurons from Astrocyte-Induced Toxicity in Amyotrophic Lateral Sclerosis. Nat. Med. 2016, 22, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, H.; Ding, Q.; Xie, Z.-H.; Chen, L.; Diao, L.; Wang, P.; Gan, L.; Crair, M.C.; Tian, N. The Immune Protein CD3zeta Is Required for Normal Development of Neural Circuits in the Retina. Neuron 2010, 65, 503–515. [Google Scholar] [CrossRef]
- Martins-Green, M.; Bixby, J.L.; Yamamoto, T.; Graf, T.; Sudol, M. Tissue Specific Expression of Yrk Kinase: Implications for Differentiation and Inflammation. Int. J. Biochem. Cell Biol. 2000, 32, 351–364. [Google Scholar] [CrossRef]
- Sorge, L.K.; Levy, B.T.; Maness, P.F. Pp60c-Src Is Developmentally Regulated in the Neural Retina. Cell 1984, 36, 249–257. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Baker, H.; Walaas, S.I.; Sudol, M. Localization of P62c-Yes Protein in Mammalian Neural Tissues. Oncogene 1991, 6, 1725–1733. [Google Scholar]
- Ho, N.; Gendron, R.L.; Grozinger, K.; Whelan, M.A.; Hicks, E.A.; Tennakoon, B.; Gardiner, D.; Good, W.V.; Paradis, H. Tubedown Regulation of Retinal Endothelial Permeability Signaling Pathways. Biol. Open 2015, 4, 970–979. [Google Scholar] [CrossRef]
- Joseph, M.S.; Bilousova, T.; Zdunowski, S.; Wu, Z.-P.; Middleton, B.; Boudzinskaia, M.; Wong, B.; Ali, N.; Zhong, H.; Yong, J.; et al. Transgenic Mice with Enhanced Neuronal Major Histocompatibility Complex Class I Expression Recover Locomotor Function Better after Spinal Cord Injury. J. Neurosci. Res. 2011, 89, 365–372. [Google Scholar] [CrossRef][Green Version]
- Adelson, J.D.; Barreto, G.E.; Xu, L.; Kim, T.; Brott, B.K.; Ouyang, Y.-B.; Naserke, T.; Djurisic, M.; Xiong, X.; Shatz, C.J.; et al. Neuroprotection from Stroke in the Absence of MHCI or PirB. Neuron 2012, 73, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.; Massieu, L. Role of Glutamate Transporters in the Clearance and Release of Glutamate during Ischemia and Its Relation to Neuronal Death. Arch. Med. Res. 2006, 37, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Hulsebosch, C.E.; Hains, B.C.; Crown, E.D.; Carlton, S.M. Mechanisms of Chronic Central Neuropathic Pain after Spinal Cord Injury. Brain Res. Rev. 2009, 60, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-G.; Chiu, K.; Lau, F.H.S.; Lee, V.W.H.; Yung, K.K.L.; So, K.-F. The Selective Vulnerability of Retinal Ganglion Cells in Rat Chronic Ocular Hypertension Model at Early Phase. Cell. Mol. Neurobiol. 2009, 29, 1143–1151. [Google Scholar] [CrossRef]
- Tezel, G. A Proteomics View of the Molecular Mechanisms and Biomarkers of Glaucomatous Neurodegeneration. Prog. Retin. Eye Res. 2013, 35, 18–43. [Google Scholar] [CrossRef]
- Yang, N.; Young, B.K.; Wang, P.; Tian, N. The Susceptibility of Retinal Ganglion Cells to Optic Nerve Injury Is Type Specific. Cells 2020, 9, 677. [Google Scholar] [CrossRef]
- Hanke, J.H.; Gardner, J.P.; Dow, R.L.; Changelian, P.S.; Brissette, W.H.; Weringer, E.J.; Pollok, B.A.; Connelly, P.A. Discovery of a Novel, Potent, and Src Family-Selective Tyrosine Kinase Inhibitor. Study of Lck- and FynT-Dependent T Cell Activation. J. Biol. Chem. 1996, 271, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.B.; Schreiner, S.J.; Choi, H.-J.; Kamens, J.; Smithgall, T.E. Selective Pyrrolo-Pyrimidine Inhibitors Reveal a Necessary Role for Src Family Kinases in Bcr-Abl Signal Transduction and Oncogenesis. Oncogene 2002, 21, 8075–8088. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pene-Dumitrescu, T.; Peterson, L.F.; Donato, N.J.; Smithgall, T.E. An Inhibitor-Resistant Mutant of Hck Protects CML Cells against the Antiproliferative and Apoptotic Effects of the Broad-Spectrum Src Family Kinase Inhibitor A-419259. Oncogene 2008, 27, 7055–7069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patel, R.K.; Weir, M.C.; Shen, K.; Snyder, D.; Cooper, V.S.; Smithgall, T.E. Expression of Myeloid Src-Family Kinases Is Associated with Poor Prognosis in AML and Influences Flt3-ITD Kinase Inhibitor Acquired Resistance. PLoS ONE 2019, 14, e0225887. [Google Scholar] [CrossRef] [PubMed]
- Karni, R.; Mizrachi, S.; Reiss-Sklan, E.; Gazit, A.; Livnah, O.; Levitzki, A. The Pp60c-Src Inhibitor PP1 Is Non-Competitive against ATP. FEBS Lett. 2003, 537, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Blake, R.A.; Broome, M.A.; Liu, X.; Wu, J.; Gishizky, M.; Sun, L.; Courtneidge, S.A. SU6656, a Selective Src Family Kinase Inhibitor, Used to Probe Growth Factor Signaling. Mol. Cell. Biol. 2000, 20, 9018–9027. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, L.F.; Allen, J.; Breed, J.; Curwen, J.; Fennell, M.; Green, T.P.; Lambert-van der Brempt, C.; Morgentin, R.; Norman, R.A.; Olivier, A.; et al. N-(5-Chloro-1,3-Benzodioxol-4-Yl)-7-[2-(4-Methylpiperazin-1-Yl)Ethoxy]-5-(Tetrahydro-2H-Pyran-4-Yloxy)Quinazolin-4-Amine, a Novel, Highly Selective, Orally Available, Dual-Specific c-Src/Abl Kinase Inhibitor. J. Med. Chem. 2006, 49, 6465–6488. [Google Scholar] [CrossRef] [PubMed]
- Heusschen, R.; Muller, J.; Binsfeld, M.; Marty, C.; Plougonven, E.; Dubois, S.; Mahli, N.; Moermans, K.; Carmeliet, G.; Léonard, A.; et al. SRC Kinase Inhibition with Saracatinib Limits the Development of Osteolytic Bone Disease in Multiple Myeloma. Oncotarget 2016, 7, 30712–30729. [Google Scholar] [CrossRef]
- Vomhof-DeKrey, E.E.; Dorsam, G.P. Stimulatory and Suppressive Signal Transduction Regulates Vasoactive Intestinal Peptide Receptor-1 (VPAC-1) in Primary Mouse CD4 T Cells. Brain Behav. Immun. 2008, 22, 1024–1031. [Google Scholar] [CrossRef]
- Oliver, J.M.; Burg, D.L.; Wilson, B.S.; McLaughlin, J.L.; Geahlen, R.L. Inhibition of Mast Cell Fc Epsilon R1-Mediated Signaling and Effector Function by the Syk-Selective Inhibitor, Piceatannol. J. Biol. Chem. 1994, 269, 29697–29703. [Google Scholar] [CrossRef]
- Kwong, J.M.K.; Caprioli, J.; Piri, N. RNA Binding Protein with Multiple Splicing: A New Marker for Retinal Ganglion Cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.R.; de Sevilla Muller, L.P.; Brecha, N.C. The RNA Binding Protein RBPMS Is a Selective Marker of Ganglion Cells in the Mammalian Retina. J. Comp. Neurol. 2014, 522, 1411–1443. [Google Scholar] [CrossRef] [PubMed]
- Wary, K.K.; Mariotti, A.; Zurzolo, C.; Giancotti, F.G. A Requirement for Caveolin-1 and Associated Kinase Fyn in Integrin Signaling and Anchorage-Dependent Cell Growth. Cell 1998, 94, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Yoo, K.-C.; An, Y.; Lee, H.-J.; Lee, M.; Uddin, N.; Kim, M.-J.; Kim, I.-G.; Suh, Y.; Lee, S.-J. FYN Promotes Mesenchymal Phenotypes of Basal Type Breast Cancer Cells through STAT5/NOTCH2 Signaling Node. Oncogene 2018, 37, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Lenassi, M.; Cagney, G.; Liao, M.; Vaupotic, T.; Bartholomeeusen, K.; Cheng, Y.; Krogan, N.J.; Plemenitas, A.; Peterlin, B.M. HIV Nef Is Secreted in Exosomes and Triggers Apoptosis in Bystander CD4+ T Cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef]
- Roversi, F.M.; Bueno, M.L.P.; Pericole, F.V.; Saad, S.T.O. Hematopoietic Cell Kinase (HCK) Is a Player of the Crosstalk Between Hematopoietic Cells and Bone Marrow Niche Through CXCL12/CXCR4 Axis. Front. Cell Dev. Biol. 2021, 9, 634044. [Google Scholar] [CrossRef] [PubMed]
- Poh, A.R.; Love, C.G.; Masson, F.; Preaudet, A.; Tsui, C.; Whitehead, L.; Monard, S.; Khakham, Y.; Burstroem, L.; Lessene, G.; et al. Inhibition of Hematopoietic Cell Kinase Activity Suppresses Myeloid Cell-Mediated Colon Cancer Progression. Cancer Cell 2017, 31, 563–575.e5. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.J.; McCarley, D.J.; Ely, C.M.; Benjamin, D.C.; Parsons, J.T. Monoclonal antibodies to Rous sarcoma virus pp60src react with enzymatically active cellular pp60src of avian and mammalian origin. J. Virol. 1984, 51, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.R.; Guilluy, C.; Xie, Z.; Sen, B.; Brobst, K.E.; Yen, S.S.; Uzer, G.; Styner, M.; Case, N.; Burridge, K.; et al. Mechanically activated Fyn utilizes mTORC2 to regulate RhoA and adipogenesis in mesenchymal stem cells. Stem Cells 2013, 31, 2528–2537. [Google Scholar] [CrossRef]
- Yousefi, O.S.; Wilhelm, T.; Maschke-Neuß, K.; Kuhny, M.; Martin, C.; Molderings, G.J.; Kratz, F.; Hildenbrand, B.; Huber, M. The 1,4-Benzodiazepine Ro5-4864 (4-Chlorodiazepam) Suppresses Multiple pro-Inflammatory Mast Cell Effector Functions. Cell Commun. Signal 2013, 11, 13. [Google Scholar] [CrossRef]
- Long, A.J.; Sampson, E.; McCarthy, R.W.; Harris, C.M.; Barnard, M.; Shi, D.; Conlon, D.; Caldwell, R.; Honor, D.; Wishart, N.; et al. Syk Inhibition Induces Platelet Dependent Peri-Islet Hemorrhage in the Rat Pancreas. Toxicol. Pathol. 2016, 44, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- Caparrós, E.; Munoz, P.; Sierra-Filardi, E.; Serrano-Gómez, D.; Puig-Kröger, A.; Rodríguez-Fernández, J.L.; Mellado, M.; Sancho, J.; Zubiaur, M.; Corbí, A.L. DC-SIGN Ligation on Dendritic Cells Results in ERK and PI3K Activation and Modulates Cytokine Production. Blood 2006, 107, 3950–3958. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Mortensen, X.; Wang, P.; Tian, N. The Effects of Immune Protein CD3ζ Development and Degeneration of Retinal Neurons after Optic Nerve Injury. PLoS ONE 2017, 12, e0175522. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. Inflammation Suspected in Eye Disorders. JAMA J. Am. Med. Assoc. 2005, 294, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Baniyash, M. TCR ζ-Chain Downregulation: Curtailing an Excessive Inflammatory Immune Response. Nat. Rev. Immunol. 2004, 4, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Ye, W.-R.; Chen, S.; Chirchiglia, D.; Wang, M. Src Family Kinases in the Central Nervous System: Their Emerging Role in Pathophysiology of Migraine and Neuropathic Pain. Curr. Neuropharmacol. 2021, 19, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.J.; Parsons, J.T. Src Family Kinases, Key Regulators of Signal Transduction. Oncogene 2004, 23, 7906–7909. [Google Scholar] [CrossRef]
- Ortiz, M.A.; Mikhailova, T.; Li, X.; Porter, B.A.; Bah, A.; Kotula, L. Src Family Kinases, Adaptor Proteins and the Actin Cytoskeleton in Epithelial-to-Mesenchymal Transition. Cell Commun. Signal 2021, 19, 67. [Google Scholar] [CrossRef]
- Serfas, M.S.; Tyner, A.L. Brk, Srm, Frk, and Src42A Form a Distinct Family of Intracellular Src-like Tyrosine Kinases. Oncol. Res. 2003, 13, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Omri, B.; Blancher, C.; Neron, B.; Marty, M.C.; Rutin, J.; Molina, T.J.; Pessac, B.; Crisanti, P. Retinal Dysplasia in Mice Lacking P56lck. Oncogene 1998, 16, 2351–2356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ingraham, C.A.; Cooke, M.P.; Chuang, Y.N.; Perlmutter, R.M.; Maness, P.F. Cell Type and Developmental Regulation of the Fyn Proto-Oncogene in Neural Retina. Oncogene 1992, 7, 95–100. [Google Scholar] [PubMed]
- Shelby, S.J.; Colwill, K.; Dhe-Paganon, S.; Pawson, T.; Thompson, D.A. MERTK Interactions with SH2-Domain Proteins in the Retinal Pigment Epithelium. PLoS ONE 2013, 8, e53964. [Google Scholar] [CrossRef]
- Groves, T.R.; Allen, A.R. Src Family Kinase Inhibitors and Their Role in the Treatment of Traumatic Brain Injuries. J. Trauma Treat. 2016, 5. [Google Scholar] [CrossRef]
- WoldeMussie, E.; Yoles, E.; Schwartz, M.; Ruiz, G.; Wheeler, L.A. Neuroprotective Effect of Memantine in Different Retinal Injury Models in Rats. J. Glaucoma 2002, 11, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Egea, M.A.; Davis, B.M.; Guo, L.; Espina, M.; Silva, A.M.; Calpena, A.C.; Souto, E.M.B.; Ravindran, N.; Ettcheto, M.; et al. Memantine-Loaded PEGylated Biodegradable Nanoparticles for the Treatment of Glaucoma. Small 2018, 14, 1701808. [Google Scholar] [CrossRef]
- Ju, W.-K.; Lindsey, J.D.; Angert, M.; Patel, A.; Weinreb, R.N. Glutamate Receptor Activation Triggers OPA1 Release and Induces Apoptotic Cell Death in Ischemic Rat Retina. Mol. Vis. 2008, 14, 2629–2638. [Google Scholar]
- Ju, W.-K.; Kim, K.-Y.; Angert, M.; Duong-Polk, K.X.; Lindsey, J.D.; Ellisman, M.H.; Weinreb, R.N. Memantine Blocks Mitochondrial OPA1 and Cytochrome c Release and Subsequent Apoptotic Cell Death in Glaucomatous Retina. Investig. Ophthalmol. Vis. Sci. 2009, 50, 707–716. [Google Scholar] [CrossRef]
- Smith, S.B. Diabetic Retinopathy and the NMDA Receptor. Drug News Perspect. 2002, 15, 226. [Google Scholar] [CrossRef]
- Barber, A.J. A New View of Diabetic Retinopathy: A Neurodegenerative Disease of the Eye. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Araszkiewicz, A.; Zozulinska-Ziolkiewicz, D. Retinal Neurodegeneration in the Course of Diabetes-Pathogenesis and Clinical Perspective. CN 2016, 14, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Chalam, K.V.; Chawla, D.K.; D’Angio, C.T.; Guillet, E.G.; Rose, S.J.; Vanderlinde, R.E.; Ambati, B.K. Elevated Gamma-Aminobutyric Acid, Glutamate, and Vascular Endothelial Growth Factor Levels in the Vitreous of Patients with Proliferative Diabetic Retinopathy. Arch. Ophthalmol. 1997, 115, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Engerman, R.L.; Case, G.L.; Kern, T.S. Retinal Glutamate in Diabetes and Effect of Antioxidants. Neurochem. Int. 2001, 38, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.-K.; Zeng, X.-X.; Ling, E.-A. Expression of Glutamate Receptors and Calcium-Binding Proteins in the Retina of Streptozotocin-Induced Diabetic Rats. Brain Res. 2004, 1018, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Santiago, A.R.; Hughes, J.M.; Kamphuis, W.; Schlingemann, R.O.; Ambrósio, A.F. Diabetes Changes Ionotropic Glutamate Receptor Subunit Expression Level in the Human Retina. Brain Res. 2008, 1198, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kusari, J.; Zhou, S.; Padillo, E.; Clarke, K.G.; Gil, D.W. Effect of Memantine on Neuroretinal Function and Retinal Vascular Changes of Streptozotocin-Induced Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5152–5159. [Google Scholar] [CrossRef] [PubMed]
- Manev, H.; Favaron, M.; Guidotti, A.; Costa, E. Delayed Increase of Ca2+ Influx Elicited by Glutamate: Role in Neuronal Death. Mol. Pharmacol. 1989, 36, 106–112. [Google Scholar]
- Dutta, R.; Trapp, B.D. Mechanisms of Neuronal Dysfunction and Degeneration in Multiple Sclerosis. Prog. Neurobiol. 2011, 93, 1–12. [Google Scholar] [CrossRef]
- Stavrovskaya, I.G.; Kristal, B.S. The Powerhouse Takes Control of the Cell: Is the Mitochondrial Permeability Transition a Viable Therapeutic Target against Neuronal Dysfunction and Death? Free Radic. Biol. Med. 2005, 38, 687–697. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs Oppose Synaptic NMDARs by Triggering CREB Shut-off and Cell Death Pathways. Nat. Neurosci. 2002, 5, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Diamond, J.S. Subunit- and Pathway-Specific Localization of NMDA Receptors and Scaffolding Proteins at Ganglion Cell Synapses in Rat Retina. J. Neurosci. 2009, 29, 4274–4286. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.L.; Hack, I.; Tter, J.H.B.; Ssle, H.W. Synaptic Localization of NMDA Receptor Subunits in the Rat Retina. J. Comp. Neurol. 2000, 420, 98–112. [Google Scholar] [CrossRef]
- Kwak, S.; Weiss, J.H. Calcium-Permeable AMPA Channels in Neurodegenerative Disease and Ischemia. Curr. Opin. Neurobiol. 2006, 16, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.H.; Lucas, S.; Strømgaard, K.; Kristensen, A.S. Evaluation of PhTX-74 as Subtype-Selective Inhibitor of GluA2-Containing AMPA Receptors. Mol. Pharmacol. 2014, 85, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Carriedo, S.G.; Yin, H.Z.; Sensi, S.L.; Weiss, J.H. Rapid Ca2+ Entry through Ca2+-Permeable AMPA/Kainate Channels Triggers Marked Intracellular Ca2+ Rises and Consequent Oxygen Radical Production. J. Neurosci. 1998, 18, 7727–7738. [Google Scholar] [CrossRef]
- Isaac, J.T.R.; Ashby, M.C.; McBain, C.J. The Role of the GluR2 Subunit in AMPA Receptor Function and Synaptic Plasticity. Neuron 2007, 54, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Sladek, A.L.; Nawy, S. Ocular Hypertension Drives Remodeling of AMPA Receptors in Select Populations of Retinal Ganglion Cells. Front. Synaptic. Neurosci. 2020, 12, 30. [Google Scholar] [CrossRef]
- Wen, X.; Cahill, A.L.; Barta, C.; Thoreson, W.B.; Nawy, S. Elevated Pressure Increases Ca2+ Influx Through AMPA Receptors in Select Populations of Retinal Ganglion Cells. Front. Cell. Neurosci. 2018, 12, 162. [Google Scholar] [CrossRef]
- Diamond, J.S. Calcium-Permeable AMPA Receptors in the Retina. Front. Mol. Neurosci. 2011, 4, 27. [Google Scholar] [CrossRef]
- Zhang, D.; Sucher, N.J.; Lipton, S.A. Co-Expression of AMPA/Kainate Receptor-Operated Channels with High and Low Ca2+ Permeability in Single Rat Retinal Ganglion Cells. Neuroscience 1995, 67, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Cueva Vargas, J.L.; Osswald, I.K.; Unsain, N.; Aurousseau, M.R.; Barker, P.A.; Bowie, D.; Di Polo, A. Soluble Tumor Necrosis Factor Alpha Promotes Retinal Ganglion Cell Death in Glaucoma via Calcium-Permeable AMPA Receptor Activation. J. Neurosci. 2015, 35, 12088–12102. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.-D.; Gao, F.; Wang, X.-H.; Miao, Y.; Wang, S.-Y.; Wu, Y.; Li, F.; Wu, J.; Cheng, X.-L.; Sun, X.-H.; et al. GluA2 Trafficking Is Involved in Apoptosis of Retinal Ganglion Cells Induced by Activation of EphB/EphrinB Reverse Signaling in a Rat Chronic Ocular Hypertension Model. J. Neurosci. 2015, 35, 5409–5421. [Google Scholar] [CrossRef] [PubMed]
- Lebrun-Julien, F.; Duplan, L.; Pernet, V.; Osswald, I.; Sapieha, P.; Bourgeois, P.; Dickson, K.; Bowie, D.; Barker, P.A.; Di Polo, A. Excitotoxic Death of Retinal Neurons in Vivo Occurs via a Non-Cell-Autonomous Mechanism. J. Neurosci. 2009, 29, 5536–5545. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, Y.-Y. Calcium Permeable-AMPA Receptors and Excitotoxicity in Neurological Disorders. Front. Neural. Circuits 2021, 15, 711564. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.A.; Grant, S.G. Supramolecular Organization of NMDA Receptors and the Postsynaptic Density. Curr. Opin. Neurobiol. 2017, 45, 139–147. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.F.; Mody, I.; Salter, M.W.; Pennefather, P.; Schneiderman, J.H. The Regulation of NMDA Receptors in the Central Nervous System. Prog. Neuropsychopharmacol. Biol. Psychiatry 1989, 13, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Brodeur, S.R.; Gish, G.; Songyang, Z.; Cantley, L.C.; Laudano, A.P.; Pawson, T. Regulation of C-Src Tyrosine Kinase Activity by the Src SH2 Domain. Oncogene 1993, 8, 1119–1126. [Google Scholar] [PubMed]
- Yu, X.M.; Askalan, R.; Keil, G.J.; Salter, M.W. NMDA Channel Regulation by Channel-Associated Protein Tyrosine Kinase Src. Science 1997, 275, 674–678. [Google Scholar] [CrossRef]
- Brugge, J.S.; Cotton, P.C.; Queral, A.E.; Barrett, J.N.; Nonner, D.; Keane, R.W. Neurones Express High Levels of a Structurally Modified, Activated Form of Pp60c-Src. Nature 1985, 316, 554–557. [Google Scholar] [CrossRef]
- Wang, Y.T.; Salter, M.W. Regulation of NMDA Receptors by Tyrosine Kinases and Phosphatases. Nature 1994, 369, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, T.; Umemori, H.; Akiyama, T.; Nakanishi, S.; Yamamoto, T. PSD-95 Promotes Fyn-Mediated Tyrosine Phosphorylation of the N-Methyl-d-Aspartate Receptor Subunit NR2A. Proc. Natl. Acad. Sci. USA 1999, 96, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Matrone, C.; Petrillo, F.; Nasso, R.; Ferretti, G. Fyn Tyrosine Kinase as Harmonizing Factor in Neuronal Functions and Dysfunctions. Int. J. Mol. Sci. 2020, 21, 4444. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Okumura-Noji, K. NMDA Receptor Subunits Epsilon 1 (NR2A) and Epsilon 2 (NR2B) Are Substrates for Fyn in the Postsynaptic Density Fraction Isolated from the Rat Brain. Biochem. Biophys. Res. Commun. 1995, 216, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Köhr, G.; Seeburg, P.H. Subtype-Specific Regulation of Recombinant NMDA Receptor-Channels by Protein Tyrosine Kinases of the Src Family. J. Physiol. 1996, 492 Pt 2, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Kalia, L.V. Src Kinases: A Hub for NMDA Receptor Regulation. Nat. Rev. Neurosci. 2004, 5, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.M.; Roder, J.C.; Davidow, J.; Salter, M.W. Src Activation in the Induction of Long-Term Potentiation in CA1 Hippocampal Neurons. Science 1998, 279, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, G.M.; Kalia, L.V.; Ng, D.; Goodfellow, N.M.; Yee, K.T.; Lambe, E.K.; Salter, M.W. Schizophrenia Susceptibility Pathway Neuregulin 1-ErbB4 Suppresses Src Upregulation of NMDA Receptors. Nat. Med. 2011, 17, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Pitcher, G.M. Dysregulated Src Upregulation of NMDA Receptor Activity: A Common Link in Chronic Pain and Schizophrenia. FEBS J. 2012, 279, 2–11. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, W.; Ali, D.W.; Pelkey, K.A.; Pitcher, G.M.; Lu, Y.M.; Aoto, H.; Roder, J.C.; Sasaki, T.; Salter, M.W.; et al. CAKbeta/Pyk2 Kinase Is a Signaling Link for Induction of Long-Term Potentiation in CA1 Hippocampus. Neuron 2001, 29, 485–496. [Google Scholar] [CrossRef]
- Lei, G.; Xue, S.; Chéry, N.; Liu, Q.; Xu, J.; Kwan, C.L.; Fu, Y.-P.; Lu, Y.-M.; Liu, M.; Harder, K.W.; et al. Gain Control of N-Methyl-d-Aspartate Receptor Activity by Receptor-like Protein Tyrosine Phosphatase Alpha. EMBO J. 2002, 21, 2977–2989. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Gingrich, J.R.; Vargas-Caballero, M.; Dong, Y.N.; Sengar, A.; Beggs, S.; Wang, S.-H.; Ding, H.K.; Frankland, P.W.; Salter, M.W. Treatment of Inflammatory and Neuropathic Pain by Uncoupling Src from the NMDA Receptor Complex. Nat. Med. 2008, 14, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Matsumura, S.; Katano, T.; Mabuchi, T.; Takagi, K.; Xu, L.; Yamamoto, A.; Hattori, K.; Yagi, T.; Watanabe, M.; et al. Fyn Kinase-Mediated Phosphorylation of NMDA Receptor NR2B Subunit at Tyr1472 Is Essential for Maintenance of Neuropathic Pain. Eur. J. Neurosci. 2005, 22, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Kunori, S.; Mabuchi, T.; Katano, T.; Nakazawa, T.; Abe, T.; Watanabe, M.; Yamamoto, T.; Okuda-Ashitaka, E.; Ito, S. Impairment of CaMKII Activation and Attenuation of Neuropathic Pain in Mice Lacking NR2B Phosphorylated at Tyr1472. Eur. J. Neurosci. 2010, 32, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Sabha, M.; Emirandetti, A.; Cullheim, S.; De Oliveira, A.L.R. MHC I Expression and Synaptic Plasticity in Different Mice Strains after Axotomy. Synapse 2008, 62, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Atwal, J.K.; Pinkston-Gosse, J.; Syken, J.; Stawicki, S.; Wu, Y.; Shatz, C.; Tessier-Lavigne, M. PirB Is a Functional Receptor for Myelin Inhibitors of Axonal Regeneration. Science 2008, 322, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Washburn, L.R.; Zekzer, D.; Eitan, S.; Lu, Y.; Dang, H.; Middleton, B.; Evans, C.J.; Tian, J.; Kaufman, D.L. A Potential Role for Shed Soluble Major Histocompatibility Class I Molecules as Modulators of Neurite Outgrowth. PLoS ONE 2011, 6, e18439. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-P.; Bilousova, T.; Escande-Beillard, N.; Dang, H.; Hsieh, T.; Tian, J.; Kaufman, D.L. Major Histocompatibility Complex Class I-Mediated Inhibition of Neurite Outgrowth from Peripheral Nerves. Immunol. Lett. 2011, 135, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Endo, S.; Takai, T.; Yamashita, T. Myelin Suppresses Axon Regeneration by PIR-B/SHP-Mediated Inhibition of Trk Activity. EMBO J. 2011, 30, 1389–1401. [Google Scholar] [CrossRef]
- Nakamura, Y.; Fujita, Y.; Ueno, M.; Takai, T.; Yamashita, T. Paired Immunoglobulin-like Receptor B Knockout Does Not Enhance Axonal Regeneration or Locomotor Recovery after Spinal Cord Injury. J. Biol. Chem. 2011, 286, 1876–1883. [Google Scholar] [CrossRef]
- Omoto, S.; Ueno, M.; Mochio, S.; Takai, T.; Yamashita, T. Genetic Deletion of Paired Immunoglobulin-like Receptor B Does Not Promote Axonal Plasticity or Functional Recovery after Traumatic Brain Injury. J. Neurosci. 2010, 30, 13045–13052. [Google Scholar] [CrossRef]
- Boulanger, L.M. Immune Proteins in Brain Development and Synaptic Plasticity. Neuron 2009, 64, 93–109. [Google Scholar] [CrossRef]
- Vorwerk, C.K.; Kreutz, M.R.; Bockers, T.M.; Brosz, M.; Dreyer, E.B.; Sabel, B.A. Susceptibility of Retinal Ganglion Cells to Excitotoxicity Depends on Soma Size and Retinal Eccentricity. Curr. Eye Res. 1999, 19, 59–65. [Google Scholar] [CrossRef]
- DeParis, S.; Caprara, C.; Grimm, C. Intrinsically Photosensitive Retinal Ganglion Cells Are Resistant to N-Methyl-d-Aspartic Acid Excitotoxicity. Mol. Vis. 2012, 18, 2814–2827. [Google Scholar]
- Wang, S.; Gu, D.; Zhang, P.; Chen, J.; Li, Y.; Xiao, H.; Zhou, G. Melanopsin-Expressing Retinal Ganglion Cells Are Relatively Resistant to Excitotoxicity Induced by N-Methyl-d-Aspartate. Neurosci. Lett. 2018, 662, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Dunkelberger, G.R.; Green, W.R. Chronic Human Glaucoma Causing Selectively Greater Loss of Large Optic Nerve Fibers. Ophthalmology 1988, 95, 357–363. [Google Scholar] [CrossRef]
- Glovinsky, Y.; Quigley, H.A.; Dunkelberger, G.R. Retinal Ganglion Cell Loss Is Size Dependent in Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 1991, 32, 484–491. [Google Scholar]
- Della Santina, L.; Ou, Y. Who’s Lost First? Susceptibility of Retinal Ganglion Cell Types in Experimental Glaucoma. Exp. Eye Res. 2017, 158, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, J.; Seilheimer, R.L.; Tao, X.; Cowan, C.S.; Frankfort, B.J.; Wu, S.M. Elevated IOP Alters the Space-Time Profiles in the Center and Surround of Both ON and OFF RGCs in Mouse. Proc. Natl. Acad. Sci. USA 2017, 114, 8859–8864. [Google Scholar] [CrossRef]
- Della Santina, L.; Inman, D.M.; Lupien, C.B.; Horner, P.J.; Wong, R.O.L. Differential Progression of Structural and Functional Alterations in Distinct Retinal Ganglion Cell Types in a Mouse Model of Glaucoma. J. Neurosci. 2013, 33, 17444–17457. [Google Scholar] [CrossRef]
- El-Danaf, R.N.; Huberman, A.D. Characteristic Patterns of Dendritic Remodeling in Early-Stage Glaucoma: Evidence from Genetically Identified Retinal Ganglion Cell Types. J. Neurosci. 2015, 35, 2329–2343. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Jo, R.E.; Ullian, E.M.; Wong, R.O.L.; Della Santina, L. Selective Vulnerability of Specific Retinal Ganglion Cell Types and Synapses after Transient Ocular Hypertension. J. Neurosci. 2016, 36, 9240–9252. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhao, Y.; Yoshida, M.; Chen, H.; Yang, J.F.; Kim, T.S.; Cang, J.; Troy, J.B.; Liu, X. Sustained Ocular Hypertension Induces Dendritic Degeneration of Mouse Retinal Ganglion Cells That Depends on Cell Type and Location. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1106. [Google Scholar] [CrossRef] [PubMed]
- Puyang, Z.; Gong, H.-Q.; He, S.-G.; Troy, J.B.; Liu, X.; Liang, P.-J. Different Functional Susceptibilities of Mouse Retinal Ganglion Cell Subtypes to Optic Nerve Crush Injury. Exp. Eye Res. 2017, 162, 97–103. [Google Scholar] [CrossRef]
- Duan, X.; Qiao, M.; Bei, F.; Kim, I.-J.; He, Z.; Sanes, J.R. Subtype-Specific Regeneration of Retinal Ganglion Cells Following Axotomy: Effects of Osteopontin and mTOR Signaling. Neuron 2015, 85, 1244–1256. [Google Scholar] [CrossRef]


| Inhibitors | Targeted SFKs | Vendor | Cat # | IC50 | Dosage | References |
|---|---|---|---|---|---|---|
| PP2 | Src, Fyn, Lck, Hck | Santa Cruz Biotechnology, Dallas, TX, USA | sc-202769 | 4–100 nM | 400 nM | [31,35] |
| A419259 | Src, Lck, Lyn, Hck, Fgr, | Sigma, Burlington, MA, USA | SML0446 | 3–61.8 nM | 3.3 mM | [32,33,34] |
| SU6656 | Src, Fyn, Yes, Lyn, Lck | Santa Cruz Biotechnology, Dallas, TX, USA | sc-203286 | 20 nM–6.88 μM | 688 μM | [36] |
| Saracatinib | Src, Fyn, Yes, Fgr, Lck, Lyn, Blk | Santa Cruz Biotechnology, Dallas, TX, USA | sc-364607 | 2.7–11 nM | 1 μM | [37,38] |
| Piceatannol | Zap70/Syk | Santa Cruz Biotechnology, Dallas, TX, USA | sc-200610A | 10 μM | 1 mM | [39,40] |
| Antibody | Antigen | Host | Vendor | Cat# | Validation | Conc. | References |
|---|---|---|---|---|---|---|---|
| Primary Abs | |||||||
| Anti-RBPMS | RNA binding protein with multiple splicing | Guinea pig | PhosphoSolutions, Aurora, CO, USA | 1832-RBPMS | WB, IHC | 1:500 | [41,42] |
| Fyn | Fyn Proto-Oncogene | Goat | Santa Cruz Biotechnology, Dallas, TX, USA | sc-16 | WB, IHC | 1:100 | [43,44] |
| Lck | Lck proto-oncogene | Rabbit | Santa Cruz Biotechnology, Dallas, TX, USA | sc-28882 | WB | 1:100 | [45] |
| Hck | Hematopoietic Cell Kinase Hck (phospho Y410) | Rabbit | Abcam, Cambridge, UK | ab61055 | WB, IHC | 1:200 | [46,47] |
| Src | Src Proto-oncogene | Mouse | Abcam, Cambridge, UK | ab231081 | IHC, WB | 1:100 | [48] |
| Yes | YES Proto-Oncogene | Rabbit | Cell signaling, Boston, MA, USA | 3201S | WB, IP | 1:100 | [49] |
| Syk | Spleen tyrosine kinase | Rabbit | Santa Cruz Biotechnology, Dallas, TX, USA | sc-1077 | WB, IHC | 1:100 | [50,51] |
| Zap70 | ζ-chain of T cell receptor-associated protein kinase 70 | Rabbit | Santa Cruz Biotechnology, Dallas, TX, USA | sc-574 | WB | 1:500 | [52] |
| Secondary Abs | |||||||
| Cyanine CyTM 3-conjugated AffiniPure Donkey Anti-Guinea Pig IgG (H and L) | Guinea pig | Donkey | Jackson ImmunoResearch, West Grove, PA, USA | 706-165-148 | 1:400 | ||
| Alexa Fluor® 488 conjugated AffiniPure Donkey Anti-Goat IgG (H and L) | Goat | Donkey | Jackson ImmunoResearch, West Grove, PA, USA | 705-545-147 | 1:400 | ||
| Alexa Fluor® 488 conjugated AffiniPure Donkey Anti-Rabbit IgG (H and L) | Rabbit | Donkey | Jackson ImmunoResearch, West Grove, PA, USA | 711-545-152 | 1:400 | ||
| Fluorescein (FITC) AffiniPure™ Donkey Anti-Mouse IgG (H+L) | Mouse | Donkey | Jackson ImmunoResearch, West Grove, PA, USA | 715-095-151 | 1:400 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, R.; Wang, P.; Tian, N. CD3ζ-Mediated Signaling Protects Retinal Ganglion Cells in Glutamate Excitotoxicity of the Retina. Cells 2024, 13, 1006. https://doi.org/10.3390/cells13121006
Du R, Wang P, Tian N. CD3ζ-Mediated Signaling Protects Retinal Ganglion Cells in Glutamate Excitotoxicity of the Retina. Cells. 2024; 13(12):1006. https://doi.org/10.3390/cells13121006
Chicago/Turabian StyleDu, Rui, Ping Wang, and Ning Tian. 2024. "CD3ζ-Mediated Signaling Protects Retinal Ganglion Cells in Glutamate Excitotoxicity of the Retina" Cells 13, no. 12: 1006. https://doi.org/10.3390/cells13121006
APA StyleDu, R., Wang, P., & Tian, N. (2024). CD3ζ-Mediated Signaling Protects Retinal Ganglion Cells in Glutamate Excitotoxicity of the Retina. Cells, 13(12), 1006. https://doi.org/10.3390/cells13121006






